Physio-Control LIFEPAK 1000 User Manual

LIFEPAK® 1000 DEFIBRILLATOR
OPERATING INSTRUCTIONS

LIFEPAK® 1000 DEFIBRILLATOR
OPERATING INSTRUCTIONS

Important
This instrument is to be used by authorized personnel only.
!USA
 Rx Only
Rx Only
!USA Device Tracking
The U.S. Food and Drug Administration requires defibrillator manufacturers and distributors to track the location of their defibrillators. If the device is located somewhere other than the shipping address or the device has been sold, donated, lost, stolen, exported, destroyed, permanently retired from use, or if the device was not obtained directly from Physio-Control, please do one of the following: register the device at http://www.physio-control.com, call the device registration phone line at 1.800.426.4448, or use one of the postage-paid address change cards located in the back of this manual to update this vital tracking information.
Responsibility for Information
It is the responsibility of our customers to ensure that the appropriate person(s) within their organization have access to this information, including cautions and warnings provided throughout this manual.
LIFEPAK, LIFENET, and QUIK-COMBO are registered trademarks of Physio-Control, Inc. ADAPTIV, CODE-STAT, cprMAX, REDI-PAK, and Shock Advisory System are trademarks of Physio-Control, Inc. Microsoft and Windows are registered trademarks of Microsoft Corporation. Ambu is a registered trademark of the Ambu Corporation. Specifications are subject to change without notice.
©2006–2012 Physio-Control, Inc. All rights reserved.
Publication Date: 03/2012 |
|
3205213-008 |

TABLE OF CONTENTS
Preface |
|
About Defibrillation ....................................................................................................... |
vi |
Indications for Use ........................................................................................................ |
vi |
Defibrillation ............................................................................................................ |
vi |
ECG Monitoring....................................................................................................... |
vi |
Operator Considerations ............................................................................................. |
vii |
About the LIFEPAK 1000 Defibrillator .......................................................................... |
vii |
Defibrillator Features .............................................................................................. |
vii |
Text Conventions ........................................................................................................ |
viii |
|
|
1 Safety |
|
Terms ......................................................................................................................... |
1-2 |
General Warnings and Cautions ................................................................................. |
1-2 |
Symbols ...................................................................................................................... |
1-3 |
|
|
2 Controls and Indicators |
|
Controls and Indicators ............................................................................................. |
2-2 |
Battery Indicators ....................................................................................................... |
2-5 |
Battery Charger Indicators ......................................................................................... |
2-6 |
|
|
3 How to Use the LIFEPAK 1000 Defibrillator |
|
Modes of Operation .................................................................................................... |
3-2 |
Defibrillation Warnings and Cautions.................................................................... |
3-2 |
Defibrillation in AED Mode .......................................................................................... |
3-3 |
Basic Steps for Using the LIFEPAK 1000 Defibrillator.......................................... |
3-3 |
Voice Prompts and Messages in AED Mode ........................................................ |
3-4 |
Special Situations for Electrode Placement.......................................................... |
3-5 |
LIFEPAK 1000 Defibrillator Operating Instructions |
iii |
©2006-2012 Physio-Control, Inc.

Defibrillation in Manual Mode .................................................................................... |
3-6 |
Analysis ................................................................................................................ |
3-6 |
Troubleshooting Tips for Defibrillation ....................................................................... |
3-7 |
ECG Monitoring (ECG Mode) ..................................................................................... |
3-9 |
Troubleshooting Tips for ECG Monitoring.......................................................... |
3-10 |
|
|
4 Data Management |
|
Managing Defibrillator Data ....................................................................................... |
4-2 |
Overview of Data Storage..................................................................................... |
4-2 |
Data Stored by the LIFEPAK 1000 Defibrillator.................................................... |
4-2 |
Overview of Connections for Transmitting Reports ............................................. |
4-3 |
|
|
5 Caring for the LIFEPAK 1000 Defibrillator |
|
Maintenance and Testing Schedule ........................................................................... |
5-2 |
Self-Test Performance ............................................................................................... |
5-2 |
Self-Tests ............................................................................................................. |
5-2 |
Auto Tests ............................................................................................................ |
5-3 |
Inspection .................................................................................................................. |
5-3 |
Cleaning ..................................................................................................................... |
5-4 |
Battery Maintenance .................................................................................................. |
5-4 |
LIFEPAK 1000 Defibrillator Nonrechargeable Battery.......................................... |
5-5 |
LIFEPAK 1000 Defibrillator Lithium-ion Rechargeable Battery ............................ |
5-5 |
Electrode Care and Storage ....................................................................................... |
5-7 |
Service ...................................................................................................................... |
5-7 |
Product Recycling Information .................................................................................. |
5-8 |
Supplies, Accessories, and Training Tools ................................................................ |
5-8 |
Warranty Information ................................................................................................. |
5-9 |
A Specifications
B Shock Advisory System
C cprMAX™ Technology
D Changing Setup Options
E User’s Checklist
Index
iv |
LIFEPAK 1000 Defibrillator Operating Instructions |

PREFACE
This section provides information about defibrillation and an overview of the LIFEPAK® 1000 defibrillator.
About Defibrillation |
page vi |
Indications for Use |
vi |
Operator Considerations |
vii |
About the LIFEPAK 1000 Defibrillator |
vii |
Text Conventions |
viii |
|
|
LIFEPAK 1000 Defibrillator Operating Instructions |
v |
©2006-2012 Physio-Control, Inc.

Preface
ABOUT DEFIBRILLATION
Defibrillation is a recognized means of terminating certain potentially fatal arrhythmias. A direct current defibrillator applies a brief, high-energy pulse of electricity to the heart muscle. The Physio-Control LIFEPAK® 1000 defibrillator is an automated external defibrillator (AED) that delivers this energy through disposable defibrillation electrodes applied to the patient's chest.
Defibrillation is only one aspect of the medical care required to resuscitate a patient with a shockable ECG rhythm. Depending on the situation, other measures may include:
•Cardiopulmonary resuscitation (CPR)
•Supplemental oxygen
•Drug therapy
It is recognized that successful resuscitation is related to the length of time between the onset of a heart rhythm that does not circulate blood (ventricular fibrillation, pulseless ventricular tachycardia) and defibrillation. The American Heart Association has identified the following as critical links in the chain of survival from sudden cardiac arrest (SCA).
•Early access
•Early CPR by first responders or bystanders
•Early defibrillation
•Early advanced life support
The physiological state of the patient may affect the likelihood of successful defibrillation. Thus, failure to resuscitate a patient is not a reliable indicator of defibrillator performance. Often, patients will exhibit a muscular response (such as jumping or twitching) during energy transfer. The absence of such a response is not a reliable indicator of actual energy delivered or defibrillator performance.
INDICATIONS FOR USE
Defibrillation
Defibrillation is a recognized means of terminating certain potentially fatal arrhythmias, such as ventricular fibrillation and symptomatic ventricular tachycardia.
The defibrillator is to be used in AED mode only on patients who are in cardiopulmonary arrest. The patient must be unresponsive, not breathing normally, and showing no signs of circulation.
The defibrillator may be used with standard defibrillation pads only on adults and children who are 8 years old or more or who weigh more than 25 kg (55 lbs). The defibrillator may be used on children who are less than 8 years old or weigh less than 25 kg (55 lbs) with Infant/Child Reduced Energy Defibrillation Electrodes.
ECG Monitoring
ECG monitoring is for use on conscious and unconscious patients of all ages for the purpose of ECG rhythm recognition and heart rate monitoring.
vi |
LIFEPAK 1000 Defibrillator Operating Instructions |

Preface
OPERATOR CONSIDERATIONS
The LIFEPAK 1000 defibrillator requires operator interaction to defibrillate the patient.
The defibrillator is intended for use by personnel who are authorized by a physician or medical director and have, at a minimum, the following skills and training.
•CPR training
•Defibrillator training equivalent to that recommended by the American Heart Association
•Training in the use of the LIFEPAK 1000 defibrillator
The LIFEPAK 1000 defibrillator is intended for use in hospital and out-of-hospital environments.
Manual mode is intended for use by personnel trained in ECG recognition who want to use the defibrillator to deliver a shock independent of AED mode. The operator has control over the charging and delivery of shocks.
ECG mode provides a nondiagnostic ECG display and is intended for use by personnel trained in ECG recognition to allow for rhythm and heart rate monitoring using standard ECG electrodes. When in ECG mode, the defibrillator’s shock capability is disabled; however, the LIFEPAK 1000 defibrillator continues to analyze the patient’s ECG for a potentially shockable rhythm.
ABOUT THE LIFEPAK 1000 DEFIBRILLATOR
The LIFEPAK 1000 defibrillator is a semiautomatic model that can be operated in either of three modes: AED mode, Manual mode, and ECG mode. The defibrillator uses the patented Physio-Control Shock Advisory System™ (SAS) to analyze the patient's electrocardiographic (ECG) rhythm and prompts you when it detects a shockable rhythm and when it does not detect a shockable rhythm. Responder interaction is required to provide therapy (defibrillation) to the patient.
Defibrillator Features
The following paragraphs introduce the LIFEPAK 1000 defibrillator features.
Heart Rhythm Analysis
The patented Physio-Control Shock Advisory System evaluates the patient’s heart rhythm.
ECG Display (optional)
This feature allows display of the ECG using the 3-wire (Lead II) cable and when using the defibrillator in AED mode. This feature is also necessary to use the defibrillator in Manual mode.
Defibrillation Waveform
The defibrillation shock, using ADAPTIV™ Biphasic technology, is delivered in the form of a biphasic truncated exponential (BTE) defibrillation waveform. LIFEPAK biphasic defibrillators measure the patient’s transthoracic impedance and automatically adjust the defibrillation waveform current, duration, and voltage to meet the needs of the individual patient. Patient impedance is measured whenever defibrillation electrodes are in contact with the patient.
LIFEPAK 1000 Defibrillator Operating Instructions |
vii |
©2006-2012 Physio-Control, Inc.

Preface
cprMAX™ Technology
The cprMAX technology is designed to allow resuscitation protocols to maximize the amount of CPR administered during treatment using the LIFEPAK 1000 defibrillator.
When used with the factory default settings enabled, the defibrillator allows AED protocols to be consistent with the 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care and European Resuscitation Council Guidelines for Resuscitation.
Data Management
The LIFEPAK 1000 defibrillator digitally records patient data, including ECG rhythm and delivered shocks. Recorded data may be transferred from the defibrillator to a PC using an infrared connection. The PC must have one of our LIFENET® products installed to collect and review the recorded patient data.
Battery Options
A nonrechargeable lithium manganese dioxide (Li/MnO2) battery or a rechargeable Lithium-ion (Li-ion) battery provides power to the defibrillator. Both batteries have indicators that show the approximate state of charge. The nonrechargeable battery is best suited for low-use applications. The rechargeable battery is best suited for high-use defibrillator applications, such as fire departments and ambulance services. It requires periodic recharging by an external battery charger. To save battery life if the defibrillator is accidentally turned on or left on, the defibrillator automatically turns off if it is not connected to a patient and no buttons are pressed for 5 minutes.
Daily Self-Test
The defibrillator performs a daily self-test every 24 hours and every time you turn on the defibrillator. This feature tests the most important circuitry in the defibrillator to give the responder a high degree of confidence that it is ready for use.
Readiness Display
The LIFEPAK 1000 defibrillator includes a readiness display. The OK symbol appears in the display if the daily self-test is completed successfully. A battery symbol that approximates the remaining state of charge is also visible. If the self-test detects that service is required, the OK symbol disappears and the service symbol appears.
TEXT CONVENTIONS
Throughout this manual, special text characters are used to indicate labels, screen messages, and voice prompts.
Operating control labels: |
CAPITAL LETTERS such as ON/OFF and SHOCK. |
Screen messages, and |
CAPITAL ITALICIZED LETTERS such as PUSH ANALYZE and |
voice prompts: |
CONNECT ELECTRODES. |
viii |
LIFEPAK 1000 Defibrillator Operating Instructions |

SAFETY
This section provides important information to help you operate the LIFEPAK 1000 defibrillator. Familiarize yourself with all of these terms, warnings, and symbols.
Terms |
page 1-2 |
General Warnings and Cautions |
1-2 |
Symbols |
1-3 |
|
|
LIFEPAK 1000 Defibrillator Operating Instructions |
1-1 |
©2006-2012 Physio-Control, Inc.

Safety
TERMS
The following terms are used either in this manual or on the LIFEPAK 1000 defibrillator.
Danger: Immediate hazards that will result in serious personal injury or death.
Warning:Hazards or unsafe practices that could result in serious personal injury or death.
Caution: Hazards or unsafe practices that could result in minor personal injury, product damage, or property damage.
GENERAL WARNINGS AND CAUTIONS
The following section provides general warning and caution statements. Other specific warnings and cautions are provided as needed in other sections of this manual.
WARNINGS!
Shock hazard.
The defibrillator delivers up to 360 joules of electrical energy. Unless properly used as described in these operating instructions, this electrical energy may cause serious injury or death. Do not attempt to operate this device unless thoroughly familiar with these operating instructions, and the function of all controls, indicators, connections, and accessories.
Shock hazard.
Do not disassemble the defibrillator. It contains no responder-serviceable components and dangerous high voltages may be present. Contact authorized service personnel.
Shock or fire hazard.
Do not immerse any portion of this device in water or other fluids. Avoid spilling any fluids on device or accessories. Do not clean with ketones or other flammable agents. Do not autoclave or sterilize this device or accessories unless otherwise specified.
Possible fire or explosion.
Do not use this device in the presence of flammable gases or anesthetics. Use care when operating this device close to oxygen sources (such as bag-valve-mask devices or ventilator tubing). Turn off gas source or move source away from patient during defibrillation.
Possible electrical interference with device performance.
Equipment operating in close proximity may emit strong electromagnetic or radio frequency interference (RFI) which could affect the performance of this device. RFI may result in improper device operation, distorted ECG, or failure to detect a shockable rhythm. Avoid operating the device near cauterizers, diathermy equipment, cellular phones, or other portable and mobile RF communications equipment. Do not rapidly key EMS radios on and off. Refer to “LIFEPAK 1000 Defibrillator Electromagnetic Compliance Guidance” for recommended distances of equipment. Contact authorized service personnel if assistance is required.
Possible electrical interference.
Using cables, electrodes, or accessories not specified for use with this device may result in increased emissions or decreased resistance to electromagnetic interference which could affect the performance of this device or of equipment in close proximity. Use only parts and accessories specified in these operating instructions.
Possible electrical interference.
This defibrillator may cause electromagnetic interference (EMI) especially during charge and energy transfers. EMI may affect the performance of equipment operating in close proximity. Verify the effects of defibrillator discharge on other equipment prior to using defibrillator in an emergency situation, if possible.
1-2 |
LIFEPAK 1000 Defibrillator Operating Instructions |

Safety
WARNINGS! (CONTINUED)
Possible device shutdown.
Always have access to a spare, fully-charged, properly maintained battery. Replace the battery when the device displays a low battery warning.
Possible improper device performance.
Using other manufacturers’ cables, electrodes, or batteries may cause the device to perform improperly and invalidates the safety agency certification and may invalidate the warranty. Use only the accessories specified in these operating instructions.
Safety risk and possible equipment damage.
Monitors, defibrillators, and their accessories (including electrodes and cables) contain ferromagnetic materials. As with all ferromagnetic equipment, these products must not be used in the presence of the high magnetic field created by a Magnetic Resonance Imaging (MRI) device. The high magnetic field created by an MRI device will attract the equipment with a force sufficient to cause death or serious personal injury to persons between the equipment and the MRI device. This magnetic attraction may also damage the equipment. Skin burns will also occur due to heating of electrically conductive materials, such as patient leads and pulse oximeter sensors. Consult the MRI manufacturer for more information.
CAUTION!
Possible equipment damage.
This device may be damaged by mechanical or physical abuse such as immersion in water or dropping the device. If the device has been abused, remove it from use and contact authorized service personnel.
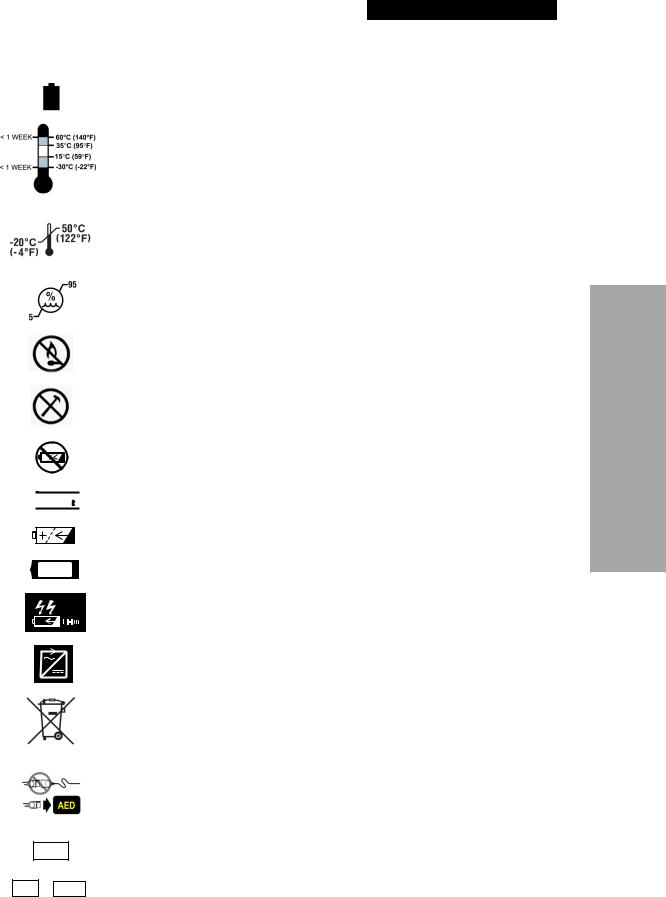
SYMBOLS
The following symbols may be found in this manual or on various configurations of the LIFEPAK 1000 defibrillator and its accessories.
Defibrillation-protected. Type BF patient connection
Attention. Consult accompanying documents
Warning. High voltage
Type BF patient connection
Menu button
Battery status symbol
LIFEPAK 1000 Defibrillator Operating Instructions |
1-3 |
©2006-2012 Physio-Control, Inc.

Safety
OK
!USA
YYYY
Service symbol
Symbol indicating self-test completed successfully
Use By or Install By date shown: yyyy-mm-dd or yyyy-mm
This end up
Fragile/breakable.
Handle with care.
Protect from water
Single use only
Mark of conformity to applicable European Directives
Canadian Standards Association certification for Canada and the United States
The features of the LIFEPAK 1000 defibrillator which could come in either direct or casual contact with the patient or caregiver during normal use are not manufactured with materials that contain latex as an intentionally added component or expected impurity.
Cable Connector
For USA audiences only
Date of manufacture
Power On/Off
Shock button
Shock symbol
1-4 |
LIFEPAK 1000 Defibrillator Operating Instructions |
 Li/MnO2
Li/MnO2 

1000
LOT
PN , MIN
Safety
Symbol indicating location of battery compartment
Recommended storage temperature: 15° to 35°C (59° to 95°F). Storage at extreme temperatures of -30° and 60°C (-22° and 140°F) is limited to seven days. If storage at these temperatures exceeds one week, the electrode shelf-life will be reduced.
Recommended shipping temperature: -20° to 50°C (-4° to 122°F).
Relative humidity range 5% to 95%
Do not place near an open flame, heat above 100°C (212°F), or incinerate
Do not crush, puncture, or disassemble battery
Do not recharge battery
Lithium manganese dioxide battery
Rechargeable battery
Battery for use with the LIFEPAK 1000 defibrillator
Battery charger for use with Lithium-ion battery
AC to DC power adapter
Do not dispose of this product in the unsorted municipal waste stream. Dispose of this product according to local regulations. See www.physio-control.com/recycling for instructions on disposing of this product.
Infant Child Reduced Energy Electrodes are not compatible with QUIK-COMBO® defibrillation and therapy cables. To use Infant/Child Electrodes, connect Infant/Child electrodes directly to the AED.
Lot number (batch code)
Part number or Manufacturer’s item number
LIFEPAK 1000 Defibrillator Operating Instructions |
1-5 |
©2006-2012 Physio-Control, Inc.

Safety
|
CAT |
|
|
Catalog number |
||
|
|
|
|
|
Reorder number |
|
|
REF |
|
||||
|
|
|
|
|
Serial number |
|
|
|
|
|
|||
|
SN |
|
||||
|
|
|
|
By prescription only |
||
|
|
|||||
Rx Only or |
Rx Only |
|||||
|
IP55 |
Enclosure ingress protection code per IEC 60529 |
||||
1-6 |
LIFEPAK 1000 Defibrillator Operating Instructions |

CONTROLS AND INDICATORS
This section provides a description of each of the LIFEPAK 1000 defibrillator primary controls and indications.
Controls and Indicators |
page 2-2 |
|
|
Battery Indicators |
2-5 |
|
|
|
|||
Battery Charger Indicators |
2-6 |
|
|
|
|
|
|
|
|
|
|
LIFEPAK 1000 Defibrillator Operating Instructions |
2-1 |
©2006-2012 Physio-Control, Inc.
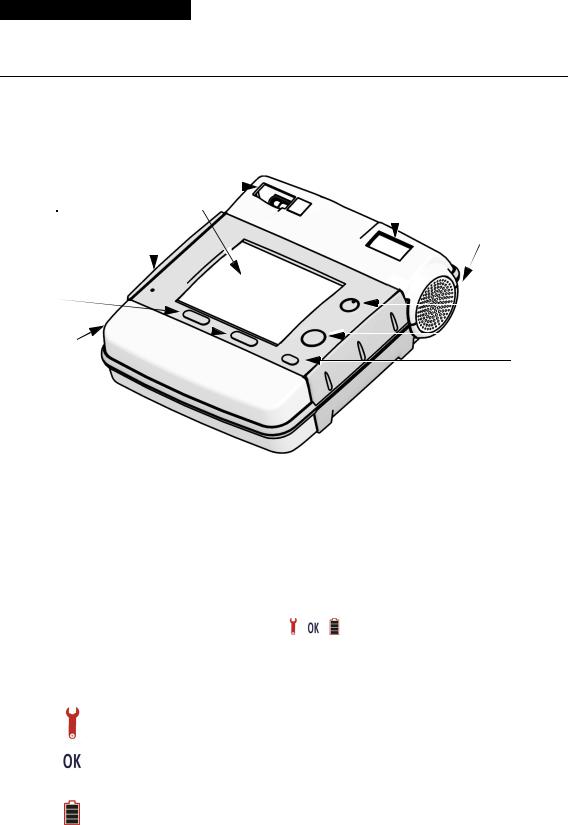
Controls and Indicators
CONTROLS AND INDICATORS
This section introduces you to the controls and indicators on the LIFEPAK 1000 defibrillator.
10 |
|
|
|
|
|
|
|
|
|
|
|
|
1 |
||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
98 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
(Left side) |
|
|
|
|
|
||||||||||||
|
|
||||||||||||||||
7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
3 |
|||
|
|
|
|
|
|
|
|
|
|
||||||||
6 |
|
|
|
|
|
|
|
|
|
|
|
|
4 |
||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
(Left side) |
5 |
|
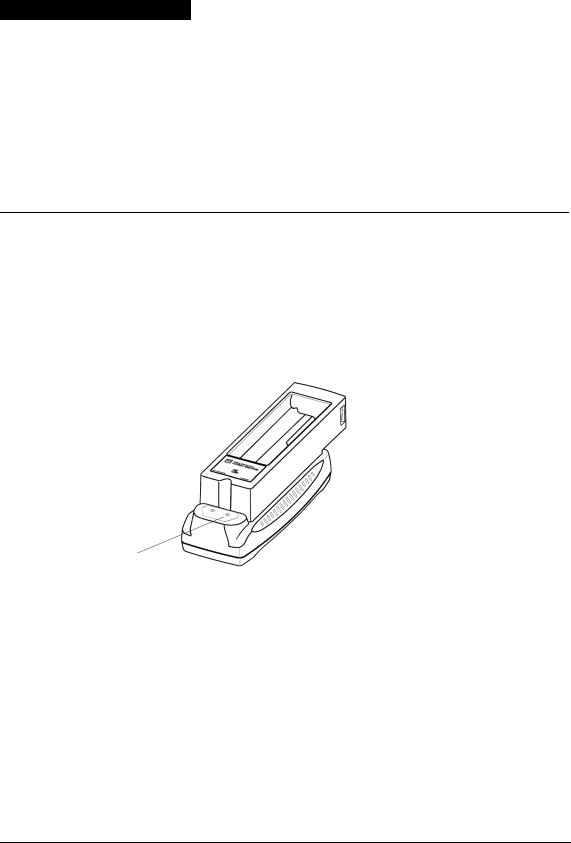
Figure 2-1 Controls and Indicator
Table 2-1 Controls and Indicators
|
Feature |
Description |
|
|
|
1 |
Readiness display |
The readiness display alerts you to the defibrillator’s readiness |
|
|
status. |
|
|
Three symbols ( , , ) allow you to determine whether the |
|
|
defibrillator is ready for use or needs attention. |
|
|
The following defines what each symbol represents and |
|
|
when/where each appears. |
|
|
|
|
|
The wrench indicator appears on the readiness display when a |
|
|
condition exists that prevents or could prevent normal |
|
|
defibrillator operation. |
|
|
|
|
|
The OK symbol indicates that the defibrillator is ready for use. |
|
|
This symbol is visible only when the defibrillator is off. |
|
|
|
|
|
The battery symbol appears on the readiness display when the |
|
|
defibrillator is off. When one bar is visible in the symbol, the |
|
|
battery is low. If the symbol is blank, the battery is extremely |
|
|
low and the OK symbol will not appear when the defibrillator is |
|
|
off. |
|
|
|
2 |
Speaker |
Provides audio voice prompts and tones. |
|
|
|
2-2 |
LIFEPAK 1000 Defibrillator Operating Instructions |

Controls and Indicators
Table 2-1 Controls and Indicators (Continued)
Feature
3
ON
ON/OFF button
Description
Green ON/OFF button turns the power on or off. The button is lit whenever the defibrillator is on.
4 |
|
Pressing the SHOCK button (when flashing) delivers a shock to |
|
|
|
|
the patient. |
|
|
|
SHOCK button |
|
|
|
|
|
|
|
|
5 |
|
Used to select operating modes (Manual or AED) and enter |
|
|
|
|
information in Setup mode. |
|
|
|
MENU button |
|
|
|
|
|
|
|
|
6 |
Battery |
Accommodates a single battery. |
|
|
|
compartment |
|
|
|
|
|
|
|
|
7 |
|
Two softkeys work in conjunction with the screen, providing a |
|
|
|
|
way for you to make selections while using the defibrillator. |
|
|
|
Softkeys |
The softkey functions vary, depending on the task you are |
|
|
|
|
|
||
|
|
performing at the time. Their function is identified by the label |
|
|
|
|
above them on the screen. |
|
|
|
|
|
|
|
8 |
IrDA port |
Infrared Data Association. This port provides wireless |
|
|
|
|
communications for transferring data from the defibrillator to a |
|
|
|
|
PC. |
|
|
|
|
|
|
|
9 |
Screen |
Displays pertinent information for use during all modes of |
|
|
|
|
operation. Figure 2-2 defines the information displayed on the |
|
|
|
|
screen. |
|
|
|
|
|
|
|
10 |
Cable receptacle |
Allows direct connection to therapy electrodes (black), ECG |
|
|
|
|
cable (green), Infant/Child electrodes (pink), and |
|
|
|
|
|
||
|
|
QUIK-COMBO therapy electrodes (gray). |
|
|
|
|
|
|
|
|
|
|
|
|
LIFEPAK 1000 Defibrillator Operating Instructions |
2-3 |
©2006-2012 Physio-Control, Inc.
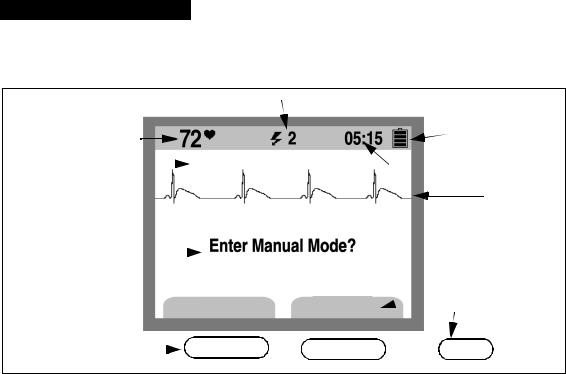
Controls and Indicators
Heart rate indicator |
Shock indicator |
|
|
|
Battery status |
|||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
symbol |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Message area |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Elapsed time |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
ECG
Prompts |
|
|
|
|
|
Enter Manual Mode? |
|
|
|
Softkey label |
|||
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
||
Softkeys |
|
|
|
|
|
YES |
|
NO |
|
|
|
Menu button |
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure 2-2 Defibrillator Screen
Heart rate indicator. The heart rate indicator displays heart rates between 20 – 300 bpm. Indicator is present in Manual mode or when the 3-wire ECG cable is used.
Battery status symbol. When the defibrillator is turned on, this symbol appears on the screen indicating the relative level of charge. One bar indicates the battery is low. When the battery is very low, the symbol is blank and a REPLACE BATTERY message appears on the screen.
ECG. The ECG appearing on the screen is a nondiagnostic ECG, obtained by means of the therapy electrodes or the Lead II ECG cable. The presence of an ECG does not ensure that the patient has a pulse.
Softkey labels. These labels define the function that can be activated by pressing the softkey. ANALYZE and DISARM are function examples.
2-4 |
LIFEPAK 1000 Defibrillator Operating Instructions |
Controls and Indicators
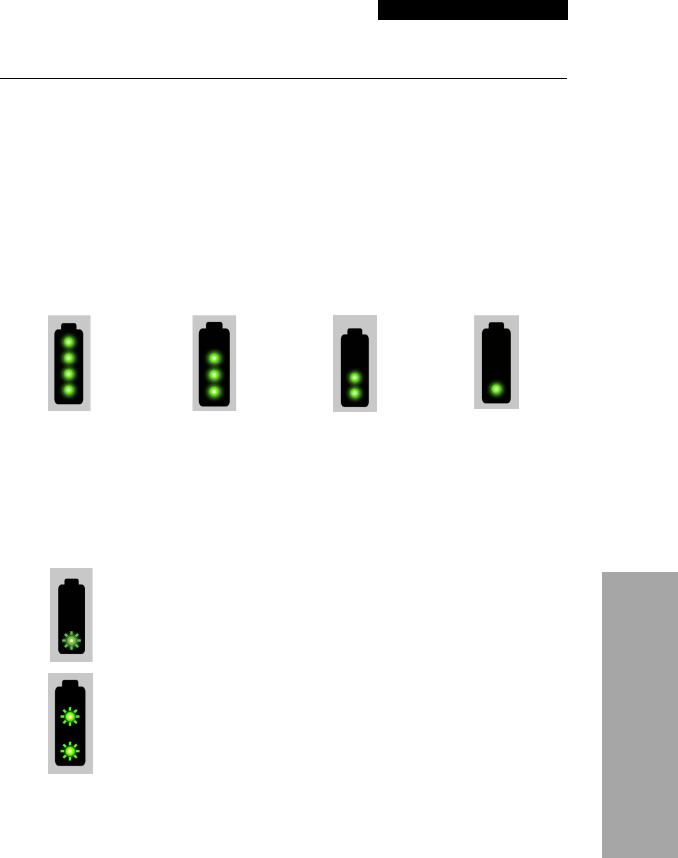
BATTERY INDICATORS
The LIFEPAK 1000 defibrillator can be powered by two types of batteries:
•A nonrechargeable Lithium manganese dioxide battery
•A rechargeable Lithium-ion battery
Battery Charge Level Indicators
Both battery types have a fuel gauge that indicates the approximate charge level of the battery when it is not installed in a defibrillator. Push the gray button below the battery symbol to check the battery’s charge level before installing it in the defibrillator.
Note: Always carry a spare, fully-charged battery.
For both battery types, the four battery indicators shown here represent approximate charge.
> 75% charge |
> 50% charge |
> 25% charge |
25% charge or less |
Figure 2-3 Battery Charge Indicators
Note: The fuel gauge on a new rechargeable battery will not function until the battery has been charged for the first time.
Battery Warning Indicators
For both battery types, a single flashing LED indicates the battery charge level is very low. The battery should be removed from service for charging (rechargeable battery) or disposal (nonrechargeable battery).
For rechargeable batteries only, two flashing LEDs indicate the battery is faulty and should be returned to your local Physio-Control representative.
Figure 2-4 Battery Warning Indicators
The nonrechargeable battery is shipped to customers fully charged. All four LEDs should illuminate when the fuel gauge is activated. Check the charge level of a new nonrechargeable battery before putting it into service. When optimally maintained, a new nonrechargeable battery can provide approximately 17 hours of “ON time” or 440 discharges at 200 joules.
The rechargeable battery is shipped to customers at approximately 40% charge and must be fully charged before use. Charge the battery fully within six months of when you receive it and at
LIFEPAK 1000 Defibrillator Operating Instructions |
2-5 |
©2006-2012 Physio-Control, Inc.
Controls and Indicators
least once every six months thereafter. When optimally maintained, a new fully-charged battery provides approximately ten hours on “ON time” or 261 discharges at 200 joules. Since all rechargeable batteries permanently lose battery capacity over time, and because battery capacity together with the level of battery charge determines how long a rechargeable battery will provide defibrillator power, you can expect that a fully-charged battery’s “ON time” will decrease with age.
BATTERY CHARGER INDICATORS
The LIFEPAK 1000 Defibrillator Battery Charger is intended for use with LIFEPAK 1000 defibrillator Lithium-ion (Li-ion) rechargeable batteries. No other batteries are compatible with this charger. For complete battery charger information, refer to the LIFEPAK 1000 Defibrillator Battery Charger Instructions for Use provided with the charger.
When power is applied to the battery charger, both LEDs on the charger flash briefly and then turn off. Before inserting a battery, inspect the battery contacts for obvious damage or foreign substances. Figure 2-5 describes the LEDs on the battery charger when a rechargeable battery is inserted.
1 
2 
LED |
Behavior |
Definition |
Explanation |
|
|
|
|
|
|
1 |
Flashing green |
Battery is |
A fully depleted battery takes approximately |
|
charging. |
4 hours to charge. |
|||
|
|
|
|
|
|
|
|
If the battery is kept in the charger, the battery will |
|
1 |
Steady green |
Battery charging is |
remain in an optimally charged condition. The |
|
complete. |
charger enters “maintenance” mode after charging |
|||
|
|
completes, automatically providing periodic top-off |
||
|
|
|
||
|
|
|
charging. |
|
|
|
|
|
|
|
|
|
To test the battery: Remove battery and check fuel |
|
|
|
|
gauge; two flashing LEDs indicate a faulty battery. |
|
2 |
Red |
Battery or charger |
To test the charger: Reinstall a functional battery; a |
|
is faulty. |
persistent red charger LED indicates a faulty |
|||
|
|
charger. Contact your authorized service personnel for assistance with a faulty battery or charger.
Figure 2-5 Battery Charger Indicators
For details on batteries and instructions for disposal, see "Battery Maintenance" on page 5-4 and "Product Recycling Information" on page 5-8.
2-6 |
LIFEPAK 1000 Defibrillator Operating Instructions |

HOW TO USE THE LIFEPAK 1000 DEFIBRILLATOR
This section provides an overview of information and instructions for using the LIFEPAK 1000 defibrillator.
Modes of Operation |
page 3-2 |
Defibrillation in AED Mode |
3-3 |
Defibrillation in Manual Mode |
3-6 |
Troubleshooting Tips for Defibrillation |
3-7 |
ECG Monitoring (ECG Mode) |
3-9 |
|
|
LIFEPAK 1000 Defibrillator Operating Instructions |
3-1 |
©2006-2012 Physio-Control, Inc.

How to Use the LIFEPAK 1000 Defibrillator
MODES OF OPERATION
You can use the LIFEPAK 1000 defibrillator for:
•Automated external defibrillation (AED mode)
•Manual defibrillation therapy (Manual mode) (Requires ECG display option)
•ECG monitoring (ECG mode) (Requires ECG display option)
Defibrillation Warnings and Cautions
WARNINGS!
Shock hazard.
The defibrillator delivers up to 360 J of electrical energy. When discharging the defibrillator, do not touch the disposable therapy electrodes.
Shock hazard.
If a person is touching the patient, bed, or any conductive material in contact with the patient during defibrillation, the delivered energy may be partially discharged through that person. Clear everyone away from contact with the patient, bed, and other conductive material before discharging the defibrillator.
Possible skin burns.
During defibrillation, air pockets between the skin and therapy electrodes may cause patient skin burns. Apply therapy electrodes so that entire electrode adheres to skin. Do not reposition the electrodes once applied. If the position must be changed, remove and replace with new electrodes.
Possible skin burns and ineffective energy delivery.
Therapy electrodes that are dried out or damaged may cause electrical arcing and patient skin burns during defibrillation. Do not use therapy electrodes that have been removed from foil package for more than 24 hours. Do not use electrodes beyond expiration date. Check that electrode adhesive is intact and undamaged. Replace therapy electrodes after 50 shocks.
Possible interference with implanted electrical device.
Defibrillation may cause implanted devices to malfunction. Place therapy electrodes away from implanted devices if possible. Check implanted device function after defibrillation, if possible.
Possible misinterpretation of data.
Do not analyze in a moving vehicle. Motion artifact may affect the ECG signal resulting in an inappropriate shock or no shock advised message. Motion detection may delay analysis. Stop vehicle and stand clear of patient during analysis.
Possible misinterpretation of data.
Do not move the AED during analysis. Moving the AED during analysis may affect the ECG signal resulting in an inappropriate shock or no shock advised decision. Do not touch the patient or the AED during analysis.
CAUTION!
Possible equipment damage.
Before using this defibrillator, disconnect all equipment that is not defibrillator-protected from the patient.
3-2 |
LIFEPAK 1000 Defibrillator Operating Instructions |

How to Use the LIFEPAK 1000 Defibrillator
DEFIBRILLATION IN AED MODE
The LIFEPAK 1000 defibrillator uses the patented Physio-Control Shock Advisory System to evaluate the patient’s heart rhythm. The LIFEPAK 1000 defibrillator has an optional feature that displays the ECG waveform and Heart Rate Indicator in AED mode. The operation in AED mode remains the same whether or not the defibrillator displays the ECG waveform. When ECG DISPLAY is set to ON, the ECG appears with all of the AED messages and prompts. When ECG DISPLAY is set to OFF, the messages and prompts fill the screen.
Basic Steps for Using the LIFEPAK 1000 Defibrillator
1Establish that the patient is in cardiopulmonary arrest (the patient must be unresponsive, not breathing normally and showing no signs of circulation).
2Press ON/OFF to turn on the defibrillator (the green LED illuminates). Voice prompts will sound, guiding you through the rescue process.
3Prepare the patient for therapy electrode placement.
•If possible, place the patient on a hard surface away from standing water.
•Remove clothing from the patient's upper torso.
•Remove excessive hair from the electrode sites. If shaving is necessary, avoid cutting the skin.
•Clean the skin and dry it briskly with a towel or gauze.
•Do not apply alcohol, tincture of benzoin, or antiperspirant to the skin.
4Open the therapy electrode packet and remove the electrodes. Slowly peel back the protective liner on the electrodes, beginning with the cable connection end. Safely discard the liner to prevent slipping.
5 Apply the therapy electrodes to the patient's chest. Starting from one end, press the electrodes firmly onto the patient's skin, as shown.
WARNING!
Anterior |
|
|
|
|
|
Excessive Energy Delivery. |
|
|
|
|
|||
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
For children less than 8 years of age or 55 lbs (25 kg), use |
|
|
|
|
|
Lateral |
Infant/Child Reduced Energy Defibrillation electrodes. Do not use |
|
|
|
|
|
Pediatric QUIK-COMBO electrodes; these electrodes do not |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
attenuate the energy delivery by this defibrillator. |
LIFEPAK 1000 Defibrillator Operating Instructions |
3-3 |
©2006-2012 Physio-Control, Inc.

How to Use the LIFEPAK 1000 Defibrillator
6Connect the electrodes to the defibrillator (if they are not already connected).
7Follow the screen messages and voice prompts provided by the defibrillator.
Voice Prompts and Messages in AED Mode
The following descriptions of voice prompts and messages are based on the default settings for AED mode. Changing the setup options may result in different AED behavior.
CONNECT ELECTRODES
STAND CLEAR, ANALYZING NOW, STAND CLEAR
PREPARING TO SHOCK
STAND CLEAR, PUSH SHOCK BUTTON
Voice prompt and message when a patient has not been connected to the defibrillator.
Voice prompt and message when a patient is connected to the defibrillator.
Do not touch or move the patient, or therapy cables, during analysis.
ECG analysis requires 6–9 seconds.
Message displayed if the defibrillator detects a shockable rhythm.
The defibrillator charges to the joule setting for that shock number.
A rising tone and a charging bar on the screen indicate that the defibrillator is charging.
Voice prompt and message when charging is complete.
The  (shock) button flashes.
(shock) button flashes.
Clear everyone away from the patient, bed, or any equipment connected to the patient.
|
Press the (shock) button to discharge the defibrillator. |
|
The energy level for shocks depends on the energy protocol setup |
|
option and the analysis decision after shocks. |
|
If the (shock) button is not pressed within 15 seconds, the |
|
defibrillator disarms the shock button, and the DISARMING... |
|
message appears on the screen. |
ENERGY DELIVERED |
Message displayed after each shock. |
START CPR |
A message and countdown timer (min:sec format) appears for the |
|
CPR time. |
NO SHOCK ADVISED |
Voice prompt and message when the defibrillator detects a |
|
nonshockable rhythm. The defibrillator will not charge, and a shock |
|
cannot be delivered. |
|
When a NO SHOCK ADVISED prompt follows a shock and CPR, |
|
the energy level will not increase for the next shock. |
3-4 |
LIFEPAK 1000 Defibrillator Operating Instructions |
 Loading...
Loading...