Pathway Activity
Proling
OncoSignal
ER AR PI3K MAPK
The Philips Pathway Activity Proling OncoSignal Test for measuring ER, AR, PI3K and MAPK pathway activity
is now available for research use in your molecular biology laboratory. The OncoSignal Test is performed using
an RT-qPCR testing plate to measure mRNA levels of selected pathway target genes and includes access to a
secure cloud-based environment for calculation and reporting of pathway activity scores.
OncoSignal is based on a unique knowledge-based approach and helps users gain insight into underlying
tumor driving cell signaling pathways. The OncoSignal Test quantitatively measures the activity of the
hormonal estrogen receptor (ER) and androgen receptor (AR) pathways, as well as the activity of PI3K and
MAPK growth factor signal transduction pathways simultaneously in a single sample, using RNA from FFPE
tissue of human origin.
OncoSignal Testing Plates
mRNA levels transcribed from target genes regulated by the pathway
transcription factor are measured by means of the OncoSignal Testing
Plates. The 96-well PCR testing plates are packaged in a box of six, for
testing of six samples. Each testing plate contains carefully designed
and optimized primers and probes for reliable detection of mRNA
levels of the pre-selected signaling pathway target genes. Upon
adding the sample RNA and one-step RT-qPCR mix to the testing
plate, the test is run on a thermocycler under a specied PCR cycling
program. Detailed protocols are provided in the Instructions for Use.
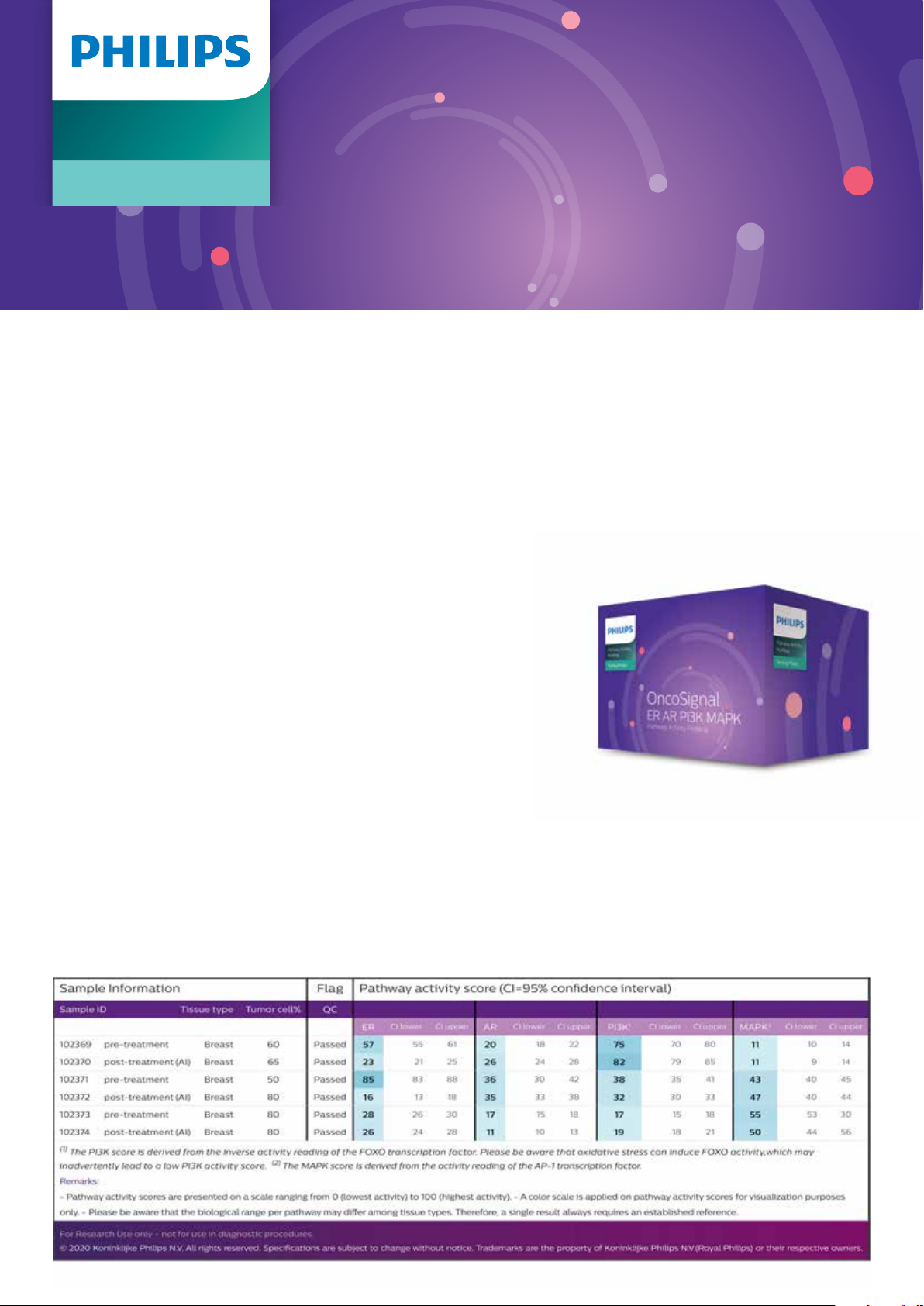
OncoSignal Report
PCR gene expression data are translated via a computational model into quantitative pathway activity scores.
Each PCR data le uploaded in the cloud-based Philips ISPM OncoSignal portal undergoes a thorough quality
check (QC) (e.g. controls for the quantity and quality of the RNA, and detection of correct plate lling), to ensure
high-quality results. The OncoSignal Report presents an overview of the pathway activity scores per sample on
a scale of 0-100 with respective 95% condence intervals. The report provides insights into the tumor driving
pathways in the samples tested and enables a direct comparison of pathway activities between samples.
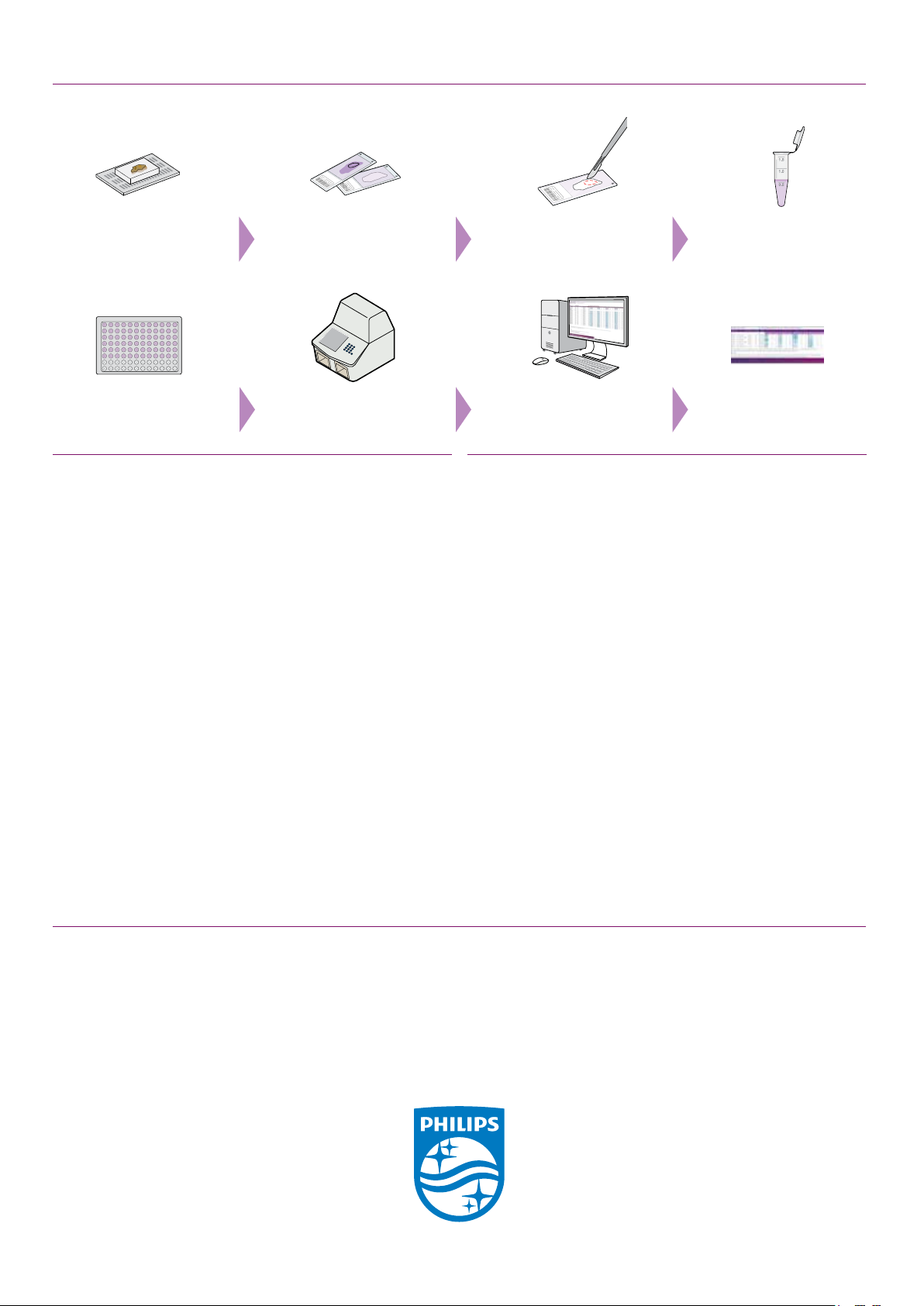
Workow OncoSignal Test
Select FFPE tumor
tissue block
1 2 3 4 5 6 7 8 9 10 11 12
A
B
C
D
E
F
G
H
Add PCR mix w ith extracted
RNA to test ing plate
Identify tumor area Macro dissect tumor area
Run the test on
a thermocycler
Features
• AR, ER, PI3K and MAPK pathway activity
• Highly reproducible quantitative pathway activity
scores on a scale of 0-100 with condence
intervals
• Translation of RT-qPCR data into pathway activity
scores in a secure cloud-based environment
• Report providing pathway activity prole per
sample
• Easy to implement in molecular biology laboratory
• Requires only 0.25 mm3 FFPE tissue per sample
• Fast turn-around-time (105 minutes)
Transfer tissue to
from unstained slide(s)
Process PCR data in
cloud-based env ironment
RNase-free tube
Generate report with
pathway activ ity scores
Additional reagents and equipment needed
• RNeasy FFPE Kit (Qiagen, cat. no. 73504)
• SuperScript III Platinum One-Step qRT-PCR Kit
(Thermo Fisher Scientic, cat.no. 11732088)
• Bio-Rad CFX96 (Touch) Real-Time PCR Detection
System
• Plate centrifuge
Applications
For information about example experimental designs,
and interpretation of the OncoSignal Report, please
request the application note.
Box with OncoSignal Testing Plates contains
• Six OncoSignal Pathway Activity Proling Testing
Plates (96-wells plates with pre-designed and
spotted qPCR assays)
• Six (+one spare) clear, adhesive PCR plate foils
• Quick Reference Guide
For Research Use Only - not for use in diagnostics
procedures.
© 2020 Koninklijke Philips N.V. All rights reserved.
Specications are subject to change without notice.
Trademarks are the property of Koninklijke Philips
N.V. (Royal Philips) or their respective owners.
DEC 2020
Order information
Please send your request to oncosignal@philips.com.
More information about the OncoSignal Pathway
Activity Proling Test can be found at
www.philips.com/oncosignal-test
Philips Electronics Nederland B.V.
High Tech Campus 5
5656 AE Eindhoven
The Netherlands
www.philips.com/oncosignal
oncosignal@philips.com
 Loading...
Loading...