Page 1
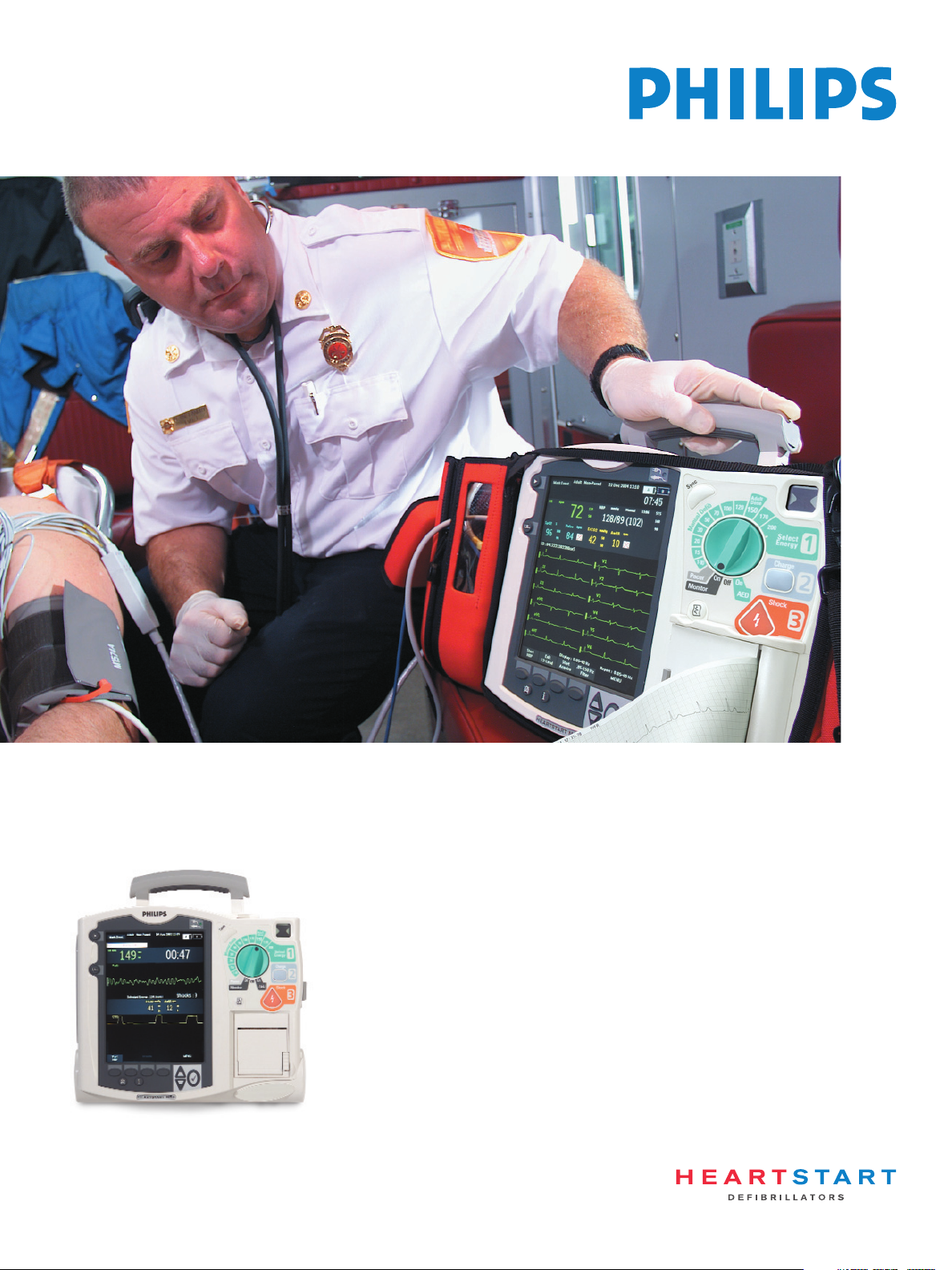
Power to see the big picture
Philips HeartStart MRx ALS Monitor
Product information
Page 2

The capabilities you need and
the performance
HeartStart MRx has the capabilities you need and
the performance you demand for rapid intervention,
thorough care and positive patient outcomes.
Page 3

you demand
All of these measurements, therapies and features, plus its
compact size, low weight (13.2 pounds), and balanced shape
make HeartStart MRx easy to carry, easy to stow, and above all,
easy to operate.
•Thoughtfully organized controls and
ports clearly separate functions,
monitoring from therapeutic.
• Monitoring starts once a patient cable is
connected to the device.
• Monitoring and therapy data are clearly
and logically arranged on-screen.
• Large numeric measurements,
waveforms,and alarm indicators enable
the user to quickly locate information.
Measures
12.4 x 7.7 x11.7 inches
• The appearance of measurements
and waveforms can be customized,
and the screen organized to the user’s
preferences.
• On-screen menus simplify navigation
for configuring data, setting and
responding to alarms, and accessing
additional functionality.
Monitoring Capabilities
• Monitoring through
defibrillation pads
• 3- and 5-lead ECG monitoring
through electrodes
• ST/AR Basic
detection
•FAST-SpO
Suppression Technology),
optional
• Noninvasive Blood Pressure
(NBP), optional
• Microstream
(etCO
• 12-Lead ECG, optional
• Q-CPR™ Measurement and
Feedback, optional
Therapies
• SMART Biphasic waveform
• Manual mode with shock
delivery through defibrillation
pads or paddles
• AED mode
• Synchronized cardioversion
• Noninvasive pacing, optional
Features
•Adjustable ECG size and
autogain
• 8.4 inch (diag.), 4-wave color
display, largest in its class
•12-lead data transmission
•Data collection and event
summary
• Strip chart printer
• Automated self-tests
•Operational checks
• Individual, adjustable volume
of QRS beeper, voice prompts,
and alerts
• Lithium ion battery (2 bays)
with capacity gauge
• “Ready-for-Use” indicator
•Configuration mode
•Diagnostic mode
• Carrying Case
• Bed rail hook
•AC and DC power modules,
optional
• Ambulance mounting bracket,
optional
™ arrhythmia
(Fourier Artifact
2
®
Capnography
), optional
2
3
Page 4

Superior Measurements
A typical monitoring view shows some
basic patient information, the date, time
and battery status. Next are the numerics
and waveforms.The bottom half of the
screen shows additional monitoring
numerics followed by their waveforms,
and soft keys for customizing the display,
setting and responding to alarms, and
selecting parameters to view additional
monitoring data.
Arrhythmia Monitoring
• Philips’ ST/AR Basic arrhythmia algorithm.
• Analyzes ECGs for heart rate, while
continuously monitoring for ventricular
arrhythmias.
• Detects 10 rhythm disturbances and
irregularities, including 5 life-threatening
arrhythmias: asystole, ventricular fibrillation,
ventricular tachycardia, extreme bradycardia,
and extreme tachycardia.
• Generates visible and audible alarms
as needed.
SpO2with Fourier Artifact
Suppression Technology (FAST-SpO
•Low-noise hardware and patented digital
processing to prevent false readings,
drop-outs and false alarms in the presence of
motion and other interferences.
• Applies rule-based analysis to technical and
physiological criteria and quality indicators to
generate the Fourier spectrum.
• Measures reliably even in the presence of low
peripheral perfusion.
Noninvasive Blood Pressure (NBP)
•ADVANTAGE®oscillometric, motion-tolerant,
noninvasive blood pressure system from
SunTech Medical Instruments.
• Measures systolic and diastolic pressure and
calculates mean arterial pressure.
Philips 12-Lead ECG
• Philips’ 12-Lead ECG algorithm.
• Removes noise and artifact before generating
interpretive statements.
• Detects and stratifies early acute coronary
syndromes, for patients with symptoms of
ST-segment elevation acute myocardial infarc-
tion (STEMI).
• 12-Lead ECG algorithm employs its Pediatric
Criteria Program, which recognizes 12 dis-
tinct age groups for patients under the age of
16.
)
2
Complete 12-lead reports can be transmitted
from HeartStart MRx to the receiving hospital,
giving the ED and/or catheterization lab a head
start in preparing for patient care. Utilizing the
latest cell phone technologies, the speed and
encryption capability of the Internet, and
Philips HeartStart 12-Lead Transfer Station
software, reports can be viewed on-line,
faxed, printed, e-mailed and forwarded to
TraceMaster ECG Management System or
a central database for storage.
In 12-lead preview mode, 12 waves are
viewable on-screen, in addition to
numeric vital sign values.
Microstream®Capnography (etCO2)
•Microstream®CO2technology from Oridion
Medical.
•No zeroing, no heating and no sensor to
interfere with the patient’s airway.
•Microstream’s FilterLine®airway adapter and
sample line inhibit the build-up of condensed
water and secretions.
•Works on both intubated and nonintubated
patients, adult and pediatric.
All waves print on the strip chart printer in 3x4 format.
Q-CPR™ Measurement
and Feedback
•Q-CPR technology by Laerdal.
• Monitors and analyzes compression rate
and depth, as well as ventilation volume and
frequency in real-time.
• Provides on-screen measurements and
indicators and audio feedback when needed.
• Reinforces CPR training with each use.
Page 5

Proven Therapies
SMART Biphasic Technology
• Philips’ patented low-energy SMART Biphasic
(truncated exponential) waveform. No other
external defibrillation waveform is supported by
more peer-reviewed clinical data.
•Impedance compensation algorithm measures
chest impedance and delivers a low-energy
shock based on the patient’s unique physical
requirements.
All monitoring connections
are located on the left side
panel. A carrying case pouch
covers and protects ports
and cable connectors from
damage.
AED Mode
•Clear, concise voice and text prompts, like
those of our industry leading automated exter-
nal defibrillators, guide the user through the
defibrillation process.
•Preset150 Joules of non-escalating energy.
Manual Defibrillation
•Charges to its highest energy level,
200 Joules, in less than 5 seconds.
•Defibrillates with either paddles or pads.
Synchronized Cardioversion
• Philips’ SMART Biphasic waveform, supported
by peer-reviewed evidence for its effectiveness
in cardioverting atrial fibrillation.
• On-screen, R-wave markers are shown above
(or on) each detected R-wave.
Noninvasive Pacing
•Demand and fixed modes.
• 40 msec pulse width.
•Adjustable rate and output (mA).
HeartStart MRx can be equipped with a set
of anterior/anterior, water resistant, external
paddles for adult and pediatric use.They
convert from adult to pediatric by removing
the outer contacts.
Sensors in the external paddles’ electrodes
assess paddle-to-patient contact and display
their readings in the Patient Contact Indicator
(PCI) located on the sternum paddle’s handle.
If pads are preferred, HeartStart MRx can be used
with Philips HeartStart Multifunction Defibrillator
Pads, which come in adult and pediatric sizes.
When connected to MRx, they can provide
ECG monitoring, synchronized cardioversion,
and noninvasive pacing, in addition to external
defibrillation.
In code view,a single large ECG wave is shown in the top
half of the display, the incident timer is prominent and all
alarms are paused. CPR numerics and the compression wave
appear in the middle.The bottom of the display accomodates
the CO
wave and active soft keys.
2
A flashing hourglass in
the “Ready-for-Use”
indicator window signals
that HeartStart MRx has
ample battery power to
monitor and deliver a
shock.When battery
power is low, ECG
capability is compromised,
or MRx detects that it
cannot pace or shock, a
red “X” replaces the hourglass and the monitor
will audibly chirp until the situation is corrected.
Therapies – defibrillation, synchronized
cardioversion, and pacing – are activated
using the therapy knob and surrounding keys.
For manual defibrillation, energy is selected
(1) using the therapy knob.With the press of
a button (2), MRx charges. Pressing the shock
button (3), MRx delivers defibrillation therapy.
5
Page 6

Feature-packed and still lightweight
Self-tests and operational checks
• Ready-for-Use indicator.
• Automated hourly,daily and weekly self-tests.
• Easy-to-run routine operational checks.
•Test results are stored in internal memory and can
be viewed on-screen and printed with the strip
chart printer.
Strip chart printer
•Integrated strip char t printer: 50 mm standard or
75 mm optional.
•Prints the primar y ECG lead with event
annotations and event summary repor ts,including
ECG rhythm strips and 12-lead ECG reports.
•When configured,prints automatically on marked
events,charge, shock and alarms.
•Prints measurements in real-time or with a
10-second delay.
Data collection, management
and reporting
•Internal memory and an optional, removable
CompactFlash®data card capture approximately
8 hours of continuous ECG waveforms and events
(including drug and therapy markers), plus 50
12-lead ECG reports.
• Stored data can be printed as an event summary
report on the device’s strip char t printer or viewed
on-screen.
• Data transferred to a PC running HeartStar t Event
Review Pro data management software can be
compiled, edited,shared and archived for quality
control and reporting.
•HeartStar t MRx can accept a data card from
Heartstream and Hear tStart FR2-series AEDs.
12-Lead Data Transmission
•Using a cell phone to establish a dial-up
connection between HeartStar t MRx and
the Internet, access Philips HeartStart 12-Lead
Tr ansfer Station and send a report.Transmission
capability is optional.
Carrying Case
• Semi-rigid structure covered with polyester.
• Modular pouches for segmenting and
organizing accessories and supplies.
•Pouches snap on and can be easily removed for
thorough cleaning.
Quick Reference Cards
•Highlight device’s key functionality and operation.
• Laminated to resist wear and stains, the card set can
be tethered to HeartStar t MRx or stored in its
carrying case.
Training materials
• Self-paced, interactive,web-based training
familiarizes the user with the features and
operation of HeartStart MRx.
•Users explore components and accessories, run
simulations of hands-on procedures,and test their
understanding of the material.
• Continuing education credit is available for
completing the program.
•Optional instructor-based training material,
in person instruction, and a training video
are also available.
Rugged, reliable, and ready for use
The 8.4-inch color display is well protected
against damage. Recessed behind a 3 mm
non-reflective polycarbonate shield, framed
and backed in an energy-absorbing foam
blanket, and supported by a rigid magnesium
casting, it endures routine impact from bumps,
knocks, and even drops.
Internal assemblies are rugged. Latched
connectors hold cables in place to ensure
uninterrupted communications between
circuit boards.And every circuit board is
aggressively reinforced, braced and fastened
at multiple points, keeping the device’s
internal structure rigid, even in high
vibration environments.
Tw o rechargeable, lithium ion batteries,
when new and fully charged, provide up to
10 hours of monitoring, more than any other
monitor/defibrillator. Depleted batteries can be
charged to full capacity in just 3 hours.
No conditioning is required. Capacity gauges
on the monitor’s screen and on each battery
show the remaining charge.
6
Page 7

Product Specifications
Physical
Defibrillator Model
Dimensions
Weight
HeartStar t MRx (M3536A)
Without external paddles: 12.4 in. (W) x 7.7 in. (D) x
11.7 in. (H) (313 mm x 195 mm x 295 mm)
With external paddles: 12.4 in. (W) x 7.7 in. (D) x
13.4 in. (H) (313 mm x 195 mm x 340 mm)
13.2 lbs. (6 kg): base unit with 1 batter y, pads and pads
cable. 13 lbs. (5.9 kg) with optional 75 mm strip chart
printer. Paddle tray and external standard paddles add less
than 2.5 lbs. (1.1 kg). Carr ying case adds 4.1 lbs. (1.86 kg).
Environmental and Physical Requirements
Solids/Water
IP24
Resistance
Temperature
Humidity
Altitude
Mechanical Shock
Vibration
Safety
Operating: 32º - 113º F (0º - 45º C)
Storage: -4º - 158º F (-20º - 70º C)
Operating: 0% to 95% relative
Operating: 0 to 15,000 ft (0 to 4,500 m)
Storage: 0 to 15,000 ft (0 to 4,500 m)
Bump: IEC 68-2-29
Freefall: IEC 68-2-32
Operating: MIL STD 810E 514.4 Category 6 Helicopter,
General Storage, UH60
Non-Operating: IEC 68-2-6 Swept Sine Vibration and IEC
68-2-64 Random Vibration
Meets EN 60601-1, UL 2601-1, CSA C22.2 No. 601-1
Display
Dimensions
Type
8.4’’ diagonal (128 mm x 171 mm)
TFT color LCD
Battery
Type
Dimensions
Weight
Charge Time
Capacity
Battery Indicators
Strip Chart Recorder
Recorder
Continuous ECG Strip
Auto Printing
Reports
Paper Size
6.3 Ah, 14.8 V, rechargeable lithium ion
6.5" (H) x 3.8" (W) x 1.6" (D) (165 mm x 95 mm x 42 mm)
1.6 lb. (0.73 kg)
Approximately 3 hours to 100%, 90 minutes to 80%
At least 5 hours of continuous 12-lead ECG, SpO
and CO
monitoring, with NBP every 15 minutes on
2
one new, fully charged battery
At least 3.5 hours of continuous 12-lead ECG, SpO
CO
monitoring, with NBP every 15 minutes and pacing
2
at 180 ppm at 160 mA on one new, fully charged batter y
Battery gauge on batter y, capacity indicator on display;
flashing RFU indicator, chirp, and ‘Low Battery’ message
appears on display for low battery condition, when
10 minutes of monitoring time and 6 maximum
energy discharges remain (with a new batter y at room
temperature, 25º C)
Standard: 50 mm (paper width) thermal array printer
Optional: 75 mm (paper width) thermal array printer
Prints primar y ECG lead with event annotations and
measurements in real-time or with 10-second delay
Recorder can be configured to print marked events,
charge, shock and alarms
Event Summary, 12-Lead, Operational Check,
Configuration, Status Log, and Device Information
1.97 in. (50 mm) W by 100 ft. (30 m) L
2.95 in. (75 mm) W by 100 ft. (30 m) L
2
2
,
, and
Resolution
Wave Viewing Time
Defibrillation
Wave form
Output Energy
Charge Time
Shock Delivery
Shock-to-Shock
Cycle Time
Patient Impedance
Range
AED Mode
480 x 640 pixels (VGA)
5 seconds (ECG)
Tr uncated Exponential Biphasic.Waveform parameters
adjusted as a function of patient impedance.
Manual (selected): 1-10, 15, 20, 30, 50, 70, 100, 120, 150,
170, 200 Joules into a 50 Ohm load
AED Mode (single energy output): 150 Joules into a 50
ohm load.
Less than 5 seconds to 200 Joules with a new, fully
charged lithium ion battery at 25º C
Via multifunction defib electrode pads or paddles
Typically less than 20 seconds
Minimum: 15 Ohm (internal defibrillation); 25 Ohm
(external defibrillation)
Maximum: 180 Ohm
Shock advisory sensitivity and specificity meet AAMI
DF-39 guidelines
Data Storage
Internal
Data Card
8 hours of continuous ECG waveforms and events,
plus 50 12-lead ECG reports
8 hours of continuous ECG waveforms and events,
plus 50 12-lead ECG reports, on a CompactFlash
memory card
ECG and Arrhythmia Monitoring
Input
Lead Fault
Pads Fault
Heart Rate Display
Heart Rate/
Arrhythmia Alarms
ECG Size
Up to 4 ECG waves displayed and up to 2 ECG waves
print simultaneously
Lead I, II, or III obtained through 3-lead ECG cable and
separate monitoring electrodes. With 5-lead cable,
obtain leads I, II, III, aVR, aVL, aVF, or V. Pads ECG
obtained through 2 multifunction defibrillation
electrode pads.
‘Lead Off ’ message and dashed line displayed, if an
electrode or lead wire becomes disconnected
Dashed line displayed if a pad becomes disconnected.
Digital readout on display 15 to 300 bpm, accuracy ±10%
HR, Asystole,VFIB/VTACH,VTACH, extreme tachycardia,
extreme bradycardia, PVC rate
2.5, 5,10, 20, 40 mm/mV, autogain
Page 8

SpO2Pulse Oximetry
Range
0 to 100%
Resolution
Alarm Range
Alarm Delay
1%
Low Limit: 50 to 99% (Adult/Pediatric)
High Limit: 51 to 100% (Adult/Pediatric)
10 seconds
Noninvasive Blood Pressure
Pressure Range
Initial Pressure
Maximum Pressure
Alarm Range
End-Tidal CO
Range
Resolution
Sample Size
Alarm Range
Systolic: 40 to 260 mmHg
Diastolic: 20 to 200 mmHg
Adult: 160 mmHg
Pediatric: 120 mmHg
280 mmHg
Systolic high limit: 30 - 270 (Adult), 35 - 180 (Pediatric)
Systolic low limit: 30 - 265 (Adult), 30 - 175 (Pediatric)
Diastolic high limit: 18 - 240 (Adult), 18 - 150 (Pediatric)
Diastolic low limit: 10 - 240 (Adult), 10 - 145 (Pediatric)
2
0 to 99 mmHg
1 mmHg (0.1 kPa)
50 ml per minute
Low Limit: 10 to 95 mmHg (Adult/Pediatric)
High Limit: 20 to 100 mmHg (Adult/Pediatric)
12-Lead ECG
Input
Display View
Strip Record
Transmission
12-Lead cable: leads I, II, III, aVR, aVL, aVF,V/C1-V/C6
All 12-lead ECG waves display simultaneously
All 12-leads print on the strip char t printer in 3x4 format
CompactFlash data card; cellular dial-up Internet connection
CPR Measurement and Feedback
Compression Depth
Compression Rate
Ventilation Volume
Ventilation Rate
Ta r get: -1.50 to -2.00 in. (-38 to -51 mm)
Ta r get: 90 to 120 cpm
Graphic indicator: empty, 1/3-full, 2/3-full, full
Ta r get for breaths delivered before 60 seconds since last
compression: 6 to 16 vpm
Ta r get for breaths delivered beyond 60 seconds since
last compression: 9 to 16 vpm
Noninvasive Pacing
Wave form
Current Pulse
Amplitude
Pulse Width
Rate
Monophasic Truncated Exponential
10 mA to 160 mA (5 mA resolution); accuracy
10 mA to 50 mA ± 5 mA, 50 mA - 160 m A ± 10%
40 ms with ± 10% accuracy
30 ppm to 180 ppm (10 ppm increments);
accuracy ± 1.5%
Modes
Refractory Period
Demand or Fixed Rate
340 msec (30 to 80 ppm); 240 msec (90 to 180 ppm)
Page 9

Q-CPR is the first and
only CPR measurement
and feedback tool
integrated into an
ALS monitor.
Page 10

Built to Perform and Backed by Philips
Our dedication to excellence in design, manufacturing and
customer support makes us a trusted supplier of patient monitors
and defibrillators, serving the healthcare community for more
than 35 years. HeartStart MRx is part of our cardiac resuscitation
family of products, which includes ALS defibrillator/ monitors and
automated external defibrillators used in private and public
environments. Each is tailored to the needs and skills of a particular
type of user, extending the reach of care from the home to the
hospital.
Warranties, services, and support
So that our HeartStart MRx customers can continue delivering
reliable and effective patient care, we provide a variety of warranty
offerings and preventative maintenance programs. Philips backs
HeartStart MRx with one year of on-site service. On-site service,
requested through our Medical Response Center, is provided by an
authorized Philips service representative.At the time of purchase, a
2-year repair and return warranty can be substituted for the
standard 1-year on-site service warranty.To extend the coverage
period of either warranty, service contracts can be purchased
annually in 1-year increments (without limit).
Philips Medical Systems is part
of Royal Philips Electronics
On the web
www.philips.com/heartstart
Via email
medical@philips.com
By fax
+31 40 27 64 887
By postal service
Philips Medical Systems
3000 Minuteman Road
Andover, MA 01810-1085
Asia
Tel: +852 2821 5888
Europe, Middle East, Africa
Tel: +31 40 27 87246
Latin America
Tel: +1 954 628 1000
Philips Medical Supplies
Philips is committed to producing and supporting the finest quality
medical equipment and supplies. Our supplies are thoughtfully
designed, tested and manufactured to deliver reliable and accurate
results from your HeartStar t MRx. For a complete list of supplies,
please visit http://shop.medical.philips.com.
North America
Tel: +1 800 934 7372
© Koninklijke Philips Electronics N.V. 2005
All rights are reserved. Reproduction in whole or in
part is prohibited without the prior written consent
of the copyright holder.
Philips is a registered trademark of Koninklijke Philips Electronics N.V.
Philips Medical Systems North America Corporation reserves the
right to make changes in specifications or to discontinue any product
at any time without notice or obligation and will not be liable for any
consequences resulting from the use of this publication.
Printed in The Netherlands.
4522 962 06831/861 * SEP 2005
Microstream and FilterLine are registered trademarks of Oridion
Medical Ltd. ADVANTAGE is a registered trademark of SunTech
Medical Instruments. Q-CPR is a trademark of Laerdal Medical
Corporation. CompactFlash is a registered trademark of SanDisk
Corporation.
 Loading...
Loading...