Philips HeartStart FR3 Series Manual To Setup, Operation, And Maintenance

HeartStart FR3 Debrillator
Guide to Setup,
Operation, and Maintenance

Administrator’s Reference CD
THIS CD CONTAINS PDF FILES. To read these les, you need
Adobe Reader, available at no charge from www.get.adobe.com/reader.
PHILIPS MEDICAL SYSTEMS

PHILIPS MEDICAL SYSTEMS
HeartStart FR3
861388 / 861389
Automated External Defibrillator
GUIDE TO SETUP, OPERATION, AND MAINTENANCE
Edition 7
IMPORTANT NOTE:
It is important to understand that survival rates for sudden cardiac arrest are
directly related to how soon victims receive treatment. For every minute of
delay, the chance of survival declines by 7% to 10%.
Treatment with CPR and defibrillation cannot assure survival. In some
patients, the underlying problem causing the cardiac arrest is simply not
survivable despite any available care.

About this edition
The information in this guide applies to the HeartStart FR3 Text
model 861388 and FR3 ECG model 861389 defibrillators. This
information is subject to change. Please contact Philips at
www.philips.com/productdocs or your local Philips representative
for information on revisions.
Edition history
Edition 7
Publication date: October 2013
Publication number: 453564358322
Notices
© 2013 Koninklijke Philips N.V. All rights reserved.
Specifications are subject to change without notice.
Trademarks are the property of Koninklijke Philips N.V. or their
respective owners. The Bluetooth® word mark and logos are
registered trademarks owned by Bluetooth SIG, Inc. and any use of
such marks by Philips Medical Systems is under license. Koninklijke
Philips N.V. is an Associate Member of the Bluetooth SIG.
Authorized EU representative
Philips Medizin Systeme Boeblingen GmbH
Hewlett-Packard Strasse 2
71034 Boeblingen, Germany
(+49) 7031 463-2254
CAUTION:
Federal law (USA) restricts this device to sale by or on the order
of a physician.
Device manufacturer
Philips Medical Systems
22100 Bothell Everett Highway
Bothell, WA 98021-8431, USA
Patents
This product is manufactured and sold under one or more of the
following United States patents:
US5591213, US5601612,
US5607454, US5611815, US5617853, US5632280,
US5650750, US5735879, US5749905, US5773961,
US5776166, US5800460, US5803927, US5836993,
US5868792, US5879374, US5889388, US5891046,
US5891049, US5899926, US5902249, US5904707,
US5951598, US5967817, US6016059, US6075369,
US6185458, US6230054, US6234816, US6272385,
US6287328, US6299574, US6317635, US6319031,
US6350160, US6356785, US6405081, US6417649,
US6441582, US6553257, US6556864, US6611708,
US6871093, US7079894,
and other patents pending.
For Technical Support
If you need technical support, please contact your local Philips
representative or go to www.philips.com/AEDsupport. Technical
information about all Philips HeartStart automated external
defibrillators, including clinical summaries of several key studies
using Philips automated external defibrillators, is also available
online at www.philips.com/productdocs, in the Technical Reference
Manuals for HeartStart Automated External Defibrillators.
PHILIPS MEDICAL SYSTEMS
Device tracking
This device is subject to tracking requirements by the
manufacturer and distributors. If the defibrillator has been sold,
donated, lost, stolen, exported, or destroyed, notify Philips Medical
Systems or your distributor.

Heartstart FR3 Defibrillator
GUIDE TO SETUP, OPERATION, AND MAINTENANCE
CONTENTS
Description and Indications for Use ...................................................................... 1
Intended User ............................................................................................................ 1
Setup and Configuration .......................................................................................... 1
Installing the FR3 Language Card and the Battery ...................................... 2
Installing the Optional FR3 Data Card and the Battery ............................ 3
Setting the Date and Time ............................................................................... 3
Connecting the HeartStart SMART Pads III ................................................. 4
Automatic User-Initiated Test ........................................................................ 5
Placing and Securing the FR3 ........................................................................... 5
Directions for Use .................................................................................................... 5
PHILIPS MEDICAL SYSTEMS
Maintenance and Cleaning ....................................................................................... 6
Routine Maintenance ......................................................................................... 6
After Each Use .................................................................................................... 7
User-Initiated Test ............................................................................................. 7
Cleaning ................................................................................................................ 8
Troubleshooting ........................................................................................................ 8
Indicators .............................................................................................................. 8
Recommended Action in an Emergency ....................................................... 8
Safety Considerations .............................................................................................. 9
Symbols and Controls .............................................................................................. 11
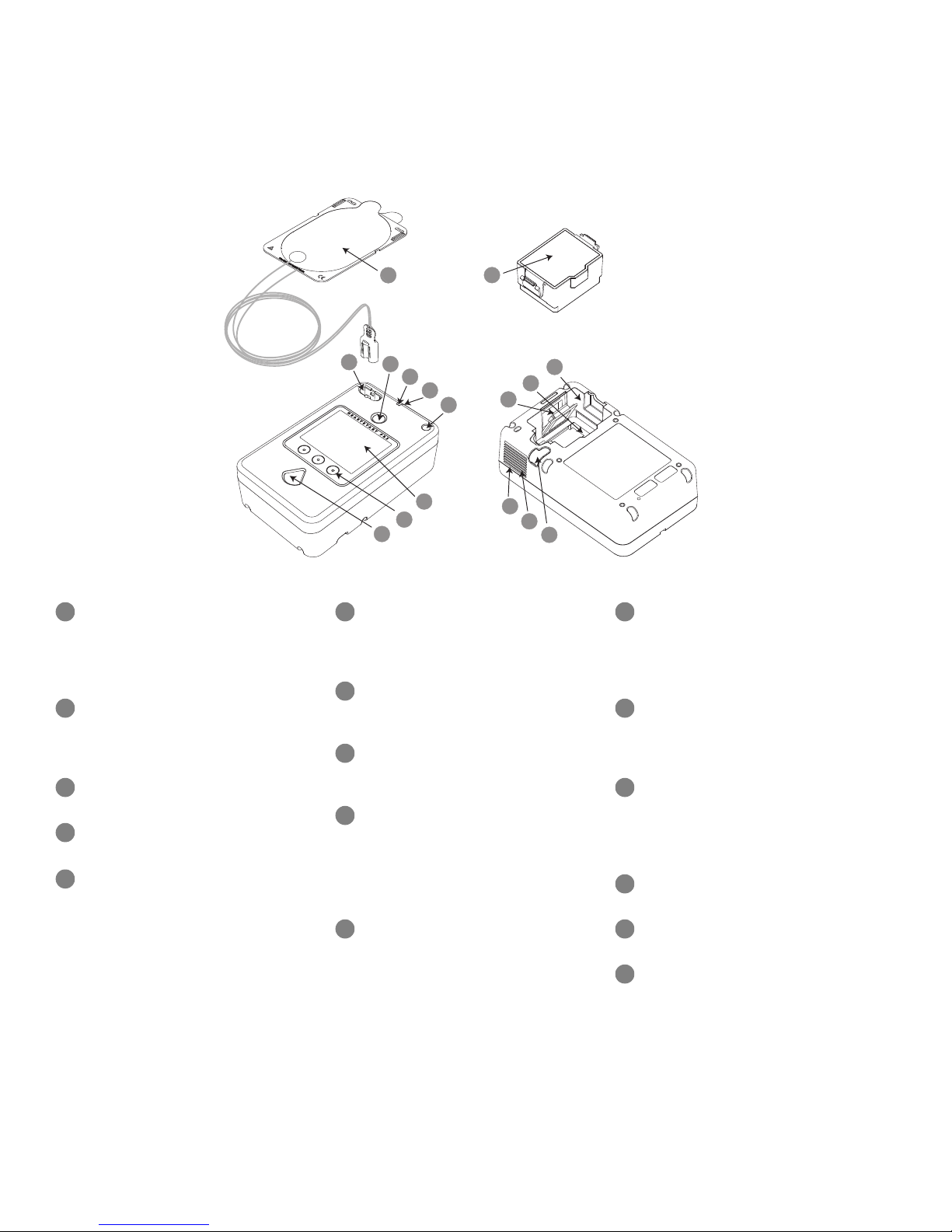
I
A
B
C
D
E
H
G
F
J
K
L
M
N
O
P
The HeartStart FR3 Defibrillator
A
B
C
D
E
F
G
H
I
J
K
L
M
N
O
P
PHILIPS MEDICAL SYSTEMS
Defibrillator pads connector
socket. Receptacle for the defibrillator pads
cable connector. A light on the socket
flashes when the FR3 is turned on to show
socket location.
Green On/Off button. Tu r n s o n th e
FR3 and starts voice and text prompts. A
second press brings up the status screen,
and then turns off the FR3.
Green Ready light. Shows the
readiness status of the FR3.
Microphone. Used optionally to
record audio during an incident.
Infant/Child Key port.
Accommodates the optional FR3
Infant/Child Key accessory to enable
pediatric treatment protocols for patients
under 55 lbs (25 kg) or 8 years old.
Screen. Displays text prompts,
graphics, and incident data. The FR3 ECG
model also displays the patient’s ECG if
enabled.
Option buttons (three). When
pressed, activates the function identified on
the screen.
Orange Shock button. Controls
shock delivery. The button flashes when the
FR3 is ready to deliver a shock.
SMART Pads III. Self-adhesive pads
supplied with attached cable and connector.
If using an optional FR3 system case and/or
the Pads Sentry, store pads in Pads Sentry
and pre-connect pads to FR3 for automatic
self-test.
Battery. Battery used to power
the FR3.
Battery compartment. Provides
electrical connection for installed battery
and contains data card slot and Bluetooth®
wireless technology transceiver module
compartment.
Data card slot. Receptacle for
optional data card accessory. Located
beneath the battery in the battery
compartment.
Bluetooth wireless technology
transceiver module compartment.
Accommodates optional transceiver module
accessory. Located behind a removable door
in battery compartment.
Speaker. Broadcasts FR3 voice
prompts and alert tones when appropriate.
Beeper. Broadcasts FR3 alert chirps
when appropriate.
Accessory port. Connection port for
CPR meter accessory.
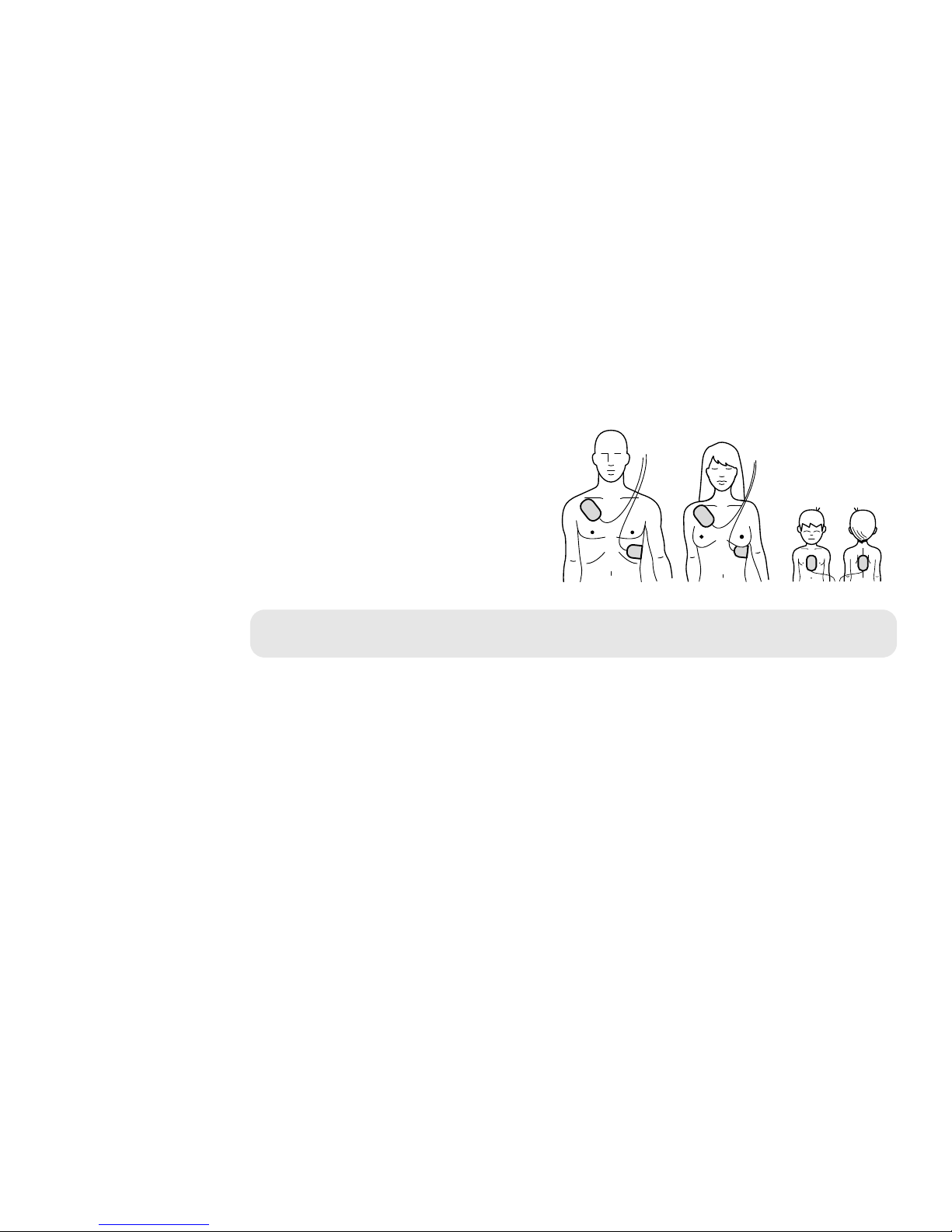
HEARTSTART FR3 DEFIBRILLATOR
Pads placement on adults.
Pads placement on children
under 55 lbs (25 kg) or
8 years old.
DESCRIPTION AND INDICATIONS FOR USE
The HeartStart FR3 Defibrillator (FR3) is a compact, lightweight, battery-powered automated external
defibrillator (AED) designed for use by trained responders to treat suspected victims of ventricular
fibrillation (VF), the most common cause of sudden cardiac arrest (SCA), and certain ventricular
tachycardias (VTs). The FR3 is used with disposable defibrillator pads applied to potential victims of
SCA with the following symptoms:
• Unresponsiveness
• Absence of normal breathing
If in doubt, apply the pads, as illustrated.
The FR3 is intended for use on adults and
children over 55 lbs (25 kg) or 8 years old.
The FR3 is also intended for children under
55 lbs (25 kg) or 8 years old when used
with the optional FR3 Infant/Child Key. If the
Infant/Child Key is not available, or you are
uncertain of the child’s age or weight, do not
delay treatment. Apply the pads as illustrated
for a child and use the defibrillator.
WARNING: Performance of the SMART CPR AUTO1 and AUTO2 settings for the CPR First
feature has not been established in patients under 55 lb (25 kg) or 8 years old.
PHILIPS MEDICAL SYSTEMS
INTENDED USER
The HeartStart FR3 is intended for use by responders who have been trained in its operation and
qualified by training in Basic Life Support (BLS), Advanced Life Support (ALS), or another physicianauthorized emergency medical response program.
SETUP AND CONFIGURATION
The FR3 is shipped with a factory-default setup optimized for compliance with Guidelines 2010 to
meet the needs of most users. However, the FR3 is extensively configurable. Any changes to default
settings must be done under supervision of the Medical Director. Depending on the kind of changes
you will be making, you will also need the following:
• Changing the FR3 primary language: the FR3 language card, provided with certain versions of the
FR3. If you do not have a language card and want to modify the default language of the FR3, or to
enable the bilingual option and select a second language, use HeartStart Configure software,
version 3.1 or higher and a data card.
• Changing the FR3 device operation, self-test options, patient care, defibrillation, and advanced use mode
parameter settings: the HeartStart Configure software, available separately.
1
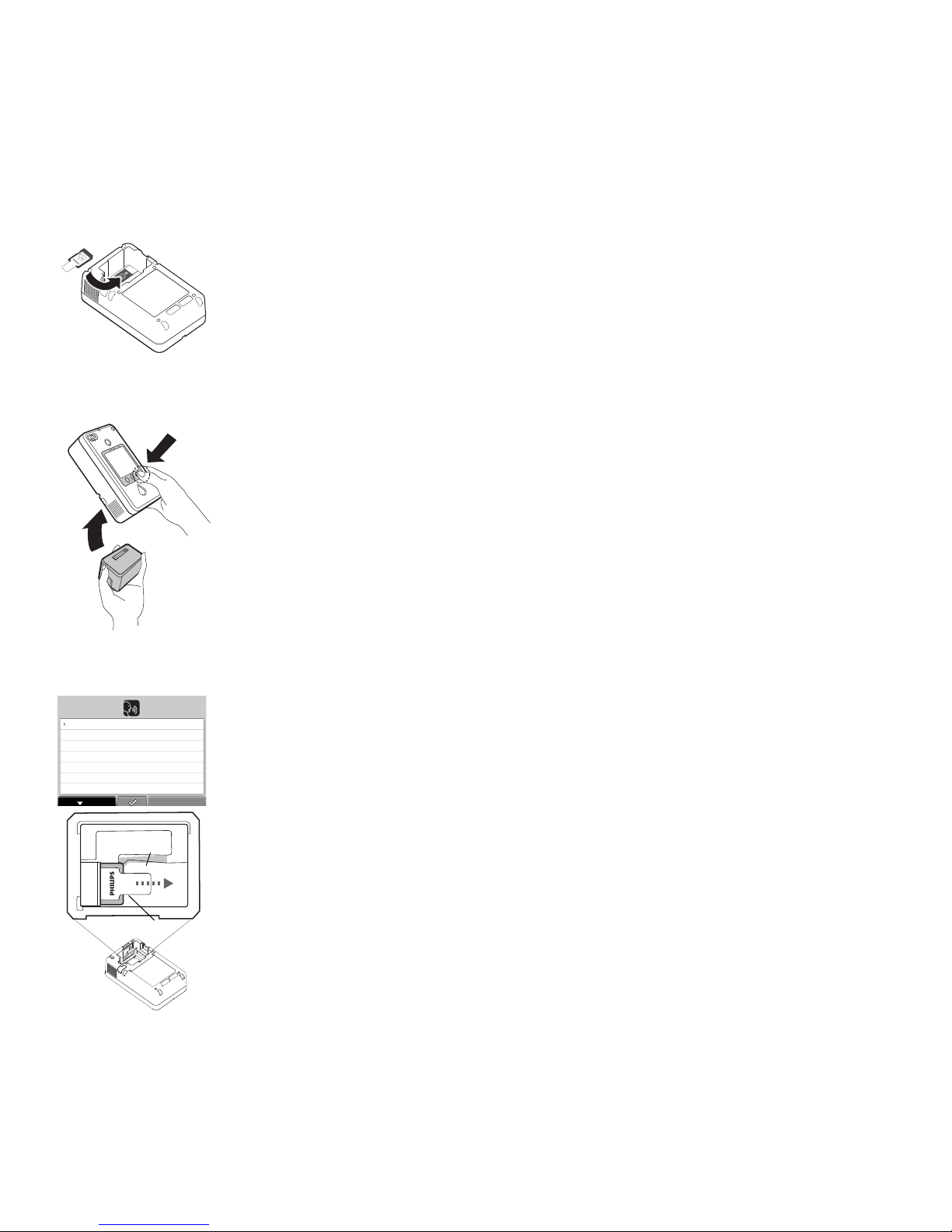
• Enabling the FR3 bilingual option and selecting a secondary language: the HeartStart Configure
(Optional) Install the FR3
language card.
(Optional) Re-install the
battery while holding down
the right option button.
86(QJOLVK
,WDOLDQR
)UDQoDLV
'HXWVFK
8.(QJOLVK
(VSDxRO
邁釈딺
Battery compartment.
Retention clip.
Language card tail.
software and the FR3 language card or a data card.
Setup with the FR3 language card (if provided) and the optional FR3 data card is discussed below. See
the FR3 Instructions for Administrators CD-ROM, provided with the FR3, for a list of accessories and
directions on setting up the FR3 with certain other optional accessories.
INSTALLING THE FR3 LANGUAGE CARD AND THE BATTERY
The primary language is the language the FR3 uses for voice and text prompts. The FR3 can operate in
any language that is provided on the FR3 language card.
If you do not want to change the default primary language, go to the following section,
“Installing the Optional Data Card and the Battery.”
To change the default primary language to a different primary language, follow these steps:
1. Insert the FR3 language card into the data card slot in the bottom of the FR3 battery
compartment. A label on the floor of the battery compartment shows the correct orientation for
inserting the card. A retention clip helps hold the card in the slot. The tail on the data card serves
as a visual reminder that a card is installed and aids in card removal.
2. Check the battery label to be sure the battery is within its install-by date. Remove the battery
from its packaging. Install the battery in the FR3 battery compartment, while holding down the right
option button. Keep the button pressed until the language selection screen is displayed. The FR3
display screen automatically provides a list of languages. There may be more than one screen of
languages.
3. Press the option button to scroll to the language you want, then press the option button to
select it. (If no selection is made within 60 seconds, the FR3 displays an error message and shuts
down after approximately 5 minutes.) The new language file automatically loads. The FR3 displays
the language name and a progress bar. During language installation, the FR3 cannot be turned off.
When the language load is complete, the FR3 will operate in the new language. Other default or
previously modified configuration settings will remain unchanged. The FR3 displays a screen for 10
seconds announcing that the setup is complete, and reminding you to remove the language card.
4. Now, remove the battery and the FR3 language card. To remove the language card, grasp the data
card by its tail, press the retention clip away from the card, and pull the card out. Store the
language card in a safe place for future use or reference.
5. Insert an FR3 data card, if desired, in the data card slot (see following section), and reinstall the
battery. As soon as the battery is installed, the FR3 automatically runs a detailed interactive userinitiated test. Press the buttons when directed, or the test will fail.
6. On completion of the test, the FR3 displays the results and then displays the main Administration
screen. If the FR3 clock has not yet been set, the FR3 will provide a reminder message. See
instructions below.
7. After setting the clock, press the green On/Off button to display the status screen and put the
defibrillator into standby.
PHILIPS MEDICAL SYSTEMS
2
 Loading...
Loading...