Philips HeartStart FR2 Service manual

eЙ~кнpн~кн=coOH=aЙСбДкбдд~нзк
INSTRUCTIONS FOR USE
M3860A, M3861A
Edition 15
qЬЙ=eЙ~кнpн~кн=coOH=aЙСбДкбдд~нзк
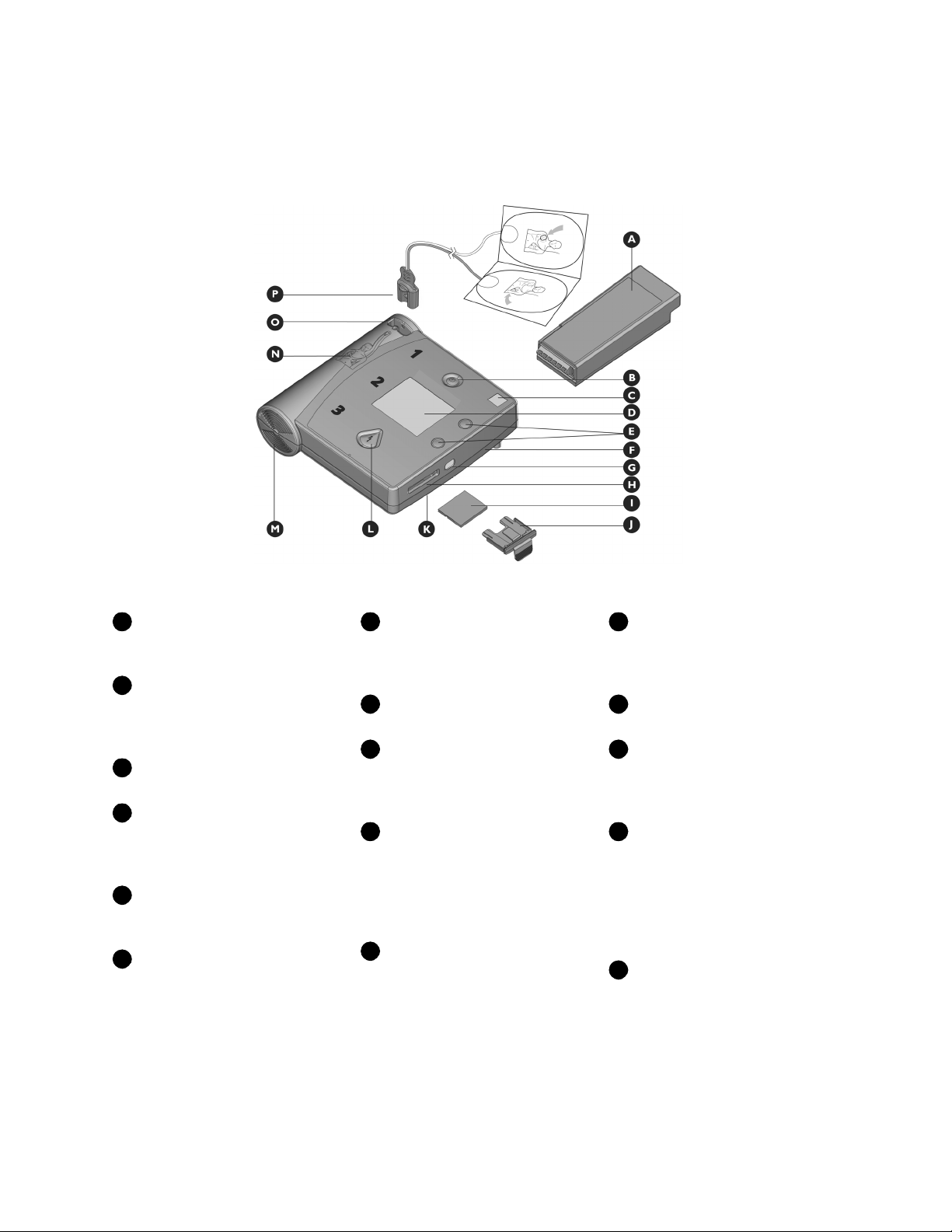
A
B
C
DEFGH
I
JKLMNOP
PHILIPS MEDICA L SYSTEMS
Battery. Standard long-life or
rechargeable battery used to
power the FR2+.
On/Off button. Turns on the
FR2+ and starts voice and text
prompts. Second press turns off
the FR2+.
Status Indicator. Shows you
the readiness status of the FR2+.
Display screen. Displays text
prompts and incident data. The
FR2+ M3860A screen also displays
the patient’s ECG.
Option buttons. Adjust the
contrast of the screen display and
control special functions.
Beeper port. Broadcasts
alert beeps when required. It is
located under the right edge of the
FR2+.
Infrared (IR) communica-
tions port.
“eye,” used to transfer data
directly to or from another device.
A special lens, or
Data card port. Receptacle
for data card tray.
Data card (optional). Used
to store and review information
about an incident, including ECG
and optional voice recording.
Data card tray. Special sleeve
that holds the data card and fits
into the data card port to help seal
the FR2+ against fluids. The tray
should be kept installed in the FR2+
even if no data card is used.
Microphone. Used optionally
to record surrounding audio
during an incident. It is located
under the right edge of the FR2+.
Shock button. Controls
shock delivery. The button flashes
when the HeartStart FR2+ is ready
to deliver a shock.
Speaker. Amplifies voice
prompts during use of the FR2+.
Pads placement diagram.
Illustrates correct placement of
adult pads. Diagrams are also shown
on the defibrillator pads.
Defibrillator pads
connector socket.
connector of the defibrillator pads
cable. An adjacent LED light flashes
to show socket location and is
covered when connector is
inserted.
Receptacle for
Defibrillator pads. Self-
adhesive pads with attached cable
and connector. (Adult pads
shown.)
eЙ~кнpн~кн=coOH=aЙСбДкбдд~нзк
nrf`h=obcbobk`b=`^oa
PHILIPS MEDICA L SYSTEMS

Intentionally blank.
PHILIPS MEDICA L SYSTEMS

eÉ~êípí~ê í=coOH
jPUSM^LjPUSN^
^мнзг~нЙЗ=bснЙке~д=aЙСбДкбдд~нзк
INSTRUCTIONS FOR USE
Edition 15
IMPORTANT NOTE:
It is important to understand that survival rates for sudden cardiac arrest
are directly related to how soon victims receive treatment. For every
minute of delay, the chance of survival declines by 7% to 10%.
Defibrillation cannot assure survival. In some patients, the underlying
problem causing the cardiac arrest is simply not survivable despite any
PHILIPS MEDICA L SYSTEMS
available care.

About this edition
The information in this guide applies to the
model M3860A/M3861A HeartStart FR2+
Defibrillator. This information is subject to
change. Please contact Philips at
www.medical.philips.com/heartstart or your
local Philips representative for information on
revisions.
CAUTION: Federal law (USA) restricts this
device to sale by or on the order of a physician.
The HeartStart FR2+ is designed to be used only
with Philips-approved accessories. The HeartStart
FR2+ may perform improperly if non-approved
accessories are used.
Device Tracking
Edition history
Edition 15
Publication date: April 2008
Publication number: 011120-0015
Printed in the U.S.A.
Copyright
© 2008 Philips Electronics North America
Corp.
No part of this publication may be
reproduced, transmitted, transcribed, stored
in a retrieval system or translated into any
human or computer language in any form by
any means without the consent of the
copyright holder.
Unauthorized copying of this publication may
not only infringe copyright but also reduce the
ability of Philips Medical Systems to provide
accurate and up-to-date information to users
and operators alike.
Authorized EU Representative
Philips Medizin Systeme Boeblingen GmbH
Hewlett-Packard Strasse 2
71034 Boeblingen, Germany
(+49) 7031 463-2254
This device is subject to tracking requirements by
the manufacturer and distributors. If the
defibrillator has been sold, donated, lost, stolen,
exported, or destroyed, notify Philips Medical
Systems or your distributor.
Device Manufacturer
The HeartStart FR2+ Defibrillator is manufactured
by Philips Medical Systems, Seattle, Washington,
USA.
Patents
This product is manufactured and sold under one
or more of the following United States patents:
U.S. Pat. No US6047212, US6317635, US5892046,
US5891049, US6356785, US5650750, US6553257,
US5902249, US6287328, US6662056, US5617853,
US5951598, US6272385, US6234816, US6346014,
US6230054,US6299574, US5607454, US5803927,
US5735879, US5749905, US5601612, US6441582,
US5889388, US5773961, US6016059, US6075369,
US5904707, US5868792, US5899926, US5879374,
US5632280, US5800460, US6185458, US5611815,
US6556864, and other patents pending.
PHILIPS MEDICA L SYSTEMS
HEARTSTART FR2+ M3860A/M3861A

PHILIPS MEDICAL S YSTEMS
`lkqbkqp
N fkqolar`qflk=ql=qeb=eb^oqpq^oq=coOH
Device description .......................................................................................... 1-1
Indications for use ........................................................................................... 1-1
Principles of operation ................................................................................... 1-2
O dbqqfkd=pq^oqba
Package contents ............................................................................................. 2-1
Setup overview ................................................................................................. 2-1
Installing the data card .................................................................................... 2-1
Installing the battery and setting the clock ................................................ 2-2
Running the Battery Insertion Test ............................................................. 2-3
Placing and securing the HeartStart FR2+ ................................................. 2-3
P rpfkd=qeb=eb^oqpq^oq=coOH
Overview ........................................................................................................... 3-1
Treating infants and children ........................................................................ 3-1
Step 1: Preparation .......................................................................................... 3-2
Step 2: ECG analysis and CPR interval ....................................................... 3-3
Step 3: Shock delivery .................................................................................... 3-4
CPR Interval .............................................................................................. 3-4
ECG display for ongoing observation ......................................................... 3-5
Q j^fkq^fkfkdI=qbpqfkdI=^ka=qolr_ibpel lqfkd
Maintenance ...................................................................................................... 4-1
Suggested maintenance schedule .......................................................... 4-1
After using the HeartStart FR2+ .......................................................... 4-2
Cleaning the HeartStart FR2+ .............................................................. 4-3
Operator’s checklist ................................................................................ 4-4
i

ii
Testing ............................................................................................................... 4-5
Battery insertion selftest ........................................................................ 4-5
Device history .......................................................................................... 4-8
Battery History ......................................................................................... 4-8
Troubleshooting Guide .................................................................................. 4-9
Status indicator summary ....................................................................... 4-9
Recommended action during an emergency ..................................... 4-9
Troubleshooting during patient use .................................................... 4-10
General troubleshooting ........................................................................ 4-12
R p^cbqv=`lkpfabo^qflkp
General dangers, warnings, and cautions ................................................... 5-1
Defibrillation warnings and cautions ........................................................... 5-3
Monitoring cautions ........................................................................................ 5-4
PHILIPS MEDICA L SYSTEMS
Maintenance cautions ..................................................................................... 5-4
S `lkcfdro^qflkI=pbqrmI=^ka=^as^k`ba=jlab=cb^qrobp
Configuration ................................................................................................... 6-1
Non-protocol parameters ..................................................................... 6-1
Automatic protocol parameters .......................................................... 6-2
Manual override parameters ................................................................. 6-5
Using setup features ....................................................................................... 6-6
Reviewing current setup ........................................................................ 6-7
Revising setup ........................................................................................... 6-7
Receiving setup ......................................................................................... 6-8
Reading setup ............................................................................................ 6-9
Sending and receiving clock settings .................................................... 6-9
Using advanced mode features .................................................................... 6-10
Using the manual analyze feature ......................................................... 6-11
Using the manual charge feature (M3860A only) ............................. 6-12
HEARTSTART FR2+ M3860A/M3861A

T a^q^=j^k^dbjbkq=^ka=obsfbt
Overview ........................................................................................................... 7-1
Recording incident data ................................................................................. 7-1
Recording data in internal memory ..................................................... 7-1
Recording data on a data card .............................................................. 7-1
Reviewing Incident Data ................................................................................ 7-2
Reviewing data from internal memory ................................................ 7-2
Reviewing data from a data card .......................................................... 7-3
iii
PHILIPS MEDICA L SYSTEMS

iv
^mmbkaf`bp
^ ^ЕЕЙллзкбЙл=Сзк=нЬЙ=eЙ~кнpн~кн=coOH=
_ qЙЕЬебЕ~д=pйЙЕбСбЕ~нбзел=
` dдзлл~ку=зС=лугДздл=~еЗ=Езенкздл=
a dдзлл~ку=зС=нЙкгл=
b `mo=cбклн=`зеСбЦмк~нбзе=
c ^ЗЗбнбзе~д=нЙЕЬебЕ~д=З~н~=кЙимбкЙЗ=Сзк=bмкзйЙ~е=ЕзеСзкгбну=
PHILIPS MEDICA L SYSTEMS
fåÇÉñ
HEARTSTART FR2+ M3860A/M3861A
N fенкзЗмЕнбзе=нз=нЬЙ=eЙ~кнpн~кн=coOH
Device description
The HeartStart FR2+ Defibrillator (“FR2+”) is a compact, lightweight, portable,
and battery powered automated external defibrillator designed for use by a
trained responder to treat ventricular fibrillation (VF), the most common cause of
sudden cardiac arrest (SCA).
The FR2+ has a Status Indicator that is always active, so you can tell at a glance if it
has passed its last selftest. The front panel of the FR2+ has an On/Off button at
the top and a Shock button at the bottom. A display screen in the center of the
panel provides text prompts and incident information. Voice prompts are provided
through a speaker located at the base of the FR2+. See the diagram on the inside
front cover for details.
The FR2+ is available in two models, the M3860A and the M3861A. They share a
set of basic features, detailed in Chapter 6. The principle differences between the
two models are identified below:
1
PHILIPS MEDICA L SYSTEMS
Configurable ECG display on screen Text prompt display on screen, no
Configurable manual charge in
advanced mode (See Chapter 6)
NOTE: The FR2+ comes with a factory default setup that can be modified. (See
Chapter 6, “Configuration, Setup, and Advanced Mode Features,” for a description of setup defaults and options.)
Model M3860A Model M3861A
ECG display
No manual charge in advanced mode
(See Chapter 6)
Indications for use
The HeartStart FR2+ is intended to be used with disposable defibrillator pads
applied to victims of sudden cardiac arrest exhibiting the following symptoms:
• Unresponsiveness
• Absence of normal breathing
If in doubt, apply the pads.
1-1

1-2
To use the FR2+ on children under 8 years or 55 pounds (25 kg), apply FR2 Infant/
child reduced-energy defibrillator pads, if available. Otherwise, apply the standard
pads.
WARNING: Performance of the SMART CPR AUTO1 and AUTO2 settings
has not been established in patients under 8 years or 55 lb. (25 kg). See
Appendix E for more information.
The FR2+ is intended for use by responders who have been trained in its
operation. The user should be qualified by training in Basic Life Support (BLS), in
Advanced Life Support (ALS), or another physician-authorized emergency medical
response program.
At the discretion of emergency care personnel, the FR2+ M3860A can also be
used with the FR2+ ECG assessment module to provide non-diagnostic display
and evaluation of the heart rhythm of a responsive or breathing patient, regardless
of age, for attended patient monitoring. While connected to the FR2+ ECG
assessment module, the FR2+ M3860A disables its shock capability.
Principles of operation
The HeartStart FR2+ Defibrillator is designed to provide external defibrillation
therapy to someone experiencing sudden cardiac arrest caused by ventricular
fibrillation (VF), the most common cause of SCA. The only effective treatment for
VF is defibrillation.The FR2+ treats VF by sending a shock across the heart, so it
can start beating regularly again.
PHILIPS MEDICA L SYSTEMS
The FR2+ is extremely easy to use. When connected to defibrillator pads that are
properly applied to the patient’s bare chest, the FR2+:
1. prompts you to take specific actions,
2. automatically analyzes the patient's heart rhythm and advises you whether or
3. arms the Shock button, if appropriate, and instructs you to press it to deliver
Detailed instructions for use are provided in Chapter 3.
HEARTSTART FR2+ M3860A/M3861A
not the rhythm is shockable, and
a biphasic electric pulse designed to defibrillate the heart.

O dЙннбеЦ=pн~кнЙЗ
Package contents
The HeartStart FR2+ Defibrillator is supplied with a standard long-life battery, two
sets of adult defibrillator pads with integrated cable and connector, and a data card
tray. Other accessories, including FR2 infant/child reduced-energy defibrillator
pads, an FR2+ rechargeable battery, and (for M3860A only, with ECG display
enabled) a three-wire FR2+ ECG assessment module, are available. See Appendix
A for a list of accessories and other recommended supplies.
2
Setup overview
Setting up the HeartStart FR2+ for use is quick and simple.
• Install a data card. (optional)
• Install a battery.
• Set the clock in the FR2+. (optional)
• Run the battery insertion selftest.
• Place the FR2+ with recommended accessories in a convenient location.
PHILIPS MEDICA L SYSTEMS
Installing the data card
The HeartStart FR2+ comes with a data card tray, which should be kept in the
data card port even if no data card is used. If a data card is to be used, install it as
follows:
1. Load the data card face up into the data card tray, with the tray’s “tongue”
fitting over the matching yellow area on the data card.
2. Press the On/Off button to turn off the HeartStart FR2+ if it is on.
3. Hold the loaded data card tray by its handle and gently insert the tray all the
way into the defibrillator’s data card port until only the tab remains outside
the case.
2-1
2-2
The data card will automatically record incident data the next time the HeartStart
FR2+ is turned on.
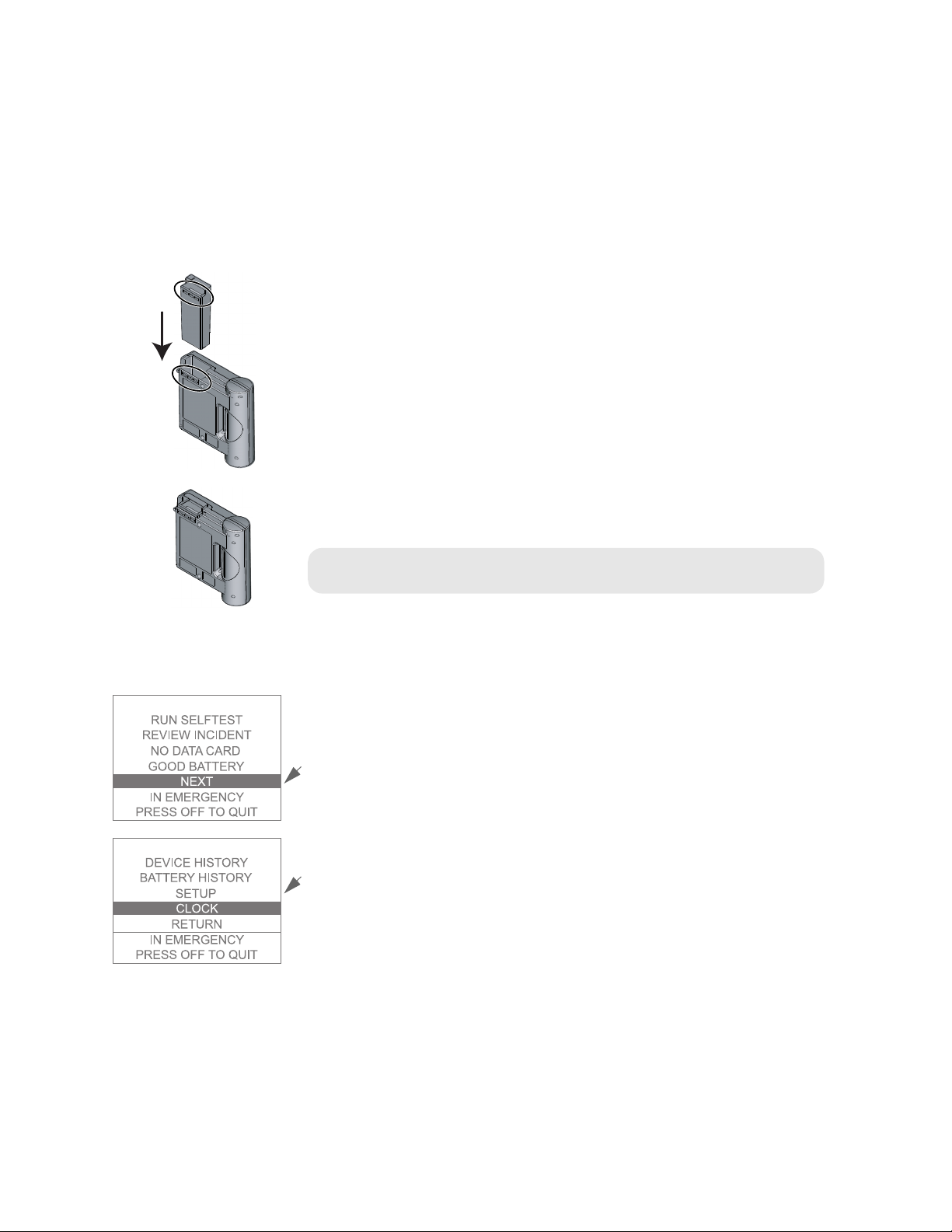
Installing the battery and setting the clock
The HeartStart FR2+ Defibrillator is shipped with an M3863A standard, long-life
battery. The battery is enclosed in a gray plastic case with a yellow latch at one
end, designed to hold the battery in place when it is correctly installed. (The
optional M3848A FR2+ rechargeable battery case is blue, and it also has a yellow
latch. Except where otherwise noted, the following information applies to both
battery types.) To install the battery:
1. Hold the battery by the latch end and slide it into the battery compartment at
the top of the HeartStart FR2+.
2. Slide the battery all the way into the opening, until the latch clicks into place.
PHILIPS MEDICA L SYSTEMS
CAUTION: Follow all instructions supplied with the battery. Install the battery
before the install-by date shown on the battery.
When the battery is installed, the FR2+ automatically turns on. The Status
Indicator displays a flashing black hourglass. The Shock button light and the
indicator light for the defibrillator pads connector socket turn on briefly. The
display screen brings up the main menu.
It is recommended that you set the FR2+’s internal clock to the correct date and
local time at this point.
1. Within 10 seconds of installing the battery, press the lower Option button to
2. Press the upper Option button to select
3. Press the lower Option button to move the highlight bar to
4. Press the upper Option button to bring up the CLOCK menu.
5. To receive clock settings from another FR2+, see directions provided in
HEARTSTARTFR2+ M3860A/M3861A
move the highlight bar on the displayed main menu to
NEXT to bring up the second menu
NEXT.
screen.
CLOCK.
Chapter 6. To manually set the time and date, follow the remaining steps.

2-3
6. Press the lower Option button to move the highlight bar to the date or time
field to be changed.
7. Using the upper Option button, scroll through the available settings to the
desired value.
8. Use the lower Option button to move to any other date or time field to be
changed, and repeat step 6.
9. When all selections have been made, use the lower Option button to move
the highlight bar to
RETURN, then press the upper Option button to return to
the second menu.
10. After ten seconds, the HeartStart FR2+ automatically starts the battery
insertion selftest.
*
If you choose not to set the clock at this point, the HeartStart FR2+ automatically
starts the battery insertion selftest within ten seconds of battery insertion. You
can remove and reinsert the battery at any time to review or adjust the clock
settings.
Running the Battery Insertion Test
2
PHILIPS MEDICA L SYSTEMS
The battery insertion selftest has two parts, an automatic part, during which the
screen displays a bar that fills in as the test continues, followed by an interactive
part. Follow the defibrillator’s prompts during the interactive part of the test.
When the FR2+ has passed the battery insertion selftest, it turns off and goes to
standby mode to be ready for use. Standby mode is indicated by the flashing black
hourglass status indicator.
NOTE:If the battery is removed from the FR2+ for more than two hours, the
clock settings will be lost and must be reset.
Placing and securing the HeartStart FR2+
Place the HeartStart FR2+ Defibrillator in an accessible area with the Status
Indicator easily visible. The defibrillator can be secured in a carrying case suitable
for use with a wallmount bracket or defibrillator cabinet. Useful accessories for
* See Chapter 4 for details about the battery insertion selftest.
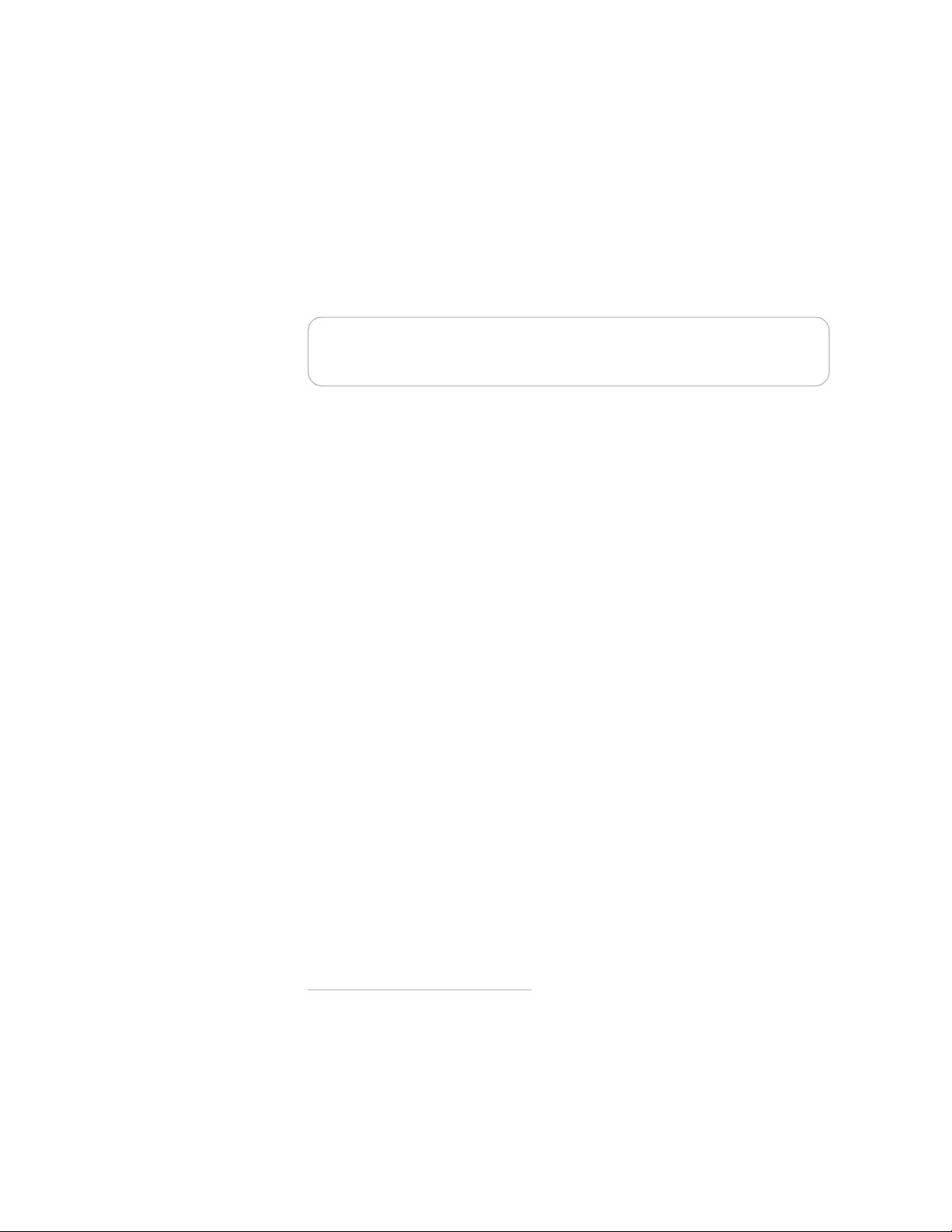
2-4
storage with the HeartStart FR2+ include a spare battery, spare pads, spare data
card (if used), and a Fast Response Kit containing a pocket mask, a disposable
razor, 2 pairs of gloves, a pair of paramedics scissors, and an absorbent wipe. See
Appendix A for a list of accessories.
NOTE: Be careful not to overpack the carrying case, to avoid placing inadvertent
pressure on the control buttons. Do not store the FR2+ with the defibrillator
pads attached. Do not open the pads package until ready for use.
With the battery installed and the FR2+ stored in appropriate environmental
*
conditions,
the FR2+ performs detailed daily, weekly, and monthly selftests to
check its readiness for use. These periodic selftests are described in Chapter 4.
While the FR2+ is in the standby mode, the Status Indicator shows the flashing
black hourglass unless the periodic selftest detects a problem. If a problem is
detected, the Status Indicator will show a flashing red X or a solid red X and the
FR2+ will chirp to alert you. Chapter 4 contains instructions for troubleshooting.
PHILIPS MEDICA L SYSTEMS
* See Appendix B for environmental specifications.
HEARTSTARTFR2+ M3860A/M3861A

P rëáåÖ=íÜÉ=eÉ~êípí~êí=coOH
Overview
This chapter describes how to use the HeartStart FR2+ Defibrillator in an
emergency. Some general things to remember are:
Try to relax and stay calm. The HeartStart FR2+ automatically provides voice
and text prompts to guide you through each step of its use.
The defibrillator pads must have good contact with the patient’s skin. The
pads have a layer of sticky, conductive gel beneath the protective backing.
It may be necessary to dry the patient’s skin or to clip or shave excessive
chest hair to provide good contact between the defibrillator pads and the
patient’s skin.
Be sure to read the Warnings and Cautions on the last page of this chapter.
Detailed directions for use based on default configuration are provided on the
following pages.
3
PHILIPS MEDICA L SYSTEMS
NOTE:These directions apply to both the model M3860A and the model
M3861A FR2+, except where otherwise noted.
Treating infants and children
WARNING: Most cardiac arrests in children are not caused by heart
problems. When responding to cardiac arrest in an infant or child:
• Provide infant/child CPR while a bystander calls EMS and brings the FR2+.
• If no bystander is available, provide 1-2 minutes of CPR before calling EMS
and retrieving the FR2+.
• If you witnessed the child's collapse, call EMS immediately and then get the
FR2+.
Alternatively, follow your local protocol.
3-1
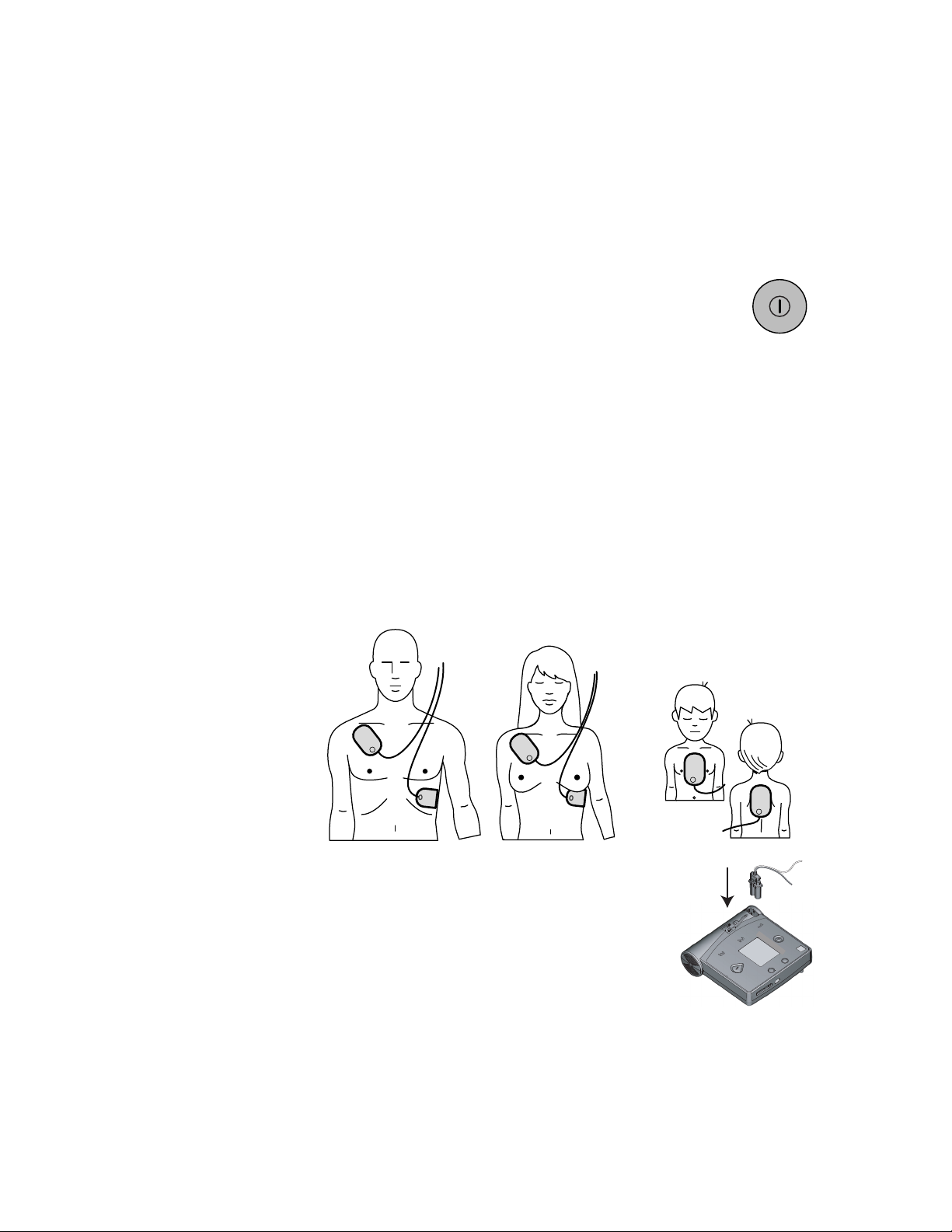
3-2
O
N
•
O
F
F
Pad placement for adults and children 8 years old
or 55 pounds or more.
Pad placement for infants and children
under 8 years old or 55 pounds.
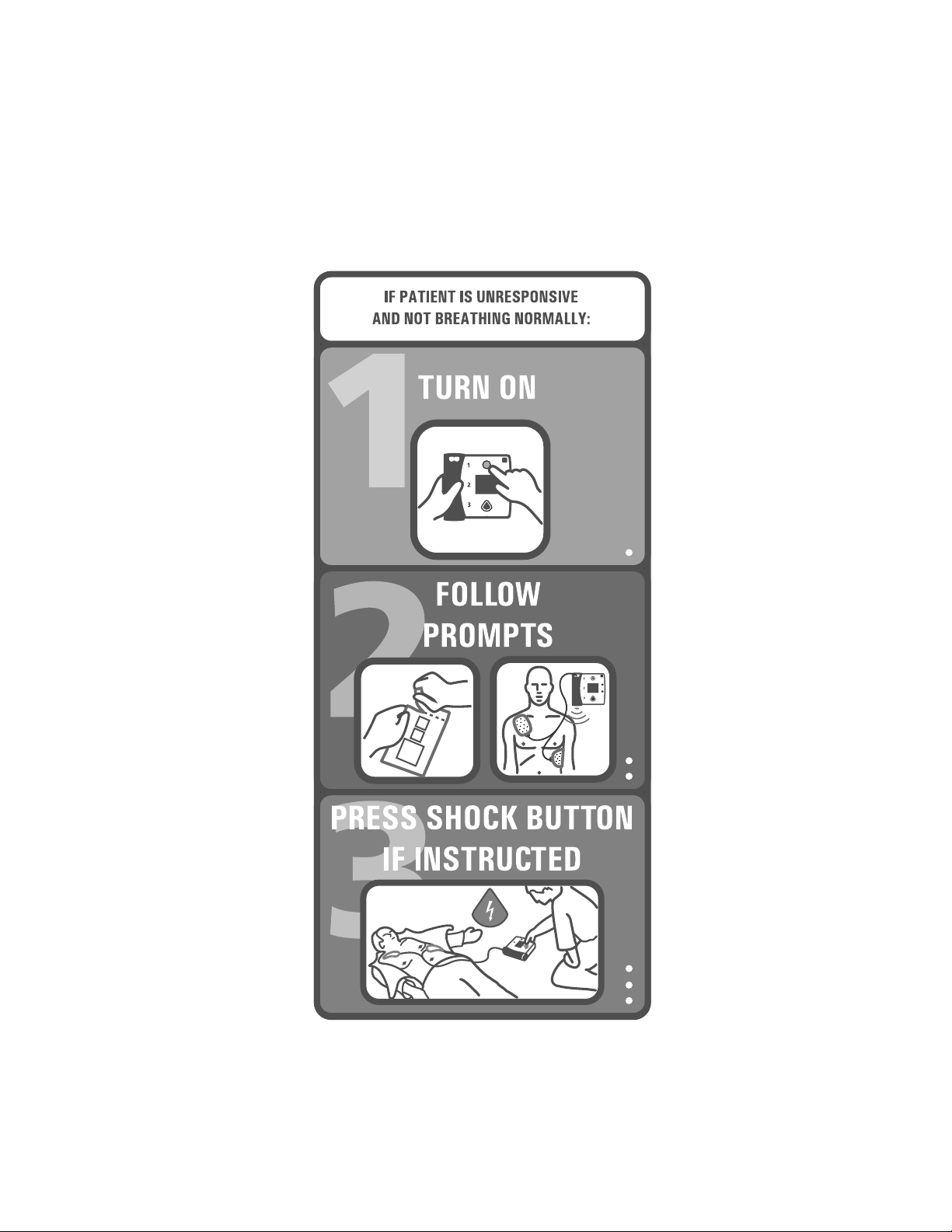
Step 1: Preparation
Press the On/Off button to turn on the HeartStart FR2+
Defibrillator. Follow the instructions provided by the FR2+ voice
and text prompts.
• Remove clothing from the patient's upper body. If needed, wipe
moisture from the patient's skin and clip or shave excessive chest hair.
• If the patient appears to be under eight years of age or 55 lb. (25 Kg), use
M3870A FR2 infant/child reduced-energy defibrillator pads, if available. If the
infant/child pads are not available, or if the child appears older/larger, use adult
defibrillator pads.
EXACT AGE/WEIGHT.
• Open the defibrillator pads package. Pull off the protective backing from the
defibrillator pads.
• Place the sticky side of each pad on the patient’s bare skin, exactly as shown on
the drawing on each pad.
DO NOT DELAY TREATMENT TO DETERMINE THE CHILD’S
PHILIPS MEDICA L SYSTEMS
HEARTSTART FR2+ M3860A/M3861A
• Insert the defibrillator pads connector firmly into the
defibrillator’s connector socket, indicated by a
flashing light at the top left of the FR2+.
Step 2: ECG analysis and CPR interval
Follow the instructions provided by the FR2+ Defibrillator’s voice and text
prompts.
As soon as the FR2+ detects that the defibrillator
pads are connected properly, it automatically begins
analyzing the patient’s heart rhythm. Do not touch the
patient during rhythm analysis.
3-3
If no shock is advised
voice and text prompts to tell you so and provides a
CPR interval with a prompt to perform CPR, if
needed. The duration of the CPR interval is determined by CPR Timer setting.
Following the CPR interval, the FR2+ reanalyzes the patient’s heart rhythm. If no
shock is again advised, the device goes into a patient care interval, during which
you can perform CPR if needed or otherwise tend to the patient. The duration of
the patient care interval is determined by the NSA Action setting.
NOTE:CPR may interfere with background heart rhythm monitoring by the
FR2+ in monitoring mode. During CPR, periodically pause for 15 seconds to
PHILIPS MEDICA L SYSTEMS
reassess the patient and allow the FR2+ to analyze the patient’s heart rhythm
without possible interference from CPR artifact.
If a shock is advised
gives the voice and text prompts to tell you that a shock is advised.
, the HeartStart FR2+ provides
, the HeartStart FR2+ charges to prepare for shock delivery. It
3
3-4
Step 3: Shock delivery
First, make sure that no one is touching the patient or the pads. While the FR2+ is
charging, it continues to analyze the patient’s heart rhythm. If the rhythm changes
and a shock is no longer appropriate, the FR2+ disarms. Voice and text prompts
advise you what action to take.
There are four ways you can tell that the defibrillator is ready to deliver a shock:
• you hear a voice prompt telling you to deliver a shock,
• you see the Shock button flashing,
• you hear a steady tone, and/or
• you see a text prompt telling you to press the orange (Shock) button.
Press the Shock button to deliver the shock.
IMPORTANT: You must press the button for a shock to be
delivered. The HeartStart FR2+ Defibrillator will not automatically
deliver a shock. This safety feature allows you to verify that the
patient is clear before a shock is delivered.
NOTE:If you do not press the Shock button within 30 seconds of being
prompted, the HeartStart FR2+ will disarm itself and provide a pause. It will
resume analyzing heart rhythm after 30 seconds or when the Resume
Analyzing key is pressed.
PHILIPS MEDICA L SYSTEMS
After you press the Shock button, a voice prompt tells you the shock was
delivered. Then the FR2+ pauses to allow you to perform CPR. The duration of
this CPR interval is determined by the CPR Timer setting.
CPR Interval
After telling you that it has paused, the FR2+ gives no more voice prompts during
the CPR interval, so that you can provide uninterrupted patient care. During the
CPR interval, the FR2+ screen shows a bar that fills in as the CPR interval is used
up. The HeartStart FR2+ M3860A also displays the ECG, if enabled, during this
period.
NOTE:It is important to perform CPR for the entire duration of the CPR
interval, until you hear the voice prompt telling you to stop CPR.
HEARTSTART FR2+ M3860A/M3861A
3-5
ECG display for ongoing observation
At the discretion of emergency care personnel, the HeartStart FR2+ M3860A with
ECG display enabled can also be used with the M3873A/M3874A FR2+ ECG
assessment module to provide a non-diagnostic ECG display of the patient’s heart
rhythm for attended patient monitoring. The system is intended for use on a
conscious or breathing patient, regardless of age. While connected to the FR2+
ECG assessment module, the FR2+'s shock capability is disabled, but the FR2+
continues to evaluate the patient's ECG. There are no known contraindications to
use of the FR2+ ECG assessment module.
The module is designed for connection to ECG electrodes per AAMI (M3873A) or
IEC (M3874A) color convention. The module’s colored leadwires are connected
to ECG electrodes, which are then placed on the patient’s bare chest, and the
module’s device connector is inserted in the FR2+’s connector socket.
NOTE:It is not necessary to turn the FR2+ Defibrillator off prior to
connecting the ECG assessment module.
Once connected, the HeartStart FR2+ displays and evaluates the patient's ECG
(Lead II). Follow all prompts from the defibrillator. If a data card is used when the
ECG assessment module is connected, all recorded events can be viewed using
PHILIPS MEDICA L SYSTEMS
one of the Event Review data management software products.
3
Check the patient if:
• indicated by the observed ECG display,
• the patient becomes unresponsive or stops breathing, or
• the FR2+ prompts
If appropriate, unplug the ECG assessment module from the FR2+, attach the
defibrillator pads to the patient, and connect the defibrillator pads to the FR2+.
Verify that the defibrillator pads are at least one (1) inch (2.5 cm) away from the
ECG electrodes.
IF NEEDED, ATTACH DEFIBRILLATION PADS.

3-6
WARNING: During defibrillation, air pockets between the skin and
defibrillator pads can cause patient skin burns. To help prevent air pockets,
make sure defibrillator pads completely adhere to the skin.
WARNING: Do not let the defibrillator pads touch each other or other ECG
electrodes, lead wires, dressings, transdermal patches, etc. Such contact can
cause electrical arcing and patient skin burns during defibrillation and may
divert defibrillating current away from the heart.
WARNING: Handling or transporting the patient during heart rhythm analysis
can cause an incorrect or delayed diagnosis. If the FR2+ gives a
SHOCK ADVISED
prompt, keep the patient as still as possible for at least 15 seconds so the
HeartStart FR2+ can reconfirm the rhythm analysis before a shock is delivered.
WARNING: CPR rates significantly above 100 compressions per minute can
cause incorrect or delayed analysis by the FR2+. Under certain circumstances,
this may result in a prompt to stop all movement so that the device can
confirm rhythm analysis.
WARNING: Defibrillation current can cause operator or bystander injury. Do
not touch the patient during defibrillation. Do not allow the defibrillator pads
to touch any metal surfaces. Disconnect the pads connector from the FR2+
before using any other defibrillator.
PHILIPS MEDICA L SYSTEMS
CAUTION: Aggressive handling of the pads in storage or prior to use can
damage the pads. Discard the defibrillator pads if they become damaged.
CAUTION: Do not place the pads directly over an implanted pacemaker or
defibrillator. A noticeable lump with a surgical scar should indicate its position.
HEARTSTART FR2+ M3860A/M3861A

Q j~бен~бебеЦI=qЙлнбеЦI=~еЗ=qкзмДдЙлЬззнбеЦ
Maintenance
Maintenance of the FR2+ is very simple, but it is a very important factor in its
dependability. When in standby mode (with the battery installed), the FR2+
performs many maintenance activities itself. These include daily and weekly
selftests to verify readiness for use and more detailed monthly selftests that also
verify the shock waveform delivery system. In addition, each time the FR2+ is first
turned on, it executes a selftest. Further, a detailed battery insertion selftest is run
whenever a battery is installed in the FR2+.
The FR2+ requires no calibration or verification of energy delivery. The FR2+ has
no user-serviceable parts.
CAUTION: Improper maintenance may damage the FR2+ or cause it to
function improperly. Maintain the FR2+ only as described in these Instructions
for Use or as designated by your program's Medical Director.
CAUTION: Electrical shock hazard. Dangerous high voltages and currents are
present. Do not open the FR2+, remove its covers, or attempt repair. There
are no user-serviceable components in the FR2+. The FR2+ should be returned
PHILIPS MEDICA L SYSTEMS
to Philips for repair.
4
Suggested maintenance schedule
The following table presents a sample maintenance schedule for the FR2+.
Different frequency intervals may be appropriate, depending upon the
environment in which the FR2+ is used. The required maintenance frequency is at
the discretion of your program’s Medical Director.
4-1
4-2
daily monthly maintenance task/response
Check the Status Indicator.
If you see the flashing black hourglass: The FR2+ is ready to use.
No action required.
If you see anything other than a flashing black hourglass, remove
and reinstall the battery to run the selftest.
• If the selftest passes and the Status Indicator shows the
flashing black hourglass, the FR2+ is ready to use.
• If the selftest fails, install a new battery and run the selftest. If
the selftest passes, the FR2+ is ready to use. If the selftest
fails, contact Philips Medical Systems.
• If the selftest does not run, check to be sure that there is no
pads connector installed in the FR2+.
Check supplies, accessories, and spares for damage and
expiration dating.
Do not use damaged or expired accessories. Replace them
immediately.
If a LOW BATTERY or REPLACE BATTERY message is
displayed: Replace the battery and run the selftest.
• Do not attempt to charge the M3863A FR2 standard battery.
It is not rechargeable.
• The M3848A FR2+ battery is rechargeable. Recharge it, using
the M3849A Charger only.
PHILIPS MEDICA L SYSTEMS
After using the HeartStart FR2+
After each use of the FR2+, in addition to the maintenance tasks described in the
table above, perform the following post-use checks before returning the FR2+ to
service:
• Check the operation of the FR2+ by removing and reinstalling the battery and
• Check the outside of the FR2+ and the connector socket for signs of dirt or
HEARTSTART FR2+ M3860A/M3861A
Check the outside of the FR2+ and the connector socket
for cracks or other signs of damage.
If you see signs of damage: Contact Philips for technical support.
running the battery insertion selftest.
contamination. If the FR2+ is dirty or contaminated, clean it according to the
guidelines provided in this manual.

4-3
• Check the data card if one has been used. If the data card has been used to
record incident data, remove and replace it with a blank data card. Deliver the
recorded data card to appropriate personnel according to your local
guidelines and medical protocol.
• Check the connector socket to make sure that defibrillator pads are
disconnected from the FR2+ when it is not in use.
• Check to make sure the data card tray is installed, even if a data card is not
being used.
Cleaning the HeartStart FR2+
The outside of the FR2+, including the defibrillator pads connector socket, can be
cleaned with a soft cloth dampened in one of several appropriate cleaning agents
(see list below). The following guidelines include some important reminders:
• Do not immerse the FR2+ in fluids.
• Make sure a battery (or the M3864A Training & Administration Pack) and a
data card tray are installed when cleaning the FR2+, to keep fluids out of the
device.
• Do not use abrasive materials, cleaners, strong solvents such as acetone or
acetone-based cleaners, or enzymatic cleaners.
PHILIPS MEDICA L SYSTEMS
• Clean the FR2+ and the connector socket with a soft cloth dampened with
one of the cleaning agents listed below.
— Isopropyl alcohol (70% solution)
—Soapy water
— Chlorine bleach (30 ml/l water)
— Ammonia-based cleaners
— Glutaraldehyde-based cleaners
— Hydrogen peroxide
4
CAUTION: Do not immerse any portion of the FR2+ in water or other fluids.
Do not allow fluids to enter the FR2+. Avoid spilling any fluids on the FR2+ or
accessories. Spilling fluids into the FR2+ may damage it or present a fire or
shock hazard. Do not sterilize the FR2+ or accessories.
4-4
Operator’s checklist
The following checklist is for your reference. You may want to photocopy it or
use it as the basis for creating your own checklist.
lmbo^qloDp=`eb`hifpq
HeartStart FR2+ Model No.: ________________________ Serial No.: _________________________________
HeartStart FR2+ Location or Vehicle ID: _________________________________________________________
Date
Scheduled frequency
HeartStart FR2+
Clean, no dirt or contamination; no
signs of damage
Supplies available
• Two sets defibrillator pads,
sealed, undamaged, within
expiration date
• Ancillary supplies (hand towel,
scissors, razor, pocket mask,
gloves)
• Spare M3863A battery, within
“Install Before” date
• Data cards, undamaged, and spare
data card tray
PHILIPS MEDICA L SYSTEMS
Status indicator
Shows alternating hourglass/square;
selftest passed.
Inspected by
Signature or initials of operator
completing the maintenance
inspection
Remarks, problems, corrective
actions
HEARTSTART FR2+ M3860A/M3861A
4-5
Testing
The HeartStart FR2+ Defibrillator has several ways of testing itself and alerting
you if it finds a problem. In addition to the selftest performed each time it is
turned on and each time a battery is installed, the FR2+ also automatically
performs periodic selftests daily.
NOTE:The FR2+ selftests are designed to check that the FR2+ is ready for use.
However, in the event that the FR2+ has been dropped or mishandled, it is
recommended that the battery be removed and reinstalled to initiate a selftest.
If the FR2+ has visible signs of damage, contact Philips for technical support.
Battery insertion selftest
Before running the battery insertion selftest, be sure that neither the defibrillator
pads nor the FR2+ ECG assessment module are connected to the device. When
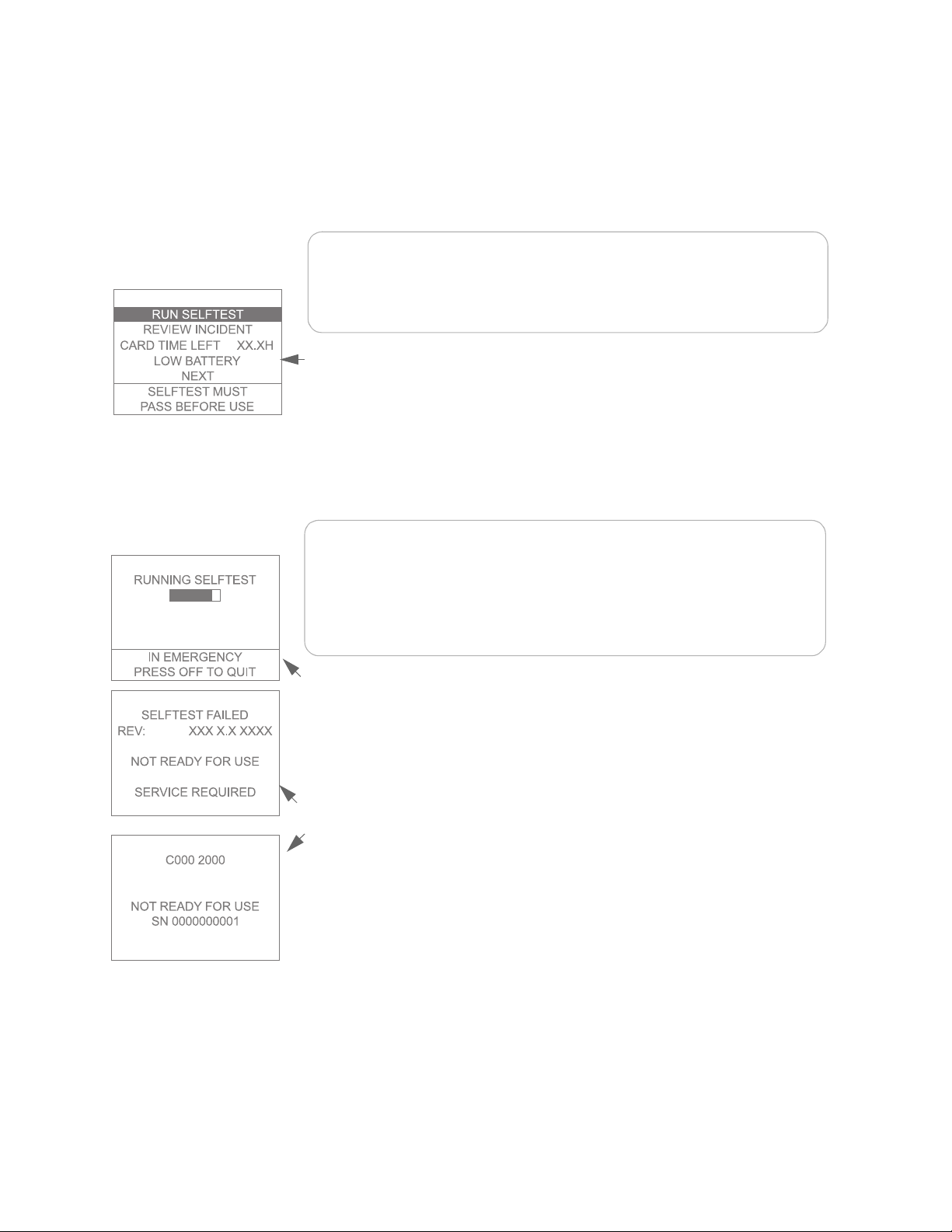
you insert the battery, the main menu is displayed and a two-part selftest will run
*
unless you make another selection from the menu
within 10 seconds. The selftest
includes an automatic part and an interactive part.
NOTE:The menu screen will not appear when a battery is inserted if:
• the defibrillator pads are attached to a patient, indicating that the
FR2+ is in use,
PHILIPS MEDICA L SYSTEMS
• the FR2+ ECG assessment module is connected to the FR2+, or
• the battery is completely depleted.
If less than five minutes have passed since the FR2+ was last used, the menu
screen will be displayed, but after 10 seconds the FR2+ will go to standby mode if
you make no selection.
4
To run the battery insertion selftest, remove and reinstall the battery. The screen
tells you whether or not a data card is installed. If it is, a screen message displays
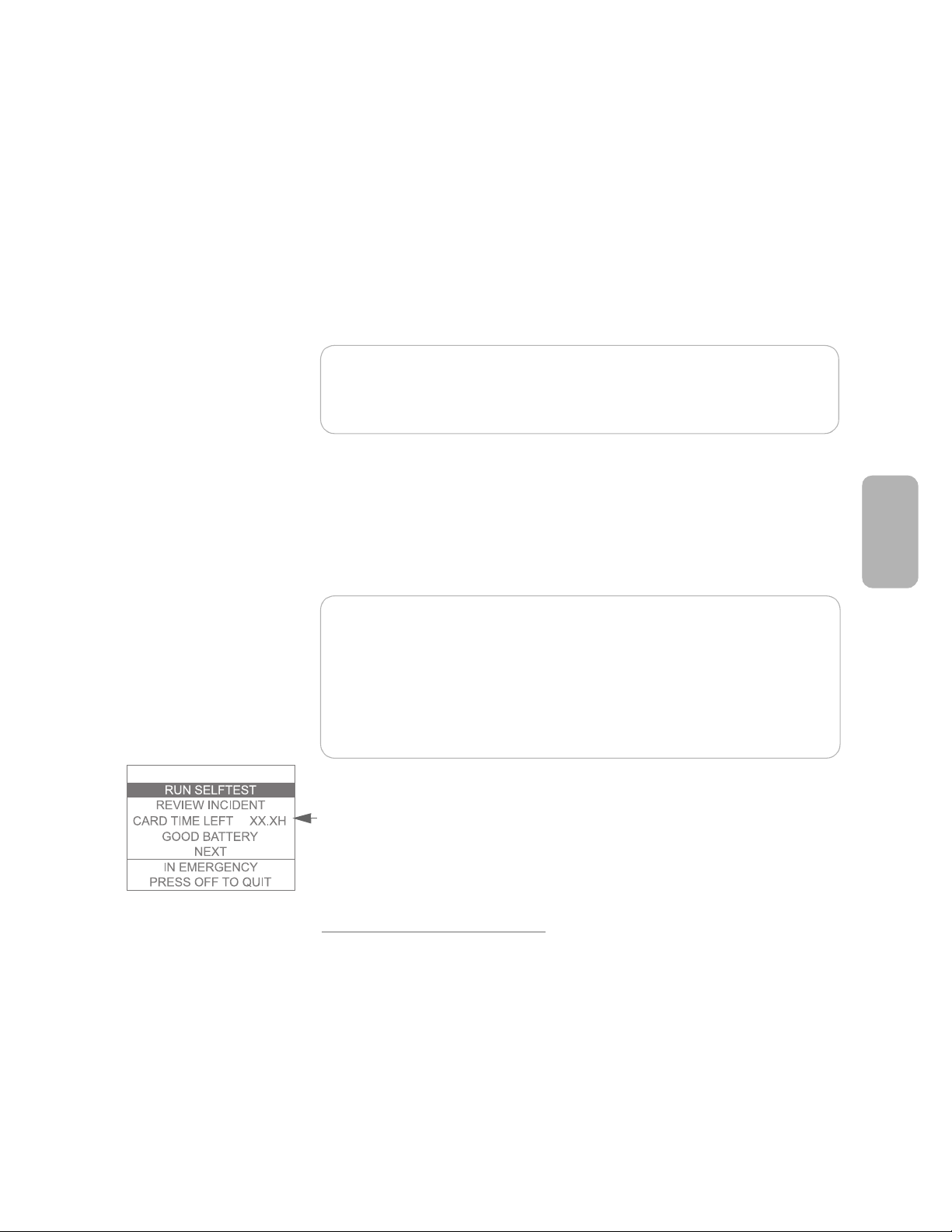
how much recording time is left until the data card is full.
* To move around the menus displayed, use the Option buttons as follows:
• Press the lower Option button to move the highlight bar from one item to another on
the menu.
• Press the upper Option button to select the highlighted item or to scroll through the
settings for that item.
4-6
NOTE:The data card is typically capable of storing a number of incidents.
However, it is recommended that it be cleared or replaced after every use.
In the unlikely event that the card fills up during an incident, no further data
can be recorded, so it is important for you to monitor the
CARD TIME LEFT
information on this screen.
Text prompts tell you if the battery power is low. If so, replace the battery. If a
previous selftest has failed, the screen displays a message that the FR2+ must pass
a selftest before being used.
It is a best practice to always have a spare battery available. However, if you do
not have a spare battery when a screen display prompts you to replace the battery
or the Status Indicator shows a flashing red X, you can continue to use the FR2+
until the battery is completely depleted. This may be necessary in an emergency.
NOTE:The M3848A FR2+ rechargeable battery should not be used as a spare
or backup battery.
NOTE:If you connect defibrillator pads (that are applied to the patient) or the
FR2+ ECG assessment module to the FR2+ during a battery insertion selftest,
the selftest will stop and the FR2+ will go to its standby mode to be ready for
use.
During the automatic part of the selftest, the screen displays a bar that fills in as
the test continues. When that part of the test is finished, the FR2+ beeps. If a data
card was inserted in the FR2+ prior to installing the battery, the results of the
selftest are automatically recorded on the data card.
If the automatic part of the selftest detects a problem:
• The screen displays a message that the selftest has failed. After a short time,
an error code is displayed. Write down the error code and serial number.
• The Status Indicator shows a flashing or solid red X.
Replace the battery with a new battery and repeat the test. If the second selftest
fails, contact Philips for technical support.
PHILIPS MEDICA L SYSTEMS
HEARTSTART FR2+ M3860A/M3861A

4-7
If the automatic part of the selftest passes:
• The screen displays a message that the selftest passed, then begins the
interactive part of the test.
The interactive part of the selftest requires you to respond to prompts in order to
make sure the display, buttons, lights, and speaker on the FR2+ are working
properly.
Text prompts guide you through a series of steps in the interactive part of the
selftest. Some ask you to observe that a feature of the FR2+ works properly.
Others ask you to take certain actions — for example, to press a button. The
screen then displays a message showing that the button’s operation has been
verified. If you do not press the button, or if you do but the button is not working,
the screen displays a message that the button’s function is not verified.
4
PHILIPS MEDICA L SYSTEMS
If something does not work correctly — for example, if lights do not come on or
you do not hear beeps when expected — make a note of the problem and contact
Philips for technical support.
NOTE:Do not use the FR2+ if any parts of the interactive selftest indicate a
problem. Be sure to note and report any problems you find.
When the interactive part of the battery insertion selftest is complete, the FR2+
turns off and goes to standby mode to be ready for use.
If it detects a problem during any selftest, the FR2+ beeps and displays a flashing
red X or a solid red X on the Status Indicator.

4-8
Device history
The FR2+ stores key information about its history in internal memory. To review
the history of your FR2+, select
you insert the battery, then select
The device history information includes:
USES — how many times the FR2+ has been used (shown in the left column of
•
numbers) and the total time in minutes it has been used (shown in the right
column of numbers);
SHOCKS — the total number of shocks it has delivered;
•
TRAINING — how many times it has been used with the Training &
•
Administration Pack for training (left column) and the total time in minutes it
has been used for training (right column); and
TESTS — how many tests have been run. Four figures are shown: daily (upper
•
left), weekly (upper right), and monthly (lower left) periodic selftests, and
battery insertion selftests (lower right).
REV — device model, software version and language.
•
NEXT from the main menu screen displayed when
DEVICE HISTORY from the next menu displayed.
PHILIPS MEDICA L SYSTEMS
Battery History
Information about use of the battery currently installed in your FR2+ is also
available. To review the history of the battery, select
displayed when you insert the battery, then select BATTERY HISTORY from the
next menu displayed.
The battery history information is read from the internal memory of the battery. It
includes:
•
•
•
HEARTSTART FR2+ M3860A/M3861A
NEXT from the menu screen
USE MINUTES — the total operating time (in minutes), including selftest time,
for this battery;
CHARGES — the total number of full defibrillation charges that have been
provided by this battery, including selftest charges;
BATTERY READINESS — a GOOD BATTERY message (M3863A) or a fuel gauge
display (M3848A) showing 25%, 50%, 75% or 100%, or a
REPLACE BATTERY message, as appropriate.
LOW BATTERY or
 Loading...
Loading...