Page 1
Instructions
Experiment
Instruction Manual
No. 012-08420A
*012-08420*
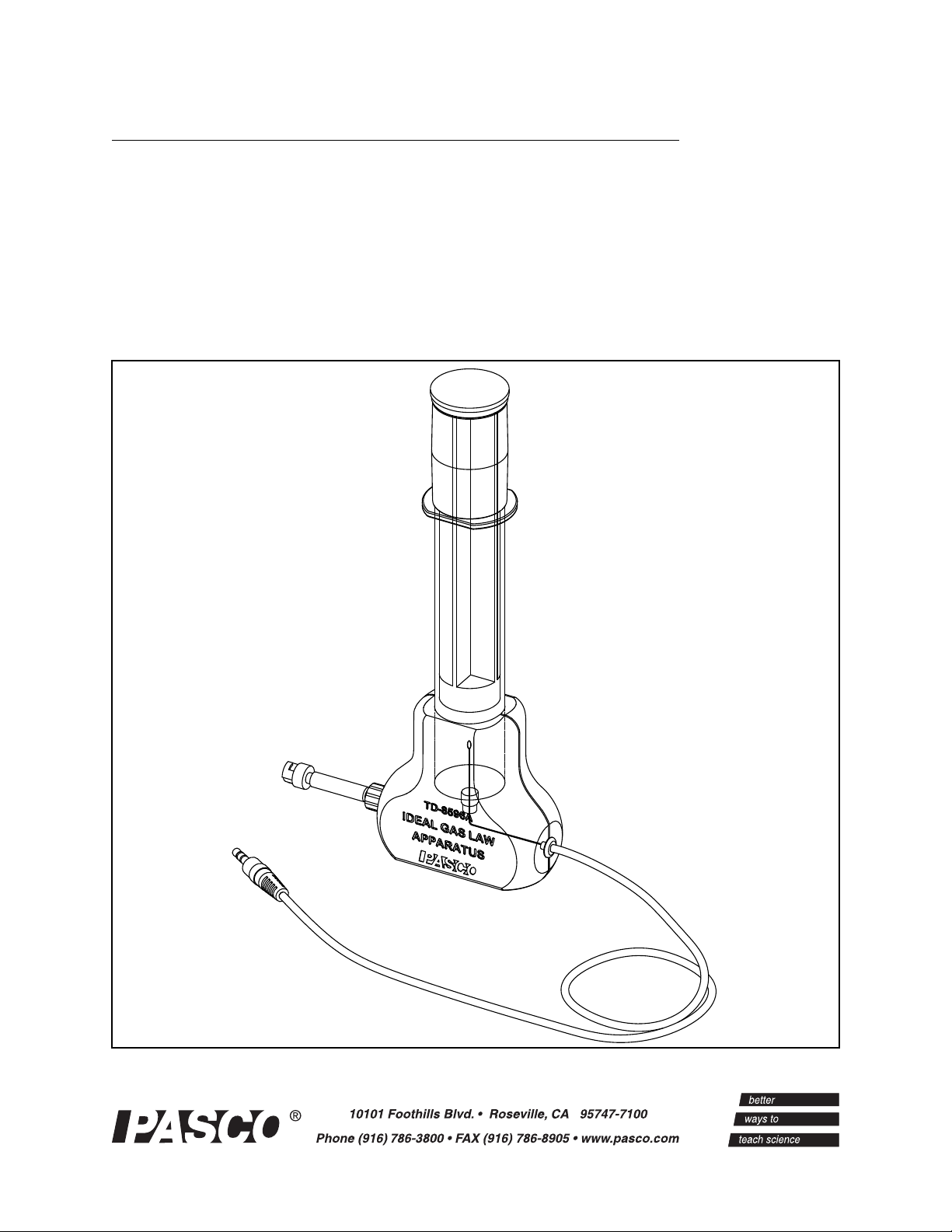
Ideal Gas Law Apparatus
TD-8596A
Page 2

Ideal Gas Law Apparatus Model No. TD-8596A
Table of Contents
Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Equipment Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Experiment 1: Ideal Gas Law . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Safety. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Technical Support . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Copyright and Warranty Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Teacher’s Notes - Experiment 1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Teacher’s Notes - Ideal Gas Law Workbook . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Page 3
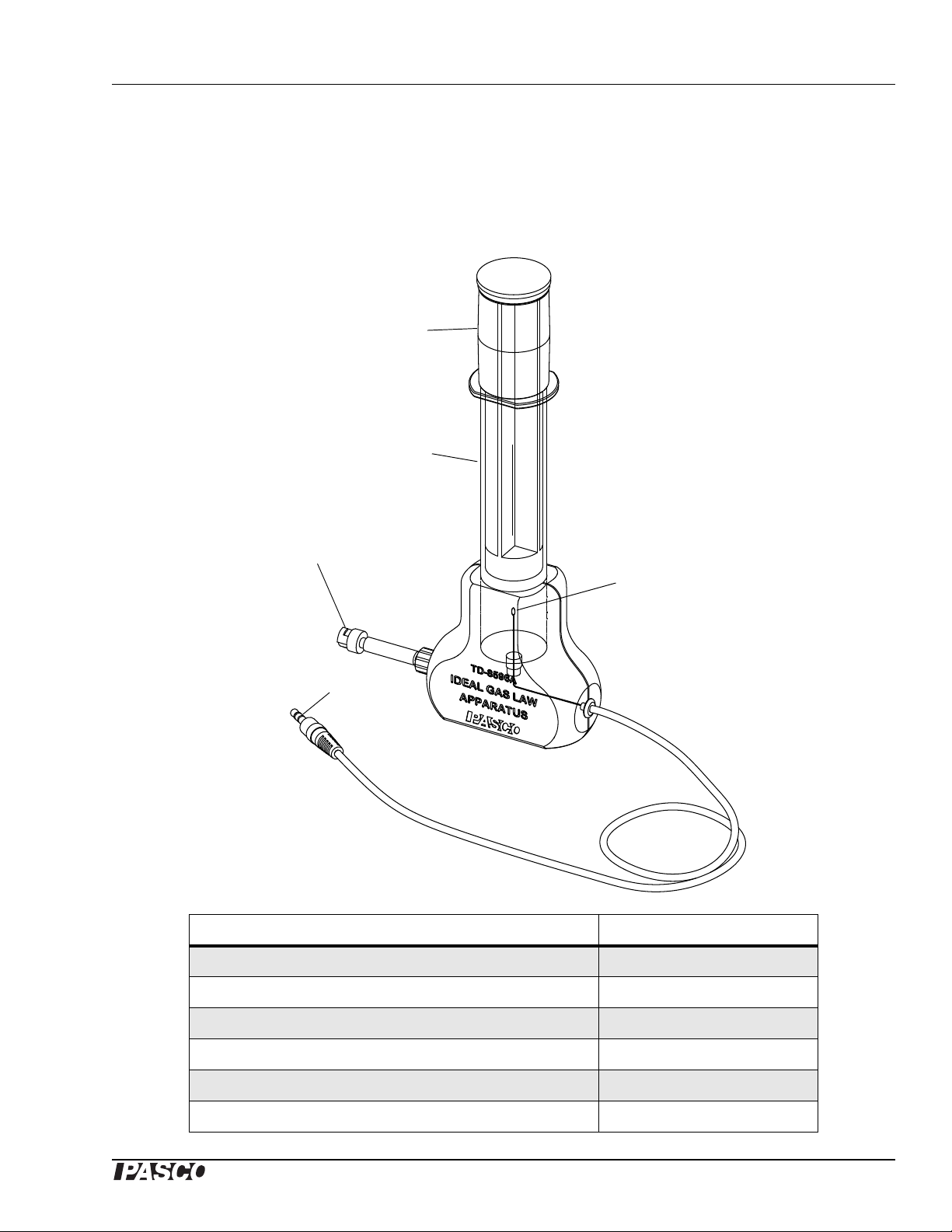
Model No. TD-8596A Ideal Gas Law Apparatus
®
4
1
5
3
2
Ideal Gas Law Apparatus
Model No. TD-8596A
Included Equipment Replacement Part Number
1. Mechanical stop
2. Syringe and plunger
3. Thermistor
4. Pressure connector (quick-release connector)
5. Temperature connector (mini stereo jack)
6. DataStudio Workbook CD (not shown)
013-08980
1
Page 4
Ideal Gas Law Apparatus Model No. TD-8596A
®
Additional Equipment Required or Recommended (*) Model Number
PASPORT interface*
Pressure/Temp sensor or
Pressure sensor and Temperature sensor
(*) PASPORT extension cable
PS-2146 or
PS-2125 and PS-2107
PS-2500
(*See the PASCO catalog or web site for more information.)
Introduction
The Ideal Gas Law Syringe allows simultaneous measurements of temperature and pressure of a
gas as it is compressed. A low thermal mass thermistor is built into the end of the syringe to
measure temperature changes inside the syringe. The response time is around a half of a second.
The plunger is equipped with a mechanical stop that protects the thermistor, and also allows for a
quick, predetermined change in volume. The temperature connector, a mini stereo jack, connects
directly to a temperature sensor, and the pressure connector, a quick-release connector, attachs
directly to a pressure sensor. As the plunger of the syringe is depressed, the volume decreases
while pressure and temperature increase.
Equipment Setup
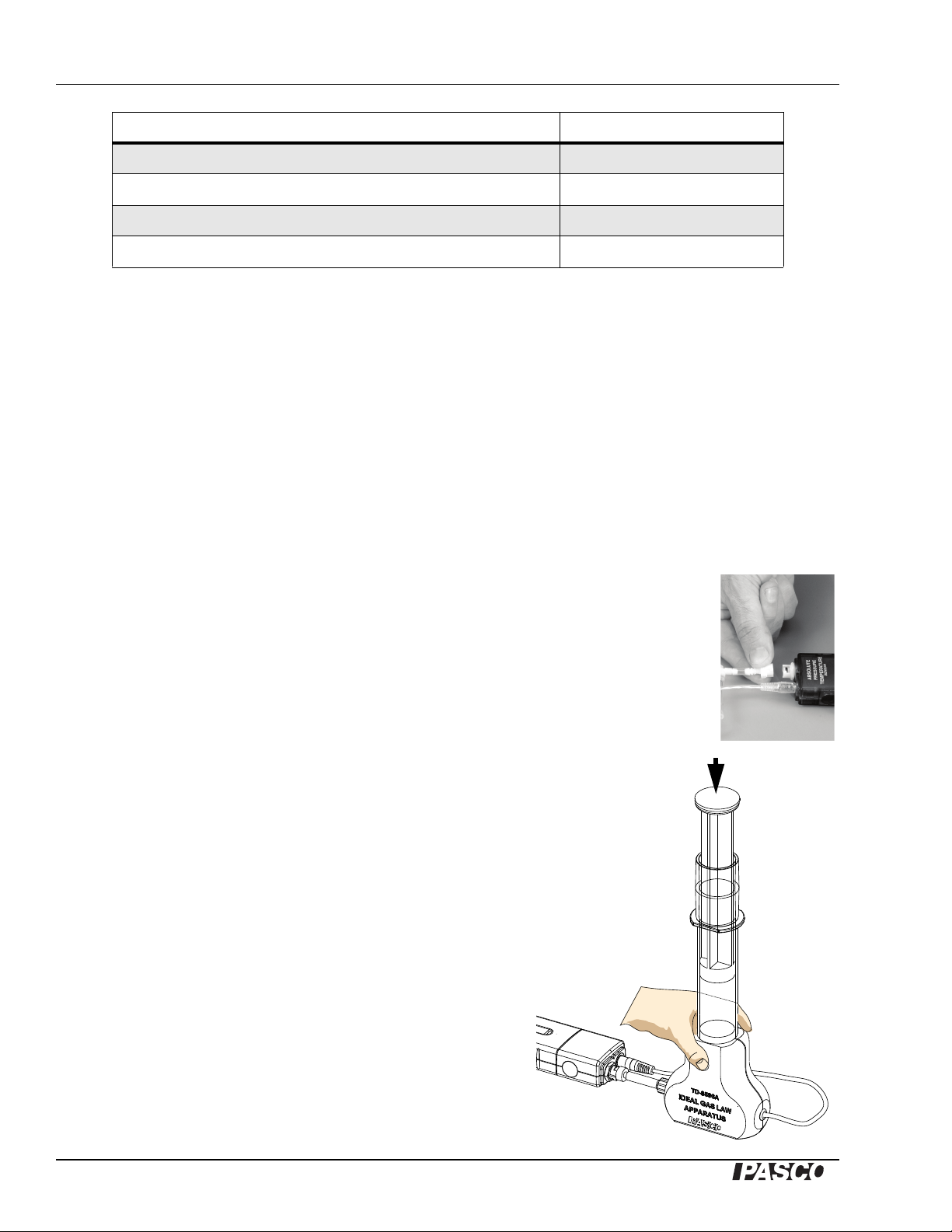
Plug the mini stereo jack into the temperature sensor. Connect the quick-release
connector to the pressure port as shown. This white plastic connector can be
disconnected and re-connected during the experiment to allow for different initial
plunger positions.
Procedure
Set the plunger for a volume of 40 cc.
Hold the base of the syringe firmly against a sturdy
horizontal surface.
Slap down on the plunger with the palm of your hand to
fully compress the gas inside the syrinnge. Hold this
position until the temperature and pressure have
equalized and are no longer changing (about 30
seconds).
Release the plunger and allow it to return on its own. (It
may not go back to 40 cc.)
NOTE: Do not use a hammer or mallet on the plunger!
Only use the palm of your hand to push the plunger
down.
2
Page 5

Model No. TD-8596A Procedure
®
Mechanical Stop
Plunger
Sensor
40 cc
Experiment 1: Ideal Gas Law
Procedure
1. With the pressure coupling disconnected,
push the plunger all the way in so that the
stop is bottomed out. Record the volume
reading on the syringe. It should be around
20 cc.
2. Set the plunger for a volume of 40 cc.
Connect the pressure coupling, and make
sure the temperature jack is also plugged
in. Hold the base of the apparatus firmly
against a sturdy horizontal surface.
3. Open the DataStudio file “Ideal Gas Law.”
4. Click Start. Fully compress the plunger
quickly so that the stop is bottomed out.
Hold this position until the temperature
and pressure have equalized and are no
longer changing. It should take less than 30 seconds for the temperature to return to room
temperature.
5. Release the plunger and allow it to expand back out on its own. (It may not go back to 40 cc.)
Wait again until the temperature and pressure have equalized and are no longer changing.
Record the final volume reading on the syringe.
6. Click Stop.
Analysis
Constant Temperature
1. Highlight an area (click and drag) on the pressure graph at the beginning of the run before you
compressed the air. You should see that data highlighted in the Data Table. Record the initial
pressure (P1) in Table 1.
2. Highlight an area on the pressure graph at the point just before you released the plunger. Note
that the temperature should be back down to almost room temperature again. Record the final
pressure (P2) in Table 1. Record the volume (V2) of the syringe when the plunger is fully
compressed. It should be around 20 cc
3
Page 6

Ideal Gas Law Apparatus Model No. TD-8596A
®
V
1
V
2
------
P
2
P
1
-----
(1)=
V1V0+
V2V0+
--------------------
P
2
P
1
-----
(2)=
Note: V140 cc
Table 1: Constant Temperature
Volume (cc) Pressure (kPa)
1 40.0
2
3. For constant temperature, the Ideal Gas Law reduces to P1V1 = P2V2, or
4. Take the ratio of the final pressure over the initial pressure P2 / P1. Take the ratio of the initial
volume over the final volume V1 / V2. Are they equal? Why not? There is actually a small
consistent error in the volume that you can account for. The calibration on the syringe does
not include the volume of air in the tubing. If we call this unknown, additional volume Vo, the
equation (1) above can be more correctly written as
Using your measured values of V1, V2, .P1 and P2, algebraically solve for and calculate the
volume Vo.
Varying Temperature
1. Highlight an area on the temperature graph at the beginning of the run before you compressed
the air, as you did before. It does not matter if it is the same pressure point or not. Record both
the initial pressure (P1) and initial temperature (T1), in Table 2.
2. Record the initial volume (V1), including your calculated value of Vo .
3. Highlight the area on the temperature graph where it peaks. Pick the place where the
temperature has peaked, not the pressure. It takes the temperature sensor about 1/2 second to
respond. Record the peak temperature (T
in Table 2. You want two values that occurred at the same time.
) and the corresponding pressure (P2) for that time
2
4
Page 7

Model No. TD-8596A Procedure
®
PV
T
--------
Constant.=
P1V
1
T
1
------------
P2V
2
T
2
------------
Percent Difference
Value #2 - Value #1
Value #1
------------------------------------------------
x 100 (%)=
Table 2: Varying Temperature
Volume (cc) Pressure (kPa) Temperature (K)
1
2
4. Record the volume (V2), (including Vo) of the fully compressed plunger.
5. The Ideal Gas Law states that the quantity.
Use your values to calculate the ratio
Use your values to calculate the ratio
6. Compare these two ratios. Are they about the same? Calculate the percent difference between
them.
Questions
1. When the syringe volume is suddenly cut in half, the pressure changes by more than a factor
of 2. Why does it momentarily spike above 200 kPa?
2. When the syringe volume is suddenly cut in half, both the temperature and the pressure go up.
After a short time, the temperature approaches room temperature, but the pressure approaches
some new, higher value. Why doesn't the pressure decrease back to its original value like the
temperature does?
3. When the plunger is released in the last part of the data run, what happens to the temperature?
Why?
5
Page 8

Ideal Gas Law Apparatus Model No. TD-8596A
®
Safety
Read the instructions before using this product. Students should be supervised by their instructors.
When using this product, follow the instructions in this manual and all local safety guidelines that
apply to you.
Technical Support
For assistance with any PASCO product, contact PASCO at:
Address: PASCO scientific
10101 Foothills Blvd.
Roseville, CA 95747-7100
Phone: (916) 786-3800
(800) 772-8700
Fax: (916) 786-3292
Web: www.pasco.com
Email: support@pasco.com
Copyright and Warranty Information
Copyright Notice
The PASCO scientific 012-08842C Ideal Gas Law Apparatus Instruction Manual is copyrighted
and all rights reserved. However, permission is granted to non-profit educational institutions for
reproduction of any part of this manual, providing the reproductions are used only for their
laboratories and are not sold for profit. Reproduction under any other circumstances, without the
written consent of PASCO scientific, is prohibited.
Limited Warranty
For a description of the product warranty, see the PASCO catalog.
6
Page 9

Model No. TD-8596A Teacher’s Notes—Ideal Gas Law
®
V
0
P2V2P1V
1
–
P1P2–
-------------------------------
=
Experiment 1:
Teacher’s Notes—Ideal Gas Law
Constant Temperature, Sample Data and Analysis
Table 1: Constant Temperature
Volume (cc) Pressure (kPa)
1 40.0 99.76
2 20.0 184.17
V0 = 3.6 cc
Among different lab groups or over several trials you may find that calculated values of V
by 1 cc or more. This may look like a very large uncertainty, but, since it is added to the total
volume of gas (40 to 60 cc) the absolute uncertainty, rather than the relative uncertainty, should be
considered.
vary
0
7
Page 10

Ideal Gas Law Apparatus Model No. TD-8596A
®
P1V
1
T
1
------------
14.4 kPa cc K=
P2V
2
T
2
------------
14.6 kPa cc K=
Varying Temperature, Sample Data and Analysis
Table 2: Varying Temperature
Volume (cc) Pressure (kPa) Temperature (K)
1 43.6 99.76 301.04
2 23.6 199.22 321.08
Percent Difference = 1%
Answers to Questions
1. When the cylinder is compressed, the pressure momentarily spikes because the temperature
of the gas increases. As the temperature drops back down, the pressure decreases.
2. The pressure does not return to its original value because volume has decreased while the
molar quantity of gas remains the same.
3. When the pressure is released the temperature drops rapidly, then slowly returns to room
temperature. The temperature drops due to sudden decompression (which is essentially
adiabatic). It returns to room temperature due to heat flow from the environment into the
syringe.
8
Page 11

Model No. TD-8596A Teacher’s Notes—Ideal Gas Law Workbook
®
Experiment 1:
Teacher’s Notes—Ideal Gas Law Workbook
With the electronic
workbook contained on the
CD-ROM, students will
explore the relationship
between the volume,
pressure and temperature of
a gas. They will compare
graphs of V versus T P for
two different quantities of
gas, and use these graphs to
calculate the number of
moles in both cases.
Have your students open the
DataStudio file “Ideal Gas
Law Workbook” and follow
the on-screen instructions.
They will collect, graph and
analyze data within the
electronic workbook.
To hand in their work, students can save a copy of the file or print the workbook after they have
finished.
These sample data are from the file “Workbook with data”.
9
Page 12

Ideal Gas Law Apparatus Model No. TD-8596A
®
n
21.3 cc kPa K
8.31 J K mol
---------------------------------------
0.0213 J K
8.31 J K mol
------------------------------------
2.56 10
3–
mol===
Answers to Questions on Page 5 of the Workbook:
3. The slope of each line is nR. From the slope and the initial pressure and temperature on Table
1, the initial volume of gas is:
Initial Volume = nRTP = (21.3 cc·kPaK) × (300 K) (102 kPa) = 62.8 cc
4. This figure is the volume of the syringe, plus the volume of the attached tubing:
Volume of tubing = 62.8 cc - 60.0 cc = 2.8 cc
5. The y-intercept of the best-fit line, 2.68 cc, is also the volume of gas in the tubing. In this case
the two values deviate by about 0.1 cc.
6. n = slope/R. Pay attention to units in this calculation.
7. The ratio of slopes is 1.51, close to the expected value of 1.5.
8. In theory the y-intercepts of
both slopes are equal because
they both represent the
volume of the tubing. In this
case they differ by 0.35 cc.
10
 Loading...
Loading...