Page 1
Instruction Manual and
Experiment Guide for the
PASCO scientific
Model TD-8592
SMALL PISTON HEAT
ENGINE APPARATUS
012-08375A
Page 2

Page 3
The exclamation point within an equilateral
triangle is intended to alert the user of the
presence of important operating and maintenance (servicing) instructions in the literature
accompanying the appliance.
Page 4

012-08375A
Small Piston Heat Engine
Table of Contents
Section Page
Copyright, Warranty, and Equipment Return ....................................................ii
Introduction ...................................................................................................... 1
Equipment ........................................................................................................ 1
Experiments
1) Operation of a Heat Engine ........................................................................ 3
2) Charles’ Law ............................................................................................. 5
3) Boyle’s Law .............................................................................................. 7
4) Combined Gas Law (Gay-Lussac’s) .......................................................... 9
5) The Mass Lifter Heat Engine ................................................................. 11–18
Technical Support ................................................................................... Back Cover
i
Page 5
Small Piston Heat Engine 012-08375A
Copyright, Warranty, and Equipment Return
Please—Feel free to duplicate this manual
subject to the copyright restrictions below.
Copyright Notice
The PASCO scientific 012-08375A Small Pistion Heat
Engine manual is copyrighted and all rights reserved.
However, permission is granted to non-profit educational
institutions for reproduction of any part of the manual,
providing the reproductions are used only for their
laboratories and are not sold for profit. Reproduction
under any other circumstances, without the written
consent of PASCO scientific, is prohibited.
Limited Warranty
PASCO scientific warrants the product to be free from
defects in materials and workmanship for a period of one
year from the date of shipment to the customer. PASCO
will repair or replace at its option any part of the product
which is deemed to be defective in material or workmanship. The warranty does not cover damage to the product
caused by abuse or improper use. Determination of
whether a product failure is the result of a manufacturing
defect or improper use by the customer shall be made
solely by PASCO scientific. Responsibility for the return
of equipment for warranty repair belongs to the customer.
Equipment must be properly packed to prevent damage
and shipped postage or freight prepaid. (Damage caused
by improper packing of the equipment for return shipment
will not be covered by the warranty.) Shipping costs for
returning the equipment after repair will be paid by
PASCO scientific.
Equipment Return
If the product requires return to PASCO scientific for any
reason, notify PASCO scientific by letter, phone, or fax
BEFORE returning the product. Upon notification, the
return authorization and shipping instructions will be
promptly issued.
➤➤
➤
NOTE: NO EQUIPMENT WILL BE
➤➤
ACCEPTED FOR RETURN WITHOUT AN
AUTHORIZATION FROM PASCO.
When returning equipment for repair, the units must
be properly packed. Carriers will not accept responsibility for damage caused by improper packing. To
be certain the unit will not be damaged in shipment,
observe the following rules:
➀ The packing carton must be strong enough for the item
shipped.
➁ Make certain there are at least two inches of packing
material between any point on the apparatus and the
inside walls of the carton.
➂ Make certain that the packing material cannot shift in
the box or become compressed, allowing the
instrument come in contact with the packing carton.
Address: PASCO scientific
10101 Foothills Blvd.
Roseville, CA 95747-7100
Credits
Editor: Sunny Bishop
Phone: (916) 786-3800
FAX: (916) 786-3292
email: techsupp@pasco.com
web: www.pasco.com
ii
Page 6
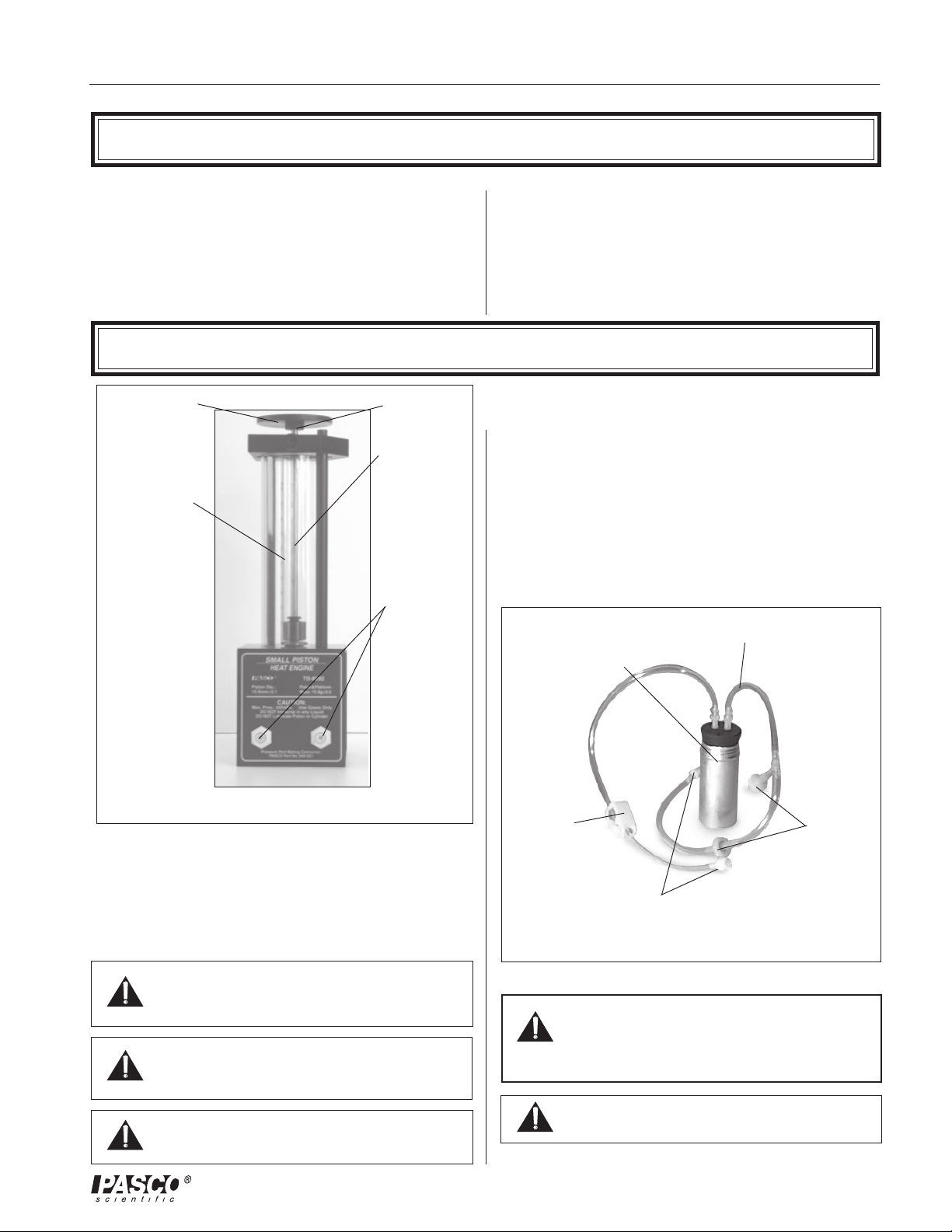
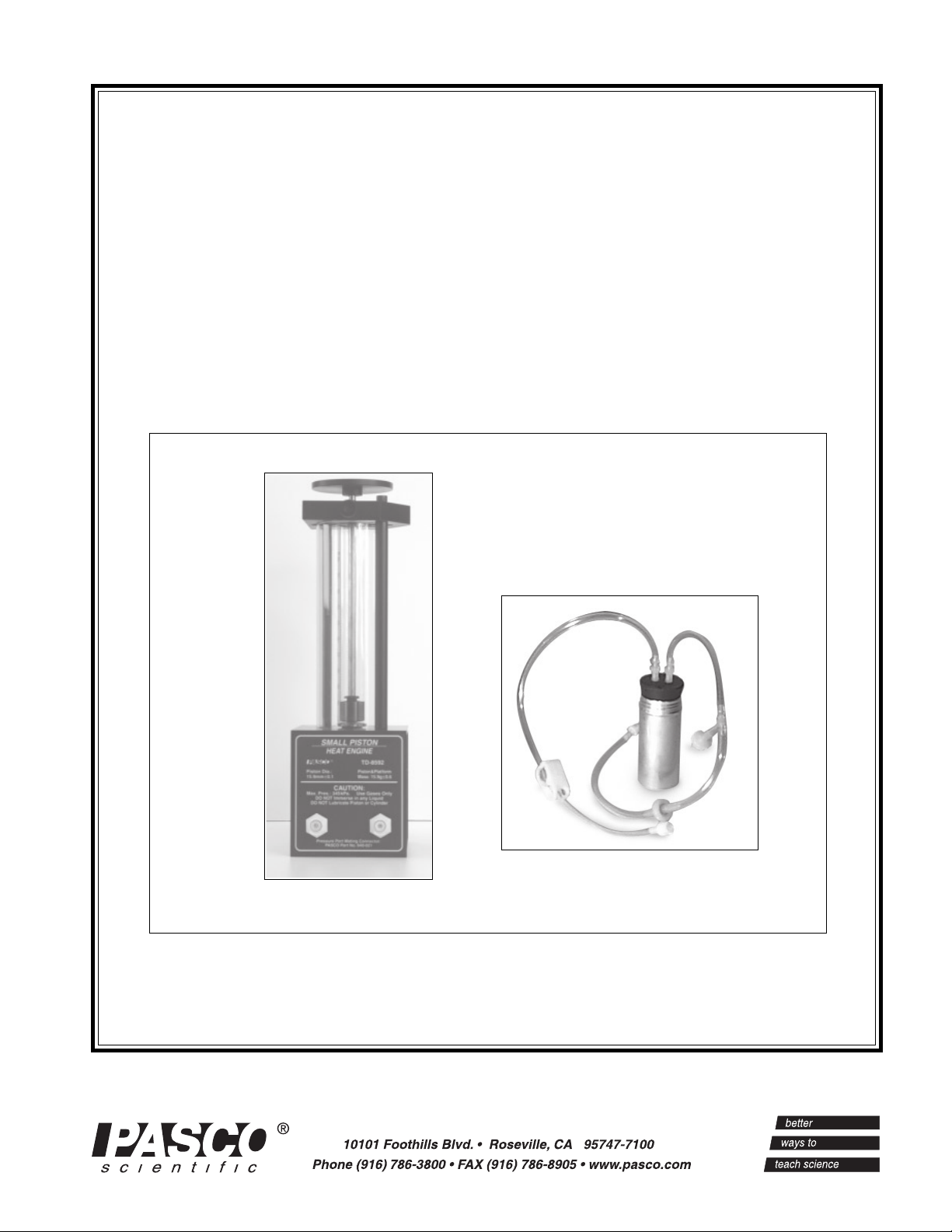
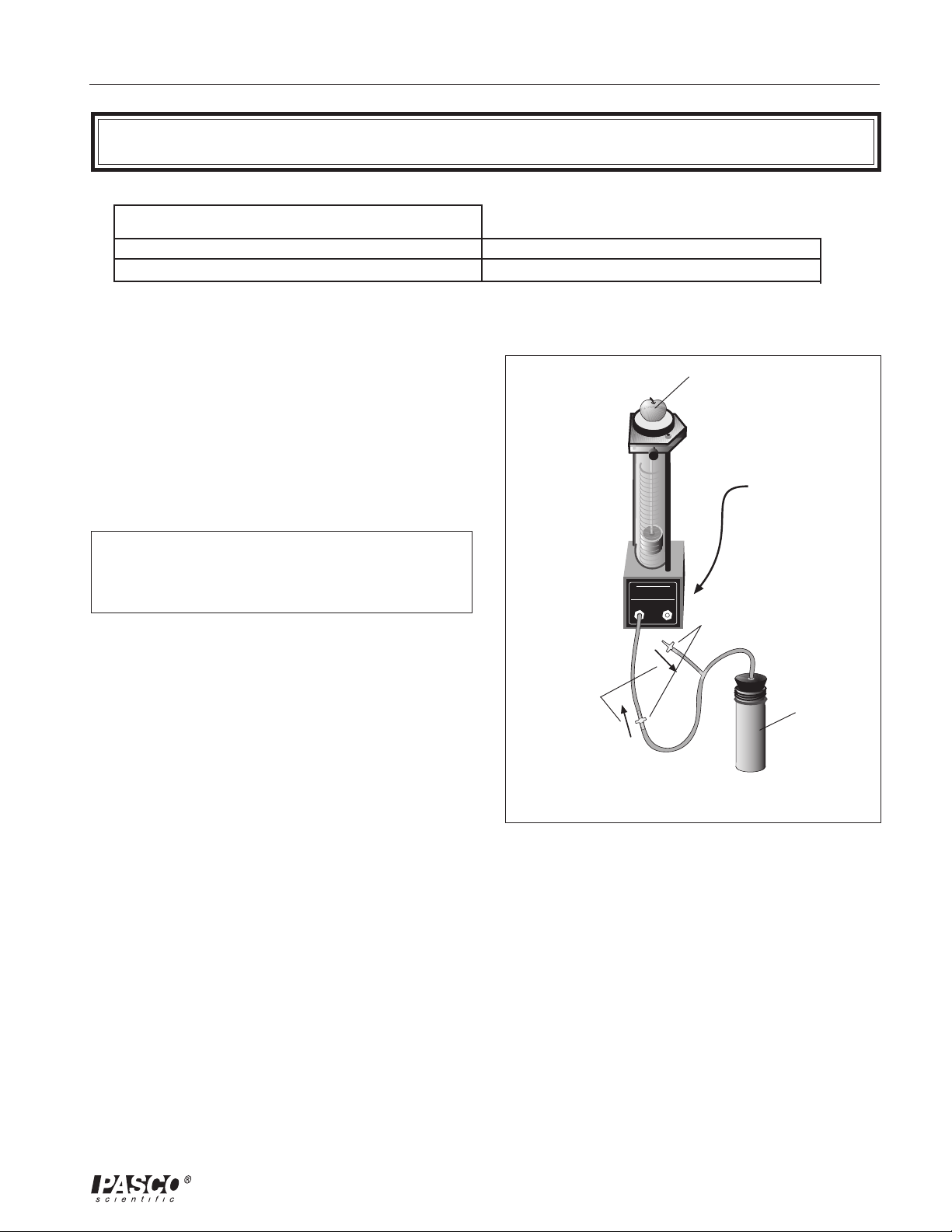
012-08375A Small Piston Heat Engine
Introduction
The PASCO TD-8592 Small Piston Heat Engine is used
for quantitative experiments involving the Ideal Gas Law
(as described below) and for investigations of a working
heat engine. The equipment allows the amount of work
done by thermal energy to be measured.
Equipment
Mass platform for
adding a load to
do work
experiments
Precision-bore
pyrex cylinder
inside a protective
plastic shield
Piston-holding
thumbscrew
Graphite piston
with mm scale for
measuring piston
displacement
Pressure port
mating
connectors
The heart of this apparatus is a nearly friction-free piston/
cylinder system. The graphite piston fits snugly into a
precision-ground Pyrex cylinder so that the system
produces almost friction-free motion and negligible
leakage.
The apparatus includes the following equipment:
• Base apparatus (Figure 1)
- piston diameter: 15.9 mm ± 0.1
- mass of piston and platform: 15.9 g ± .06
• Air chamber (Figure 2)
• Three hose configurations: one with one-way check
valves and one with a clamp (Figure 2), and one
plain piece of tubing (not shown)
• One each, one-holed and two-holed rubber stopper
Air chamber for
immersing in hot
or cold water
Tubing for connecting
chamber to cylinder
Figure 1. Base apparatus with piston
The Small Piston Heat Engine is designed with two
pressure ports with quick-connect fittings for connecting
to the air chamber tubing.
The apparatus can be connected to a Low Pressure Sensor
for use with PASCO computer interfaces.
Do not apply lubricant to the piston or
cylinder.
Do not immerse the base apparatus in
liquid.
Note: Use only non-caustic/non-toxic
gases such as air or helium.
clamp
pressure port mating connectors
Figure 2. Air chamber and tubing
one-way
check valves
Always release the tubing clamps prior to
storage to avoid permanently deforming the
tubing.
Maximum Pressure: 345 kPa.
1
Page 7

Small Piston Heat Engine
Notes:
012-08375A
2
Page 8
012-08375A Small Piston Heat Engine
Experiment 1: Operation of a Heat Engine
Equipment Required:
• Small Piston Heat Engine
• Mass, 50 – 100 g mass
Equipment Setup
• Container of hot water
• Container of ice water
➀ Using the one-holed stopper, connect the tubing
with the one-way valves to the air chamber and
to a connecting port on the base assembly.
➁ Close the shut-off valve on the tubing from the
unused port.
➂ Set a mass of 50 to 100 g on the mass platform.
➤ Note: Use a maximum mass of 100 grams in
the experiment. A larger mass will cause the
valve seals to leak.
Procedure
➀ Move the air chamber from an ice water bath to a
hot water bath. You will note that the air in the
chamber quickly expands through the tubing and
moves the piston up. Note also that the one-way
check valve in the tubing connecting the base
apparatus and the air chamber permits air to enter
the cylinder, while the other one-way checkvalve
prevents air from leaving through the branched
tube.
mass
m
Close the shut-off
valve on the
tubing from the
unused port.
HEAT ENGINE
GAS LAW APPARATUS
TD-8572
Piston Dia.:
Piston&Platform
32.5mm±0.1
Mass:
35.0g±0.6
CAUTION:
Max. Pres.: 345kPa. Use Gases Only
DO NOT
Immerse in any Liquid
DO NOT Lubricate Piston or Cylinder
Pressure Port Mating Connector:
PASCO Part No. 640-021
direction of
air flow
one-way check
valves
Figure 1.1. Setup for the Heat Engine
air chamber
➁ Move the air chamber back to the cold bath and note that external air is sucked into the air chamber through the
one-way valve located at the end of the branched tube. Note also that the one-way valve in the connecting
tube prevents the air from escaping from the piston, so the height of the piston remains the same.
➂ Repeat steps 1 and 2 until the mass has been completely lifted. The greater the temperature differential be-
tween the hot and cold water baths, the greater the lift achieved through each cycle through them.
Note: For a more detailed, quantitative investiagtion of the heat engine operation, see Experiment 5 (page 11).
3
Page 9

Small Piston Heat Engine
Notes:
012-08375A
4
Page 10

012-08375A Small Piston Heat Engine
Experiment 2: Charles’ Law
Equipment Required:
• Small Piston Heat Engine
• Thermometer
Theory
Charles’ law states that at a constant pressure, the volume of a fixed mass or quantity of gas varies
directly with the absolute temperature:
V = cT (at constant pressure, P, where temperature, T, is
expressed in degrees Kelvin)
Setup
• Container of hot water
• Ice
➀ Using the one-holed stopper and plain tubing, connect the base apparatus and the air chamber.
➁ Close the shut-off valve on the tubing from the unused port.
➂ Turn the base apparatus on its side. (In this position, the force acting on the apparatus is the
atmospheric pressure and is equal throughout the range of operation of the piston.)
Close the shut-off valve on
the tubing from the unused
port.
m
r
o
f
t
a
l
P
&
n
o
t
s
TD-8572
i
P
HEAT ENGINE
GAS LAW APPARATUS
Piston Dia.:
Figure 2.1: Experiment setup
6
.
0
±
g
0
.
5
3
:
s
s
a
M
32.5mm±0.1
kPa. Use Gases Only
CAUTION:
Max. Pres.: 345
Immerse in any Liquid
Pressure Port Mating Connector:
DO NOT
DO NOT Lubricate Piston or Cylinder
CO Part No. 640-021
AS
P
Add ice.
Do not allow the tip of the
thermometer to touch the
bottom of the container.
Hot
Procedure
➀
Place the air chamber in a container of hot water. After the chamber equilibrates to the
temperature, record the temperature and the height of the piston.
➁ Add ice to the container and record the temperature and pressure at regular time intervals.
➂ Calculate the gas volumes at the various piston positions you measured and make a graph of plots of tempera-
ture versus volume. (Hint: The diameter of the piston is 15.9± 0.1 mm.)
5
Page 11

Small Piston Heat Engine
Notes:
012-08375A
6
Page 12

012-08375A Small Piston Heat Engine
Experiment 3: Boyle’s Law
Equipment Required:
• Small Piston Heat Engine
• Pressure Sensor (CI-6532A)
*For details on setting up and operating the Pressure Sensor with DataStudio software, please
consult the instruction sheet for the Pressure Sensor and the DataStudio online help.
Theory
Boyle’s law states that the product of the volume of a gas times its pressure is a constant at a fixed
temperature:
Therefore, at a fixed temperature, the pressure will be inversely related to the volume, and the
relationship will be linear:
Setup
PV = a
P=
a
V
• Science Workshop computer interface*
• DataStudio software
➀ With the platform raised to its uppermost
position, connect the Pressure Sensor to a port on
the base apparatus with a short piece of tubing
(Figure 3.1).
➁ Close the shut-off valve on the tubing from the
unused port.
➂ Connect the Pressure Sensor to the computer
interface and set up DataStudio to record pressure. Be sure that you set up the keyboard
sampling option so you can enter height data by
hand. (See “Manual Sampling” or “Keyboard
Sampling” in the DataStudio online help.)
Procedure
➀ Record the height of the piston and the pressure
when the platform is raised to its highest position.
➁ Press the platform down to a series of levels and
record the height and pressure at each level.
➂ Convert the height measurements to gas volume
measurements. (Hint: The diameter of the piston
is 32.5 mm.)
To computer interface
HEAT ENGINE
GAS LAW APPARATUS
TD-8572
Piston Dia.:
Piston&Platform
32.5mm±0.1
35.0g±0.6
Mass:
CAUTION:
Max. Pres.: 345kPa. Use Gases Only
DO NOT
Immerse in any Liquid
TO
INTERFACE
PRESSURE
SENSOR
0-700 kPa
(ABSOLUTE)
:
T
1
R
R
2
O
CI-6532
0
O
-
T
P
0
C
4
E
E
6
O
R
N
.
C
U
N
O
S
O
N
S
S
C
T
E
A
R
G
R
0
P
A
N
P
I
SERIE
P
0
DRY AIR ONLY
T
O
A
5
C
M
6
S
E
A
P
INTERFAC
PORT
PRESSURE
700 kPa MAX
DO NOT Lubricate Piston or Cylinder
Pressure Port Mating Connector:
PASCO Part No. 640-021
Pressure Sensor
Figure 3.1. Experiment setup
Close the shut-off
valve on the tubing
from the unused
port.
➃ Prepare a graph of pressure versus volume.
➤ Note: At pressures greater than 120 kPa, the relationship between pressure and volume may
not be linear because of air leakage from the valves and ports at higher pressures.
7
Page 13

Small Piston Heat Engine
Notes:
012-08375A
8
Page 14

012-08375A Small Piston Heat Engine
HEAT ENGINE
GAS LAW APPARATUS
Piston Dia.:
32.5mm±0.1
TD-8572
CAUTION:
Max. Pres.: 345kPa. Use Gases Only
DO NOT
Immerse in any Liquid
DO NOT Lubricate Piston or Cylinder
Pressure Port Mating Connector:
PASCO Part No. 640-021
Piston&Platform
Mass:
35.0g±0.6
PRESSURE
SENSOR
0-700 kPa
(ABSOLUTE)
CI-6532
P
R
E
SS
U
RE
P
O
R
T
700
k
P
a
M
A
X
D
R
Y A
IR
O
N
LY
P
R
E
S
S
U
R
E
P
O
R
T
M
A
T
I
N
G
C
O
N
N
E
C
T
O
R
:
P
A
S
C
O
P
A
R
T
N
O
.
6
4
0
-
0
2
1
I
N
T
E
R
F
A
C
E
P
A
S
C
O
P
A
S
CO
6
500
S
E
R
I
E
T
O
IN
T
E
R
F
A
C
E
Experiment 4: Combined Gas Law (Gay-Lussac’s )
Equipment Required:
• Small Piston Heat Engine
• Pressure Sensor (CI-6532)
• Science
Workshop® computer interface*
• Temperature Sensor (CI-6505)
*For details on setting up and operating the Pressure Sensor and Temperature Sensor with DataStudio
software, please see the instruction sheets for the Pressure and Temperature Sensors and the
DataStudio online help.
Theory
Charles’ law states that the volume,V, is proportional to the temperature, T. Boyle’s law states that the volume,
V, is proportional to the pressure, P, where pressure=1/P. Combining these, we have:
The combined gas law predicts that for a given mass of gas, if V is held constant, P is proportional
to T.
V=
aT
P
• Hot plate
• Beaker with water
• Ice
• DataStudio software
Setup
➀ Secure the piston just above its lowest
position by tightening the thumb screw.
➁ Connect the Pressure Sensor to a port on the
base apparatus with a short piece of tubing.
➂ Connect the air chamber fitted with the
two-holed stopper to the other port of the base
apparatus with a piece of tubing.
➃ Insert the Temperature Sensor into the other
hole of the rubber stopper.
Use a silicon lubricant on the end of
the Temperature Probe to aid insertion
and to prevent damage to the probe.
➄ Connect the Pressure Sensor and Temperature
Sensor to the computer interface, and set up the
DataStudio software program to graph temperature versus pressure.
Tighten the
piston-holding
thumb screw.
to
computer
interface
Pressure
Sensor
Gas Law Apparatus
To computer interface
hot plate
Figure 4.1. Experiment Setup
Temperature
Sensor
➤ Note: You can substitute a thermometer in the water container for the Temperature Sensor.
Be sure to keep the tip of the thermometer from touching the bottom of the container.
9
Page 15

Small Piston Heat Engine
➅ Place the air chamber in the Pyrex container and turn on the hot plate.
Procedure
➀
Record the temperature and pressure as the water heats.
➁ Display a graph of temperature versus pressure in DataStudio software.
Notes:
012-08375A
10
Page 16

012-08375A Small Piston Heat Engine
Experiment 5: The Mass Lifter Heat Engine
1
The Small Piston Heat Engine is ideal for use in the calculus-based experiment 18.10 of the Workshop
Physics Activity Guide. Following is a slightly modified reprint of the experiment:
Equipment Required:
• Small Piston Heat Engine (1)
• Beakers (2), 1000 ml (to use as reservoirs)
• Ruler (1)
• Barometer pressure gauge (1)
• Calipers (1 set)
• Mass set, 20 g, 50 g, 100 g, 200 g
• Hot plate(1)
• Vat to catch water spills (1)
Optional:
• A computer-based laboratory system with barometer sensor
Your working group has been approached by the Newton Apple Company about testing a heat engine that lifts
apples that vary in mass from 50 g to 100 g from a processing conveyer belt to the packing conveyer belt that is
10 cm higher. The engine you are to experiment with is a "real" thermal engine that can be taken through a
four-stage expansion and compression cycle and that can do useful mechanical work by lifting small masses
from one height to another. In this experiment, we would like you to verify experimentally that the useful
mechanical work done in lifting a mass, m, through a vertical distance, y, is equal to the net thermodynamic
work done during a cycle as determined by finding the enclosed area on a P-V diagram. Essentially you are
comparing useful mechanical “ma
y” work (which we hope you believe in and understand from earlier studies)
g
with the accounting of work in an engine cycle as a function of pressure and volume changes given by the
expression:
W
=PdV
net
Although you can prove mathematically that this relationship holds, the experimental verification will allow you
to become familiar with the operation of a real heat engine.
m
y
b
c
P
m
0
a
d
V
Figure 5.1. Doing useful mechanical
work by lifting a mass, m, through a
height, y.
1
Priscilla W. Laws, et al. Workshop Physics Activity Guide, 1996 by John Wiley & Sons, Inc.
Figure 5.2 Doing thermodynamic
work in a heat engine cycle.
Reprinted by permission of John Wiley & Sons, Inc.
11
Page 17

Small Piston Heat Engine
The Incredible Mass Lifter Engine
The heat engine consists of a hollow cylinder with a graphite piston that can move along the axis of the cylinder
with very little friction. The piston has a platform attached to it for lifting masses. A short length of flexible
tubing attaches the cylinder to an air chamber (consisting of a small can sealed with a rubber stopper that can be
placed alternately in the cold reservoir and the hot reservoir. A diagram of this mass lifter is shown in Figure
5.2.
m
HEAT ENGINE
GAS LAW APPARATUS
Piston Dia.:
32.5mm±0.1
CAUTION:
Max. Pres.: 345kPa. Use Gases Only
DO NOT
Immerse in any Liquid
DO NOT Lubricate Piston or Cylinder
Pressure Port Mating Connector:
PASCO Part No. 640-021
TD-8572
Piston&Platform
Mass:
012-08375A
Close the shut-off valve
on the tubing from the
unused port.
35.0g±0.6
Cold
Hot
Figure 5.3. A schematic diagram of the incredible mass lifter heat engine
If the temperature of the air trapped inside the cylinder, hose, and can is increased, then its volume will
increase, causing the platform to rise. Thus, you can increase the volume of the trapped air by moving
the can from the cold to the hot reservoir. Then, when the apple has been raised through a distance y, it
can be removed from the platform. The platform should then rise a bit more as the pressure on the
cylinder of gas decreases a bit. Finally, the volume of the gas will decrease when the air chamber is
returned to the cold reservoir. This causes the piston to descend to its original position once again. The
various stages of the mass lifter cycle are shown in Figure 5.3.
Before taking data on the pressure, air volume, and height of lift with the heat engine, you should set it
up and run it through a few cycles to get used to its operation. A good way to start is to fill one container
with room temperature water and another with hot tap water or preheated water at about 60–70°C. The
engine cycle is much easier to describe if you begin with the piston resting above the bottom of the
cylinder. Thus, we suggest you raise the piston a few centimeters before inserting the rubber stopper
firmly in the can. Also, air does leak out of the cylinder slowly. If a large mass is being lifted, the
leakage rate increases, so we suggest that you limit the added mass to something between 50 g and 100
g. After observing a few engine cycles, you should be able to describe each of the points a, b, c, and d
12
Page 18

012-08375A Small Piston Heat Engine
m
m
y
y
m
Point A Point B Point C Point D
0
Cold Cold Hot Hot
m
0
0
Figure 5.4. A simplified diagram of the mass lifter heat engine at different stages of its cycle
of a cycle carefully, indicating which of the transitions between points are approximately adiabatic and which are isobaric. You can observe changes in the volume of the gas directly and you
can predict how the pressure exerted on the gas by its surroundings ought to change from point to
point by using the definition of pressure as force per unit area.
5.1 Activity: Description of the Engine Cycle
a. Predicted transition a➔b: Close the system to outside air but leave the can in the cold reservoir.
Make sure the rubber stopper is firmly in place in the can. What should happen to the height of
the platform when you add a mass? Explain the basis of your prediction.
b. Observed transition a➔b: What happens when you add the mass to the platform? Is this what
you predicted?
c. Predicted transition b
reservoir ?
d. Observed transition b
the platform with the added mass on it. Is this what you predicted? (This is the engine
power stroke!)
➔
c: What do you expect to happen when you place the can in the hot
➔
c: Place the can in the hot reservoir and describe what happens to
13
Page 19

Small Piston Heat Engine
e. Predicted transition c➔d: Continue to hold the can in the hot reservoir and predict what will happen
if the added mass that is now lifted is removed from the platform and moved onto an upper conveyor belt. Explain the reasons for your prediction.
➔
f. Observed transition c
d: Remove the added mass and describe what actually happens. Is this what
you predicted?
➔
g. Predicted transition d
a: What do you predict will happen if you now place the can back in the
cold reservoir? Explain the reasons for your prediction.
➔
h. Observed transition d
a: Now it's time to complete the cycle by cooling the system down to its
original temperature for a minute or two before placing a new mass to be lifted on it. Place the can
in the cold reservoir and describe what actually happens to the volume of the trapped air. In
particular, how does the volume of the gas actually compare to the original volume of the trapped
air at point a at the beginning of the cycle? Is it the same or has some of the air leaked out?
012-08375A
i. Theoretically, the pressure of the gas should be the same once you cool the system back to its
original temperature. Why?
Determining Pressures and Volumes for a Cycle
To calculate the thermodynamic work done during a cycle of this engine, you will need to be able to
plot a P-V diagram for the engine based on determinations of the volumes and pressures of the
trapped air in the cylinder, tubing, and can at the points a, b, c, and d in the cycle.
5.2 Activity: Volume and Pressure Equations
a. What is the equation for the volume of a cylinder that has an inner diameter of d and a length L?
b. Use the definition of pressure to derive the equation for the pressure on a gas being contained by a
vertical piston of diameter d if the total mass on the piston including its own mass and any added
mass is denoted as M. Hints: (1) What is the definition of pressure? (2) What is the equation
needed to calculate the gravitational force on a mass, M, close to the surface of the Earth? (3) Don't
forget to add in the atmospheric pressure, P
, acting on the piston and hence the gas at sea level.
atm
Now that you have derived the basic equations you need, you should be able to take your engine
through another cycle and make the measurements necessary for calculating both the volume and
the pressure of the air and determining a P-V diagram for your heat engine. Instead of calculating
the pressures, if you have the optional equipment available, you might want to measure the pressures with a barometer or a barometer sensor attached to a computer-based laboratory system.
14
Page 20

012-08375A Small Piston Heat Engine
5.3 Activity: Determining Volume and Pressure
a. Take any measurements needed to determine the volume and pressure of air in the system at
all four points in the engine cycle. You should do this rapidly to avoid air leakages around the
piston and summarize the measurements with units in the space below.
b. Next you can use your measurements to calculate the pressure and volume of the system at point
a. Show your equations and calculations in the space below and summarize your results with
units. Don't forget to take the volume of the air in the tubing and can into account!
P
=
a
V
=
a
c. Use the measurements at point b to calculate the total volume and pressure of the air in the
system at that point in the cycle. Show your equations and calculations in the space below and
summarize your results with units.
P
=
b
V
=
b
d. What is the height, y, through which the added mass is lifted in the transition from b to c?
e. Use the measurements at point c to calculate the total volume and pressure of the air in the
system at that point in the cycle. Show your equations and calculations in the following
space and summarize your results with units.
P
=
c
V
=
c
f. Remove the added mass and make any measurements needed to calculate the volume and
pressure of air in the system at point d in the cycle. Show your equations and calculations in the
space below and summarize your results with units.
15
Page 21

Small Piston Heat Engine
Pd=
V
=
d
g. We suspect that transitions from a
➔
b and from c➔d are approximately adiabatic. Explain why.
012-08375A
h. You should have found that the transitions from b
➔
c and from d➔a are isobaric. Explain why this
is the case.
Finding Thermodynamic Work from the Diagram
In the next activity, you should draw a P- V diagram for your cycle and determine the thermodynamic work for your engine.
5.4 Activity: Plotting and Interpreting a
P-V
Diagram
a. Fill in the appropriate numbers on the scale on the graph frame that follows and plot the P-V
diagram for your engine cycle. Alternatively, generate your own graph using a computer
graphing routine and affix the result in the space below.
b. On the graph in part a, label each of the points on the cycle (a, b, c, and d). Indicate on the graph
which of the transitions (a
➔
b, b➔c, etc.) are adiabatic and which are isobaric.
16
Page 22

012-08375A Small Piston Heat Engine
Next you need to find a way to determine the area enclosed by the P-V diagram. The enclosed
area doesn't change very much if you assume that P is approximately a linear function of V for
the adiabatic transitions. By making this approximation, the figure is almost a parallelogram so
you can obtain the enclosed area using one of several methods. Three of the many possibilities
are listed below. Creative students have come up with even better methods than these, so you
should carefully think about your method of analysis carefully.
Method I
Since the pressure doesn't change from point b to point c, you can take the pressure of those two
points as a constant pressure between points. The same holds for the transition from d to a. This
gives you a figure that is approximately a parallelogram with two sets of parallel sides. You can
look up and properly apply the appropriate equation to determine the net thermodynamic work
performed.
Method II
Display your graph with a grid and count the boxes in the area enclosed by the lines connecting
points a, b, c, and d. Then multiply by the number of joules each box represents. You will need
to make careful estimates of fractions of a box when a "leg" of a cycle cuts through a box.
Method III
Fit a straight line to each of the starting and ending points for the four transitions in the cycle.
Each equation will give you a function relating P and V. Perform an integral for each of these
equations, since
b
PdV = PdV
a
c
+PdV
b
+PdV
d
c
a
+PdV
d
5.5 Activity: Comparing Thermodynamic and Useful Mechanical Work
a. Choose a method for computing the thermodynamic work in joules, describe it in the space
below, and show the necessary calculations. Report the result in joules.
b. What is the equation you need to use to calculate the useful mechanical work done in lifting the
mass from one level to another?
c. Use the result for the height that the mass is lifted in the power stroke of the engine to calculate
the useful mechanical work performed by the heat engine.
d. How does the thermodynamic work compare to the useful mechanical work? Please use the
correct number of significant figures in your comparison (as you have been doing all along,
right?)
17
Page 23

Small Piston Heat Engine
The Incredible Mass Lifter Engine Is Not So Simple
Understanding the stages of the engine cycle on a P-V diagram is reasonably straightforward. However, it is
difficult to use equations for adiabatic expansion and compression and the ideal gas law to determine the
temperature (and hence the internal energy of the air throughout the cycle. There are several reasons for this.
First, air is not an ideal gas. Second, the mass lifter engine is not well insulated and so the air that is warmed in
the hot reservoir transfers heat energy through the cylinder walls. Thus, the air in the can and in the cylinder are
probably not at the same temperature. Third, air does leak out around the piston, especially when larger masses
are added to the platform. This means that the number of moles of air decreases over time. You can observe
this by noting that in the transition from point d to point a, the piston can actually end up in a lower position
than it had at the beginning of the previous cycle. However, the Incredible Mass Lifter Engine does help us
understand typical stages of operation of a real heat engine.
➤ Note: The previous experiment was intended to help students consolidate the concepts of pressure and
volume by taking their own data for height and mass in each part of the cycle and then calculating the
pressures using the basic definition of pressure vs. force per unit area. An alternate method for doing this
experiment is to use the Science Workshop computer interface with the Pressure Sensor (CI-6532) in
conjunction with either a Motion Sensor (CI-6529) or Rotary Motion Sensor (CI-6538) to detect pressure,
volume, and height automatically with a computer.
012-08375A
18
Page 24

Technical Support
Feedback
If you have any comments about the product or manual,
please let us know. If you have any suggestions on
alternate experiments or find a problem in the manual,
please tell us. PASCO appreciates any customer feedback. Your input helps us evaluate and improve our
product.
To Reach PASCO
For technical support, call us at 1-800-772-8700 (toll-free
within the U.S.) or (916) 786-3800.
Fax: (916) 786-3292
E-mail: techsupp@pasco.com
Web: www.pasco.com
Contacting Technical Support
Before you call the PASCO Technical Support staff, it
would be helpful to prepare the following information:
➤ If your problem is with the PASCO apparatus, note:
- Title and model number (usually listed on the
label);
- Approximate age of apparatus;
- A detailed description of the problem/sequence of
events. (In case you can’t call PASCO right away,
you won’t lose valuable data.);
- If possible, have the apparatus within reach when
calling to facilitate description of individual parts.
➤ If your problem relates to the instruction manual, note:
- Part number and revision (listed by month and year
on the front cover);
- Have the manual at hand to discuss your questions.
 Loading...
Loading...