Page 1
Instruction Manual
®
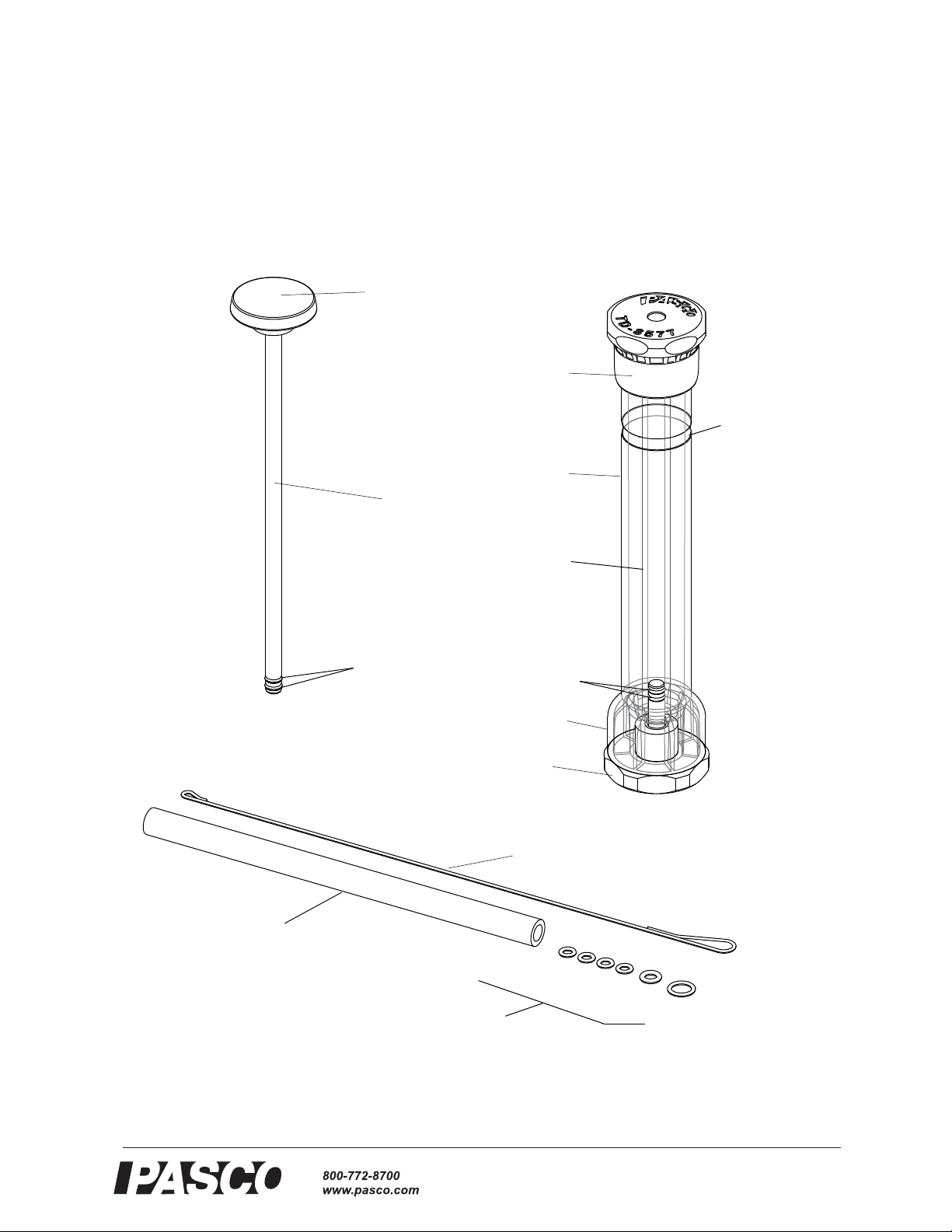
Outer Safety
Tube
(polycarbonate)
Piston
Removable
Base
Bottom
Removable Top
Knob
Loading/Cleaning
Rod
“O” rings
“O” rings
Replacement
Glass Tube
Small O-ring (4)
Inner Glass
Tube
Medium O-ring (1)
IMPORTANT!
Piston Starting
Position label
Large O-ring (1)
Replacement
O-rings (6)
PASCO Compression Igniter
TD-8577
n
o
i
t
i
s
o
P
t
o
g
n
n
i
t
S
r
t
a
P
i
s
012-13028B
Page 2

PASCO Compression Igniter 012-13028B Introduction
®
PVconstant=
PVP0V
0
=
PVP015V
=
P 15
P
0
=
The ignition temperature of
paper is about 451 °F.
See the science fiction
novel Fahrenheit 451
by
Ray Bradbury.
P0V
0
nRT0 and =
PV nRT=
Included Equipment Included Equipment
PASCO Compression Igniter Replacement Glass Tube*
Loading/Cleaning Rod Replacement O-rings* (6)
Lubricant, silicone compound, (2 packets), not shown Material Safety Data Sheet for Lubricant
Additional Item Needed Cotton, tissue paper, “flash paper”
* The TD-8498A Replaceable Glass Tubes includes two replacement tubes and twelve O-rings.
Introduction
The TD-8577 PASCO Compression Igniter (or “fire syringe”) illustrates what happens inside the cylinder of a diesel engine. It demon s trat es the high temperatures produced during adiabatic compression of air by igniting a small amount of cotton, tissue
paper, or magician’s “flash paper” inside a replaceable, thick-wall glass tube. The
glass tube is encased in a polycarbonate safety tube that protects users from injury if
the glass tube breaks. The piston has two rubber “O” rings at its end that provide a
tight seal against the inside of the glass tube. When the piston is rapidly pressed down
into the glass tube, the temperature inside the tube rises quickly.
The Compression Igniter comes with one replacement glass tube and six O-rings. The
large O-ring is a replacement for the ring at the top of the inner glass tube. The
medium O-ring is a replacement for the ring at the top of the piston rod where it joins
the knob. The small O-rings are replacements for the rings on the end of the piston
rod and for the rings on the post that is part of the removable base.
Theory
The compression of air inside a hollow cylinder is an adiabatic process since the
compression is so fast that there is no time for thermal energy to leave or enter the
cylinder.
For an adiabatic compression of gas, , where P is the pressure of the
gas, V is the volume of the gas, and is the ratio of specific heats of the gas (specific
heat at constant pressure divided by specific heat at constant volume)
Therefore, when the gas in the cylinder is at its original volume V
and then is compressed to a small volume V and pressure P,
With the compression igniter, it is possible to compress the gas in the cylinder by a
factor of about 15, so V
= 15V. With this assumption, the final pressure at maximum
0
compression can be calculated in terms of the initial (atmospheric) pressure:
and pressure P0
0
2
The quantity that is of interest is the final temperature: Is it high enough to ignite
paper? To arrive at the temperature, assume that the gas is air and that it is an ideal
gas. For an ideal gas,
Page 3
Model No.TD-8577 012-13028B Operation
®
PV
P
0V0
T
T
0
----------------
=
T
PV
P
0V0
------------
T
0
15P0V
P
0
15V
-------------------- -
T
0
==
P
i
s
t
o
n
S
t
a
r
t
i
n
g
P
o
s
i
t
i
o
n
Combustible
material
Piston
Push knob
down rapidly
Grip
tube
firmly
Watch
here
Piston Starting
Position label
Bottom
Top
Safety Tube
Glass Tube
Insert combustible
material here.
Removable
Base
where n is the number of moles of gas, R is the gas constant, and T is the absolute
(thermodynamic) temperature. Solving the initial equation for nR and substituting the
expression into the final equation gives:
Solving for the final temperature, T, and substituting the final pressure, P, derived
from the adiabatic equation and the final volume, V, which is 1/15 the initial volume
gives:
Simplifying this expression gives T = 15
1.4, so this gives T = 15
(297 K), then T = 15
0.4
T0. Assuming that the initial temperature is about 24 °C
0.4
(297 K) = 877 K. Converting to celsius, T = 604 °C. Since
( - 1)
T0. For air, which is mainly diatomic , =
604 °C = 1120 °F, the temperature is high enough to ignite paper.
Operation
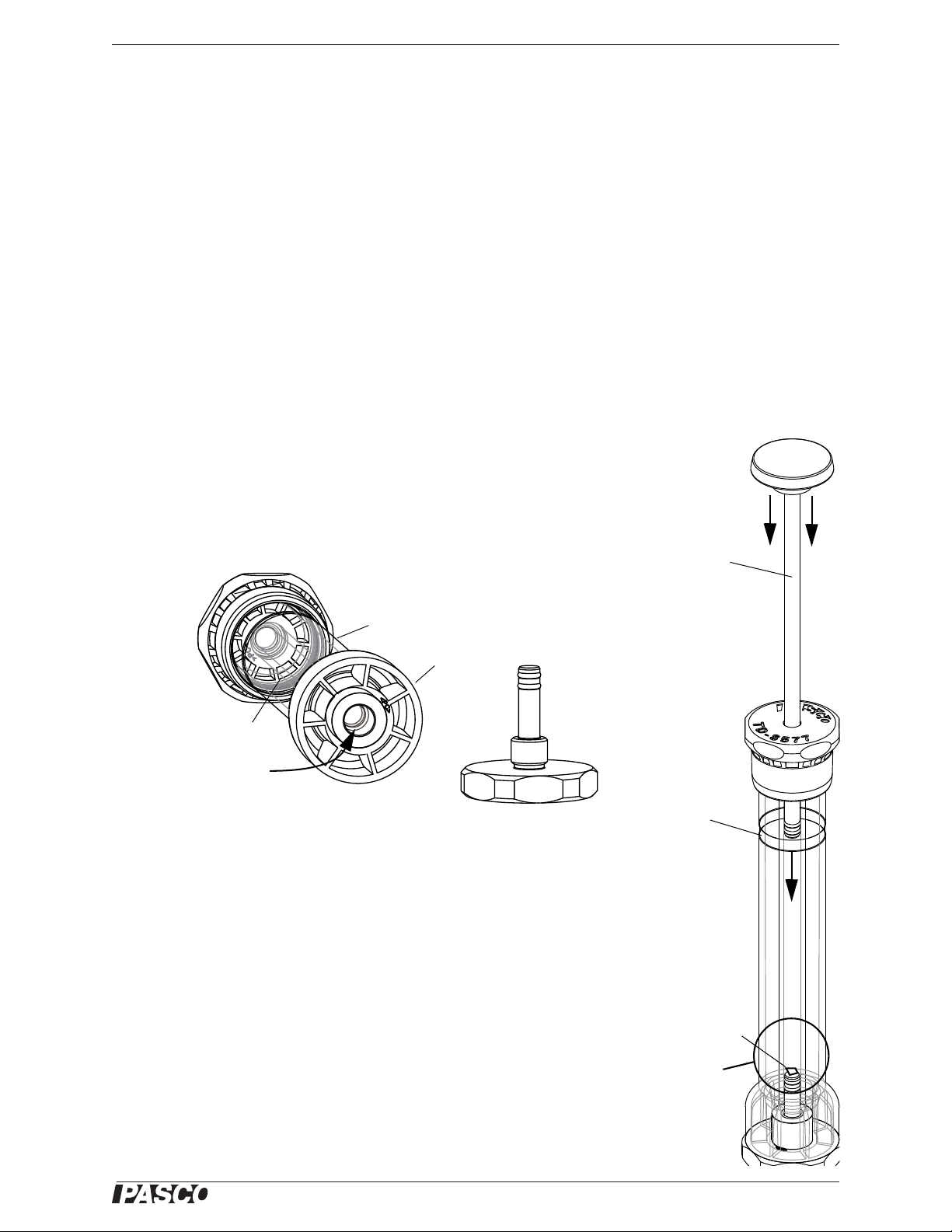
1. Turn the removable base counterclockwise to unscrew it from the bottom of the
igniter. Remove the base and set it aside.
2. Insert a small piece (~ 1 cm
pulled from the end of a cotton swab) into the bottom of the glass tube.
2
) of tissue paper or a few strands of cotton fiber (e.g.,
3. Use the loading/cleaning rod to push the material up into the lower part of the
glass tube. Carefully remove the loading/cleaning rod without pulling the material out of the lower part of the glass tube.
4. IMPORTANT: Insert the piston into the top of the igniter and push it in far
enough that the end of the piston lines up with or is slightly below the
“Piston Starting Position” label on the outer tube 2.5 cm (1 inch) below the top.
5. Replace the removable base by screwing it clockwise into the bottom.
6. Place the base of the igniter on a sturdy horizontal surface and securely hold the
tube with one hand. Make sure that the combustible material in the bottom of the
glass tube is visible.
7. Watch the piece of combustible material at the bottom of the glass cylinder.
8. With the palm of your other hand, quickly slap down on the knob of the piston so
the end of the piston goes down into the cylinder rapidly.
3
Page 4

PASCO Compression Igniter 012-13028B Caution
®
• Do not ‘clump’ or compress the combustible material. Allow it to remain
flat and spread out. The more surface area it has, the better.
• If the material does not ignite, try reducing the amount of material you put
into the glass tube.
• If you are using a piece of tissue paper, remove the paper and tear it in half.
Put one half of the paper back into the glass tube and try again.
• If you are using cotton and it does not ignite, reduce the number of strands
of cotton and try again.
Wad of tissue
paper
Loading/Cleaning
Rod
Glass
Tube
.
Maintenance
Cleaning
To remove fragments of burned material from the igniter, unscrew the removable base from the bottom of the igniter. Use the loading/cleaning rod to scrape
any residue out of the lower part of the glass tube.
To clean the glass tube, remove the piston and the base. Use the loading/cleaning rod to push a small wad of tissue paper through the glass tube.
After cleaning, screw the removable base back into the bottom of the igniter.
Do not use a wrench or other tool to tighten the removable base or the top.
Instead, they should be only as tight as you can make them using your hand.
Lubrication
The “O” rings on the piston and the removable base are lubricated at the factory . If the “O” rings on the piston become dry , tear or cut the tip off the packet
of lubricant. Squeeze a small drop (1 to 2 mm diameter) of lubricant onto an
applicator (such as a toothpick, cotton-tipped swab, etc.), and rub the lubricant
onto the two “O” rings. Store the open packet in a plastic bag or a small jar.
CAUTION: Do not use a different lubricant than the kind provided.
Storage
Store the compression igniter with the piston removed from the inner glass
tube.
Caution
The inner cylinder in the Compression Igniter is thick-wall glass, but it can
break if the Compression Igniter is dropped or if the piston is not far enough
down into the glass tube when the igniter is used. Before using the igniter, be
sure that the bottom of the piston is at least to the “Piston Starting Position”
label.
Be careful when handling the Compression Igniter, and always store the igniter
with the piston removed from the glass tube.
The Compression Igniter is shipped with a replacement inner glass tube packed
separately to minimize the possibility of damage during transit.
4
Page 5

Model No.TD-8577 012-13028B Caution
®
P
i
s
t
o
n
S
t
a
r
t
i
n
g
P
o
s
i
t
i
o
n
Safety Tube
Glass Tube
Post
Base
Insert the glass
tube over the post
and O-rings.
Top
Glass
Tube
1. Remove
the top.
2. Clear the
broken glass.
3. Insert the
replacement
glass tube.
4. Replace
the top.
Replacing a Glass Tube
Top
O-ring
installed
O-ring
Removable
Top
Top View
Side View
(cutaway)
Replacing a Large O-ring into the Top
Replacing the Inner Glass Tube
If the glass tube in the Compression Igniter should break, replace it with the
included replacement glass tube*.
• Hold the igniter over a receptacle to catch any falling glass and remove the
piston.
• Unscrew the removable base from the bottom, and unscrew the top from
the safety tube.
• Carefully remove any broken glass from inside the safety tube and dispose
of the glass properly.
• Check the condition of the O-rings and replace them if necessary. NOTE: If
the O-rings show no abrasion damage, they do not need replacing.
• Screw the removable base into the bottom of the igniter. Leave the top off
the igniter.
• Carefully insert the replacement glass tube into the igniter so that the bottom end of the glass tube fits over the post at the bottom of the igniter.
• Replace the top on the igniter. Be careful to line up the upper end of the
Replacing a Large O-ring
inner glass tube with the hole in the top.
*
The TD-8498A Replaceable Glass Tubes kit includes two more replacement tubes and
twelve O-rings
• To replace the large O-ring in the top of the igniter, unscrew the top from
the safety tube.
• Remove the old O-ring. (You may need to use a small, flat-blade screwdriver to remove the ring.)
• Place the replacement O-ring in the groove at the bottom of the hole. Make
sure that the O-ring is flat against the bottom of the hole..
• Replace the top on the igniter. Line up the top end of the glass tube with the hole
in the top and then turn the top clockwise to screw it onto the igniter.
5
Page 6

PASCO Compression Igniter 012-13028B Technical Support
®
Specifications
Inside diameter of glass
tube = 0.797 cm
Compressible length of glass
tube = 18.308 cm
Compression ratio
(approximate) = 15 to 1
Technical Support
For assistance with any PASCO product, contact PASCO at:
Address: PASCO scientific
10101 Foothills Blvd.
Roseville, CA 95747-7100
Phone: +1 916-786-3800 (worldwide)
800-772-8700 (U.S.)
Web: www.pasco.com
Email: support@pasco.com
For more information about the P ASCO Compression Igniter and the latest revision of
this Instruction Manual, visit the PASCO web site at www.pasco.com and enter
TD-8577 in the Search window.
Limited Warranty
For a description of the product warranty, see the PASCO catalog.
Copyright
The PASCO scientific 012-13028B PASCO Compression Igniter Instruction Manual is copyrighted with all rights reserved. Permission
is granted to non-profit educational institutions for reproduction of any part of this manual, providing the reproductions are used only
in their laboratories and classrooms, and are not sold for profit. Reproduction under any other circumstances, without the written consent of PASCO scientific, is prohibited.
Trademarks
PASCO and PASCO scientific are trademarks or registered trademarks of PASCO scientific, in the United S t ates and/or in other countries. For more information visit www.pasco.com/legal.
Material Safety Data Sheet
Please see the next four pages.
6
Page 7

Model No.TD-8577 012-13028B Material Safety Data Sheet
®
DOW CORNING CORPORATION
Material Safety Data Sheet
Page: 1 of 7
Version: 1.5
Revision Date: 2011/01/28
DOW CORNING(R) 111 VALVE LUBRICANT & SEALANT
1. PRODUCT AND COMPANY IDENTIFICATION
Dow Corning Corporation
South Saginaw Road
Midland, Michigan 48686
24 Hour Emergency Telephone:
Customer Service:
Product Disposal Information:
CHEMTREC:
(989) 496-5900
(989) 496-6000
(989) 496-6315
(800) 424-9300
MSDS No.: 01889834 Revision Date: 2011/01/28
Generic Description: Silicone grease.
Physical Form: Grease
Color: Translucent white
Odor: Odorless
NFPA Profile: Health 0 Flammability 1 Instability/Reactivity 0
Note: NFPA = National Fire Protection Association
2. HAZARDS IDENTIFICATION
POTENTIAL HEALTH EFFECTS
Acute Effects
Eye: Direct contact may cause tempor ary redness and discomfort.
Skin: No significant irritation expected f rom a single short-term exposure.
Inhalation: No significant effects expected fr om a single short-term exposure.
Oral: Low ingestion hazar d in normal use.
Prolonged/Repeated Exposure Effects
Skin: No known applicable inform ation.
Inhalation:
No known applicable information.
Oral: No known applicable information.
Signs and Symptoms of Overexposure
No known applicable information.
Medical Conditions Aggravated by Exposure
No known applicable information.
DOW CORNING CORPORATION
Material Safety Data Sheet
Page: 2 of 7
Version: 1.5
Revision Date: 2011/01/28
DOW CORNING(R) 111 VALVE LUBRICANT & SEALANT
The above listed potential effects of overexposure are based on actual data, results of studies performed upon similar compositions,
component data and/or expert review of the product. Please refer to Section 11 for the detailed toxicology information.
3. COMPOSITION/INFORMATION ON INGREDIENTS
None present. This is not a hazardo us material as defined in the OSHA Hazard Communication S tandard.
4. FIR ST AID ME ASURES
Eye: If irritation occurs, flush eye(s) with lukewarm gently flowing water for 5 minutes. Obtain
medical attention.
Skin: No health effects expected. If irr itation does occur flush with lukewarm, gently flowing wat er
for 5 minutes. If irritation pers ists, obtain medical advice.
Inhalation: If symptoms are experienced rem ove source of contamination or move victim to fresh air . If
irritation persists, obtain medic al advice.
Oral: If irritation or disc omfort occur, obtain medical advice.
Notes to Physician: Treat accor ding to person's condition and specifics of exposure.
5. FIRE FIGHTING MEASURES
Flash Point: > 214 °F / > 101.1 °C (Closed Cup)
Autoignition Temperature: Not determined.
Flammability Limits in Air: Not determ ined.
Extinguishing Media: On large fires use dry chemic al, foam or water spray. On small fires use carbon dioxide
(CO2), dry chemical or water spra y. Water can be used to cool fire exposed containers.
Fire Fighting Measures: Self-contained breathing apparatus and protect ive clothing should be worn in fighting large
fires involving chemicals. Deter mine the need to evacuate or isolate the area accord ing to
your local emergency plan. Use water spray to keep fire exposed containers cool.
Unusual Fire Hazards: None.
6. ACCIDENTAL RELEASE MEASURES
7
Page 8

PASCO Compression Igniter 012-13028B Material Safety Data Sheet
®
Suitable Respirator: None should be needed.
Personal Protective Equipment for Spills
Personal Protective Equipment for Routine Handling
Engineering Controls
8. EXPOSURE CONTROLS / PERSON AL PROTECTION
Component Exposure Limits
There are no components with work place exposure limits.
Use with adequate ventilation. Av oid eye contact.
Use reasonable care and store awa y from oxidizing materials. This material in its finel y divided form presents an
explosion hazard. Follow NFPA 654 (for chemical dusts) or 484 (for metal dusts) as appropria te for managing dust
hazards to minimize secondary explos ion potential.
Note: See Section 8 for Personal Protective Equipment for Spills. Call (989) 496-5900 , if additional information is
required.
7. HANDLING AND STORAGE
Local Ventilation: None should be needed.
General Ventilation: Recommended.
Eyes: Use proper protection - safety glass es as a minimum.
Skin: Washing at mealtime and end of shift is adequate.
Suitable Gloves: Handle in accordance with good indus trial hygiene and safety practices.
Inhalation: No respiratory protection should be needed.
Containment/Clean up: Observe all personal protection eq uipment recommendations described in Sections 5 and 8.
DOW CORNING(R) 111 VALVE LUBRICANT & SEALANT
Wipe up or scrape up and contain for salvage or disposal. Clean area as appropriate since
spilled materials, even in sm all quantities, may present a slip hazard. Final clean ing may
require use of steam, solvents or det ergents. Dispose of saturated absorbant or cleaning
materials appropriately, since spontan eous heating may occur. Local, state and federal laws
and regulations may apply to rele ases and disposal of this material, as well as those materials
and items employed in the cleanup of releases. You will need to determine which federal,
state and local laws and regulatio ns are applicable. Sections 13 and 15 of this MSDS provide
information regarding certain feder al and state requirements.
DOW CORNING CORPORATION
Material Safety Data Sheet
Page: 3 of 7
Revision Date: 2011/01/28
Version: 1.5
Materials to Avoid: Oxidizing material can cause a reac tion.
Hazardous Decomposition Products
Thermal breakdown of this product d uring fire or very high heat conditions may evolve t he following decomposition
products: Carbon oxides and traces of incompletely burned carbon compounds. Silicon dioxid e. Formaldehyde.
Hazardous polymerization will not oc cur.
Note: The above information is not intended for use in preparing product specifications. Contact Dow Corning before writing
specifications.
10. STABILITY AND REACTIVITY
Flammability Limits in Air: Not determined.
Autoignition Temperature: Not determined.
Flash Point: > 214 °F / > 101.1 °C (Closed Cup)
Volatile Content: Not determined.
pH: Not determined.
Solubility in Water: Not determined.
Vapor Density: Not determined.
Vapor Pressure @ 25°C: Not determined.
Boiling Point: Not determined.
Freezing/Melting Point: Not determined.
Viscosity: Not determined.
Specific Gravity @ 25°C: 1.1
9. PHYSICAL AND CHEMICAL PROPERT IES
Physical Form: Grease
Color: Translucent white
Odor: Odorless
Chemical Stability: Stable.
Hazardous
Polymerization:
Conditions to Avoid: None.
Precautionary Measures: Avoid eye contac t. Use reasonable care.
Note: These precautions are for room temperature handling. Use at elevated temperature or aerosol/spray applications may require
added precautions.
DOW CORNING(R) 111 VALVE LUBRICANT & SEALANT
No respiratory protection should be n eeded.
Eyes: Use proper protection - safety glass es as a minimum.
Skin: Washing at mealtime and end of shift is adequate.
Inhalation/Suitable
Respirator:
DOW CORNING CORPORATION
Material Safety Data Sheet
Page: 4 of 7
Revision Date: 2011/01/28
Version: 1.5
8
Page 9

Model No.TD-8577 012-13028B Material Safety Data Sheet
®
Hazard Parameters (LC50 or EC50) High Medium Low
Acute Aquatic Toxicity (mg/L) <=1 >1 and <=100 >100
Not subject to DOT.
DOT Road Shipment Information (49 CFR 172.101)
Ocean Shipment (IMDG)
14. TRANSPORT INFORMATION
State or local laws may impose ad ditional regulatory requirements regarding dispo sal. Call (989) 496-6315, if additional
information is required.
When a decision is made to discard t his material, as received, is it classified as a hazardo us waste? No
RCRA Hazard Class (40 CFR 261)
13. DISPOSAL CONSIDERATIONS
Acute Terrestrial Toxicity <=100 >100 and <= 2000 >2000
This table is adapted from "Environmental Toxicology and Risk Assessment", ASTM STP 1179, p.34, 1993.
This table can be used to classify the ecotoxicity of this product when ecotoxicity data is listed above. Please read the other information presented in the
section concerning the overall ecological safety of this material.
Complete information is not yet a vailable.
Environmental Effects
Fate and Effects in Waste Water T reatment Plants
Complete information is not yet a vailable.
12. ECOLOGICAL INFORMATION
Environmental Fate and Distribution
Complete information is not yet a vailable.
11. TOXICOLOGICAL INFORMATION
Special Hazard Information on Components
No known applicable inform ation.
DOW CORNING(R) 111 VALVE LUBRICANT & SEALANT
DOW CORNING CORPORATION
Material Safety Data Sheet
Ecotoxicity Classification Criteria
Page: 5 of 7
Revision Date: 2011/01/28
Version: 1.5
Not subject to IMDG code.
Not subject to IATA regulations.
15. REGULATORY INFORMATION
Call Dow Corning Transportation, (989) 496-8577, if additional information is required.
Air Shi pment (I ATA)
DOW CORNING(R) 111 VALVE LUBRICANT & SEALANT
TSCA Status: All chemical substances in this m aterial are included on or exempted from listing on th e TSCA
Massachusetts
and Toxic Enforcement Act of 1986 (Pr oposition 65) as being known to cause cancer, birth defects or other
reproductive harm.
None known.
Warning: This product contains the f ollowing chemical(s) listed by the State of California under the Saf e Drinking Water
Supplemental State Compliance Information
California
None present or none present in regulated quantities.
Reactive: No
Section 313 Toxic Chemicals (40 CFR 372):
Note: Chemicals are listed under the 313 Toxic Chemicals section only if they meet or exceed a reporting threshold.
Section 311/312 Hazard Class (40 CFR 370):
None.
Pressure: No
Chronic: No
Acute: No
Fire: No
EPA SARA Title III Chemical Listings
Section 302 Extremely Hazardous Substances (40 CFR 355):
None.
Section 304 CERCLA Hazardous Substances (40 CFR 302):
Contents of this MSDS compl y with the OSHA Hazard Communication Standard 29 CFR 1910.1200.
Inventory of Chemical Substances.
DOW CORNING CORPORATION
Material Safety Data Sheet
Page: 6 of 7
Revision Date: 2011/01/28
Version: 1.5
9
Page 10

Model No.TD-8577 012-13028B Material Safety Data Sheet
®
DOW CORNING CORPORATION
Material Safety Data Sheet
Page: 7 of 7
Version: 1.5
Revision Date: 2011/01/28
DOW CORNING(R) 111 VALVE LUBRICANT & SEALANT
CAS Number Wt % Component Name
7631-86-9 7.0 - 13.0 Silica, amorphous
New Jersey
CAS Number Wt % Component Name
63148-62-9 70.0 - 90.0 Polydim ethylsiloxane
7631-86-9 7.0 - 13.0 Silica, amorphous
70131-67-8 5.0 - 10.0 Dimethyl siloxane, hydroxy-terminated
Pennsylvania
CAS Number Wt % Component Name
63148-62-9 70.0 - 90.0 Polydim ethylsiloxane
7631-86-9 7.0 - 13.0 Silica, amorphous
70131-67-8 5.0 - 10.0 Dimethyl siloxane, hydroxy-terminated
16. OTHER INFORMATION
Prepared by: Dow Corning Corporatio n
These data are offered in good fait h as typical values and not as product specifications. No warranty, either expressed or
implied, is hereby made. The recommended in dustrial hygiene and safe handling procedures are believed to be generall y
applicable. However, each user shou ld review these recommendations in the specific c ontext of the intended use and
determine whether they are appropriate.
(R) indicates Registered Tradem ark
10
 Loading...
Loading...