Page 1

Instruction Manual and
Experiment Guide for the
PASCO scientific
Model TD-8572
HEAT ENGINE/ GAS LAW
APPARATUS
10101 Foothills Blvd. Roseville, CA 95678-9011 USA
®
Phone (916) 786-3800 FAX (916) 786-8905 web: www.pasco.com
better
ways to
teach science
Page 2

The exclamation point within an equilateral
triangle is intended to alert the user of the
presence of important operating and maintenance (servicing) instructions in the literature
accompanying the appliance.
Page 3

012-06014C Heat Engine/Gas Laws Apparatus
Table of Contents
Section Page
Copyright, Warranty, and Equipment Return ....................................................ii
Introduction......................................................................................................1
Equipment ........................................................................................................1
Experiments
1) Operation of a Heat Engine........................................................................3
2) Charles’ Law .............................................................................................5
3) Boyle’s Law ..............................................................................................7
4) Combined Gas Law (Gay-Lussac’s) ..........................................................9
5) The Mass Lifter Heat Engine................................................................. 11–18
Technical Support ................................................................................... Back Cover
®
i
Page 4
Heat Engine/Gas Laws Apparatus 012-06014C
®
Copyright, Warranty, and Equipment Return
Please—Feel free to duplicate this manual
subject to the copyright restrictions below.
Copyright Notice
The PASCO scientific 012-06014B Heat Engine/Gas Law
Apparatus manual is copyrighted and all rights reserved.
However, permission is granted to non-profit educational
institutions for reproduction of any part of the manual
providing the reproductions are used only for their
laboratories and are not sold for profit. Reproduction
under any other circumstances, without the written
consent of PASCO scientific, is prohibited.
Limited Warranty
PASCO scientific warrants the product to be free from
defects in materials and workmanship for a period of one
year from the date of shipment to the customer. PASCO
will repair or replace at its option any part of the product
which is deemed to be defective in material or workmanship. The warranty does not cover damage to the product
caused by abuse or improper use. Determination of
whether a product failure is the result of a manufacturing
defect or improper use by the customer shall be made
solely by PASCO scientific. Responsibility for the return
of equipment for warranty repair belongs to the customer.
Equipment must be properly packed to prevent damage
and shipped postage or freight prepaid. (Damage caused
by improper packing of the equipment for return shipment
will not be covered by the warranty.) Shipping costs for
returning the equipment after repair will be paid by
PASCO scientific.
Equipment Return
Should the product have to be returned to PASCO
scientific for any reason, notify PASCO scientific by
letter, phone, or fax BEFORE returning the product. Upon
notification, the return authorization and shipping instructions will be promptly issued.
➤➤
➤
NOTE: NO EQUIPMENT WILL BE
➤➤
ACCEPTED FOR RETURN WITHOUT AN
AUTHORIZATION FROM PASCO.
When returning equipment for repair, the units must
be packed properly. Carriers will not accept responsibility for damage caused by improper packing. To
be certain the unit will not be damaged in shipment,
observe the following rules:
➀ The packing carton must be strong enough for the item
shipped.
➁ Make certain there are at least two inches of packing
material between any point on the apparatus and the
inside walls of the carton.
➂ Make certain that the packing material cannot shift in
the box or become compressed, allowing the
instrument come in contact with the packing carton.
Address: PASCO scientific
10101 Foothills Blvd.
Roseville, CA 95747-7100
Credits
Editor: Sunny Bishop
Phone: (916) 786-3800
FAX: (916) 786-3292
email: techsupp@pasco.com
web: www.pasco.com
ii
Page 5
012-06014C Heat Engine/Gas Law Apparatus
Introduction
The PASCO TD-8572 Heat Engine/Gas Law Apparatus is
used for quantitative experiments involving the Ideal Gas
Law (as described below) and for investigations of a
working heat engine. The equipment allows the amount
of work done by thermal energy to be measured.
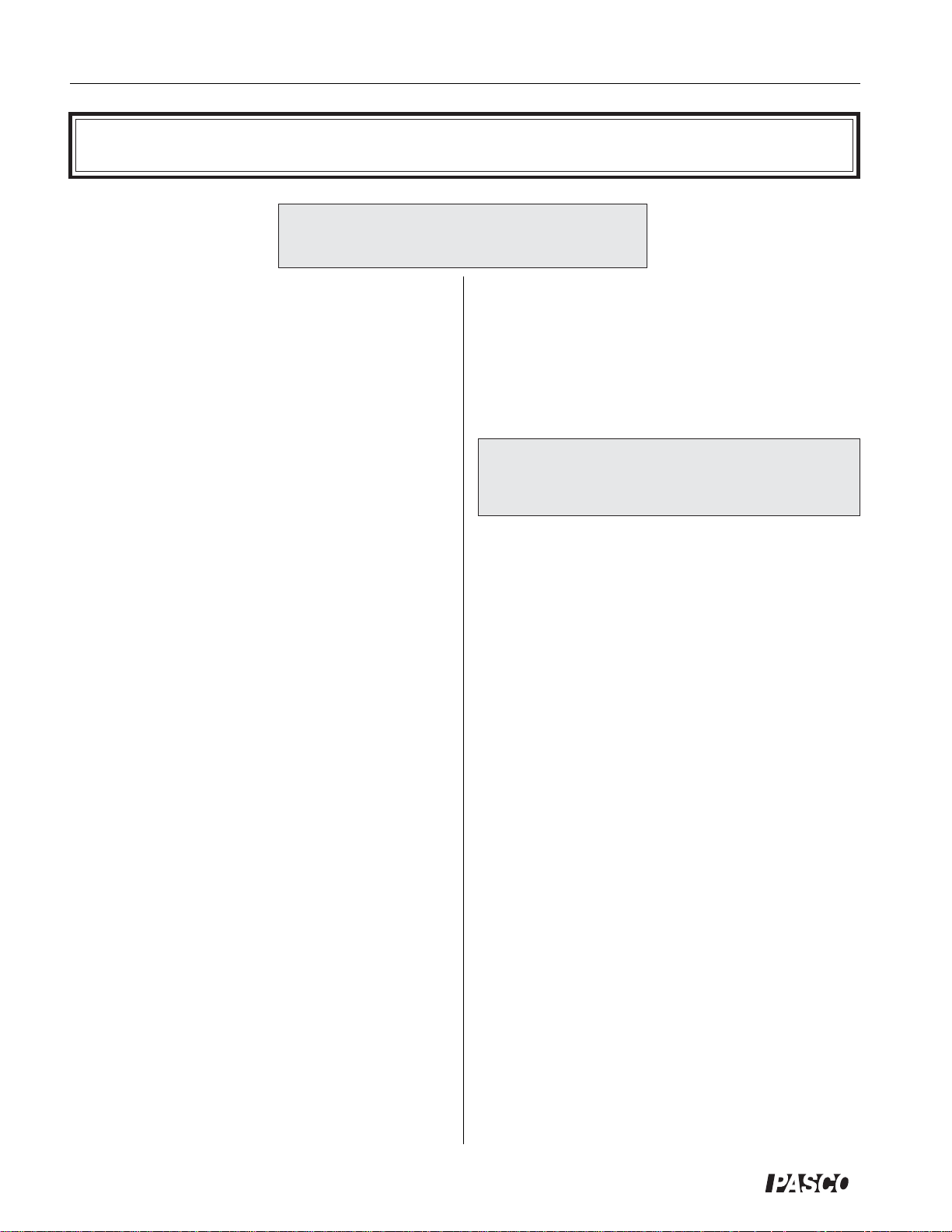
Equipment
mass platform for adding
a load to do work
experiments
piston-holding
thumbscrew
precision-bore pyrex
cylinder inside a
protective plastic shield
two port shut-off
valves
millimeter scale for
measuring piston
displacement
graphite piston
pressure port
mating
connectors
The heart of this apparatus is a nearly friction-free piston/
cylinder system. The graphite piston fits snugly into a
precision-ground Pyrex cylinder so that the system
produces almost friction-free motion and negligible
leakage.
The apparatus includes the following equipment
• base apparatus (Figure 1)
- piston diameter: 32.5 mm ± 0.1
- mass of piston and platform: 35.0 g ± .06
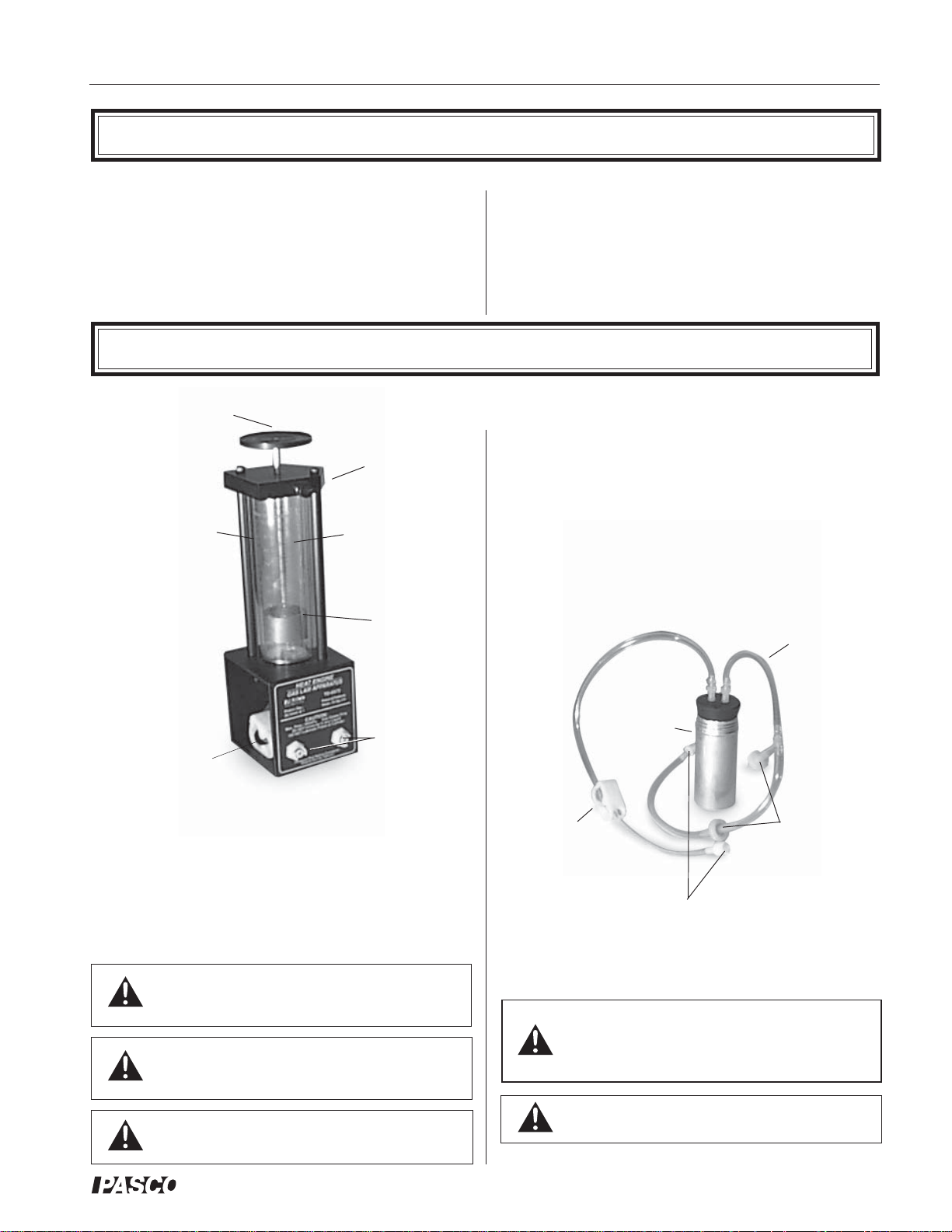
• air chamber (Figure 2)
• 3 hose configurations: one with one-way check
valves and one with a clamp (Figure 2), and one
plain piece of tubing (not shown)
• 1 each, one-holed and two-holed rubber stopper
tubing for
connecting
chamber to
cylinder
air chamber for
immersing in hot
or cold water
Figure 1. Base apparatus
The Heat Engine/Gas Law Apparatus is designed with
two pressure ports with quick-connect fittings for connecting to the air chamber tubing.
The apparatus can be connected to a Low Pressure Sensor
for use with PASCO computer interfaces.
Do not apply lubricant to the piston or
cylinder.
Do not immerse the base apparatus in
liquid.
Note: Use only non-caustic/non-toxic gases
such as air or helium.
®
clamp
pressure port mating connectors
one-way
check valves
Figure 2. Air chamber and tubing
Always release the tubing clamps prior to
storage to avoid permanently deforming the
tubing.
Maximum Pressure: 345 kPa.
1
Page 6

Heat Engine/Gas Law Apparatus 012-06014C
Notes:
2
®
Page 7
012-06014C Heat Engine/Gas Law Apparatus
HEAT ENGINE
GAS LAW APPARATUS
Piston Dia.:
32.5mm±0.1
TD-8572
CAUTION:
Max. Pres.: 345kPa. Use Gases Only
DO NOT
Immerse in any Liquid
DO NOT Lubricate Piston or Cylinder
Pressure Port Mating Connector:
PASCO Part No. 640-021
Piston&Platform
Mass:
35.0g±0.6
m
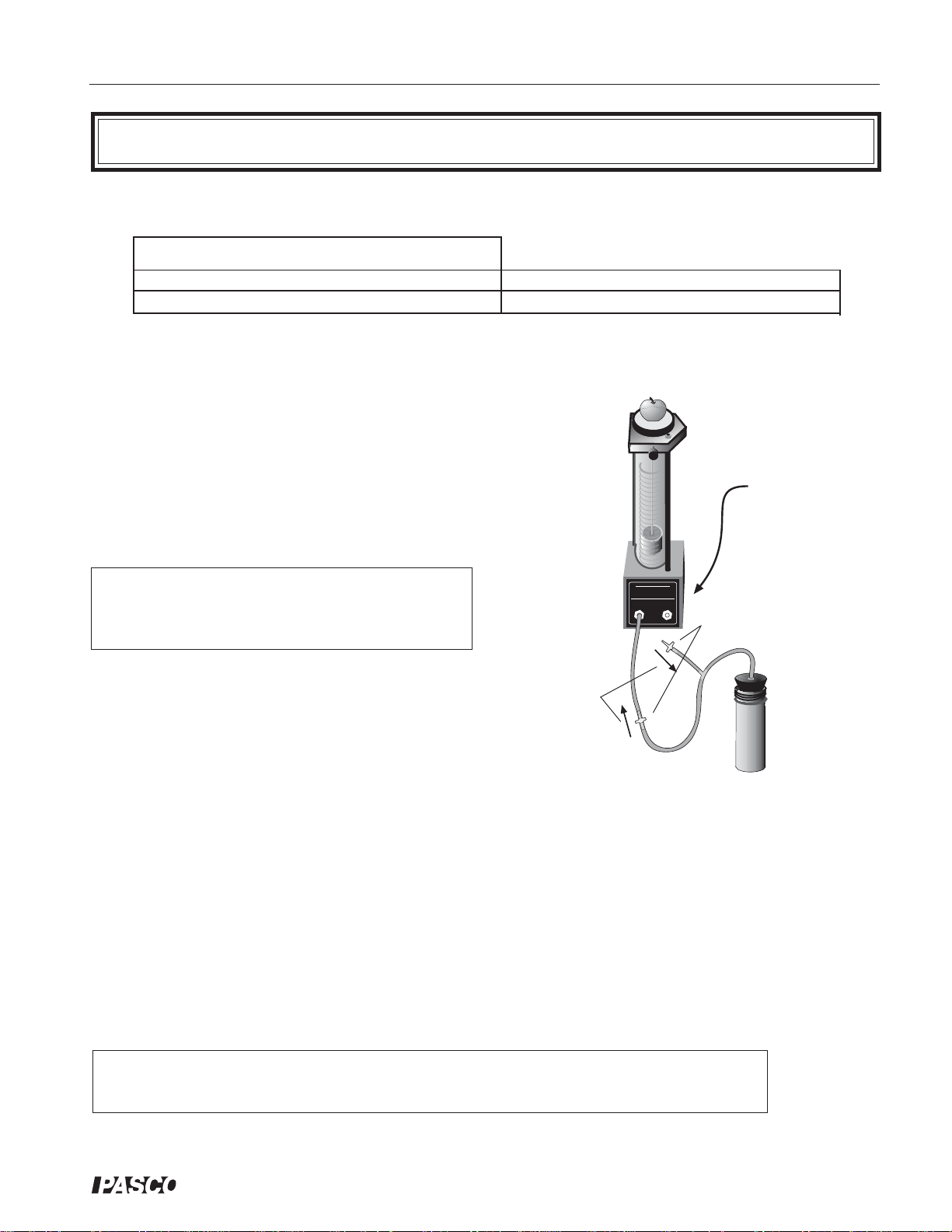
Experiment 1: Operation of a Heat Engine
Equipment Required:
• Heat Engine/Gas Law Apparatus
• 100 – 200 g mass
Equipment Setup
➀ Using the one-holed stopper, connect the tubing
with the one-way valves to the air chamber and
to a connecting port on the base assembly.
• container of hot water
• container of ice water
Procedure
➁ Close the shut-off valve on the tubing from the
unused port.
➂ Set a mass of 100 to 200 g on the mass platform.
➤ Note: Use a maximum mass of 200 grams in
the experiment. A larger mass will cause the
valve seals to leak.
➀ Move the air chamber from an ice water bath to a
direction of
air flow
one-way check
hot water bath. You will note that the air in the
chamber quickly expands through the tubing and
moves the piston up. Note also that the one-way
check valve in the tubing connecting the base
apparatus and the air chamber permits air to enter
Figure 1.1. Setup for the Heat Engine
the cylinder, while the other one-way check valve
prevents air from leaving through the branched
tube.
➁ Move the air chamber back to the cold bath and note that external air is sucked into the air
chamber through the one-way valve located at the end of the branched tube. Note also that the
one-way valve in the connecting tube prevents the air from escaping from the piston, so the
height of the piston remains the same.
Close the shut-off
valve on the
tubing from the
unused port.
valves
➂ Repeat steps 3 and 4 until the mass has been completely lifted.
➤ Note: The greater the temperature differential between the hot and cold water baths, the
greater the lift achieved through each cycle through them.
®
3
Page 8

Heat Engine/Gas Law Apparatus 012-06014C
➤ Note: For a more detailed, quantitative investigation of the operation of a heat engine, see
Experiment 5 (page 11).
4
®
Page 9

012-06014C Heat Engine/Gas Law Apparatus
Experiment 2: Charles’ Law
Equipment Required:
• Heat Engine/Gas Law Apparatus
• thermometer
Theory
Charles’ law states that at a constant pressure, the volume of a fixed mass or quantity of gas varies
directly with the absolute temperature:
V = cT (at constant P and where T is expressed in degrees Kelvin)
Setup
• container of hot water
• ice
➀ Using the one-holed stopper and plain tubing, connect the base apparatus and the air chamber.
➁ Close the shut-off valve on the tubing from the unused port.
➂ Turn the base apparatus on its side. (In this position, the force acting on the apparatus is the
atmospheric pressure and is equal throughout the range of operation of the piston.)
Close the shut-off valve on
the tubing from the unused
port.
y
l
.6
rm
n
0
r
S
±
e
O
2
tfo
g
d
d
i
s
U
:
7
n
la
r
i
.0
u
e
l
5
P
o
T
5
s
t
y
q
i
8
3
c
&
a
E
1
C
-
L
A
e
:
n
2
r
G
n
s
y
0
D
N
o
to
n
-
R
s
:
n
e
I
o
0
a
T
n
is
s
a
4
A
C
N
o
P
M
t
6
U
G
n
g
i
s
.
P
i
O
n
o
i
I
e
N
P
t
P
N
.
s
a
T
r
e
t
a
t
E
r
M
e
a
a
P
A
U
t
r
c
k
m
P
i
o
T
r
A
5
m
W
P
b
O
I
4
C
u
A
C
e
3
A
T
r
L
S
:
1
u
:
.
.
E
O
.
T
s
s
0
L
PA
a
s
N
i
O
e
±
H
e
r
r
S
N
D
O
m
P
P
D
n
.
A
O
m
o
x
D
5
t
.
a
G
s
i
2
M
3
P
Add ice.
Do not allow the tip of the
thermometer to touch the
bottom of the container.
Hot
Procedure
➀
Place the air chamber in a container of hot water. After the chamber equilibrates to the
temperature, record the temperature and the height of the piston.
➁ Add ice to the container and record the temperature and pressure at regular time intervals.
➂ Calculate the gas volumes at the various piston positions you measured and make a graph of plots
of temperature versus volume. (Hint: The diameter of the piston is 32.5 mm.)
®
5
Page 10

Heat Engine/Gas Law Apparatus 012-06014C
Notes:
6
®
Page 11

012-06014C Heat Engine/Gas Law Apparatus
P
P
A
S
C
O
Experiment 3: Boyle’ s Law
Equipment Required:
• Heat Engine/Gas Law Apparatus
• Pressure Sensor (CI-6532)
*For details on setting up and operating the Pressure Sensor with Science Workshop, please
consult the instruction sheet for the Pressure Sensor and the User’s Guide for Science Workshop.
Theory
Boyle’s law states that the product of the volume of a gas times its pressure is a constant at a fixed
temperature:
PV = a
Therefore, at a fixed temperature, the pressure will be inversely related to the volume, and the
relationship will be linear:
=
Setup
• Science Workshop computer interface*
a
V
➀ With the platform raised to its uppermost posi-
tion, connect the Pressure Sensor to a port on the
base apparatus with a short piece of tubing
(Figure 3.1).
➁ Close the shut-off valve on the tubing from the
unused port.
➂ Connect the Pressure Sensor to the computer
interface and set up Science Workshop to record
pressure. Be sure that you set up the keyboard
sampling option so you can enter height data by
hand. (Consult the Science Workshop User’s
Guide, “Keyboard Sampling,” for details.)
Procedure
➀ Record the height of the piston and the pressure
when the platform is raised to its highest position.
➁ Press the platform down to a series of levels and
record the height and pressure at each level.
➂ Convert the height measurements to gas volume
measurements. (Hint:
The diameter of the piston is 32.5 mm.)
to computer interface
HEAT ENGINE
GAS LAW APPARATUS
TD-8572
Piston Dia.:
Piston&Platform
32.5mm±0.1
35.0g±0.6
Mass:
CAUTION:
Max. Pres.: 345kPa. Use Gases Only
DO NOT
Immerse in any Liquid
E
C
O
A
T
F
R
E
E
T
N
R
I
U
R
S
O
a
S
P
S
E
k
T
N
R
0
U
E
0
L
P
7
S
O
-
0
S
B
A
(
Y
L
N
O
T
R:
CI-6532
OR
TO
R
0-021
I
O
RE P
. 64
A
C
U
NNEC
S
NO
E
Y
I
CO
A
R
R
RESS
E
0
P
ART
C
P
S
A
0
D
P
TING
F
A
5
R
M
E
6
SCO
T
E
N
PA
I
E
R
U
X
S
T
A
S
R
M
E
O
a
R
P
P
P
k
0
0
7
DO NOT Lubricate Piston or Cylinder
)
E
Pressure Port Mating Connector:
PASCO Part No. 640-021
Pressure Sensor
Figure 3.1. Experimental setup
Close the shut-off
valve on the tubing
from the unused
port.
➃ Prepare a graph of pressure versus volume.
➤ Note: The relationship between pressure and volume may not be linear at pressures greater
than 120 kPa because of air leakage from the valves and ports at higher pressures.
®
7
Page 12

Heat Engine/Gas Law Apparatus 012-06014C
8
®
Page 13

012-06014C Heat Engine/Gas Law Apparatus
P
A
S
C
O
Experiment 4: Combined Gas Law (Gay-Lussac’ s )
Equipment Required:
• Pressure Sensor (CI-6532)
• Science
Workshop computer interface*
• Temperature Sensor (CI-6505)
*For details on setting up and operating the Pressure Sensor and the Temperature Sensor with
Science Workshop, please consult the instruction sheets for the Pressure Sensor and the Temperature Sensor and the User’s Guide for Science Workshop.
Theory
Charles’ law states that V is proportional to T, and Boyle’s law states that V is proportional to 1/P.
Combining these, we have:
The combined gas law predicts that for a given mass of gas, if V is held constant, P is proportional
to T.
V=
aT
P
• hot plate
• Pyrex beaker with water
• ice
Setup
➀ The Gas Law Apparatus is not used in this
experiment. Use a short piece of tubing to
connect the pressue sensor to the air chamber
fitted with the 2-hole stopper.
➁ Insert the Temperature Sensor into the other
hole of the rubber stopper.
➂ Connect the Pressure Sensor and the Tem-
perature Sensor to the computer interface, and
set up the Science Workshop program to
graph temperature versus pressure.
Use a silicon lubricant on the end of
the Temperature Probe to aid
insertion and to prevent damage to
the probe.
➃ Place the air chamber in the Pyrex container
and turn on the hot plate.
to computer interface
to
computer
interface
E
C
O
A
T
F
R
E
E
T
N
R
I
U
R
S
O
a
)
S
P
S
E
E
k
T
N
R
0
U
E
0
L
P
7
S
O
0
S
B
A
(
2
3
5
Y
L
N
I-6
:
T
1
R
O
R
2
O
C
0
O
-
T
R
P
0
C
I
4
E
E
6
O
R
N
.
A
C
U
N
O
S
O
N
S
E
Y
S
C
I
T
E
A
R
R
G
R
R
E
0
P
A
C
N
P
I
S
P
A
0
D
T
F
O
A
5
R
C
M
E
6
S
T
E
A
N
P
I
E
R
U
X
S
T
A
S
R
E
M
O
a
R
P
P
P
k
0
0
7
Pressure
hot plate
Sensor
Figure 4.1. Experimental setup
Temperature
Sensor
➤ Note: You can substitute a thermometer in the water container for the Temperature Sensor.
Be sure to keep the tip of the thermometer from touching the bottom of the container.
®
9
Page 14

Heat Engine/Gas Law Apparatus 012-06014C
Procedure
Record the temperature and pressure as the water heats.
➀
➁ Display a graph of temperature versus pressure in Science Workshop.
10
®
Page 15

012-06014C Heat Engine/Gas Law Apparatus
Experiment 5: The Mass Lifter Heat Engine
The Heat Engine/Gas Law Apparatus is ideal for use in the calculus-based experiment 18.10 of the
Workshop Physics Activity Guide. Following is a slightly modified reprint of the experiment:
Equipment Required:
• Heat Engine/Gas Law Apparatus
• 2 Pyrex beakers, 1000 ml (to use as reservoirs)
• 1 ruler
• 1 barometer pressure gauge
Optional:
• a computer-based laboratory system with barometer sensor
Your working group has been approached by the Newton Apple Company about testing a
heat engine that lifts apples that vary in mass from 100 g to 200 g from a processing
conveyer belt to the packing conveyer belt that is 10 cm higher. The engine you are to
experiment with is a "real" thermal engine that can be taken through a four-stage expansion and compression cycle and that can do useful mechanical work by lifting small
masses from one height to another. In this experiment we would like you to verify experimentally that the useful mechanical work done in lifting a mass, m, through a vertical
distance, y, is equal to the net thermodynamic work done during a cycle as determined by
finding the enclosed area on a P-V diagram. Essentially you are comparing useful mechanical “magy” work (which we hope you believe in and understand from earlier studies)
with the accounting of work in an engine cycle as a function of pressure and volume
changes given by the expression:
W
=PdV
net
• 1 calipers
• 1 mass set, 20 g, 50 g, 100 g, 200 g
• 1 hot plate
• 1 vat to catch water spills
1
Although you can prove mathematically that this relationship holds, the experimental
verification will allow you to become familiar with the operation of a real heat engine.
m
y
b
c
P
m
0
a
V
Figure 5.1. Doing useful mechanical work
by lifting a mass, m, through a height, y.
1
Priscilla W. Laws, et al. Workshop Physics Activity Guide, 1996 by John Wiley & Sons, Inc.
Figure 5.2 Doing thermodynamic
work in a heat engine cycle.
Reprinted by permission of John Wiley & Sons, Inc.
®
11
d
Page 16

Heat Engine/Gas Law Apparatus 012-06014C
The Incredible Mass Lifter Engine
The heat engine consists of a hollow cylinder with a graphite piston that can move along the axis of the
cylinder with very little friction. The piston has a platform attached to it for lifting masses. A short
length of flexible tubing attaches the cylinder to an air chamber (consisting of a small can sealed with a
rubber stopper that can be placed alternately in the cold reservoir and the hot reservoir. A diagram of
this mass lifter is shown in Figure 5.2.
m
Close the shut-off valve
on the tubing from the
unused port.
HEAT ENGINE
GAS LAW APPARATUS
TD-8572
Piston Dia.:
Piston&Platform
32.5mm±0.1
35.0g±0.6
Mass:
CAUTION:
Max. Pres.: 345kPa. Use Gases Only
DO NOT
Immerse in any Liquid
DO NOT Lubricate Piston or Cylinder
Pressure Port Mating Connector:
PASCO Part No. 640-021
Cold
Hot
Figure 5.2. A schematic diagram of the incredible mass lifter heat engine.
If the temperature of the air trapped inside the cylinder, hose, and can is increased, then its
volume will increase, causing the platform to rise. Thus, you can increase the volume of the
trapped air by moving the can from the cold to the hot reservoir. Then, when the apple has
been raised through a distance y, it can be removed from the platform. The platform should
then rise a bit more as the pressure on the cylinder of gas decreases a bit. Finally, the volume
of the gas will decrease when the air chamber is returned to the cold reservoir. This causes
the piston to descend to its original position once again. The various stages of the mass lifter
cycle are shown in Figure 5.3.
Before taking data on the pressure, air volume, and height of lift with the heat engine, you
should set it up and run it through a few cycles to get used to its operation. A good way to
start is to fill one container with room temperature water and another with hot tap water or
preheated water at about 60–70°C. The engine cycle is much easier to describe if you begin
with the piston resting above the bottom of the cylinder. Thus, we suggest you raise the
piston a few centimeters before inserting the rubber stopper firmly in the can. Also, air does
leak out of the cylinder slowly. If a large mass is being lifted, the leakage rate increases, so
we suggest that you limit the added mass to something between 100 g and 200 g. After
observing a few engine cycles, you should be able to describe each of the points a, b, c, and
d of a cycle carefully, indicating which of the transitions between points are approximately
adiabatic and which are isobaric. You can observe changes in the volume of the gas directly
and you can predict how the pressure exerted on the gas by its surroundings ought to change
from point to point by using the definition of pressure as force per unit area.
12
®
Page 17

012-06014C Heat Engine/Gas Law Apparatus
m
m
y
y
m
Point A Point B Point C Point D
0
Cold Cold Hot Hot
m
0
0
Figure 5.3. A simplified diagram of the mass lifter heat engine at different stages of its cycle.
5.1 Activity: Description of the Engine Cycle
a. Predicted transition a➔b: Close the system to outside air but leave the can in the cold
reservoir. Make sure the rubber stopper is firmly in place in the can. What should happen
to the height of the platform when you add a mass? Explain the basis of your prediction.
b. Observed transition a
➔
b: What happens when you add the mass to the platform? Is this
what you predicted?
c. Predicted transition b
➔
c: What do you expect to happen when you place the can in the hot
reservoir ?
d. Observed transition b
➔
c: Place the can in the hot reservoir and describe what happens to
the platform with the added mass on it. Is this what you predicted? (This is the engine
power stroke!)
➔
e. Predicted transition c
d: Continue to hold the can in the hot reservoir and predict what
will happen if the added mass that is now lifted is removed from the platform and moved
onto an upper conveyor belt. Explain the reasons for your prediction.
®
13
Page 18

Heat Engine/Gas Law Apparatus 012-06014C
f. Observed transition c➔d: Remove the added mass and describe what actually happens. Is
this what you predicted?
➔
g. Predicted transition d
a: What do you predict will happen if you now place the can back in
the cold reservoir? Explain the reasons for your prediction.
➔
h. Observed transition d
a: Now it's time to complete the cycle by cooling the system down to
its original temperature for a minute or two before placing a new mass to be lifted on it. Place
the can in the cold reservoir and describe what actually happens to the volume of the trapped
air. In particular, how does the volume of the gas actually compare to the original volume of
the trapped air at point a at the beginning of the cycle? Is it the same or has some of the air
leaked out?
i. Theoretically, the pressure of the gas should be the same once you cool the system back to its
original temperature. Why?
Determining Pressures and Volumes for a Cycle
In order to calculate the thermodynamic work done during a cycle of this engine, you will
need to be able to plot a P-V diagram for the engine based on determinations of the volumes
and pressures of the trapped air in the cylinder, tubing, and can at the points a, b, c, and d in
the cycle.
5.2 Activity: Volume and Pressure Equations
a. What is the equation for the volume of a cylinder that has an inner diameter of d and a length
L?
b. Use the definition of pressure to derive the equation for the pressure on a gas being contained
by a vertical piston of diameter d if the total mass on the piston including its own mass and
any added mass is denoted as M. Hints: (1) What is the definition of pressure? (2) What is
the equation needed to calculate the gravitational force on a mass, M, close to the surface of
the Earth? (3) Don't forget to add in the atmospheric pressure, P
hence the gas at sea level.
, acting on the piston and
atm
Now that you have derived the basic equations you need, you should be able to take your
engine through another cycle and make the measurements necessary for calculating both the
volume and the pressure of the air and determining a P-V diagram for your heat engine.
Instead of calculating the pressures, if you have the optional equipment available, you might
want to measure the pressures with a barometer or a barometer sensor attached to a computerbased laboratory system.
14
®
Page 19

012-06014C Heat Engine/Gas Law Apparatus
5.3 Activity: Determining Volume and Pressure
a. Take any measurements needed to determine the volume and pressure of air in the
system at all four points in the engine cycle. You should do this rapidly to avoid air
leakages around the piston and summarize the measurements with units in the space
below.
b. Next you can use your measurements to calculate the pressure and volume of the system
at point a. Show your equations and calculations in the space below and summarize your
results with units. Don't forget to take the volume of the air in the tubing and can into
account!
=
P
a
V
=
a
c. Use the measurements at point b to calculate the total volume and pressure of the air in the
system at that point in the cycle. Show your equations and calculations in the space below
and summarize your results with units.
P
=
b
V
=
b
d. What is the height, y, through which the added mass is lifted in the transition from b to c?
e. Use the measurements at point c to calculate the total volume and pressure of the air in the
system at that point in the cycle. Show your equations and calculations in the following
space and summarize your results with units.
P
=
c
V
=
c
®
15
Page 20

Heat Engine/Gas Law Apparatus 012-06014C
f. Remove the added mass and make any measurements needed to calculate the volume and
pressure of air in the system at point d in the cycle. Show your equations and calculations in
the space below and summarize your results with units.
P
=
d
V
=
d
➔
g. We suspect that transitions from a
b and from c➔d are approximately adiabatic. Explain
why.
h. You should have found that the transitions from b
➔
c and from d➔a are isobaric. Explain
why this is the case.
Finding Thermodynamic Work from the Diagram
In the next activity you should draw a P- V diagram for your cycle and determine the thermodynamic work for your engine.
5.4 Activity: Plotting and Interpreting a
P-V
Diagram
a. Fill in the appropriate numbers on the scale on the graph frame that follows and plot the P-V
diagram for your engine cycle. Alternatively, generate your own graph using a computer
graphing routine and affix the result in the space below.
16
®
Page 21

012-06014C Heat Engine/Gas Law Apparatus
b . On the graph in part a, label each of the points on the cycle ( a, b, c, and d). Indicate on
the graph which of the transitions (a➔b, b➔c, etc.) are adiabatic and which are isobaric.
Next you need to find a way to determine the area enclosed by the P- V diagram. The
enclosed area doesn't change very much if you assume that P is approximately a linear
function of V for the adiabatic transitions. By making this approximation, the figure is
almost a parallelogram so you can obtain the enclosed area using one of several methods.
Three of the many possibilities are listed below. Creative students have come up with even
better methods than these, so you should think about your method of analysis carefully.
Method I
Since the pressure doesn't change from point b to point c, you can take the pressure of
those two points as a constant pressure between points. The same holds for the transition
from d to a. This gives you a figure that is approximately a parallelogram with two sets of
parallel sides. You can look up and properly apply the appropriate equation to determine
the net thermodynamic work performed.
Method II
Display your graph with a grid and count the boxes in the area enclosed by the lines
connecting points a, b, c, and d. Then multiply by the number of joules each box represents. You will need to make careful estimates of fractions of a box when a "leg" of a
cycle cuts through a box.
Method III
b
PdV = PdV
a
c
+PdV
b
+PdV
d
c
a
+PdV
d
Fit a straight line to each of the starting and ending points for the four transitions in the
cycle. Each equation will give you a function relating P and V. Perform an integral for
each of these equations, since
5.5 Activity: Comparing the Thermodynamic and Useful Mechanical Work
a. Choose a method for computing the thermodynamic work in joules, describe it in the space
below, and show the necessary calculations. Report the result in joules.
®
17
Page 22

Heat Engine/Gas Law Apparatus 012-06014C
b. What is the equation you need to use to calculate the useful mechanical work done in lifting
the mass from one level to another?
c. Use the result for the height that the mass is lifted in the power stroke of the engine to
calculate the useful mechanical work performed by the heat engine.
d. How does the thermodynamic work compare to the useful mechanical work? Please use the
correct number of significant figures in your comparison (as you have been doing all along,
right?)
The Incredible Mass Lifter Engine Is Not So Simple
Understanding the stages of the engine cycle on a P-V diagram is reasonably straightforward. However, it is difficult to use equations for adiabatic expansion and compression and
the ideal gas law to determine the temperature (and hence the internal energy of the air
throughout the cycle. There are several reasons for this. First, air is not an ideal gas.
Second, the mass lifter engine is not well insulated and so the air that is warmed in the hot
reservoir transfers heat energy through the cylinder walls. Thus, the air in the can and in the
cylinder are probably not at the same temperature. Third, air does leak out around the
piston, especially when larger masses are added to the platform. This means that the
number of moles of air decreases over time. You can observe this by noting that in the
transition from point d to point a, the piston can actually end up in a lower position than it
had at the beginning of the previous cycle. However, the Incredible Mass Lifter Engine
does help us understand typical stages of operation of a real heat engine.
➤ Note: The previous experiment was intended to help students consolidate the concepts
of pressure and volume by taking their own data for height and mass in each part of the
cycle and then calculating the pressures using the basic definition of pressure vs. force
per unit area. An alternate method for doing this experiment is to use the Science
Workshop computer interface with the Pressure Sensor (CI-6532) in conjunction with
either a Motion Sensor (CI-6529) or Rotary Motion Sensor (CI-6538) to detect pressure,
volume, and height automatically with a computer.
18
®
Page 23

Technical Support
Feedback
If you have any comments about the product or manual,
please let us know. If you have any suggestions on
alternate experiments or find a problem in the manual,
please tell us. PASCO appreciates any customer feedback. Your input helps us evaluate and improve our
product.
To Reach PASCO
For technical support, call us at 1-800-772-8700 (toll-free
within the U.S.) or (916) 786-3800.
fax: (916) 786-3292
e-mail: techsupp@pasco.com
web: www.pasco.com
Contacting Technical Support
Before you call the PASCO Technical Support staff, it
would be helpful to prepare the following information:
➤ If your problem is with the PASCO apparatus, note:
- Title and model number (usually listed on the
label);
- Approximate age of apparatus;
- A detailed description of the problem/sequence of
events. (In case you can’t call PASCO right away,
you won’t lose valuable data.);
- If possible, have the apparatus within reach when
calling to facilitate description of individual parts.
➤ If your problem relates to the instruction manual, note:
- Part number and revision (listed by month and year
on the front cover);
- Have the manual at hand to discuss your questions.
 Loading...
Loading...