Page 1
®
Instruction Manual and
Basic Calorimetry Set
TD-8557A
Experiment Guide
012-03060F
Al
Cu
W
Page 2

Basic Calorimetry Set
Table of Contents
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Notes on Calorimetry and Specifications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Experiment 1: What is a Calorie? . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Experiment 2: Specific Heat . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Experiment 3: Latent Heat of Vaporization . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Experiment 4: Latent Heat of Fusion. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Technical Support . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
ii
®
Page 3
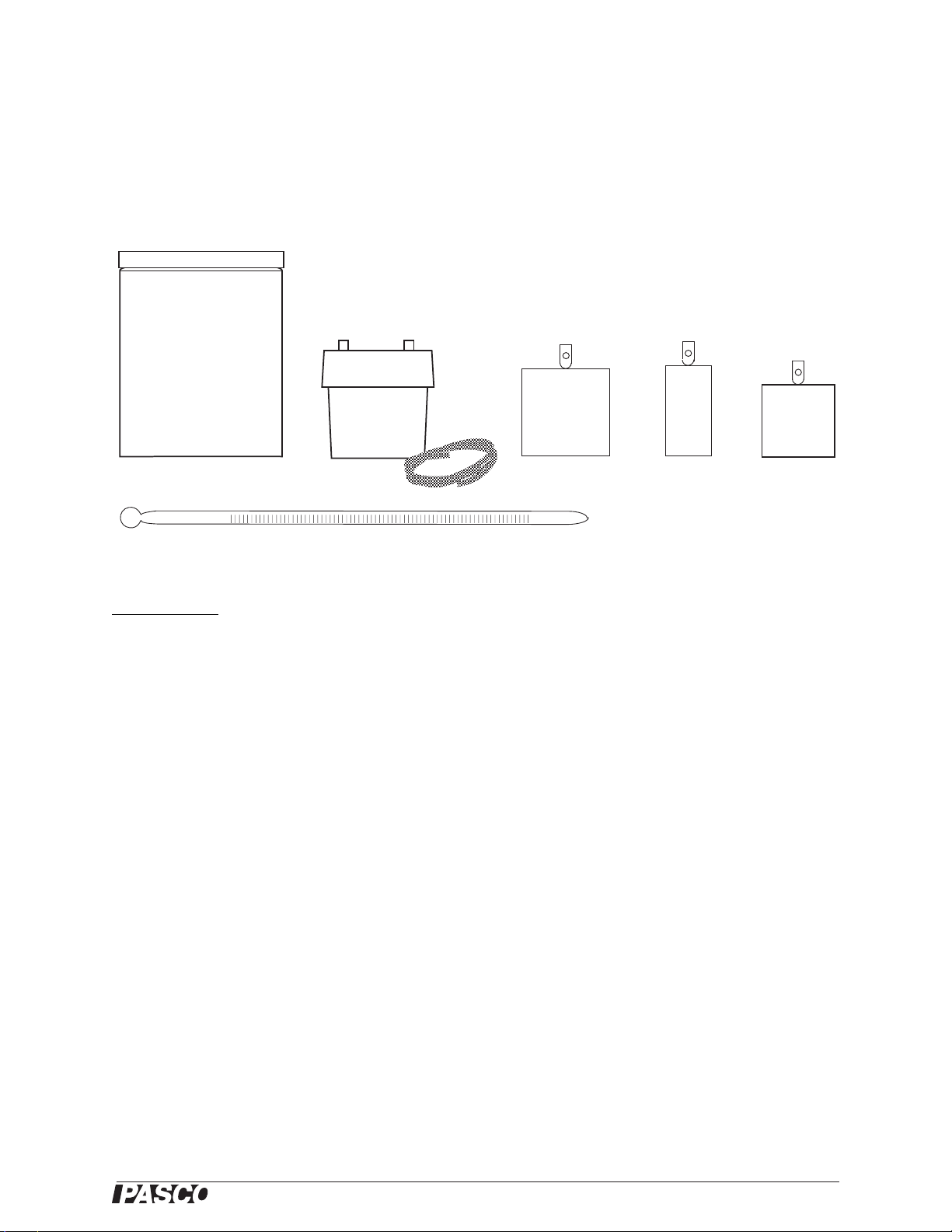
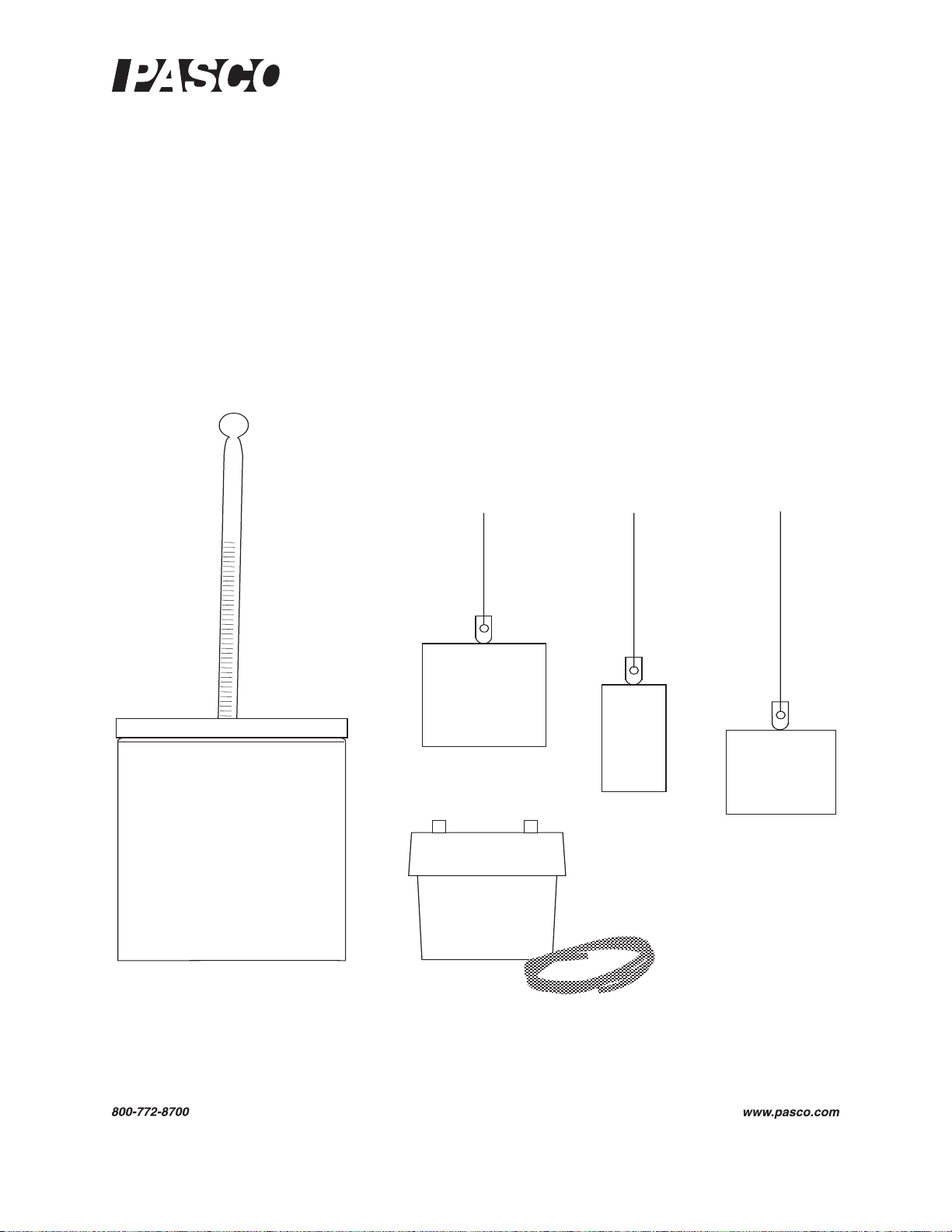
Basic Calorimetry Set
TD-8557A
1
Included Parts
1. Calorimeter with lid, 6 pieces
2. Water trap with plastic tubing
3. Aluminum sample
4. Copper sample
5. Tungsten sample
6. Thermometer
2
6
3
4
5
Introduction
Understanding calorimetry is the first step into the field of thermodynamics, the study of the role of heat in physical
processes. With the addition of a balance, ice, and a heat source, such as PASCO’s Model TD-8556 Steam Generator,
this Basic Calorimetry Set provides the equipment necessary to perform a variety of calorimetry experiments. Four
important, introductory experiments are described in this manual:
Experiment 1: What is a Calorie? An introduction to the ideas of temperature and heat, and a demonstration
of the conservation of energy.
Experiment 2: Thermal Capacity and Specific Heat The specific heats of aluminum, copper, and tungsten
are measured.
Experiment 3: Latent Heat of Vaporization The role of heat transfer in the conversion of steam into water is
investigated.
Experiment 4: Latent Heat of Fusion The role of heat transfer in the conversion of ice into water is investigated.
®
1
Page 4

Basic Calorimetry Set
Notes on Calorimetry
A calorimeter is a vessel or device that thermally isolates an experiment from its surroundings. Ideally, this means that the results of an experiment performed in a calorimeter are
independent of the temperature of the surroundings, because no heat flows into or out of
the calorimeter.
However, no calorimeter is perfect, and there is always some unwanted and unaccountable
heat flow affecting the results of any calorimetric experiment. To minimize unwanted heat
flow, always plan the experiment to follow these rules:
1. The time between the taking of initial and final temperatures is minimal.
In other words, do the critical portion of the experiment quickly, so there is minimal time
for unwanted heat flow between measurements. (Don’t rush; just plan carefully.)
2. Whenever possible, room temperature is approximately midway between
the beginning and ending temperatures of the experiment. When the experi-
mental temperature is colder than room temperature, heat flows from the surroundings
into the calorimeter. When the experimental temperature is hotter than room temperature,
heat flows from the calorimeter into the surroundings. If the experimental temperature
varies above and below room temperature by equal amounts, the heat gained and lost to
the environment will be approximately equal, minimizing the net affect on the experiment.
3. Mass measurements of liquids are made as near the critical temperature
measurements as possible. This reduces the effects of mass loss by evaporation.
Measuring liquid masses by taking appropriate differences is a useful technique (see the
instructions in the individual experiments).
NOTE: In applying the above rules, it is often helpful to perform a quick preliminary experiment to
determine the best choice for initial masses and temperatures.
Calorimeter
The calorimeter cup has two holes in its lid for inserting a thermometer, tubing from a
Steam Generator, etc. The rim of the cup has a pouring notch that makes it easier to pour
liquids out of the calorimeter.
Item Approximate Value
Mass with lid 26 g
Mass without lid 21 g
Outside diameter 10 cm
Inside diameter 7.5 cm
Height with lid 12.3 cm
Height without lid 11.4 cm
Volu me
2
500 cm
3
®
Page 5

Basic Calorimetry Set
Experiment 1: What is a Calorie?
Equipment Needed
• Calorimeters, 2 pieces
• Thermometer
•Balance
• Hot and cold water
Introduction
When two systems or objects of different temperature come into contact, energy in the
form of heat is transferred from the warmer system into the cooler. This transfer of heat
raises the temperature of the cooler system and lowers the temperature of the warmer system. Eventually the two systems reach some common, intermediate temperature, and the
heat transfer stops.
The standard unit for measuring heat transfer is the calorie. A calorie is defined as the
amount of energy required to raise the temperature of one gram of water from 14.5° C to
15.5° C. However, for our purposes, we can generalize this definition by simply saying
that a calorie is the amount of energy required to raise the temperature of one gram of
water one degree Celsius (the variation with temperature is slight).
In this experiment, you will combine hot and cold water of known temperature and mass.
Using the definition of the calorie, you will be able to determine the amount of heat energy
that is transferred in bringing the hot and cold water to their final common temperature,
and thereby determine if heat energy is conserved in this process.
Procedure
1. Determine the mass of the empty calorimeter, M
2. Fill the calorimeter about 1/3 full with cold water. Measure the mass of the calorime-
ter and water together to determine M
cal + water cold
3. Fill a second calorimeter approximately 1/3 full of hot water. The water should be at
least 20° C above room temperature. Weigh the calorimeter and water together to
determine M
4. Measure T
cal + water hot
and T
hot
. Record your result
, the temperatures the hot and cold water, and record your
cold
results.
5. Immediately after measuring the temperatures, add the hot water to the cold water and
stir with the thermometer until the temperature stabilizes. Record the final temperature of the mixture, T
final
.
6. Measure the final mass of the calorimeter and mixed water, M
. Record your result in Table 1.1.
cal
. Record your result.
.
final
7. Repeat the procedure twice with different masses of water at different temperatures.
(You might try adding cold water to hot instead of hot to cold.)
3
®
Page 6

Basic Calorimetry Set
Data
Table 1.1: Data
Trial 1 Trial 2 Trial 3
M
cal
M
cal + water cold
M
cal + water hot
T
cold
T
hot
T
final
M
final
Calculations
From your data, make the calculations necessary to determine the mass of the cold and hot
water (M
(∆T
cold
water cold
and ∆T
and M
). Enter your results in Table 1.2.
hot
), and also the temperature changes undergone by each
water hot
Using the equations shown below, calculate ∆H
cold
and ∆H
, the heat gained by the cold
hot
and hot water, respectively. Enter your results in the table.
∆H
∆H
M
water cold
M
water hot
∆T
cold
∆T
hot
∆H
cold
∆H
hot
cold
hot
= (M
= (M
water cold
water hot
)(∆T
)(∆T
)(1 cal/g K)
cold
)(1 cal/g K)
hot
Table 1.2: Calculations
Trial 1 Trial 2 Trial 3
Questions
1. Which had more thermal energy, the two cups of water before they were mixed
together or after they were mixed? Was energy conserved?
2. Discuss any unwanted sources of heat loss or gain that might have had an effect on
the experiment.
3. If 200 g of water at 85° C were added to 150 g of water at 15° C, what would be the
final equilibrium temperature of the mixture?
4
®
Page 7

Basic Calorimetry Set
Experiment 2: Specific Heat
Equipment Needed
• Calorimeter
• Thermometer
• Samples of aluminum, copper, and tungsten
•Balance
• Boiling water
•Cool water
• Thread
• Antifreeze, approximately 100 g
Introduction
The Specific Heat of a substance, usually indicated by the symbol c, is the amount of heat
required to raise the temperature of one gram of the substance by 1° C (or 1 K). From the
definition of the calorie given in Experiment 1, it can be seen that the specific heat of
water is 1.0 cal/g K. If an object is made of a substance with specific heat equal to c
then the heat, ∆H, required to raise the temperature of that object by an amount ∆T is:
sub
,
∆H = (mass of object) (c
sub
) (∆T)
In Part 1 of this experiment you will measure the specific heats of aluminum, copper, and tungsten.
In Part 2 you will measure the
CAUTION: This experiment involves the use of boiling water and the handling of HOT metal
objects. Work carefully.
specific heat of antifreeze.
Part 1: The Specific Heats of Aluminum, Copper, and Tungsten
1. Measure M
Record your result in Table 2.1.
2. Measure the masses of the aluminum, copper, and tungsten samples. Record these
masses in Table 2.1 in the row labeled M
3. Attach a thread to each of the metal samples and suspend each of the samples in boiling water. Allow a few minutes for the samples to heat thoroughly.
4. Fill the calorimeter approximately 1/2 full of cool water—use enough water to fully
cover any one of the metal samples.
5. Measure T
table.
, the mass of the calorimeter you will use (it should be empty and dry).
cal
.
sample
, the temperature of the cool water. Record your measurement in the
cool
6. Immediately following your temperature measurement, remove one of the metal samples from the boiling water, quickly wipe it dry, then suspend it in the cool water in
the calorimeter (the sample should be completely covered but should not touch the
bottom of the calorimeter).
7. Swirl the water and record T
, the highest temperature attained by the water as it
final
comes into thermal equilibrium with the metal sample.
8. Immediately after taking the temperature, measure and record M
, the total mass of
total
the calorimeter, water, and metal sample.
®
5
Page 8

Basic Calorimetry Set
Part 2: The Specific Heat of Antifreeze
Repeat Part 1 of this experiment, but instead of using the metal samples, heat approximately 100 g of antifreeze to approximately 60° C. Measure and record the temperature,
then quickly pour the antifreeze into a calorimeter containing cool water and stir until the
highest stable temperature is reached (about 1 minute). Record your data and calculations
on a separate sheet of paper. You will need the following data:
• M
• M
• T
• M
• T
, the mass of the calorimeter,
cal
the mass of the calorimeter plus water,
water
the temperature of the cool water,
cool
, the mass of the calorimeter plus water plus antifreeze
total
, the temperature of the water plus antifreeze.
final
Data and Calculations
Table 2.1: Data and Calculations (Part 1)
M
cal
M
sample
T
cool
T
final
M
total
M
water
∆T
water
∆T
sample
c
Trial 1 Trial 2 Trial 3
Part 1
For each metal tested, use the equations shown below to determine M
water used, ∆T
the metal sample, and ∆T
, the temperature change of the water when it came into contact with
water
, the temperature change of the metal sample when it came
sample
, the mass of the
water
into contact with the water. Record your results in Table 2.1.
M
∆T
∆T
water
water
sample
= M
= T
- (M
total
- T
final
cool
= 100° C - T
cal
+ M
final
sample
)
From the law of energy conservation, the heat lost by the metal sample must equal the heat
gained by the water:
Heat lost by sample = (M
is the specific heat of water, which is 1.0 cal/g K.
c
water
sample
) (c
sample
) (∆T
sample
) = (M
water
) (c
water
) (∆T
) = Heat gained by water
water
Use the above equation, and your collected data, to solve for the specific heats of alumi-
num, copper, and tungsten
. Record your results in the bottom row of Table 2.1.
6
®
Page 9

Basic Calorimetry Set
Part 2
= ____________
M
cal
= ____________
M
water
= ____________
T
cool
= ____________
M
total
= ____________
T
final
Perform calculations similar to those performed in part 1 to determine c
antifreeze
, the spe-
cific heat of antifreeze.
c
antifreeze
= ____________
Questions
1. How do the specific heats of the samples compare with the specific heat of water?
2. Discuss any unwanted heat loss or gain that might have effected your results.
3. From your measured specific heat for antifreeze, which should be the better coolant
for an automobile engine, antifreeze or water? Why is antifreeze used as an engine
coolant?
®
7
Page 10

Basic Calorimetry Set
NOTES
8
®
Page 11

Basic Calorimetry Set
Experiment 3: Latent Heat of Vaporization
Equipment Needed
• Calorimeter
• Thermometer
• Steam Generator
Tubing -
25 cm
Tubing -
35 cm
• Water Trap
•Tubing
•Balance
If a steam generator is not available, a distillation flask and Bunsen
burner is adequate. A second flask can be used as a water trap.
Introduction
When a substance changes phase, the arrangement of its
molecules changes. If the new arrangement has a higher
Glass tube
to restrict
opening
Steam
Generator
internal energy, the substance must absorb heat in order to
make the phase transition. Conversely, if the new arrangement has a lower internal energy, heat will be released as
Figure 3.1
the transition occurs.
In this experiment you will determine how much more energy is contained in one gram of
steam at 100°C, than in one gram of water at the same temperature. This value is called the
Latent Heat of Vaporization of water.
CAUTION: This experiment involves the use of boiling water and steam. Work carefully.
Water
Tr ap
Procedure
1. Measure Trm, the room temperature.
2. Set up a steam generator with a water trap as shown in Figure 3.1. The tube lengths
should be approximately as shown in the figure.
3. Determine M
4. Fill the calorimeter approximately 1/2 full of cool water about 10° C below room
temperature.
5. Turn on the steam generator and wait for the steam to flow freely for at least a minute.
6. Measure T
water plus calorimeter.
7. Immediately immerse the free end of the short tube into the cool water in the calorimeter. Stir the water continuously with the thermometer.
IMPORTANT: The bottom of the water trap should be kept higher than the water level in the calorimeter to avoid water being pulled from the calorimeter back into the water trap.
8. When the water temperature, T, gets as far above room temperature as it was initially
below room temperature, remove the steam tube. Continue stirring the water and
record the highest stable temperature attained by the water (T
, the mass of a the empty, dry calorimeter.
cal
initial
and M
cal + water
, the temperature of the cool water and the mass of the
final
).
IMPORTANT: Always remove the steam tube from the water before turning off the steam generator
heat. (Can you explain why?)
9
®
Page 12

Basic Calorimetry Set
9. Immediately determine M
, the mass of calorimeter plus water plus (condensed)
final
steam.
Data
Trm = ____________
= ____________
M
cal
= ____________
T
initial
M
cal + water
T
final
M
final
= ___________
= ____________
= ____________
Calculations
When steam condenses in cool water, heat energy is released into the water in two ways.
First, the latent heat of vaporization is released. With this release of heat, the steam is converted into water, but the newly converted water is still at boiling temperature, 100° C.
Second, the newly converted water releases heat as it comes into thermal equilibrium with
the cooler water at a final equilibrium temperature, T
According to the principle of the conservation of energy, the total heat released by the
steam equals the total heat absorbed by the cooler water. Stated mathematically:
final
.
(M
M
M
T
H
)(Hv) + (M
steam
= M
steam
= M
water
= 100 °C
steam
= the latent heat of vaporization per gram of water
v
- M
final
cal + water
Use your data and the above information to determine H
NOTE: The thermometer also absorbs a certain amount of heat during the experiment. As a good
approximation, assume that the heat capacity of the thermometer is equivalent to that of 1 g of water
(i.e., add 1 g to M
water
)(1 cal/g K)(T
steam
cal + water
- M
the above equation).
= __________________
= __________________
cal
steam
- T
final
) = (M
v
)(1 cal/g K)(T
water
.
final
- T
initial
Hv = ____________
Questions
1. Why would an injury caused by 1 g of steam at 100° C do more damage than an
injury caused by 1 g of water at 100° C?
2. Speculate on how the heat of vaporization might influence climate and weather systems.
)
3. In what way does water used to cook food serve as a refrigerant? (Hint: What happens
when the water all boils away?)
®
10
Page 13

Basic Calorimetry Set
Experiment 4: Latent Heat of Fusion
Equipment Needed
• Calorimeter
• Thermometer
• Ice in water (at melting point)
•Warm water
Introduction
Just as steam has a higher internal energy content than water, so water has a higher internal
energy content than ice. It takes a certain amount of energy for the water molecules to
break free of the forces that hold them together in the crystalline formation of ice. This
same amount of energy is released when the water molecules come together and bond to
form the ice crystal.
In this experiment, you will measure the difference in internal energy between one gram
of ice at 0° C and one gram of water at 0° C. This difference in energy is called the latent
heat of fusion of water.
Procedure
1. Measure Trm, the room temperature.
2. Determine M
, the mass of the empty, dry calorimeter.
cal
3. Fill the calorimeter approximately 1/2 full of warm water about 15° C above room
temperature.
4. Measure M
5. Measure T
cal + water
initial
, the mass of the calorimeter and water.
, the initial temperature of the warm water.
6. Add small chunks of ice to the warm water, wiping the excess water from each piece
of ice immediately before adding. Add the ice slowly, stirring continuously with the
thermometer until each chunk melts.
7. When the temperature of the mixture is as much below room temperature as the warm
water was initially above room temperature and all the ice is melted, measure the final
temperature of the water (T
8. Immediately after measuring T
.
M
final
).
final
, weigh the calorimeter and water to determine
final
Suggested Additional Experiment
Repeat the above experiment, but, instead of ordinary ice, use the material which is packaged in metal or plastic containers to be frozen and used in picnic coolers.
Data
T
= ________________
rm
= ________________
M
cal
M
cal + water
T
initial
11
= ________________
= ________________
®
Page 14

Basic Calorimetry Set
T
= ________________
final
= ________________
M
final
Calculations
According to the principle of the conservation of energy, the quantity of heat absorbed by
the ice as it melts and then heats up to the final equilibrium temperature must equal the
quantity of heat released by the warm water as it cools down to the final equilibrium temperature. Mathematically:
)(Hf) + (M
(M
ice
= M
M
ice
final
Use your data and the above information to determine H
)(1 cal/g K)(T
ice
- M
cal + water
- 0° C) = (M
final
water
= __________________
)(1 cal/g K)(T
, the latent heat of fusion per
f
initial
- T
final
)
gram of water.
= the latent heat of fusion per gram of water
H
f
Questions
1. What advantage might the commercially packaged coolant material have over ice
other than that it produces less mess? (If you didn’t perform the optional part of the
experiment, what properties would a material need in order to be a better coolant than
ice?)
2. Design an experiment to determine which of two substances (for instance, ice and
packaged coolant) will keep an insulated food cooler
a. cool for the longest time, and
b. at a lower temperature.
®
12
Page 15

Basic Calorimetry Set
Technical Support
For assistance with any PASCO product, contact PASCO at:
Address: PASCO scientific
10101 Foothills Blvd.
Roseville, CA 95747-7100
Phone: 916-786-3800 (worldwide)
800-772-8700 (U.S.)
Fax: (916) 786-7565
Web: www.pasco.com
Email: support@pasco.com
For more information about the Basic Calorimetry Set and the latest revision of this Instruction Manual, visit:
www.pasco.com/go?TD-8557A
Limited Warranty For a description of the product warranty, see the PASCO catalog. Copyright The PASCO scientific
012-03060E Basic Calorimetry Set Instruction Manual is copyrighted with all rights reserved. Permission is granted to non-profit educational institutions for reproduction of any part of this manual, providing the reproductions are used only in their laboratories and
classrooms, and are not sold for profit. Reproduction under any other circumstances, without the written consent of PASCO scientific,
is prohibited. Trademarks PASCO and PASCO scientific are trademarks or registered trademarks of PASCO scientific, in the
United States and/or in other countries. All other brands, products, or service names are or may be trademarks or service marks of,
and are used to identify, products or services of, their respective owners. For more information visit www.pasco.com/legal.
13
®
 Loading...
Loading...