Page 1
Includes
Teacher's Notes
and
Typical
Experiment Results
Instruction Manual and
Experiment Guide for
the PASCO scientific
Model TD-8552
ELECTRICAL
EQUIVALENT
OF HEAT
012-02833D
5/94
ELECTRICAL
ENERGY
1987 PASCO scientific $5.00
HEAT
Page 2

Page 3

012-02833D Electrical Equivalent of Heat
T able of Contents
Section Page
Copyright and Warranty ..................................................................................ii
Equipment Return............................................................................................ii
Introduction .....................................................................................................1
Equipment........................................................................................................1
Maintenace.......................................................................................................2
Experiments:
Experiment 1: The Electrical Equivalent of Heat......................................3
Experiment 2: Efficiency of an Incandescent Lamp .................................5
Teacher's Guide ............................................................................................... 7
i
Page 4
Electrical Equivalent of Heat 012-02833D
Copyright, Warranty and Equipment Return
Please—Feel free to duplicate this manual
subject to the copyright restrictions below.
Copyright Notice
The PASCO scientific Model TD-8552 Electrical
Equivalent of Heat manual is copyrighted and all
rights reserved. However, permission is granted to
non-profit educational institutions for reproduction of
any part of this manual providing the reproductions
are used only for their laboratories and are not sold for
profit. Reproduction under any other circumstances,
without the written consent of PASCO scientific, is
prohibited.
Limited Warranty
PASCO scientific warrants this product to be free
from defects in materials and workmanship for a
period of one year from the date of shipment to the
customer. PASCO will repair or replace, at its option,
any part of the product which is deemed to be defective in material or workmanship. This warranty does
not cover damage to the product caused by abuse or
improper use. Determination of whether a product
failure is the result of a manufacturing defect or
improper use by the customer shall be made solely by
PASCO scientific. Responsibility for the return of
equipment for warranty repair belongs to the customer. Equipment must be properly packed to prevent
damage and shipped postage or freight prepaid.
(Damage caused by improper packing of the equipment for return shipment will not be covered by the
warranty.) Shipping costs for returning the equipment,
after repair, will be paid by PASCO scientific.
Equipment Return
Should the product have to be returned to PASCO
scientific for any reason, notify PASCO scientific by
letter, phone, or fax BEFORE returning the product.
Upon notification, the return authorization and
shipping instructions will be promptly issued.
ä
NOTE: NO EQUIPMENT WILL BE
ACCEPTED FOR RETURN WITHOUT AN
AUTHORIZATION FROM PASCO.
When returning equipment for repair, the units
must be packed properly. Carriers will not accept
responsibility for damage caused by improper
packing. To be certain the unit will not be
damaged in shipment, observe the following rules:
➀ The packing carton must be strong enough for the
item shipped.
➁ Make certain there are at least two inches of
packing material between any point on the
apparatus and the inside walls of the carton.
➂ Make certain that the packing material cannot shift
in the box or become compressed, allowing the
instrument come in contact with the packing
carton.
Address: PASCO scientific
10101 Foothills Blvd.
Roseville, CA 95747-7100
Phone: (916) 786-3800
FAX: (916) 786-3292
email: techsupp@pasco.com
web: www.pasco.com
ii
Page 5
012-02833D Electrical Equivalent of Heat
Introduction
The PASCO Model TD-8552 Electrical Equivalent of
Heat Apparatus provides an experimental determination of the quantitative relationship between electrical
energy and heat. Conversely, if the electrical equivalent of heat is accepted as a given, this apparatus can
provide a convincing demonstration of the conservation of energy. With either approach, the experiment
is easily extended to determine the energy efficiency
of an incandescent lamp.
Instructions for two experiments, along with student
worksheets, are on pages 3-6. In Experiment 1, the Electrical Equivalent of Heat is experimentally determined. An
incandescent lamp is immersed in a known quantity of water
and a few drops of India ink are added to the water so it is
opaque to visible light. The temperature of the water is
measured. The lamp is then illuminated with a fixed current
Equipment
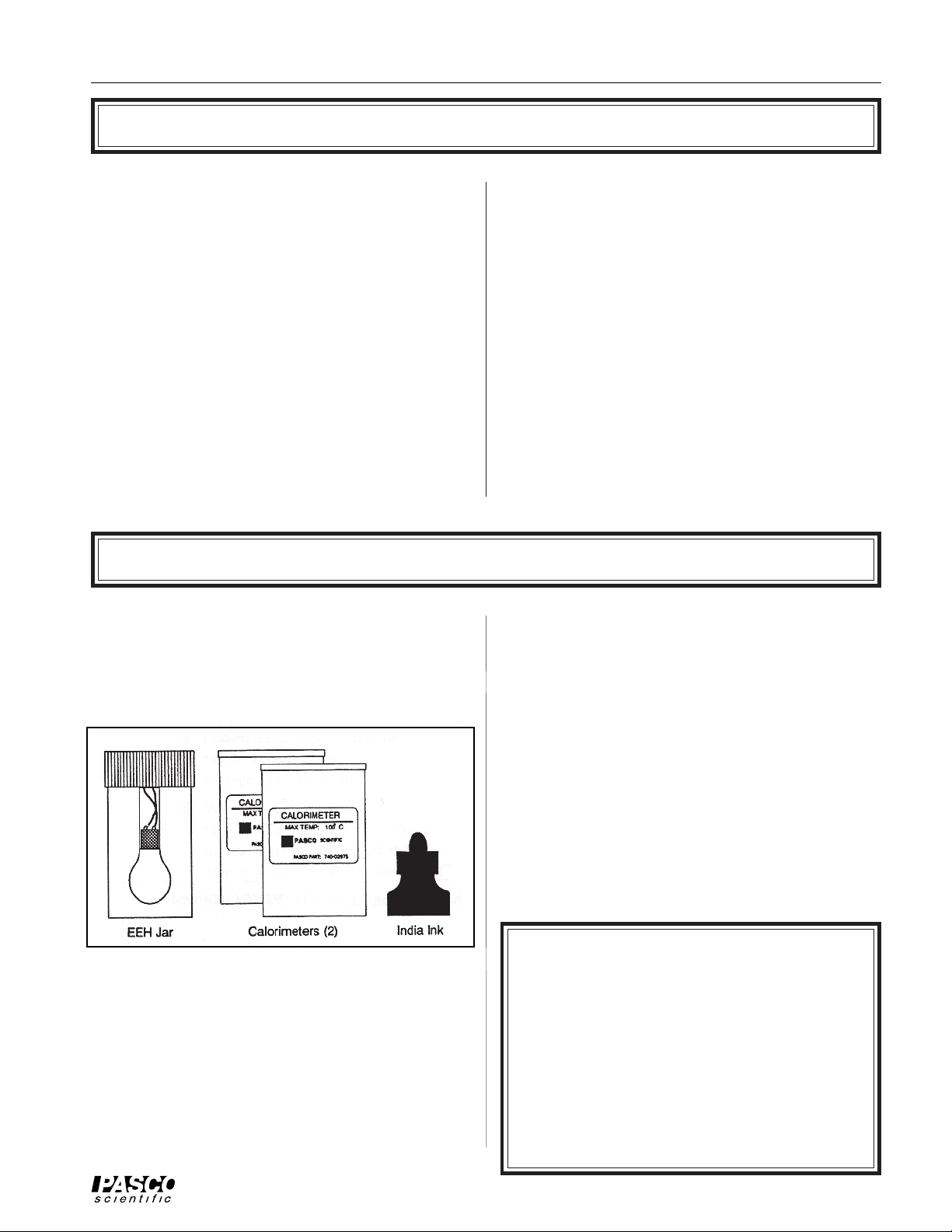
Your Model 8552 Electrical Equivalent of Heat
apparatus includes the items shown in Figure 1: a
transparent Electrical Equivalent of Heat Jar (EEH Jar)
with a built-in 35 Watt incandescent lamp, two
styrofoam Calorimeters, and a bottle of India ink.
and voltage for a measured time interval, so the electrical
energy into the lamp can be calculated. By monitoring the
temperature of the water, the heat produced by the lamp can
also be calculated. The ratio between the electrical energy
that flows into the lamp and the heat produced by the lamp
determines the electrical equivalent of heat.
In Experiment 2, the efficiency of the incandescent lamp is
measured. The details are similar to Experiment 1, but no
india ink is added to the water. Without the ink, the thermal
energy and infrared radiation from the lamp are absorbed
into the water, but the visible light escapes. To determine
the amount of energy that was released as light, the heat
transferred into the water is subtracted from the total
electrical energy that flowed into the lamp . The ratio
between the light energy and the electrical energy gives the
efficiency of the bulb.
➁ A digital Volt-Ammeter (a separate voltmeter and
ammeter are best) for measuring the power input to
the lamp. (Such as PASCO Model SE-9589.)
➂ A clock or stopwatch to determine the electrical en-
ergy that flows into the lamp (energy = power x
time).
Figure 1 Equipment
Additional Equipment Needed:
In addition to the equipment included with your
Electrical Equivalent of Heat apparatus, you will need
the following items to perform the experiments in this
manual:
➀ A regulated power supply capable of delivering up
to 3 A at 12 V. (Such as PASCO Model SF-9584.)
➃ A thermometer, or PASCO's TD-8559 Thermistor
Probe.*
➄ A balance for accurately determining the mass of
the water heated by the bulb.
(* A digital ohmmeter (SE-9589) is recom-
mended for use with the Thermistor Probe.)
➤ IMPORTANT: When using the Electrical
Equivalent of Heat Apparatus, always observe
the following precautions:
➀ Do not fill the water beyond the line indicated
on the EEH Jar. Filling beyond this level can
significantly reduce the life of the lamp.
➁ Illuminate the lamp only when it is immersed
in water.
➂ Never power the incandescent lamp at a
voltage in excess of 13 V.
1
Page 6

Electrical Equivalent of Heat 012-02833D
Maintenance
Replacing the Incandescent Lamp
A. The Easy Way
Order the Lamp Assembly directly from PASCO
scientific (Part Number 003-02956). Remove the old
assembly as shown below and replace it with the
PASCO replacement.
Bottom of Lid
1. Remove the lid of
the EEH Jar
2. Unsolder and disconnect
wires from banana plug
terminals.
B. The Hard Way
The incandescent lamp is a common one that can be
purchased at most auto parts stores (Bulb 1157). Follow
the procedure shown below, then, WEARING
GLOVES TO PROTECT YOUR HANDS, pull the
lamp out of the plastic tube. You will have to solder
wires to the replacement lamp. When you install the
new lamp, seal it in position with RTV Silicone Rubber.
Be sure the seal is water tight. Replace the lamp
assembly and resolder the wires to the banana plug
terminals.
Lamp Assembly
Top of Lid
4. Reverse the procedure to
replace the entire Lamp
3. Unscrew Lamp Assembly
from lid of EEH Jar.
Assembly (PASCO Part
Number 003-02956)
Figure 2 Replacing the Lamp
Replacement Parts
The following replacement parts can be ordered from
PASCO scientific. Call for prices (Toll-free 1-800772-8700).
Part PASCO Number
EEH:
Jar 650-026
Lamp Assembly only 003-02956
Top Assembly 003-03124
(includes Modified Lid
and Lamp Assembly)
Modified Lid Components:
Modified Lid Cover 648-02953
Lid Plate 648-02952
Label 646-02834
Part PASCO Number
Screw (6-32X3/8 P.H.)610-014
Black Connector 517-010
Red Connector 517-009
Lamp Assembly Components:
Bulb 526-019
Bulb Holder 648-02954
Standoff 648-02955
Calorimeter 740-02975
India Ink 725-003
2
Page 7

012-02833D Electrical Equivalent of Heat
Experiment 1: The Electrical Equivalent of Heat
➀ Measure and record the room temperature (T
➁ Weigh the EEH Jar (with the lid on), and record its mass (M
).
r
).
j
➂ Remove the lid of the EEH Jar and fill the jar to the indicated water line with cold wa-
ter. DO NOT OVERFILL. The water should be approxmately 10°C below room tem-
perature, but the exact temperature is not critical.
➃ Add about 10 drops of India ink to the water; enough so the lamp filament is just
barely visible when the lamp is illuminated.
➄ Using leads with banana plug connectors, attach your power supply to the terminals of
the EEH Jar. Connect a voltmeter and ammeter as shown in Figure 1.1 so you can measure both the current (I) and voltage (V) going into the lamp. NOTE: For best results,
connect the voltmeter leads directly to the binding posts of the jar.
➅ Turn on the power supply and quickly adjust the power supply voltage to about 11.5
volts, then shut the power off. DO NOT LET THE VOLTAGE EXCEED 13
VOLTS.
➆ Insert the EEH Jar into one of the styrofoam Calorimeters.
➇ Insert your thermometer or thermistor probe through the hole in the top of the EEH Jar.
Stir the water gently with the thermometer or probe while observing the temperature.
When the temperature warms to about 6 or 8 degrees below room temperature, turn the
power supply on.
➤ NOTE: You may want to turn the lamp on to help the cold water reach this
starting temperature. If you do, be sure that you turn the lamp off for several minutes
before you begin your measurements, so you are sure the water temperature is even
throughout the jar. Record the starting time (t
) and the temperature (Ti).
i
➈ Record the current, I, and voltage, V. Keep an eye on the ammeter and voltmeter
throughout the experiment to be sure these values do not shift significantly. If they do
shift, use an average value for V and I in your calculations.
➉ When the temperature is as far above room temperature as it was below room tempera-
ture (T
- Ti = Temperature - Tr), shut off the power and record the time (tf). Continue
r
stirring the water gently. Watch the thermometer or probe until the temperature peaks
and starts to drop. Record this peak temperature (T
).
f
Weigh the EEH Jar with the water, and record the value (Mjw).
–
+
Voltmeter
13 V Max!
+–
Power Supply
Ammeter
–
+
Figure 1.1 Electrical Connections
3
Page 8

Electrical Equivalent of Heat 012-02833D
Data
Tr = _________________________________________
Mj = _________________________________________
Mjw = ________________________________________
V = _________________________________________
I = _________________________________________
ti = _________________________________________
t
= _________________________________________
f
Ti = _________________________________________
Tf = _________________________________________
Calculations
In order to determine the electrical equivalent of heat (Je), it is necessary to determine both
the total electrical energy that flowed into the lamp (E) and the total heat absorbed by the
water (H).
E, the electrical energy delivered to the lamp:
E = Electrical Energy into the Lamp = V . I . t = __________________________
t = t
- ti = the time during which power was applied to the lamp = ________
f
H, the heat transferred to the water (and the EEH Jar):
H = (Mw +Me)(1 cal/gm C)(Tf-Ti) = __________________________________
M
= Mjw - Mj = Mass of water heated = ____________________________
w
M
= 23 grams. Some of the heat produced by the lamp is absorbed by the EEH Jar. For
e
accurate results, therefore, the heat capacity of the jar must be taken into acount (The heat
capacity of the EEH Jar is equivalent to that of approximately 23 grams of water.)
Je, the Electrical Equivalent of Heat:
Je = E/H = _______________________________________________________
Questions
➀ What effect are the following factors likely to have on the accuracy of your determination
of Je, the Electrical Equivalent of Heat? Can you estimate the magnitude of the effects?
a. The inked water is not completely opaque to visible light.
b. There is some transfer of thermal energy between the EEH Jar and the room atmosphere.
(What is the advantage of beginning the experiment below room temperature and ending
it an equal amount above room temperature?)
➁ How does J
compare with J, the mechanical equivalent of heat. Why?
e
4
Page 9

012-02833D Electrical Equivalent of Heat
Experiment 2: Efficiency of an Incandescent Lamp
Repeat Experiment 1, except do not use the India ink (step 4) or the styrofoam Calorimeter
(step 7). Record the same data as in Experiment 1, and use the same calculations to determine E and H. (Convert H to Joules by multiplying by Je from the first lab.)
In performing the experiment with clear water and no Calorimeter, energy in the form of
visible light is allowed to escape the system. However, water is a good absorber of infrared
radiation, so most of the energy that is not emitted as visible light will contribute to H, the
thermal energy absorbed by the water.
The efficiency of the lamp is defined as the energy converted to visible light divided by the
total electrical energy that goes into the lamp. By making the assumption that all the energy
that doesn't contribute to H is released as visible light, the equation for the efficiency of the
lamp becomes:
Data
Efficiency = (E - H
Tr = ________________________________________
Mj = ________________________________________
Mjw = ________________________________________
V = ________________________________________
I = ________________________________________
ti = ________________________________________
t
= ________________________________________
f
Ti = ________________________________________
Tf = ________________________________________
)/E.
j
5
Page 10

Electrical Equivalent of Heat 012-02833D
Calculations
In order to determine the efficiency of the lamp, it is necessary to determine both the total
electrical energy that flowed into the lamp (E) and the total heat absorbed by the water (H).
E, the electrical energy delivered to the lamp:
E = Electrical Energy into the Lamp = V . I . t = __________________________
t = tf - ti = the time during which power was applied to the lamp = ________
H, the heat transferred to the water (and calorimeter):
H = (Mw +Me)(1 cal/gm C)(Tf-Ti) = __________________________________
Mw = Mjw - Mj = Mass of water heated = ____________________________
Hj = H Je = ____________________________________________________
M
= 23 grams. Some of the heat produced by the lamp is absorbed by the EEH Jar. For
e
accurate results, therefore, the heat capacity of the jar must be taken into acount (The heat
capacity of the EEH Jar is equivalent to that of approximately 23 grams of water.)
Efficiency:
E-H
j
= _________________________________________________________
E
Questions
➀ What effect are the following factors likely to have on the accuracy of your determination of
the efficiency of the lamp? Can you estimate the magnitude of the effects?
a. Water is not completely transparent to visible light.
b. Not all the infrared radiation is absorbed by the water.
c. The styrofoam Calorimeter was not used, so there is some transfer of thermal energy
between the EEH Jar and the room atmosphere.
➁ Is an incandescent lamp more efficient as a light bulb or as a heater?
6
Page 11

012-02833D Electrical Equivalent of Heat
T eacher’s Guide
Experiment 1: The Electrical Equivalent of Heat
Notes on Procedure
➀ This measurement is not critical.
➇ It is important that the water temperature is uniform
when you begin. If you use the bulb to bring the
water temperature up to its starting temperature, let
the system rest for a few minutes, then start.
Notes on Calculations
Je = 4.184
This is also the conversion between Joules and
calories: 1cal = 4.184J.
The result obtained experimentally should be
within 5% of this value.
Experiment 2: Efficiency of an Incandescent Lamp
Notes on Calculations
It is critical that you change your value of H from
calories to Joules for the efficiency calculations. If
the students have gotten good results for Je in the
first experiments (within 5% of 4.184) have them
use that value for the conversion. Otherwise, or if
they haven’t done experiment 1, use
Je = 4.184.
Notes on Efficiency
The efficiency will vary depending on the voltage
and the bulb. Generally-accepted values for the
efficienty of incandescent lighting are on the order
of 10-15%. With our test bulb at 11.6V, the efficiency was measured as being 13%.
Notes on Questions
➀ a. Leakage of visible light will have negligible ef-
fect, since most of the output of the bulb is not
visible. In addition, much of the visible light that
escapes is reflected back into the water by the
white inside walls of the calorimeter cup.
b. This is the most significant source of error. Be-
ginning and ending equal distances above and
below room temperature will tend to create selfcancelling errors.
➁ J
is the same as J, since mechanical and electrical
e
energy are equivalent.
Questions
➀ a. Absorbtion of visible light will decrease the
measured efficiency.
b. Transmission of infrared will increase the mea-
sured efficiency.
c. Conductive and Convective heat losses will in-
crease the measured efficiency.
➁ The bulb is
a light source.
much more efficient as a heater than as
7
Page 12

Electrical Equivalent of Heat 012-02833D
Notes
8
Page 13

012-02833D Electrical Equivalent of Heat
T echnical Support
Feed-Back
If you have any comments about this product or this
manual please let us know. If you have any suggestions on alternate experiments or find a problem in the
manual please tell us. PASCO appreciates any customer feed-back. Your input helps us evaluate and
improve our product.
To Reach PASCO
For Technical Support call us at 1-800-772-8700 (tollfree within the U.S.) or (916) 786-3800.
Contacting Technical Support
Before you call the PASCO Technical Support staff it
would be helpful to prepare the following information:
• If your problem is with the PASCO apparatus, note:
Title and Model number (usually listed on the label).
Approximate age of apparatus.
A detailed description of the problem/sequence of
events. (In case you can't call PASCO right away,
you won't lose valuable data.)
If possible, have the apparatus within reach when
calling. This makes descriptions of individual parts
much easier.
• If your problem relates to the instruction manual,
note:
Part number and Revision (listed by month and year
on the front cover).
Have the manual at hand to discuss your questions.
9
Page 14

 Loading...
Loading...