Page 1
Includes
Teacher's Notes
and
Typical
Experiment Results
Instruction Manual and
Experiment Guide for
the PASCO scientific
Model TD-8551A
MECHANICAL
EQUIVALENT
OF HEAT
012-04331E
5/94
© 1990 PASCO scientific $5.00
Page 2

Page 3

012-04331E Mechanical Equivalent of Heat
T able of Contents
Section..........................................................................................................Page
Copyright and Warranty ..................................................................................ii
Equipment Return............................................................................................ii
Introduction .....................................................................................................1
Equipment........................................................................................................1
Measuring Temperature with the Thermistor..................................................2
History .............................................................................................................2
Operation .........................................................................................................3
Measuring the Mechanical Equivalent of Heat:
Experiment.................................................................................................4
Calculations ...............................................................................................6
Worksheet..................................................................................................7
Maintenace.......................................................................................................8
Thermistor Specifications:
Temperature versus Resistance .................................................................9
Biography: Benjamin Thompson—Count Rumford of Bavaria...................10
Teacher’s Guide..............................................................................................11
i
Page 4
Mechanical Equivalent of Heat 012-04331E
Copyright, Warranty and Equipment Return
Please—Feel free to duplicate this manual
subject to the copyright restrictions below.
Copyright Notice
The PASCO scientific Model TD-8551A Mechanical
Equivalent of Heat manual is copyrighted and all rights
reserved. However, permission is granted to non-profit
educational institutions for reproduction of any part of
this manual providing the reproductions are used only for
their laboratories and are not sold for profit. Reproduction under any other circumstances, without the written
consent of PASCO scientific, is prohibited.
Limited Warranty
PASCO scientific warrants this product to be free from
defects in materials and workmanship for a period of one
year from the date of shipment to the customer. PASCO
will repair or replace, at its option, any part of the product
which is deemed to be defective in material or workmanship. This warranty does not cover damage to the product
caused by abuse or improper use. Determination of
whether a product failure is the result of a manufacturing
defect or improper use by the customer shall be made
solely by PASCO scientific. Responsibility for the return
of equipment for warranty repair belongs to the customer. Equipment must be properly packed to prevent
damage and shipped postage or freight prepaid. (Damage caused by improper packing of the equipment for
return shipment will not be covered by the warranty.)
Shipping costs for returning the equipment, after repair,
will be paid by PASCO scientific.
Equipment Return
Should the product have to be returned to PASCO
scientific for any reason, notify PASCO scientific by
letter, phone, or fax BEFORE returning the product.
Upon notification, the return authorization and
shipping instructions will be promptly issued.
ä
NOTE: NO EQUIPMENT WILL BE
ACCEPTED FOR RETURN WITHOUT AN
AUTHORIZATION FROM PASCO.
When returning equipment for repair, the units
must be packed properly. Carriers will not accept
responsibility for damage caused by improper
packing. To be certain the unit will not be
damaged in shipment, observe the following rules:
➀ The packing carton must be strong enough for the
item shipped.
➁ Make certain there are at least two inches of
packing material between any point on the
apparatus and the inside walls of the carton.
➂ Make certain that the packing material cannot shift
in the box or become compressed, allowing the
instrument come in contact with the packing
carton.
Address: PASCO scientific
10101 Foothills Blvd.
Roseville, CA 95747-7100
Phone: (916) 786-3800
FAX: (916) 786-3292
email: techsupp@pasco.com
web: www.pasco.com
ii
Page 5
012-04331E Mechanical Equivalent of Heat
Introduction
The principle of the conservation of energy tells us that if a
given amount of work is transformed completely into heat,
the resulting thermal energy must be equivalent to the
amount of work that was performed. Of course, since work
is normally measured in units of Joules and thermal energy
is normally measured in units of Calories, the equivalence is
not immediately obvious. A quantitative relationship is
needed that equates Joules and Calories. This relationship is
called the Mechanical Equivalent of Heat.
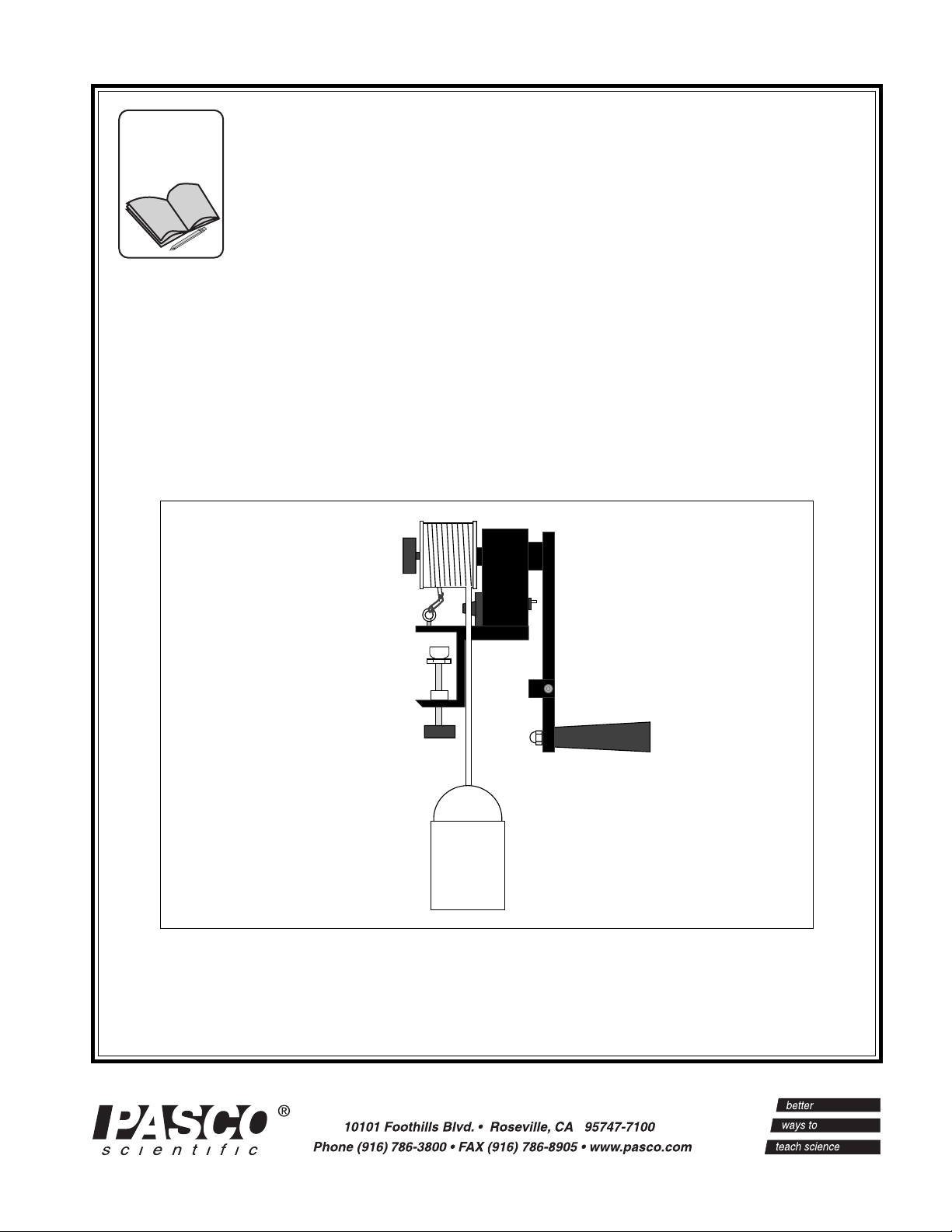
The PASCO scientific Model TD-8551A Mechanical
Equivalent of Heat apparatus allows accurate determination
of the Mechanical Equivalent of Heat (to within 5%). The
apparatus is shown in Figure 1. A measurable amount of
work is performed by turning the crank, which turns the
aluminum cylinder. A nylon rope is wrapped several times
around the cylinder so that, as the crank is turned, the
friction between the rope and the cylinder is just enough to
support a mass hanging from the other end of the rope. This
insures that the torque acting on the cylinder is constant and
measurable. A counter keeps track of the number of turns.
As the cylinder turns, the friction between the cylinder and
the rope converts the work into thermal energy, which raises
the temperature of the aluminum cylinder. A thermistor is
embedded in the aluminum so that, by measuring the
Aluminum Cylinder
with embedded
Thermistor
Counter
Crank
Nylon Rope
Mass
(≅ 10 kg)
Figure 1 Mechanical Equivalent of Heat Apparatus
resistance of the thermistor, the temperature of the cylinder
can be determined. By monitoring the temperature change of
the cylinder, the thermal energy transferred into the cylinder
can be calculated. Finally, the ratio between the work
performed and the thermal energy transferred into the
cylinder determines J, the mechanical equivalent of heat.
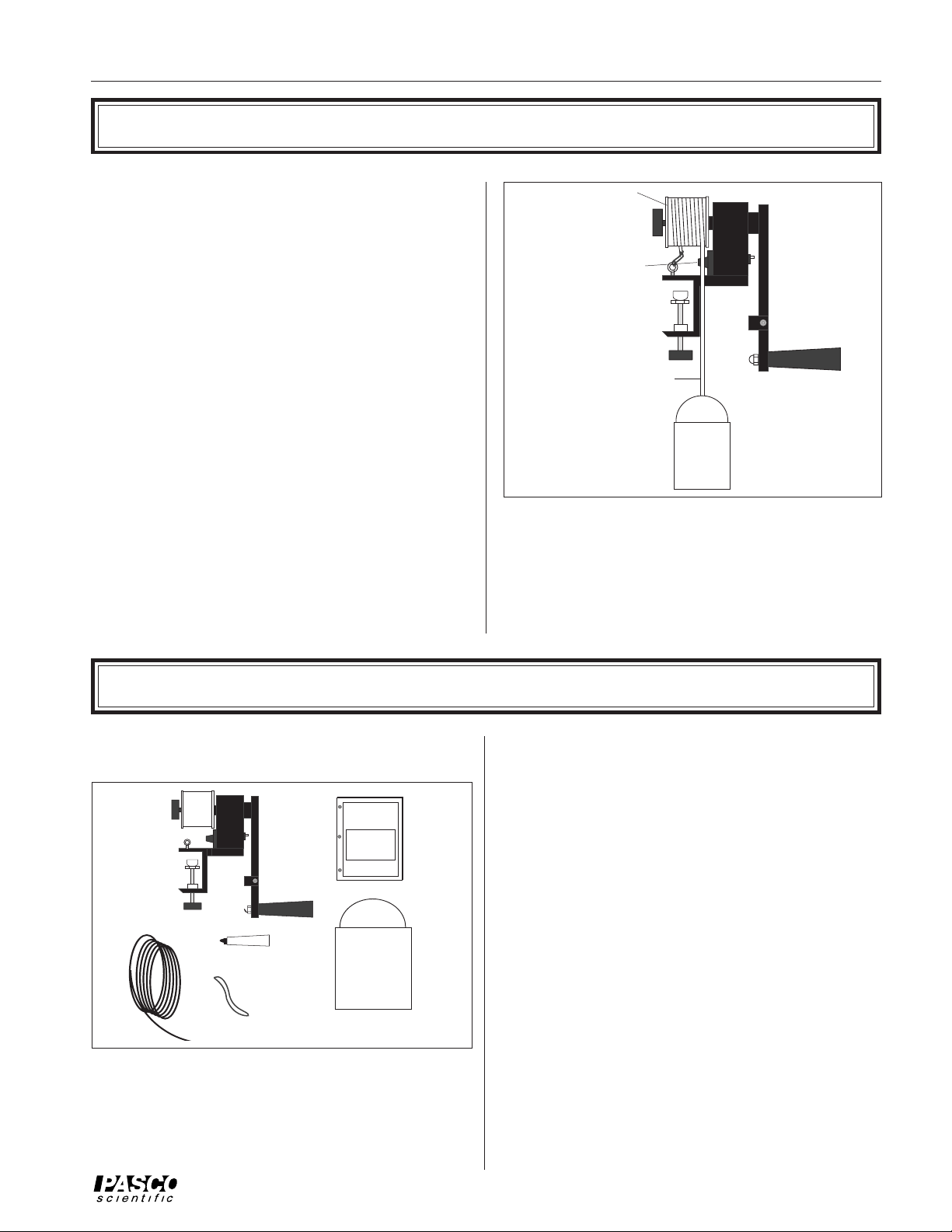
Equipment
The TD-8551A Mechanical Equivalent of Heat apparatus
includes the items shown in Figure 2.
Mechanical
Equivalent
of Heat
Apparatus
Nylon Rope
Powdered
Graphite
Rubber Band
Figure 2 Equipment
➤ IMPORTANT: In addition to the Mechanical
Equivalent of Heat apparatus, several other items are
needed to measure the mechanical equivalent of heat.
These items include:
MANUAL
Instruction
Manual
Mass
Container
• Digital Ohmmeter for measuring the resistance of the ther-
mistor in the aluminum cylinder. (An analog meter can be
used, but accuracy will be significantly sacrificed.)
• Refrigerator (or some ice), for cooling the aluminum cyl-
inder below room temperature.
• known Mass of approximately 10 kg which can be sus-
pended from the nylon rope. (The apparatus comes with a
container which can be filled with sand or dirt for the 10 kg
mass; if this is done, you will need an accurate balance for
measuring this mass. Of course, you can fill the container
by adding sand in measured increments of 1-2 kg.)
• Thermometer for measuring room temperature is conven-
ient, though the thermistor can be used for this purpose.
• Calipers and a Balance for measuring the mass and diame-
ter of the aluminum cylinder if you wish these measurements to be part of the experimental process. (Approximate
values are Mass: 200 ± 1.5 grams; Diameter: 4.763 ± 0.02
cm; Diameter including thickness of nylon rope:
4.94 ± 0.05 cm. These values can be used, but there is
some variation, so your results will be more accurate if you
make the measurements yourself.)
1
Page 6
Mechanical Equivalent of Heat 012-04331E
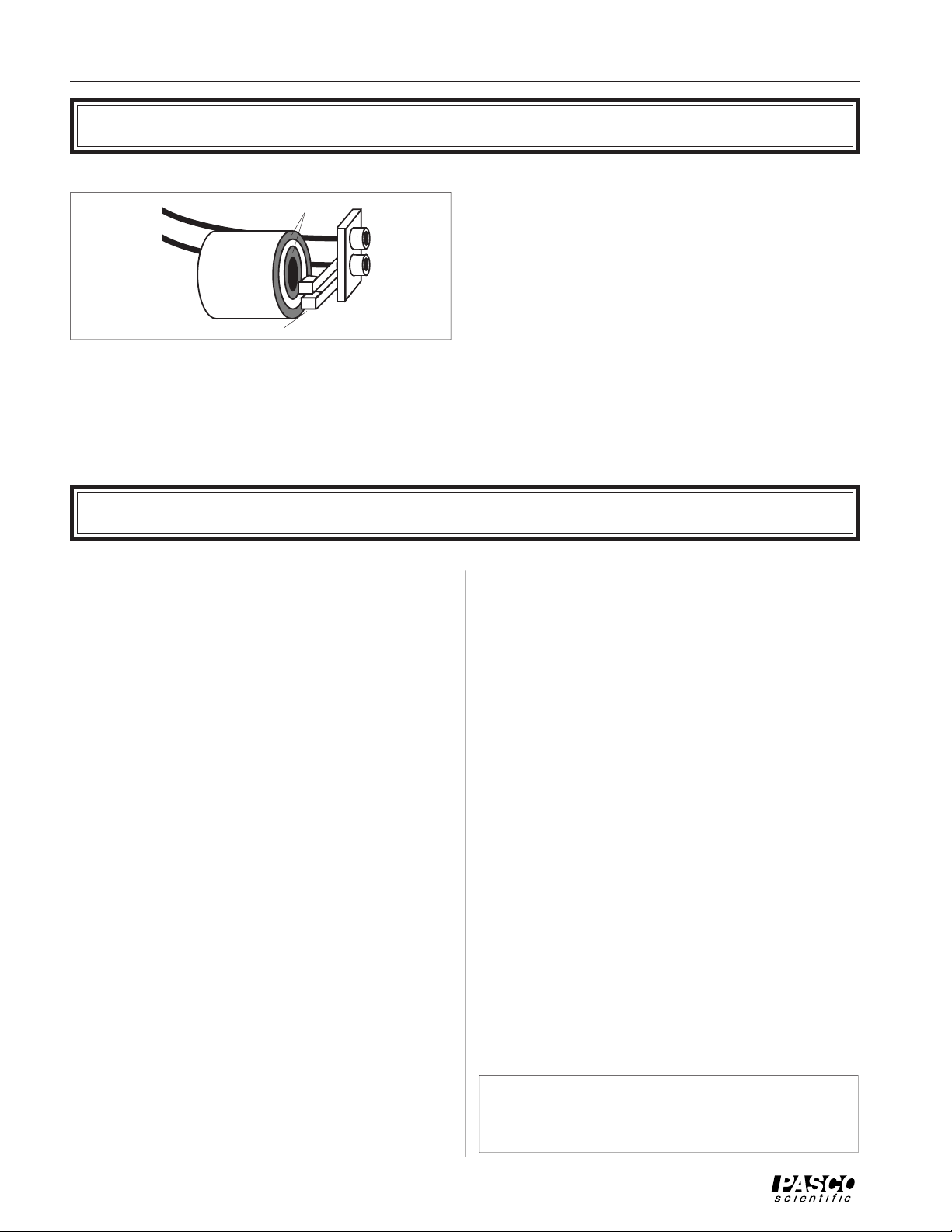
Measuring Temperature with the Thermistor
To
Ohmmeter
Figure 3 Measuring the Cylinder Temperature
Slip Rings
Banana
Jacks
Brushes
To measure the temperature of the aluminum cylinder, a
thermistor is embedded inside. A thermistor is a temperature dependent resistor. If the resistance of the thermistor is
known, its temperature can be very accurately and reliably
determined. The leads of the thermistor in the cylinder are
History
It may not seem strange to us today that there is a thing
called energy that is conserved in all physical interactions.
Energy is a concept we have all grown up with. A hundred
and fifty years ago it was not so evident that there should be
an intimate, quantitative relationship between such apparently unrelated phenomena as motion and heat. The
discovery that heat and motion can be seen as different
forms of the same thing—namely energy—was the first and
biggest step toward understanding the concept of energy
and its conservation.
Count Rumford of Bavaria, in 1798, was the first to realize
that work and heat were related phenomena. At that time, it
was commonly believed that heat resulted from the flow of
a massless fluid-like substance called caloric. It was
believed that this substance resided in objects, and that
when they were cut, ground, or otherwise divided into
smaller pieces, the pieces could not hold as much caloric as
the original object. The resulting release of caloric was
what we experience as heat.
While boring cannon for the Bavarian government,
Rumford noticed that heat was produced even when the
boring equipment had become so dulled from use that it was
no longer boring into the iron. The heat therefore was not
dependent on the breaking up of the metal into smaller
pieces. In fact, this meant that a limitless amount of heat
could be produced from the iron and boring equipment, an
idea that was inconsistent with the belief that heat was the
result of the release of a substance that resided in the
material. Rumford realized that a connection existed
between the motion of the bore and the heat. He even took
soldered to the copper slip rings (see Figure 3) on the side of
the cylinder. The brushes provide an electrical connection
between the slip rings and the banana plug connectors. By
plugging an ohmmeter into these connectors, the resistance
of the thermistor, and therefore it's temperature, can be
monitored, even when the cylinder is turning.
Although the temperature dependence of the thermistor is
accurate and reliable, it is not linear. You will therefore
need to use the table of Temperature versus Resistance that
is affixed to the base of the Mechanical Equivalent of Heat
apparatus to convert your resistance measurements into
temperature readings. A more complete version of this
table, covering a greater temperature range, is given at the
end of this manual.
his reasoning a step further, stating his belief that only if
heat were a form of motion would it demonstrate the
properties he had observed.
It was not until the experiments of Joule in 1850, however,
that Rumford's ideas about the nature of heat gained popular
acceptance. Joule performed a variety of experiments in
which he converted a carefully measured quantity of work,
through friction, into an equally carefully measured quantity
of heat. For example, in one experiment Joule used falling
masses to propel a paddle wheel in a thermally insulated,
water-filled container. Measurements of the distance
through which the masses fell and the temperature change
of the water allowed Joule to determine the work performed
and the heat produced. With many such experiments, Joule
demonstrated that the ratio between work performed and
heat produced was constant. In modern units, Joule's results
are stated by the expression:
1 calorie = 4.186 Joule.
Joule's results were within 1% of the value accepted today.
(The calorie is now defined as equal to 4.184 Joule.)
It was this series of experiments that led Joule, along with
several others, to the more general theory that energy is
conserved in all physical processes.
➤ NOTE: See the short biography at the end of
this manual for more information on the life of
Benjamin Thompson—Count Rumford, of Bavaria.
2
Page 7

012-04331E Mechanical Equivalent of Heat
Operation
Step by step instructions for using the Mechanical
Equivalent of Heat Apparatus are given on the following
pages. However, the apparatus will last longer and give
better results if you follow the guidelines listed below:
➀ Before performing the experiment, spray the surface
of the aluminum cylinder lightly with the included dry
powdered graphite.
The graphite ensures that the rope slides smoothly on
the cylinder, making it easier to provide a steady,
even torque, and greatly decreasing the wear on the
aluminum cylinder.
After several applications, the friction rope will become impregnated, so you needn't continue to apply
the lubricant at every use.
Aluminum Cylinder
Dry powdered
graphite
Figure 4 Lubricate Cylinder
Be sure the table is level.
➁ Mount the Mechanical Equivalent of Heat on a
level table.
If the apparatus is not level the rope will tend to slip
and bunch up on the cylinder, which makes it difficult
to maintain a steady torque.
➂ When turning the crank, never raise the mass higher
than about 3 cm from the floor (no higher than you
would care to have it fall on your little toe).
If the mass is raised higher, the crank can snap back
when released, which is not healthy for the equipment, or for nearby people. Also, if it is allowed to
climb, the rope will likely start overlapping the next
turn which makes it climb even higher, producing a
dangerous situation
Figure 5 Level Table
Do not raise mass
more than about 3
centimeters above
floor.
Figure 6 Don't Raise Mass too High
3
Page 8

Mechanical Equivalent of Heat 012-04331E
Experiment: Measuring the Mechanical Equivalent of Heat
➤ IMPORTANT:
Unscrew Knob and remove Cylinder
➀ For best results, read this procedure through thoroughly before
attempting the experiment
➁ A tube of powdered graphite lubricant is supplied with the
equipment. Spraying the aluminum cylinder lightly with this
before beginning the experiment will greatly reduce the wear
on the aluminum surface.
➤ NOTE: An experimental worksheet is provided at the end of
this section for recording data and calculations.
➀ Clamp the apparatus securely to the edge of a level table or
bench, as shown in Figure 7.
➁ Unscrew the black knob and remove the aluminum cylinder.
Place the cylinder in a refrigerator or freezer, or pack it in ice,
to bring the temperature down to at least 10 C° below room
temperature.
The cylinder is cooled so that, when it is heated by friction, the midpoint of the high and low
temperatures will be at room temperature. In the first half of the experiment, therefore, heat
will be transferred from the room air into the cooler cylinder. As the cylinder heats beyond
room temperature though, heat will be transferred out of the cylinder back into the room
atmosphere. By letting the change in cylinder temperature be symmetrical about the room
temperature, the quantity of heat transferred to and from the cylinder and room should be
approximately equal.
Figure 7 Clamp to Table and
Remove Cylinder
➂ While the cylinder is cooling, plan the desired temperature variation of the experiment. Ideally,
the temperature variation of the cylinder should be from 7-9 C° below room temperature to the
same amount above room temperature. Therefore, measure and record the room temperature,
and then determine and record the initial and final temperatures you wish the cylinder to reach
during the experiment. (You can record your data on the data sheet provided at the end of this
section.)
➃ Using the table of Resistance versus Temperature for the thermistor, determine the resistance
value which will correspond to each of your recorded temperatures. (A table covering most
temperature ranges is listed on the apparatus. A more complete table can be found near the end
of this manual.) Also determine the resistance measurement which corresponds to 1 C° below
the final temperature. You will want to start cranking more slowly as the temperature approaches this point, so that the final, equilibrium temperature will be close to your chosen final
temperature.
➄ When the cylinder is sufficiently cool, slide it back on the crank shaft. Be sure that the copper
plated board is facing toward the crank. Also make sure that the pins on the drive shaft fit into
the slots on the plastic ring on the cylinder, then replace the black knob and tighten securely.
➅ Plug the leads of the ohmmeter into the banana plug connectors as shown in Figure 8. Set the
ohmmeter to a range that is appropriate to the thermistor resistances that correspond to your
chosen temperature range.
➆ Wrap the nylon rope several turns around the aluminum cylinder (4-6 turns should work well)
as shown in Figure 9. Be sure that the rope lies flat against the cylinder and hangs down the
slot provided in the base plate. Tie one end of the rope, the end nearest to the crank, to the 10
kg mass as shown.
4
Page 9

012-04331E Mechanical Equivalent of Heat
➤NOTE: When the cylinder is cold, water may
condense on its surface. Dry the cylinder
thoroughly with a cloth or paper towel before
wrapping the rope, so that all of the heat goes
into heating the cylinder and not into evaporating
the condensed water.
⑧ Set the counter to zero by turning the black knob
on the counter.
⑨ Watch the ohmmeter carefully. When the
resistance reaches the value corresponding to
your starting temperature, start cranking (clockwise, facing the crank side of the apparatus).
➤ IMPORTANT: There should be only enough
turns of rope around the cylinder so that the
frictional pull on the rope is just enough to lift the
3 - 6 Turns of Rope
hanging mass about 3 cm off the floor - no
higher! To accomplish this, wrap the rope three
or four turns and crank. Add turns as needed to
support the mass while cranking with only very
slight tension on the free end. Attach the rubber
band to the free end of the rope. Now, without
cranking and while keeping the rope taught by
the rubber band, tie the other end of the rubber
band to the eyebolt on the baseplate. If you find
that the large hanging mass continues to rise
more than 3 cm as you turn the crank, remove
one turn from the cylinder nearest the free end. If
Friction
Rope
the large hanging mass continues to rest on the
floor, add another turn of rope around the
cylinder at the free end.
Crank rapidly until the temperature indicated by
the thermistor is 1° C less than your designated
stopping temperature, then crank very slowly
while watching the ohmmeter. When the
Figure 9 Add Friction Rope and Hanging Mass
temperature reaches your stopping value, stop
cranking. Continue watching the ohmmeter until the thermistor temperature reaches a maximum
(the resistance will be a minimum) and starts to drop. Record the highest temperature attained as
your final temperature.
Figure 8 Hook up the Ohmmeter
Tension on
Hanging
Mass
Ohmmeter
Banana Plug
Connectors
Cylinder (front view)
Constant
free end
Rubber Band
Base
Hanging Mass
on this end
➉ Record N, the number on the counter—the number of full turns of the crank.
11 Measure and record m, the mass of the aluminum cylinder.
12 With a pair of calipers, measure D, the diameter of the aluminum cylinder. Record the radius of the
D
cylinder in the worksheet (
R =
).
2
5
Page 10

Mechanical Equivalent of Heat 012-04331E
Calculations
Calculating W, the Work Performed
The work performed on the cylinder by turning the crank equals τ, the torque acting on
the cylinder, times θ, the total angle through which the torque acts. It would be difficult
to directly measure the torque delivered by the crank. However, since the motion of the
cylinder is more or less constant through the experiment, we know that the torque
provided by the crank must just balance the torque provided by the friction from the
rope. The torque provided by the rope friction is easily calculated. It is just:
τ
= MgR
where M is the mass hanging from the rope, g is the acceleration due to gravity, and R is the
radius of the cylinder.
Each time the crank is turned one full turn, this torque is applied to the cylinder through an
angle 2π. The total work performed therefore is:
W = τθ = MgR (2πN);
where M is the mass hanging from the rope;
g is the acceleration due to gravity (9.8 m/s2);
R is the radius of the aluminum cylinder;
and N is the total number of times the crank was turned.
Calculating Q, the Heat produced
The heat (Q) produced by friction against the aluminum cylinder can be determined from the
measured temperature change that occurred. The calculation is:
Q = m c (Tf - Ti);
where m is the mass of the aluminum cylinder;
c is the specific heat of aluminum (0.220 cal/gC∞);
is the final temperature of the cylinder;
T
f
is the initial temperature of the cylinder, just before cranking.
and T
i
Calculating J, the Mechanical Equivalent of Heat
J is just the ratio of the work performed to the heat produced. Therefore:
J = W/Q
6
Page 11

012-04331E Mechanical Equivalent of Heat
Worksheet
Data
Temperature (∞C) Corresponding Thermistor Resistance (Ω)
Room Temperature
Initial Temperature (T
Final Temperature (T
Ideal (pre-selected value)
Actual (Highest Temp)
- 1∞C
T
f
(Begin Slow Cranking)
Mass Hanging from Rope: M =__________________________
Mass of Aluminum Cylinder: m = _______________________
Radius of Cylinder: R = _______________________________
Number of turns of crank : N = _________________________
Calculations
Work performed on cylinder: W = τ θ = MgR(2πN) = __________________
)
i
)
f
Heat absorbed by cylinder: Q = mc (T
- Ti) = ________________________
f
Mechanical Equivalent of Heat: J = W/Q = ___________________________
(Acceleration due to gravity: g = 9.8 m/s
2;
Specific Heat of Aluminum: c = 0.220 cal/g∞C)
Suggested Questions
➀ Compare your value of J with the accepted value (check your textbook).
➁ Discuss any sources of error that you feel might have affected your results. Are some of these avoidable?
What affect would they have on your calculated value for J? Can you estimate the magnitude of the effects?
➂ Is it experimentally possible that the heat absorbed by the cylinder could be greater than the work performed
on it? Explain.
➃ Can your value of J be used for determining how much mechanical energy can be produced from a specified
amount of thermal energy? Why or why not?
7
Page 12

Mechanical Equivalent of Heat 012-04331E
Maintenance
The Mechanical Equivalent of Heat apparatus requires no
regular maintenance except to lubricate the aluminum
cylinder periodically to ensure that the friction rope slides
freely.
If the slip-ring or brushes become dirty enough so they
do not conduct well and affect the thermistor resistance,
just clean them with alcohol (if you have no alcohol
handy, a damp rag will probably do the trick).
Assorted Replacement Parts List
Part No. Description
648-04336 Friction Cylinder
555-04303 Commutator PCB
150-027 Thermistor 100K +/- 2°C
620-039 Cylinder Handscrew
003-02861 Brush Assembly
621-020 Crank Handle
699-050 Mechanical Counter
003-04382 Cord Assembly
8
Page 13

012-04331E Mechanical Equivalent of Heat
Thermistor Specifications:
Temperature Versus Resistance
Res. Temp.
(Ω) (∞C)
351,020 0
332,640 1
315,320 2
298,990 3
283,600 4
269,080 5
255,380 6
242,460 7
230,260 8
218,730 9
207,850 10
197,560 11
187,840 12
178,650 13
169,950 14
161,730 15
153,950 16
146,580 17
139,610 18
133,000 19
126,740 20
120,810 21
115,190 22
109,850 23
104,800 24
100,000 25
95,447 26
91,126 27
87,022 28
83,124 29
79,422 30
75,903 31
72,560 32
69,380 33
Res. Temp.
(Ω) (∞C)
66,356 34
63,480 35
60,743 36
58,138 37
55,658 38
53,297 39
51,048 40
48,905 41
46,863 42
44,917 43
43,062 44
41,292 45
39,605 46
37,995 47
36,458 48
34,991 49
33,591 50
32,253 51
30,976 52
29,756 53
28,590 54
27,475 55
26,409 56
25,390 57
24,415 58
23,483 59
22,590 60
21,736 61
20,919 62
20,136 63
19,386 64
18,668 65
17,980 66
17,321 67
Res. Temp.
(Ω) (∞C)
16,689 68
16,083 69
15,502 70
14,945 71
14,410 72
13,897 73
13,405 74
12,932 75
12,479 76
12,043 77
11,625 78
11,223 79
10,837 80
10,467 81
10,110 82
9,767.2 83
9,437.7 84
9,120.8 85
8,816.0 86
8,522.7 87
8,240.6 88
7,969.1 89
7,707.7 90
7,456.2 91
7,214.0 92
6,980.6 93
6,755.9 94
6,539.4 95
6,330.8 96
6,129.8 97
5,936.1 98
5,749.3 99
5,569.3 100
9
Page 14

Mechanical Equivalent of Heat 012-04331E
The Incredible Career of Count Rumford
One of the most incredible men associated with science
was Benjamin Thompson, later titled Count Rumford.
Aside from making as many enemies as friends, this man
amassed a large list of honorary titles and contributed
significantly to scientific knowledge. He never let an
opportunity for advancement escape him and many
claimed he had "no real love or regard for his fellow
men." Nevertheless he was one of the first American
scientists and his career was probably the strangest of all
American success stories.
Thompson was born into a Massachusetts farming family
in 1763. He was a strange boy who fancied he could
build a perpetual motion machine and took great interest
in eclipses. He became an itinerant teacher and was hired
by a wealthy family in Rumford, Massachusetts. After
endearing himself to nearly everyone, Benjamin married
the daughter of the household and was accepted into high
society. So favorably did he impress the local military
officers that he was made a major at age 19. This undeserved honor made him quite unpopular with the local
citizenry. In fact as the political climate ripened for
revolution, Thompson was arrested "upon suspicion of
being inimical to the liberties of this Country." Perhaps
he was a spy, but most likely he was indifferent to the
revolutionary cause. When released he left his wife and
fled to England.
His charming manner and good looks won the friendship
of the War Minister and soon he was elected to the Royal
Society and named Under Secretary in the War Department. He returned to America to command the Queen's
Horse Dragoons against the colonists. During this time
he strangely enough began systematic lunar observations
and extensive experiments with gunpowder and shell
velocity.
At age 30 he returned to England and traveled to Bavaria.
He won the friendship of the duke of Bavaria and in due
time was made a Count of the Holy Roman Empire—
Count Rumford. Thompson was bright enough and had
enough power to apply his cherished ideas of enlightened
despotism; he established a successful welfare system in
Munich.
This was the time he made his greatest contribution to
science. While watching a cannon being bored he noted
the extreme amount of heat produced. After careful
experiments he was able to deduce that heat was molecular motion, not a fluid. This was a breakthrough.
Count Rumford was a careful observer. He installed a
glass door in his fireplace, watched the flame carefully,
and soon designed better stoves and better chimneys. He
built up quite a reputation as a nutritionist; he wrote
several essays on the benefits of coffee over tea. Many
credit him with inventing the folding bed and he made
many improvements in the design of lamps. His main
scientific accomplishment in later life was his large role
in founding the Royal Institution in 1800. It was Count
Rumford who hired Humphrey Davy as lecturer at the
Institution and it was Count Rumford's money that kept
the Institution going in the beginning. Soon, however,
the Institution became too theoretical for Thompson and
he severed connection with it to move to France. He
died in 1814 of a fever. He left his gold watch to Sir
Humphrey Davy and much of his money to Harvard
University.
Although much of what Benjamin Thompson did in his
lifetime was simply not cricket, he was an "enlightened
philanthropist" and did more for society and science than
most men.
Benjamin Thompson
1763-1814
Reference: Count Rumford of Massachusetts
Thompson, James Alden
Farrar & Rinehart, New York 1935
Written by Steven Janke
10
Page 15

012-04331E Mechanical Equivalent of Heat
T eacher’s Guide
Experiment: Measuring the
Mechanical Equivalent of Heat
Procedure
➁ It is often helpful to bring the cylinder down to several
degrees
allows you time to determine the number of turns of
rope needed before actually taking data.
⑨ It is best to crank the cylinder as rapidly as possible.
This minimizes the time in which heat can escape to
the environment
below the desired starting temperature. This
Questions
➀ The accepted value of J is 4.184 Joules/calorie. It is
reasonable to expect results within 2% of this value.
(Typical results are J = 4.144 Joules/calorie)
➁ Some sources of error might be loss of heat to the en-
vironment, inaccurate measurement of temperature,
the fact that not all of the drum is aluminum (and thus
parts of it have a different specific heat), and nonuniform temperature in the drum. If the experiment is
done carefully, these are negligible.
➂ No. If the heat absorbed by the cylinder was more
than the work done on it, PASCO scientific would be
selling perpetual motion machines instead of real
physics apparatus. It is possible that students may
measure the heat as being more than the work done,
but this is experimental error.
➃ Not directly. There are many other factors that will
come into the calculations, including Carnot efficiency.
11
Page 16

Mechanical Equivalent of Heat 012-04331E
Notes
12
Page 17

012-04331E Mechanical Equivalent of Heat
T echnical Support
Feed-Back
If you have any comments about this product or this
manual please let us know. If you have any suggestions
on alternate experiments or find a problem in the manual
please tell us. PASCO appreciates any customer feedback. Your input helps us evaluate and improve our
product.
To Reach PASCO
For Technical Support call us at 1-800-772-8700 (tollfree within the U.S.) or (916) 786-3800.
Contacting Technical Support
Before you call the PASCO Technical Support staff it
would be helpful to prepare the following information:
• If your problem is with the PASCO apparatus, note:
Title and Model number (usually listed on the label).
Approximate age of apparatus.
A detailed description of the problem/sequence of
events. (In case you can't call PASCO right away, you
won't lose valuable data.)
If possible, have the apparatus within reach when calling. This makes descriptions of individual parts much
easier.
• If your problem relates to the instruction manual, note:
Part number and Revision (listed by month and year on
the front cover).
Have the manual at hand to discuss your questions.
13
Page 18

 Loading...
Loading...