Page 1
Thank you for purchasing the OMRON RS2 Wrist Blood Press ure
Wrist Blood
Pressure Monitor
Model RS2
Instruction Manual
IM-HEM-6121-E-02-01/2013
5338844-7B
Introduction
Important Safety Information
1. Overview
A
E
C
D
B
2. Preparation
3. Using the Unit
Cuff Wrapping Guide
START INFLATE DEFLATE COMPLETE
Monitor.
The OMRON RS2 is a compact and easy to use blood pressure
monitor, operating on the oscillometric principle. It measures your
blood pressure and pulse rate simply and quickly. For comfortable
controlled inflation without the need of pressure pre-setting or
re-inflation the device uses its advanced “IntelliSense” technology.
Intended Use
This product is designed to measure the blood pressure and pulse
rate of people within the range of the designated wrist cuff, following
the instructions in this instruction manual.
It is mainly designed for general household use. Please read the
Important Safety Information in this instruction manual before using
the unit.
Please read this instruction manual thoroughly before using the
unit.
Please keep for future reference.
For specific information about your own blood pressure,
CONSULT YOUR DOCTOR.
Consult your doctor prior to using in pregnancy or if diagnos ed with
arrhythmia or arteriosclerosis.
Please read this section carefully before using the unit.
Warning:
• Indicates a potentially hazardous situation which, if not avoided,
could result in death or serious injury.
(General Usage)
• Always consult your doctor. Self-diagnosis of measurement results
and self-treatment are dangerous.
• People with severe blood flow problems, or blood disorders, should
consult a doctor before using the unit, as cuff inflation can cause
internal bleeding.
(Battery Usage)
• If battery fluid should get in your eyes, immediately rinse with
plenty of clean water. Consult a doctor immediately.
Caution:
• Indicates a potentially hazardous situation which, if not avoided,
may result in minor or moderate injury to the user or patient or
damage to the equipment or other property.
(General Usage)
• Do not leave the unit unattended with infants or persons who
cannot express their consent.
• Do not use the unit for any purpose other than m easuring blood
pressure.
• Do not use a mobile phone or other devices that emit
electromagnetic fields, near the unit. This may result in incorrect
operation of the unit.
• Do not disassemble the unit or wrist cuff.
• Do not operate the unit in a moving vehicle (car, airplane).
(Battery Usage)
• If battery fluid should get on your skin or clothing, immediately rinse
with plenty of clean water.
• Use only two “AAA” alkaline (LR03) batteries with this unit.
• Do not insert the batteries with their polarities incorrectly aligned.
• Replace old batteries with new ones immediately. Replace both
batteries at the same time.
• Remove the batteries if the unit will not be used for three months or
more.
• Do not use new and used batteries together.
General Precautions
• Do not apply strong shocks and vibrations to or drop the unit.
• Do not take measurements after bathing, drinking alcohol or
caffeine, smoking, exercising or eating.
• Do not inflate the wrist cuff when it is not wrapped around your
wrist.
• Read and follow the “Important information regarding Electro
Magnetic Compatibility (EMC)” in the Technical Data Section.
• Read and follow the “Correct Disposal of This Product” in the
Technical Data Section when disposing of the device and any used
accessories or optional parts.
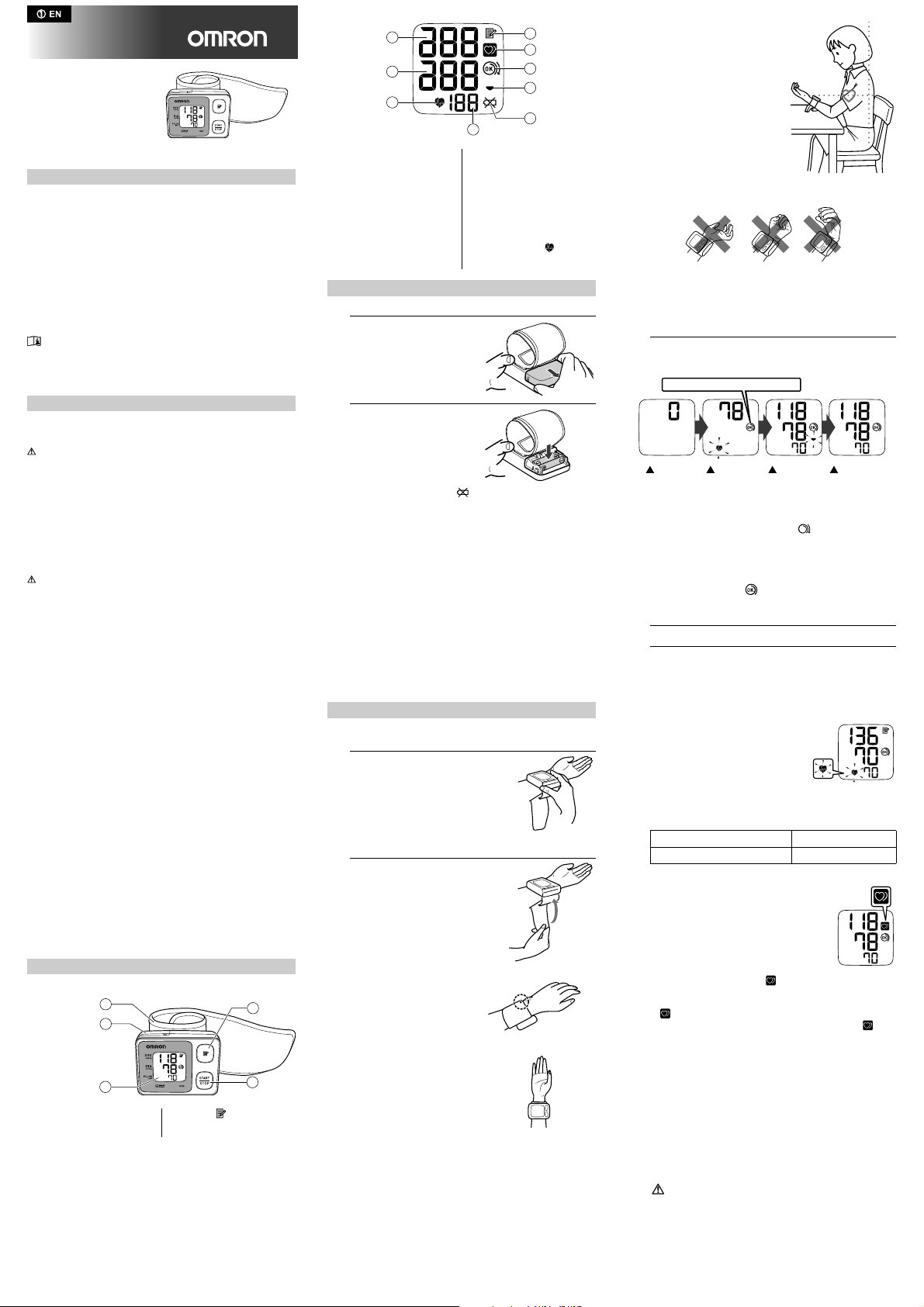
Main unit:
A. Wrist cuff
B. Battery compartment E. START/STOP button
C. Display
D. Memory ( ) button
Display:
F
G
H
F. Systolic blood pressure
G. Diastolic blood pressure
H. Heartbeat symbol
1. Flashes during measurement
2. If flashing after measurement
completed or when viewing
results stored in the memory,
indicates blood pressure out of
recommended range*.
I. Pulse display and Memory
number
I
J. Memory symbol
K. Irregular heartbeat symbol
L. Cuff wrapping guide
M. Deflation symbol
N. Battery low symbol
* Note: If your systolic or diastolic
pressure is outside the standa rd
range (above 135/85 mmH g) the
Heartbeat symbol ( ) will blink.
Please refer to Section 3.3 .
J
K
L
M
N
2.1 Installing/Replacing the Batteries
Remove the battery cover.
1.
Insert two 1.5V “AAA”
2.
alkaline (LR03) batteries
as indicated in the battery
compartment and then
replace the battery cover.
Notes:
• If the battery low symbol ( ) appears on the display, turn off
the unit then replace both batteries at the same time.
• The measurement values continue to be stored in memory
even after the batteries are replaced.
Disposal of used batteries should be carried out in accordance
with the national regulations for the disposal of batteries.
3.1 Applying the Wrist Cuff
Do not apply over clothing.
Place the wrist cuff over
1.
your wrist.
Your palm should face upward.
Wrap the wrist cuff around
2.
your wrist.
Wrap the wrist cuff securely
around the wrist for taking
accurate measurements.
Make sure that the wrist cuff
does not cover the protruding
part of the wrist bone (ulna) on
the outside of the wrist.
Note: You can take a
measurement on either
your left or right wrist.
3.2 How to Sit Correctly
To take a measurement, you need
to be relaxed and comfortably
seated, under c omfortable room
temperature. No bathing, drinking
alcohol or caffeine, smoking,
exercising or eating 30 minutes
before taking a measurement.
• Sit on a chair with your feet flat on the
floor.
• Sit upright with your back straight.
• The cuff should be at the same level
as your heart.
• Relax your wrist and hand. Do not bend your wrist back, clench
your fist, or bend your wrist forward.
3.3 Taking a Reading
Notes:
• To cancel a measurement, press the START/STOP button at
any time during measurement.
• Remain still while taking a measurement.
Press the START/STOP button.
1.
All the symbols appear on the display.
The wrist cuff will start to inflate automatically.
Cuff Wrapping Guide
The Cuff Wrapping Guide is a unique feature that
indicates if the cuff is not wrapped tightly enough
around the wrist. Even when the is displayed, a
blood pressure reading will be taken.
Note: This reading is NOT reliable due to the incorrect
wrapping of the cuff. Please wrap the cuff again, taking
care to wrap it correctly and take the measurement
again. When the is displayed, the cuff is correctly
wrapped tightly enough on the wrist and the reading is
accurate and reliable.
Undo the wrist cuff and remove the unit.
2.
Press the START/STOP button to turn off the
3.
monitor.
The monitor automatically stores the measurement in its
memory.
It will automatically turn off after two minutes.
Important:
• If your systolic or diastolic pressure is
outside the standard range, the
heartbeat symbol will blink when the
measurement result is displayed.
Recent research suggests that the
following values can be used as a
guide to high blood pressure for
measurements taken at home.
Systolic Blood Pressure Above 135 mmHg
Diastolic Blood Pressure Above 85 mmHg
This criteria is for home blood pressure measurement.
• Your blood pressure monitor includes an
irregular heartbeat feature. Irregular
heartbeats can influence the results of the
measurement. The irregular heartbeat
algorithm automatically determines if the
measurement is usable or needs to be
repeated. If the measurement results are
affected by irregular heartbeats but the result
is valid, the result is shown together with the
irregular heartbeat symbol ( ).
If the irregular heartbeats cause the measurement to be
invalid, no result is shown. If the irregular heartbeat symbol
( ) is shown after you have taken a measurement, repeat
the measurement. If the irregular heartbeat symbol ( ) is
shown frequently, please make your doctor aware of it.
Notes:
• Wait 2 - 3 minutes before taking another blood
pressure measurement. Waiting between readings
allows the arteries to return to the condition prior to
taking the blood pressure measurement.
• The blood pressure can differ between the right arm
and the left arm, and therefore also the measured
blood pressure values can be different. OMRON
recommends to always use the same arm for
measurement. If the values between the two arms
differ substantially, please check with your physician
which arm to use for your measurement.
Warning:
Self-diagnosis of measured results and treatment are
dangerous. Please follow the instructions of your doctor.
Page 2
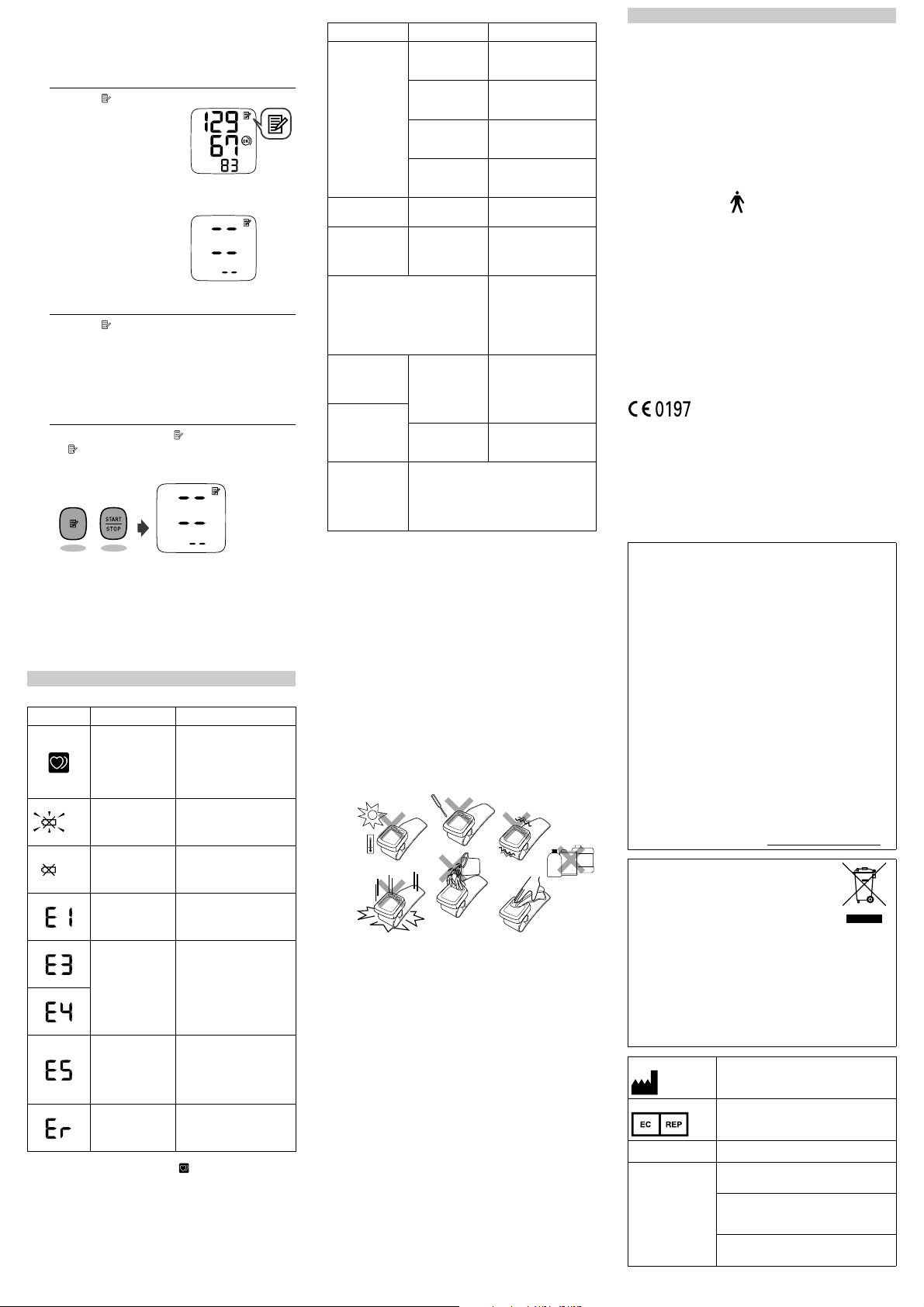
3.4 Using the Memory Function
4. Troubleshooting and Maintenance
5. Technical Data
The unit automatically stores up to 30 sets of measurement
values (blood pressure and pulse rate). If the memory is full,
the monitor will delete the oldest readings.
To View the Readings Stored in Memory
Press the button.
1.
The Memory number
appears for a second
before the pulse rate is
displayed. The newest
set is numbered “1”.
Note: If there are no
measurements results
stored in the memory, the
screen to the right is
displayed.
Press the button repeatedly to view the
2.
readings stored in memory.
To Delete All the Values Stored in Memory
When the memory symbol ( ) appears, first press
the button. Then while holding it down, press the
START/STOP button simultaneously for about 2 - 3
seconds.
First Second
Note: You cannot partially delete the stored readings.
4.1 The Icons and Error Messages
Error Display Cause Remedy
Remove the wrist cuff. Wait
2 - 3 minutes and then take
Irregular heartbeats
are detected.
The batteries are
Blink
low.
The batteries are
Lit
exhausted.
Wrist cuff not
applied correctly.
Movement during
measurement.
Wrist cuff not
applied correctly, or
movement during
measurement.
Device error.
Note: The irregular heartbeat symbol ( ) may also be displayed
with error messages.
another measurement.
Repeat the steps in section
3.3. If this error continues to
appear, contact your doctor.
You should replace them with
new ones ahead of time.
Refer to section 2.1.
You should replace them with
new ones at once.
Refer to section 2.1.
Apply the wrist cuff correctly.
Refer to section 3.1.
Repeat Measurement while
remaining still and refraining
from talking during the
measurement.
Refer to section 3.3.
Apply the wrist cuff correctly
and repeat measurement
while remaining still and
refraining from talking during
the measurement.
Refer to section 3.1 and 3.3.
Contact your OMRON retail
outlet or distributor.
4.2 Troubleshooting
Problem Cause Remedy
The wrist cuff is not
at heart level.
The cuff is not
The reading is
extremely low
(or high).
Wrist cuff pressur e
does not rise.
Wrist cuff deflate s
too soon.
The blood pressure is different
each time. The reading is
extremely low (or high).
The unit loses
power during
measurement.
Nothing happens
when you press
the buttons.
Other problems.
wrapped snugly
around the wrist.
The arms and
shoulders are
tense.
Movement or
talking during
measurement.
Air is leaking from
the wrist cuff.
The wrist cuff is
loose.
The batteries are
exhausted.
The batteries have
been inserted
incorrectly.
• Press the START/STOP button and repeat
measurement.
• If the problem continues, try replacing the
batteries with new ones.
If this still does not solve the problem, contact
your OMRON retail outlet or distributor.
Measure while in the
correct posture.
Refer to section 3.2.
Wrap the cuff correctly.
Refer to section 3.1.
Relax and try taking the
measurement again.
Refer to section 3.3.
Remain still and do not
talk during Measurement.
Refer to section 3.3.
Consult your OMRON
retail outlet or distributor.
Apply the cuff correctly so
that it is firmly wrapped
around the wrist. Refer to
section 3.1.
Blood pressure readings
constantly vary with time
of day and how relaxed
you are. Take several
deep breaths and try to
remain relaxed before
taking a measurement.
Replace the batteries with
new ones.
Refer to section 2.1.
Insert the batteries with
the correct (+/-) polarity.
Refer to section 2.1.
4.3 Maintenance
To protect your unit from damage, please observe the
following:
• Do not subject the main unit and the cuff to
extreme temperatures, humidity, moisture or
direct sunlight.
• Do not disassemble the unit.
• Do not subject the unit to strong shocks or
vibrations (for example, dropping the unit on the
floor).
• Do not use volatile liquids to clean the main unit.
• Do not wash the cuff or immerse it in water.
• Do not use petrol, thinners or similar solvents to
clean the cuff.
• Do not carry out repairs of any kind yourself. If a
defect occurs, consult your OMRON retail outlet
or distributor as mentioned on the packaging.
• The unit should be cleaned with a soft, dry cloth.
• Use a soft, moistened cloth and soap to clean
the cuff.
• Keep the unit in its storage case when not in
use.
• Fold the cuff into the storage case.
Do not store the unit in the following situations:
• If the unit is wet.
• Locations exposed to extreme temperatures,
humidity, direct sunlight, dust or corrosive
vapours.
• Locations exposed to vibrations, shocks or
where it will be at an angle.
Calibration and Service
• The accuracy of this blood pressure monitor has
been carefully tested and is designed for a long
service life.
• It is generally recommended to have the unit
inspected every two years to ensure correct
functioning and accuracy. Please consult your
authorised OMRON dealer or the OMRON
Customer Service at the address given on the
packaging or attached literature.
Product Description Wrist Blood Pressure Monitor
Model OMRON RS2 (HEM-6121-E)
Display LCD Digital Display
Measurement Method Oscillometric method
Measurement Range Pressure: 0 to 299 mmHg
Accuracy Pressure: ±3 mmHg
Inflation Automatic inflation by pump
Deflation Automatic rapid deflation
Memory 30 Measurements
Power Source 2 “AAA” alkaline (LR03) batteries 1.5V
Battery Life Approx. 300 measurements with new
Applied Part = Type B
Protection Against
Electric Shock
Operating temperature/
Humidity
Storage temperature/
Humidity
Console Weight Approximately 101g without batteries
Outer Dimensions Approximately 78 (w) mm x 60 (h) mm x
Measurable
circumference
Cuff Material Nylon and polyester
Package Content Main unit, storage case, battery set,
Note: Subject to technical modification without prior notice.
• This device fulfils the provisions of EC directive 93/42/EEC
(Medical Device Directive).
• This blood pressure monitor is designed according to the European
Standard EN1060, Non-invasive sphygmomanometers Part 1:
General Requirements and Part 3: Supplementary requirements
for electromechanical blood pressure measuring systems.
• This OMRON product is produced under the strict quality system of
OMRON HEALTHCARE Co. Ltd., Japan. The Core component for
OMRON blood pressure monitors, which is the Pressure Sensor, is
produced in Japan.
Important information regarding Electro Magnetic
Compatibility (EMC)
With the increased number of electronic devices such as PC’s and
mobile (cellular) telephones, medical devices in use may be
susceptible to electromagnetic interference from other devices.
Electromagnetic interference may result in incorrect operation of
the medical device and create a potentially unsafe situation.
Medical devices should also not interfere with other devices.
In order to regulate the requirements for EMC (Electro Magnetic
Compatibility) with the aim to prevent unsafe product situations, the
EN60601-1-2:2007 standard has been implemented. This standard
defines the levels of immunity to electromagnetic interferences as
well as maximum levels of electromagnetic emissions for medical
devices.
This medical device manufactured by OMRON HEALTHCARE
conforms to this EN60601-1-2:2007 standard for both immunity and
emissions.
Nevertheless, special precautions need to be observed:
• Do not use mobile (cellular) telephones and other devices, w hich
generate strong electrical or electromagnetic fields, near the
medical device. This may result in incorrect operation of the unit
and create a potentially unsafe situation. Recommendation is to
keep a minimum distance of 7 m. Verify correct operation of the
device in case the distance is shorter.
Further documentation in accordance with EN60601-1-2:2007 is
available at OMRON HEALTHCARE EUROPE at the address
mentioned in this instruction manual.
Documentation is also available at www.omron-healthcare.com.
Correct Disposal of This Product
(Waste Electrical & Electronic Equipment)
This marking shown on the product or its
literature, indicates that it should not be
disposed of, with other household wastes at the
end of its working life.
To prevent possible harm to the environment or human health from
uncontrolled waste disposal, please separate this product from
other types of wastes and recycle it responsibly to promo te the
sustainable reuse of material resources.
Household users should contact either the retailer where they
purchased this product, or their local government office, for details
of where and how they can return this item for environmentally safe
recycling.
Business users should contact their supplier and check the terms
and conditions of the purchase contract. This product should not be
mixed with other commercial wastes for disposal.
Manufacturer
EU-representative
Production facility
Subsidiary
Pulse: 40 to 180/min.
Pulse: ±5% of display reading
alkaline batteries at a room temperature of
23°C
Internally powered ME equipment
+10 to +40°C / Maximum: 30 to 85% RH
-20 to +60°C / Maximum: 10 to 95% RH /
700 to 1060hPa
21 (d) mm (without the wrist cuff)
Approximately 13.5 to 21.5 cm
instruction manual, guarantee card
OMRON HEALTHCARE Co., Ltd.
53, Kunotsubo, Terado-cho, Muko, Kyoto,
617-0002 JAPAN
OMRON HEALTHCARE EUROPE B.V.
Scorpius 33, 2132 LR H oofddorp
THE NETHERLANDS
www.omron-healthcare.com
OMRON (DALIAN) CO., LTD.
Dalian, CHINA
OMRON HEALTHCARE UK LTD.
Opal Drive, Fox Milne, Milton Keynes, MK15 0DG
U.K.
OMRON MEDIZINTECHNIK
HANDELSGESELLSCHAFT mbH
John-Deere-Str. 81a, 68163 Mannheim, GERMANY
www.omron-medizintechnik.de
OMRON SANTÉ FRANCE SAS
14, rue de Lisbonne, 93561
Rosny-sous-Bois Cedex, FRANCE
Made in China
 Loading...
Loading...