NORTH AMERICAN DRÄGER Evita 4 Neoflow User manual

D
MEDICAL
NeoFlow
Neonatal Mode
Addendum to
Operating Instructions
Evita 4 (as of software 2.n)
Evita 2 dura (as of software 3.10)
NOTICE
Proprietary Information
This document contains information in which Draeger Medical, Inc.
claimed proprietary rights. The information may not be reproduced in
whole or in part except as authorized in writing by Dräger. This information is the property of Draeger Medical, Inc., it is provided solely for the
use intended.
Repairs/Modifications
Repairs on this device shall be performed only by DraegerService or its
Authorized Service Centers. Information about repairs can be obtained
from Dräger or Authorized Dealers. Draeger Medical, Inc. will not be responsible for injury to persons or damage to property arising directly or
indirectly out of unauthorized repairs or modifications to this device.
Furthermore, any unauthorized repairs or modifications void any warranty extended by Dräger.
This document is provided for your information only. It will not be
exchanged or updated without request.
Trademarks
The Draeger name and logo are registered trademarks of Dräger.
AutoFlow is a registered trademark of Dräger.
NeoFlow is a trademark of Dräger.
Dräger Medical AG & Co. KGaA
All rights reserved, Subject to modifications

Contents
Contents
Important Safety Information READ THIS FIRST........4
Operator's Responsibility for Patient Safety..................4
Limitation of Liability..................................................... 4
Warranty......................................................................5
Definitions.................................................................... 6
General WARNINGS and CAUTIONS......................... 6
Precautions During Preparation.................................... 7
Precautions During Operation...................................... 7
Precautions During Care.............................................. 8
Precautions During Maintenance.................................. 8
Intended Use.............................................................. 9
Preparation................................................................10
Installation.................................................................. 10
Before First Use......................................................... 10
Preparing For Use......................................................10
Ventilator Check Evita 4............................................. 11
Ventilator Check Evita 2 dura......................................11
Calibrating the Neonatal Flow Sensor.........................12
Exchanging a Neonatal Flow Sensor Element............. 13
Troubleshooting........................................................30
Technical Data.......................................................... 31
Performance Characteristics...................................... 31
Measured Value Displays............................................31
Monitoring..................................................................32
Materials Used........................................................... 33
Special Features of Neonatal Ventilation..................34
Measurement of Leak Flow.........................................34
Measurement of Airway Pressure............................... 36
Trigger Response.......................................................37
AutoFlow................................................................. 38
Glossary.................................................................... 40
Ordering Information................................................ 41
Index......................................................................... 42
Operation.................................................................. 14
Selecting Neonatal Mode with Evita 4.........................14
Selecting Neonatal Mode with Evita 2 dura................. 14
Volume Controlled Ventilation in Neonatal Mode......... 15
Back-up Ventilation in Neonatal Mode.........................16
Pressure Support Ventilation (PSV)............................ 16
Apnea Ventilation in Neonatal Mode............................17
Flow Monitoring During Neonatal Ventilation............... 18
Flow Monitoring During Pediatric Ventilation............... 19
Nebulizing Medication Aerosols.................................. 20
O2 Concentration When Using Nebulizer....................23
Oxygenation For Bronchial Suction.............................23
Configuration of Ventilation...................................... 24
Selecting the Patient Range........................................24
Start-up Defaults for Ventilation Parameters
and Alarm Limits With Evita 4..................................... 24
Start-up Defaults for Ventilation Parameters
and Alarm Limits With Evita 2 dura............................. 26
Care...........................................................................27
Dismantling the Neonatal Flow Sensor........................27
Disinfecting/Cleaning/Sterilizing................................. 27
Maintenance...............................................................29
Operating Instructions Evita 4 / Evita 2 dura NeoFlow
3

Important Safety Information
Operator's Responsibility for Patient Safety
Limitation of Liability
Important Safety Information
Operator's Responsibility for Patient Safety
For correct and effective use of the product and in
order to avoid hazards it is mandatory to carefully
read and to observe all portions of this manual.
The design of the intensive care ventilator this device is
intended to be used with, accompanying literature, and
the labeling on the equipment, take into consideration
that the purchase and use of the equipment are
restricted to trained professionals, and that certain
inherent characteristics of the equipment are known to
the trained operator. Instructions, warnings, and
caution statements are limited, therefore, largely to the
specifics of the Draeger design. This publication
excludes references to various hazards which are
obvious to a medical professional and operator of
respiratory care equipment, to the consequences of
misuse of such equipment, and to potentially adverse
effects in patients with abnormal conditions. Product
modification or misuse can be dangerous. Draeger
Medical, Inc. disclaims all liability for the consequences
of product alterations or modifications, as well as for
the consequences which might result from uses of the
product not covered by its intended use or from the
combination of this product with other products
whether supplied by Draeger or by other manufacturers
if such a combination is not endorsed by Draeger Medical, Inc.
Limitation of Liability
Draeger Medical, Inc.'s liability, whether arising out of
or related to manufacture and sale of the goods, their
installation, demonstration, sales representation, use,
performance, or otherwise, including any liability based
upon Draeger Medical, Inc.'s Product Warranty, is subject to and limited to the exclusive terms and conditions
as set forth, whether based upon breach of warranty or
any other cause of action whatsoever, regardless of
any fault attributable to Draeger Medical, Inc. and regardless of the form of action (including, without limitation, breach of warranty, negligence, strict liability, or
otherwise).
THE STATED EXPRESSED WARRANTlES ARE IN
LlEU OF ALL OTHER WARRANTIES, EXPRESSED
OR IMPLIED, INCLUDING, WITHOUT LIMITATION,
WARRANTlES OF MERCHANTABILITY, FITNESS
FOR ANY PARTICULAR PURPOSE, OR
NONINFRINGEMENT.
Draeger Medical, Inc. shall not be liable for, nor shall
buyer be entitled to recover any special incidental, or
consequential damages or for any liability incurred by
buyer to any third party in any way arising out of or relating to the goods.
The operators of ventilator systems must recognize
their responsibility for choosing appropriate safety
monitoring that supplies adequate information on equipment performance and patient condition. Patient safety
may be achieved through a wide variety of different
means ranging from electronic surveillance of equipment performance and patient condition to simple,
direct observation of clinical signs. The responsibility
for the selection of the best level of patient monitoring
lies solely with the equipment operator.
4
Operating Instructions Evita 4 / Evita 2 dura NeoFlow

Warranty
Important Safety Information
Warranty
All Draeger products are guaranteed to be free of
defects for a period of one year from date of delivery.
The following are exceptions to this warranty:
1. The defect shall be a result of workmanship or
material. Defects caused by misuse, mishandling,
tampering, or by modifications not authorized by
Draeger Medical, Inc. or its representatives are not
covered.
2. Rubber and plastic components and materials are
warranted to be free of defects at time of delivery.
3. Oxygen sensor capsules have a six-month limited
warranty from the date of delivery.
Any product which proves to be defective in workmanship or material will be replaced, credited, or repaired
with Draeger Medical, Inc. holding the option. Draeger
Medical, Inc. is not responsible for deterioration, wear, or
abuse. In any case, Draeger Medical, Inc. will not be liable beyond the original selling price.
Application of this warranty is subject to the following
conditions:
1. Draeger Medical, Inc. or its authorized representative must be promptly notified, in writing, upon detection of the defective material or equipment.
2. Defective material or equipment must be returned,
shipping prepaid, to Draeger or its authorized
representative.
3. Examination by Draeger Medical, Inc. or its authorized representative must confirm that the defect is
covered by the terms of this warranty.
4. Notification in writing of defective material or equipment must be received by Draeger Medical, Inc. or
its authorized representative no later than two (2)
weeks following expiration of this warranty.
In order to assure complete protection under this
warranty, the Customer Registration Card and/or Periodic Manufacturer's Service Record (if applicable)
must be returned to Draeger within ten (10) days of
receipt of the equipment.
The above is the sole warranty provided by Draeger
Medical, Inc. No other warranty expressed or implied is
intended. Representatives of Draeger are not authorized to modify the terms of this warranty.
Operating Instructions Evita 4 / Evita 2 dura NeoFlow
Draeger Medical, Inc., Telford, PA
5
Important Safety Information
Definitions
General WARNINGS and CAUTIONS
Definitions
WARNING !
A WARNING statement refers to conditions
with a possibility of personal injury if disregarded.
CAUTION !
A CAUTION statement designates the possibility
of damage to equipment if disregarded.
NOTE: A NOTE provides additional information
intended to avoid inconveniences during operation.
Inspection = examination of actual condition
Service = measures to maintain specified
condition
Repair = measures to restore specified
condition
Maintenance = inspection, service, and repair,
where necessary
General WARNINGS and CAUTIONS
WARNING !
Strictly follow Operator's Instruction Manuals!
Any use of the product requires full under-
standing and strict observation of all portions
of these instructions as well as the Operating
Instructions of the Evita 4 and Evita 2 dura
ventilator. The equipment is only to be used
for the purpose specified under "Intended
Use" (page 9). Observe all WARNINGS and
CAUTIONS as rendered throughout the
manuals and on labels on the equipment.
WARNING !
DANGER, risk of explosion if used in the
presence of flammable anesthetics.
The equipment is neither approved nor certified for use in areas where combustible or
explosive gas mixtures with air or with nitrous
oxide are likely.
WARNING !
Electrical connections to equipment which is
not listed in these Operating Instructions
should only be made after consultation with
the respective manufacturers or a qualified
expert.
Preventive = Maintenance measures at regular
Maintenance intervals
Typing conventions in this manual
Controls ("hard" keys and screen keys / fields / knobs)
are designated as »Control Name«, e.g.
»Calibration«
Screen pages are indicated as »Screen page«, e.g.
»Alarm limits«
On-screen messages are printed in bold, e.g.
Calibration ok
CAUTION !
Restriction of Distribution
Federal Law and Regulations in the United
States and Canada restrict this device to sale
by or on the order of a physician.
Operating Instructions Evita 4 / Evita 2 dura NeoFlow
CAUTION !
Accessories
Use only accessories listed in the Ordering
Information (page 41).
6
Precautions During Preparation
Important Safety Information
Precautions During Preparation
Precautions During Operation
WARNING !
Installation of the NeoFlow option into Evita 4
or Evita 2 dura ventilators may only be performed by factory trained and authorized
service personnel. Follow installation instructions in the respective documentation.
WARNING !
The operator of the ventilator must still assume full responsibility for proper ventilation
while flow monitoring is not available during
calibration.
Precautions During Operation
WARNING !
The alarm limit »Paw WWWW« must always be set,
so that a warning is triggered if the airway
pressure increases with reduced compliance
or in the event of sudden changes in the size
of the leak.
WARNING !
Minute volume during neonatal ventilation
cannot be monitored without the neonatal
flow sensor!
The operator of the ventilator must still
assume full responsibility for proper ventilation while flow monitoring is not available
during neonatal ventilation.
WARNING !
Effect of aerosols on sensors, filters,
and heat and moisture exchangers!
The measuring function of the flow sensor
may be impaired.
The flow resistance of filters is liable to
increase and may impair ventilation.
Do not put a microbial filter on the nebulizer
outlet when in use!
Operating Instructions Evita 4 / Evita 2 dura NeoFlow
WARNING !
Do not use a heat/moisture exchanger
simultaneously with a nebulizer or heated
humidifier!
Risk of increased breathing resistance due to
condensation.
WARNING !
The nebulizer function integrated in Evita 4
and Evita 2 dura is designed for nebulizers
with a nebulizing flow of 6 L/min at 29 psi
(2 bar), for example nebulizer 84 12 935
(white central body). Other nebulizers may
cause deviations in tidal volume and
inspiratory O2 concentration!
WARNING !
While flow monitoring is not available during
nebulizing, the operator of the ventilator must
still assume full responsibility for proper
ventilation.
CAUTION !
Do not ventilate with the neonatal flow sensor.
Instead, use the expiratory flow sensor for
ventilation.
Secretion collecting in the flow sensor may
cause corrosion.
CAUTION !
The wires of the flow sensor are hot. If the
neonatal flow sensor is left in the ventilation
system for some time during nebulizing without
being cleaned, deposits from the medicament
sprays may build up and impair flow
measurement.
In the worst case, these deposits could catch
fire.
Disconnecting the flow sensor cable is not
sufficient to prevent this.
It is therefore important to remove the complete
flow sensor before medicament nebulizing.
7
Important Safety Information
Precautions During Maintenance
Precautions During Care
WARNING !
Follow all accepted hospital procedures for
disinfecting parts contaminated by body fluids
(protective clothing, eyewear, etc.).
CAUTION !
Certain components of the ventilator consist of
materials that are sensitive to certain organic
solvents sometimes used for cleaning and
disinfecting (e.g., phenols, halogen releasing
compounds, oxygen releasing compounds,
strong organic acids, etc.). Exposure to such
substances may cause damage that is not always
immediately apparent. Sterilization with ethylene
oxide (EtO) is also not recommended.
CAUTION !
Do not allow any liquid into the connector of the
flow sensor cable.
Precautions During Maintenance
WARNING !
To avoid any risk of infection, clean and
disinfect ventilator and accessories according
to established hospital procedures before
any maintenance - this applies also when
returning ventilators or parts for repair.
WARNING !
Preventive Maintenance work on the Evita 4
and Evita 2 dura ventilators and their
components may be performed by factory
trained and authorized personnel only.
WARNING !
Never operate a ventilator if it has suffered
physical damage or does not seem to operate
properly. In this case always refer servicing to
factory trained and authorized personnel.
CAUTION !
Do not process flow sensor element in cleaning
and disinfection equipment.
Do not use compressed air, brushes or similar
tools to clean flow sensor element as this would
possibly damage the thin wires in the flow sensor.
CAUTION !
Maintenance
In case of malfunction of this component, contact
your local DraegerService or our Factory
Authorized Technical Service Center.
The devices must be inspected and serviced
(preventive maintenance) by factory trained and
authorized technical service representatives at
regular 6 month intervals. A record must be kept
on this preventive maintenance. We recommend
obtaining a service contract through your vendor.
Maintenance or repair of Evita ventilators shall be
performed only by Draeger authorized technical
service representatives.
Operating Instructions Evita 4 / Evita 2 dura NeoFlow
8

Intended Use
NeoFlow – neonatal mode with base flow.
NeoFlow extends the patient range of Evita 4 and Evita 2
dura ventilators to infants and neonates with a minimum
body weight of 1,1 lbs (0.5 kg).
A flow sensor specifically designed for neonatal applications extends the range of flow monitoring for Evita 4 and
Evita 2 dura ventilators during pediatric and infant ventilation. This neonatal flow sensor is positioned at the
patient wye connector.
Intended Use
Operating Instructions Evita 4 / Evita 2 dura NeoFlow
9
Preparation
Installation
Before First Use
Preparing for Use
Preparation
Installation
WARNING !
Installation of the NeoFlow option into Evita 4
or Evita 2 dura ventilators may only be performed by factory trained and authorized
service personnel. Follow installation instructions in the respective documentation.
Before First Use
Please refer to page 24 for instructions on configuring
NeoFlow.
Preparing For Use
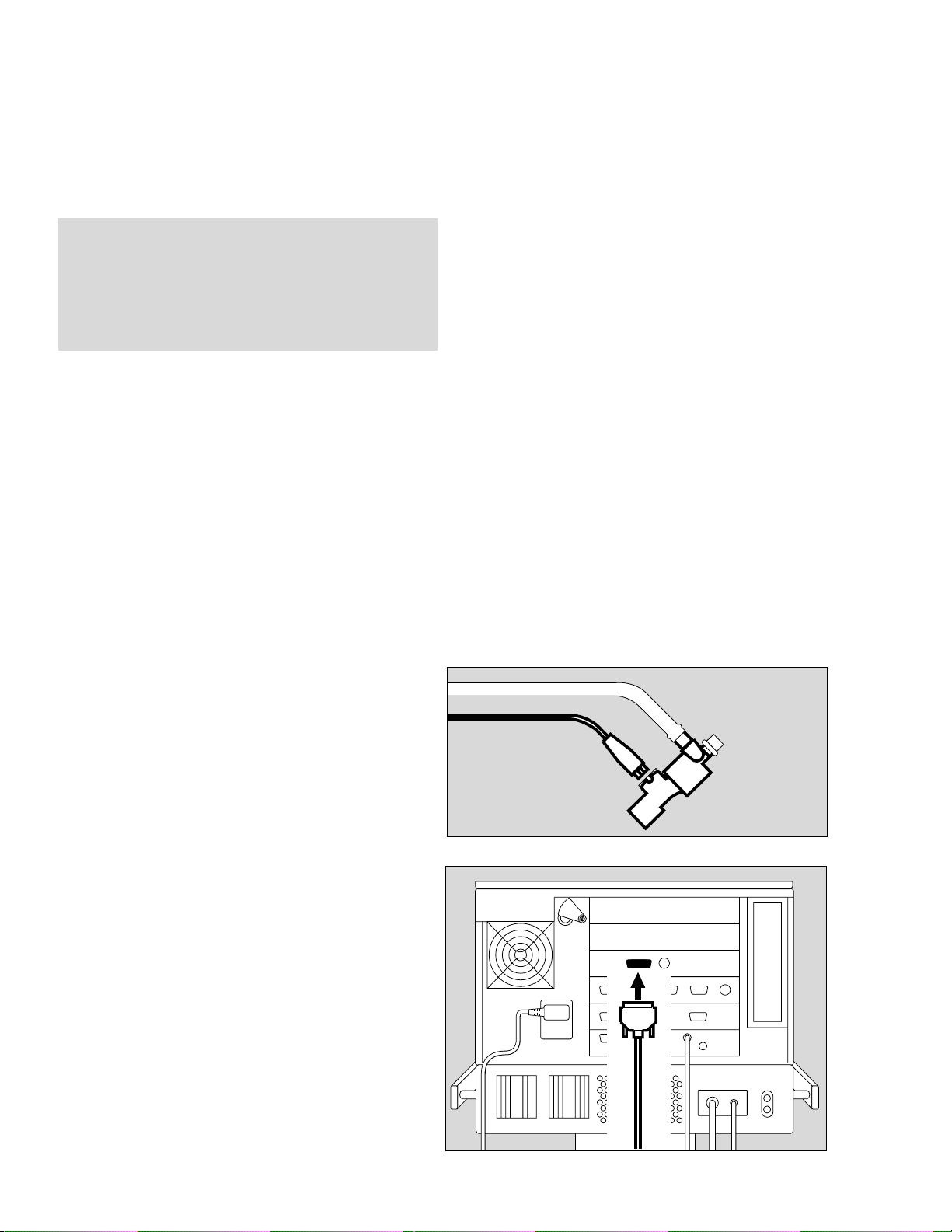
Installing the neonatal flow sensor
Prepare ventilator circuit as described under
"Ventilating Infants" in the Evita 4 and Evita 2 dura
Operating Instructions, respectively.
1 Connect wye to ventilator circuit.
2 Insert neonatal flow sensor to the ET-tube connector
end of the wye.
3 Insert flow sensor cable connector into sensor socket.
● Route sensor cable to the ventilator along the venti-
lator circuit.
4 Plug flow sensor connector into the appropriate
socket on the rear panel of the ventilator and tighten
thumbscrews.
1
3
2
00129556
Operating Instructions Evita 4 / Evita 2 dura NeoFlow
10
4
00229556
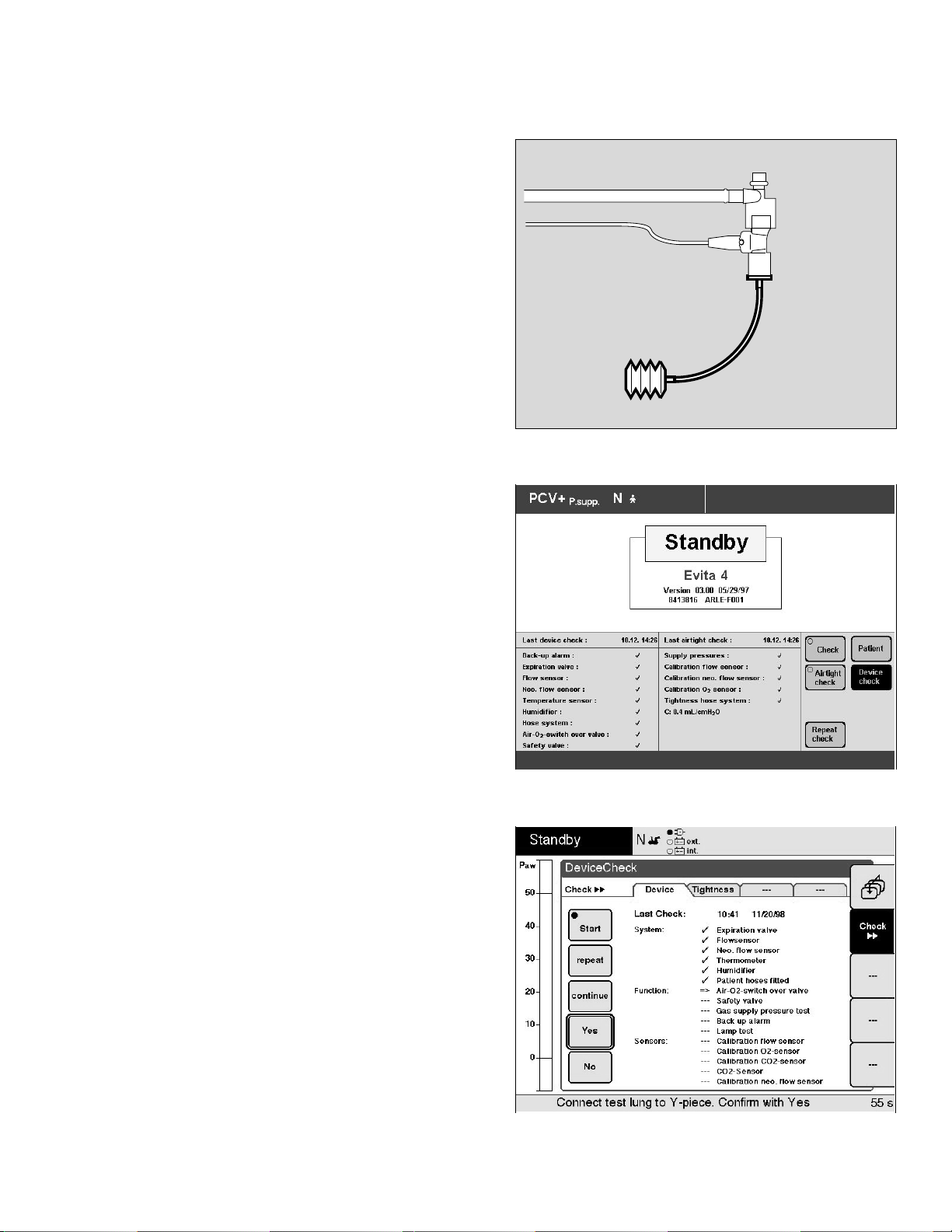
● Connect infant test lung with tracheal tube and
connector to the patient end of the neonatal flow sensor.
Ventilator Check Evita 4
Preparation
Ventilator Check Evita 4
Ventilator Check Evita 2 dura
0032955600229563
The NeoFlow option expands the Evita 4 ventilator
pre-use check procedure by the following function:
– Calibration neo. flow sensor
Ventilator Check Evita 2 dura
The NeoFlow option expands the Evita 2 dura ventilator
pre-use check procedure by the following function:
– Calibration neo. flow sensor
00129563
Operating Instructions Evita 4 / Evita 2 dura NeoFlow
11
Preparation
Calibrating the Neonatal Flow Sensor
Calibrating the Neonatal Flow Sensor
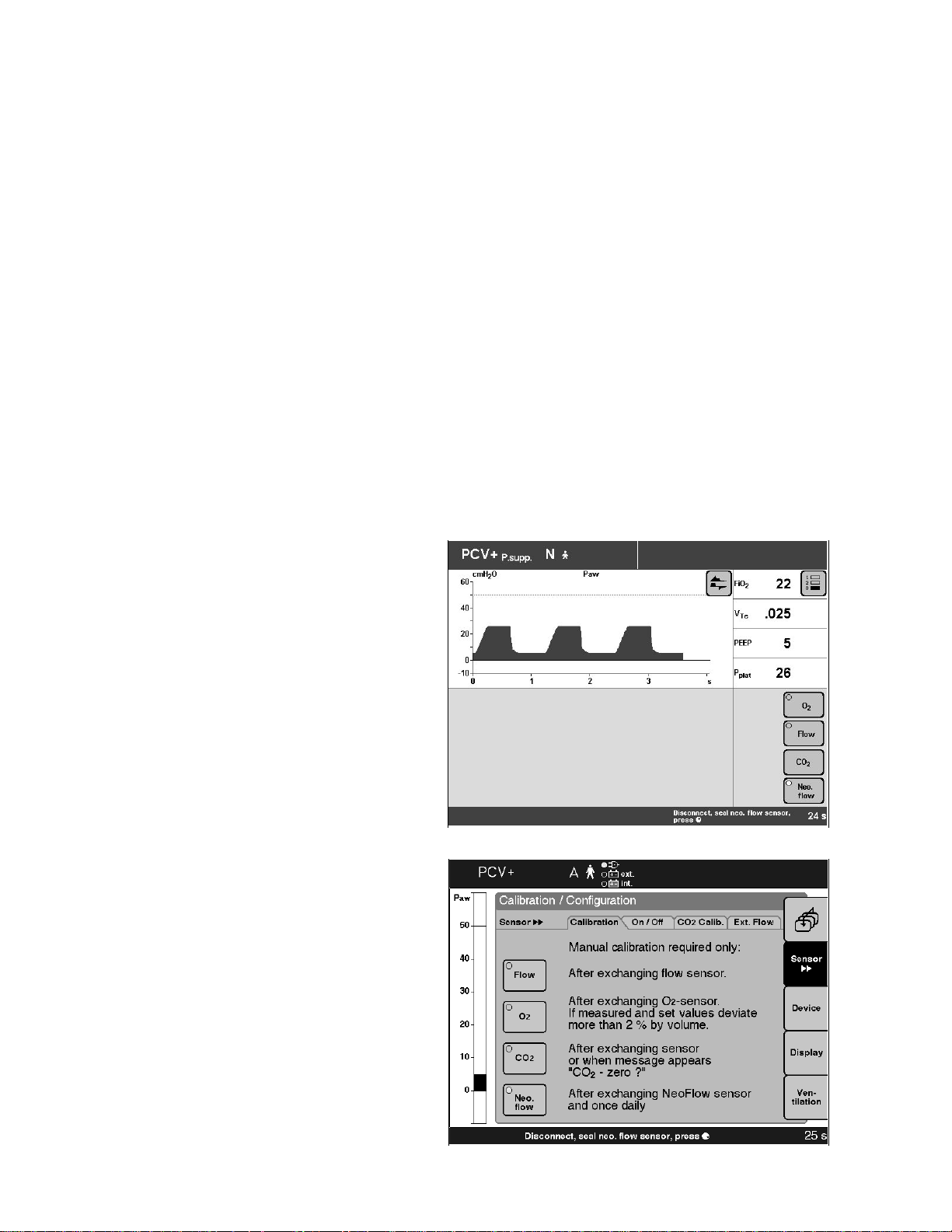
Calibrate neonatal flow sensor
– Before use, as part of the ventilator check.
– After replacing the neonatal flow sensor.
– At least once every 24 hours.
NOTE: The last calibration value obtained is saved until
the next calibration, even when the ventilator is switched
off.
Before each calibration, the neonatal flow sensor is
automatically cleaned by superheating the hot wire in the
anemometer.
NOTE: Recalibration is not necessary if the neonatal flow
sensor has been temporarily disconnected.
Starting calibration with Evita 4
● Press the »Calibration« key.
● Touch »Neo.flow« screen key.
The "LED" in the screen key will turn yellow.
Starting calibration with Evita 2 dura
● Press »Calib./Config.« key.
● Select screen key »Neo. flow« = turn dial knob.
● Start calibration = press dial knob.
Operating Instructions Evita 4 / Evita 2 dura NeoFlow
12
00429563 00329563
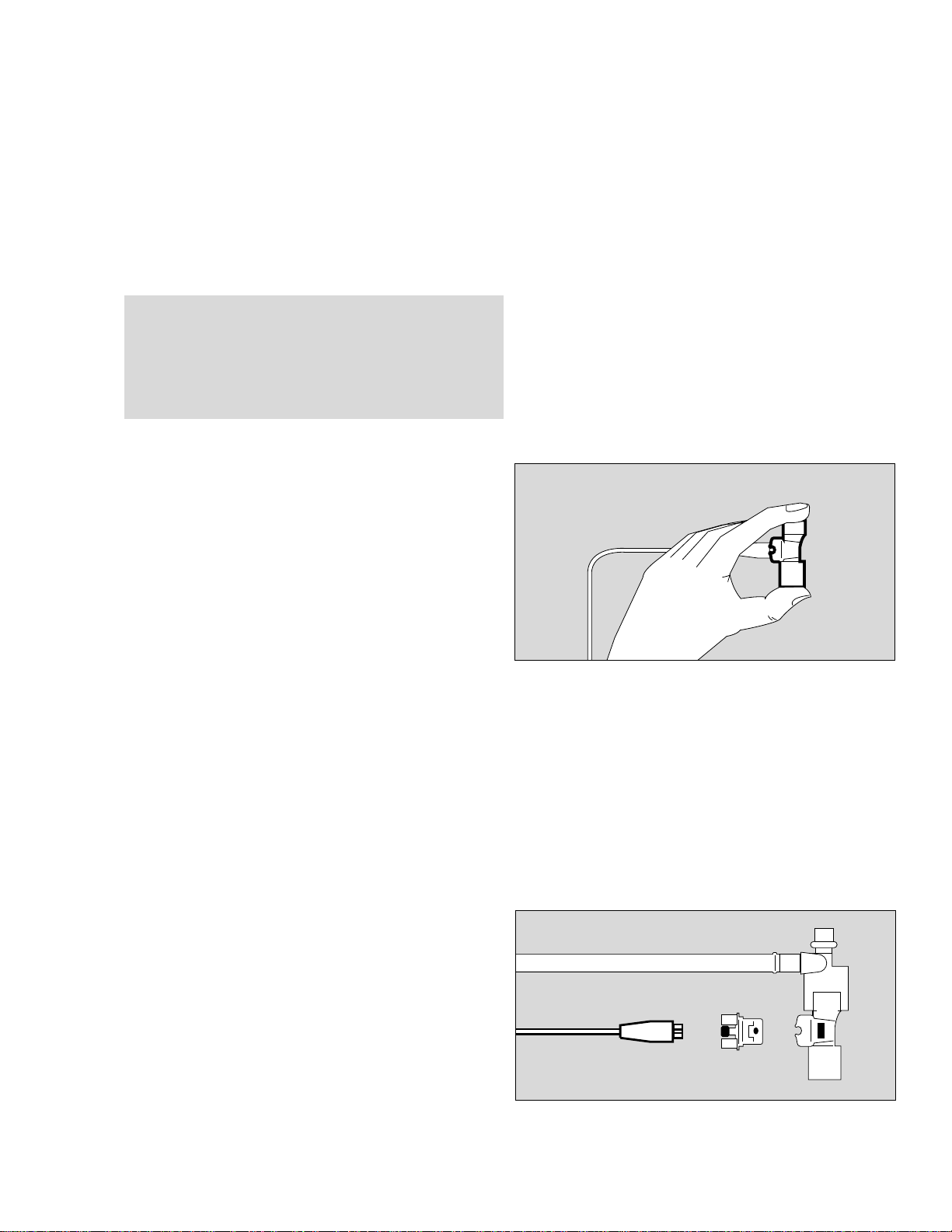
Calibration Procedure
● Disconnect neonatal flow sensor at ET-tube
connector and
● remove sensor from the wye.
● Connect patient ET-tube connector directly to the wye
for the duration of calibration.
WARNING !
The operator of the ventilator must still assume full responsibility for proper ventilation
while flow monitoring is not available during
calibration.
● Wearing a sterile glove, hold the neonatal flow sensor
so that both sides are sealed and flow = 0,
as required for calibration.
Preparation
Exchanging a Neonatal Flow Sensor Element
● Start calibration = press dial knob.
Calibration is completed after approx. 1 second.
If the message Calibration ok is displayed:
● Disconnect ET- tube connector from wye.
Re-install neonatal flow sensor in the wye.
Reconnect tube connector.
If calibration is unsuccessful:
● Repeat calibration. If necessary replace neonatal flow
sensor element. Check sensor lead.
Exchanging a Neonatal Flow Sensor Element
In the event of an error message:
Neo. flow measurement malfunction
1 Disconnect flow sensor cable from neonatal flow
sensor.
2 Press buttons on both sides while pulling flow sensor
element out of its housing.
Insert new sensor until it engages.
3 Be sure to line up oval markings engraved on flow
sensor element and sensor housing. Do not force
sensor element.
00829556
3
3
2
1
1 Reconnect flow sensor cable.
Operating Instructions Evita 4 / Evita 2 dura NeoFlow
● Calibrate neonatal flow sensor, see page 12.
00929556
13
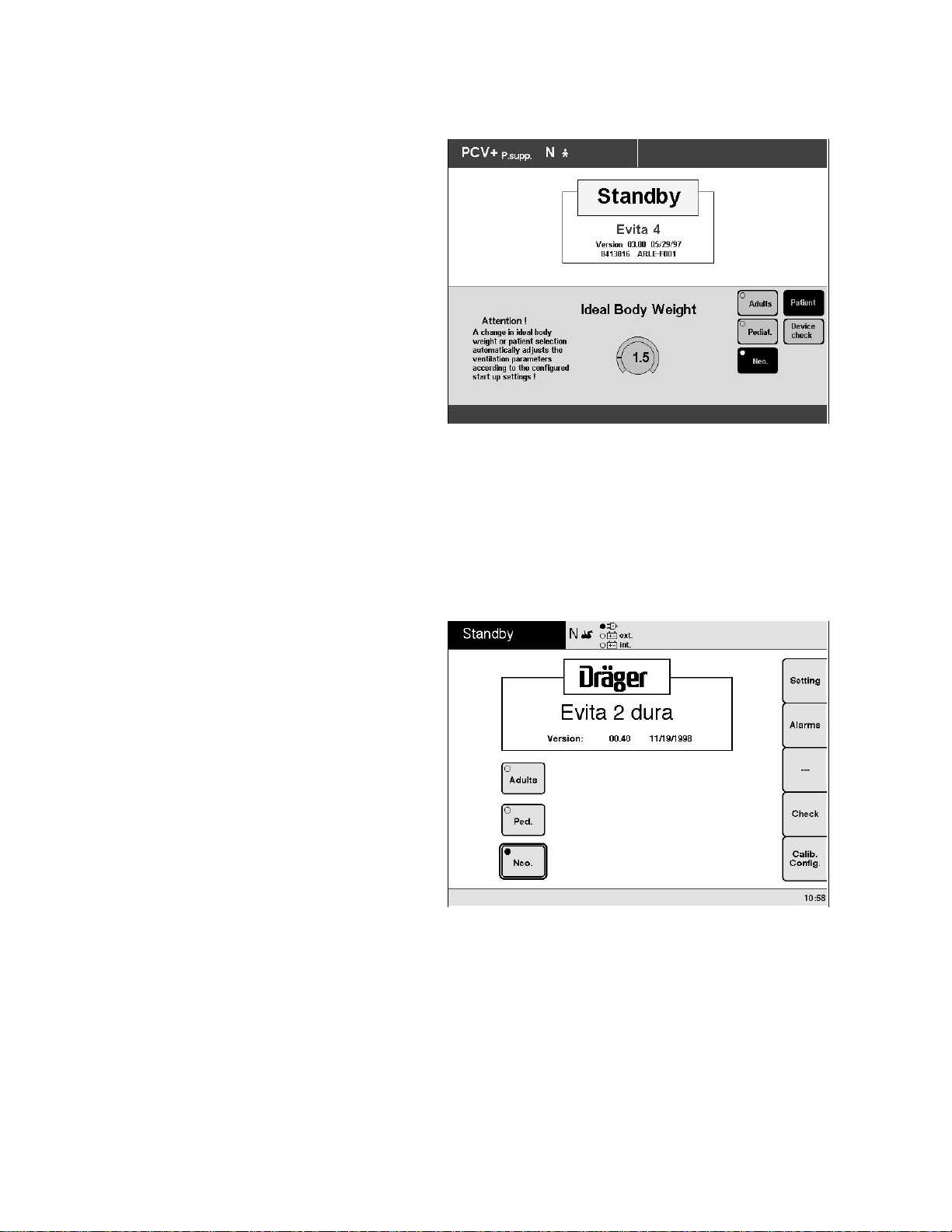
Operation
Selecting Neonatal Mode With Evita 4
Selecting Neonatal Mode With Evita 2 dura
Operation
Selecting Neonatal Mode With Evita 4
The appropriate patient mode can be selected immediately after switching the ventilator on or while in standby
mode. Mode selections from the Evita 4 menu are:
Adults = Adult
Ped. = Pediatric
Neo. = Neonatal
The patient ranges can be configured – see "Configuring
ventilation, Selecting the patient range" on page 24.
● Touch the »Neo.« screen key.
Display (example for neonatal mode):
In the top line of the screen, after the ventilation mode
identifier, the letter N = Neonatal mode is displayed.
Selecting Neonatal Mode With Evita 2 dura
The appropriate patient mode can be selected immediately after switching the ventilator on or while in standby
mode. Mode selections from the Evita 2 dura menu are:
Adults = Adult
Ped. = Pediatric
Neo. = Neonatal
The ranges for each mode in this menu can be
configured – see "Configuring Ventilation, Selecting the
Patient Range" on page 24.
● Select »Neo.« screen key = turn dial knob.
Display (example for neonatal mode):
00529563
In the top line of the screen, the letter N = Neonatal
mode is displayed.
14
Operating Instructions Evita 4 / Evita 2 dura NeoFlow
00629563
 Loading...
Loading...