Page 1

M317E 04.2.CF.2(1/2)
Microscope
ECLIPSE 50i
ECLIPSE 55i
Instructions
Page 2

Page 3
Introduction
Thank you for purchasing this Nikon product.
This instruction manual is intended for users of the Nikon ECLIPSE 50i and 55i microscopes.
To ensure correct use, please read this manual carefully before operating the product.
• This manual may not be reproduced or transmitted in whole or in part without Nikon's express consent.
• The contents of this manual are subject to change without notice.
• Although every effort has been made to ensure the accuracy of this manual, errors or inconsistencies
may remain. If you note any points that are unclear or incorrect, please contact your nearest Nikon
representative.
• Some of the products described in this manual may not be included in the set you have purchased.
• Make sure you have read the manuals for any other products attached to or to be used with this product
(super high-pressure mercury lamp power supply, high-intensity light source, etc.).
Warning/Caution symbols used in this manual
Although Nikon products are designed to provide the utmost safety, ignoring safety precautions or improper use may
result in personal injury or property damage, as well as voiding the terms of the warranty. To ensure safe use, please
read the instruction manual carefully and thoroughly before trying to operate the instrument. Do not discard this
manual. Store in a convenient location near the product for ready reference.
In this manual, safety precautions are indicated by the following symbols. For safe, correct use of the product, always
follow the instructions indicated by these symbols.
Symbol Meaning
WARNING
CAUTION
Disregarding instructions indicated by this symbol may result in death or serious
injury.
Disregarding instructions indicated by this symbol may result in injury or property
damage.
1
Page 4
Introduction
Meaning of symbols used on the product
When appearing on the product, the symbols below indicate the need for caution at all times during use.
Consult the instruction manual and read the relevant instructions before attempting to use or adjust any part to which
the symbol has been affixed.
Caution! Biohazard
This symbol found on the stage indicates the following:
•
WARNING: Contact between sample and the product may result in biohazard risks.
•
To avoid biohazard contamination, avoid touching the contaminated portion with bare hands..
•
Decontaminate the contaminated part according to the standard procedure specified for your
laboratory.
Caution for heat
This symbol found on the lamphouse of the ECLIPSE 50i indicates the following:
•
The lamp and surrounding areas (including the lamphouse) become very hot during and
immediately after a period of illumination.
•
Risk of burns. Do not touch the lamp or surrounding areas during or immediately after a
period of illumination.
•
Make sure the lamp and surrounding areas have cooled sufficiently before attempting to
replace the lamp
Caution
This symbol found on the wiring cover of the ECLIPSE 55i indicates the following:
• Always connect the specified battery and cables to the appropriate terminals. Failure to do so
may result in malfunction.
• Do not remove the wiring cover.
Do not remove the cover except for when assembling the product or replacing batteries.
Using the system without the cover may cause a short, resulting in abnormal heat.
Always use the product with the wiring cover on.
2
Page 5
Safety Precautions
Please follow the safety precautions given below.
1. Intended use of the product
2. Do not disassemble.
3. Read the instruction manuals carefully.
4. Power cord for ECLIPSE 50i and the power cord for AC adapter
WARNING
This product is intended primarily for microscopic observations and image capture of cells and tissue set
on glass slides using diascopic (transmitted) and episcopic (reflected) illumination.
It is intended for use in experimentation and observation of cells and tissue in the fields of pathology
and cytology in hospital and other laboratory settings.
Disassembly may result in malfunctions and/or electrical shock and will void the terms of the warranty.
Never attempt to disassemble any part other than the parts described in this manual. If you experience
problems with the product, contact your nearest Nikon representative.
To ensure safety, carefully read this manual and the manual provided with any other equipment used
with this product. Observe all warnings and cautions given at the beginning of each manual.
When the J-FL 50i55i Epi-fluorescence attachment is mounted on the product:
The mercury lamp (or xenon lamp) for Epi-fl microscopy requires special care during handling. Make
sure you have read the instruction manual for the light source (super high-pressure mercury lamp
power supply or high-intensity light source).
Use one of the power cords specified. Using the wrong power cord may result in fire or other hazards.
The product is classified as subject to Class I protection against electrical shock. Make sure it is
connected to an appropriate ground terminal.
Refer to Chapter 8 for the power cords specified.
To prevent electric shock, always turn off the main power switch (press it to the "{" position) of the
product before attaching or detaching the power cord.
5. Use the specified AC adapter (when using the ECLIPSE 55i) (when using J-CY cytodiagnostic
unit).
The ECLIPSE 55i and the J-CY cytodiagnostic unit are powered by an AC adapter. Use only the
specified adapter model meeting the requirements. Using any other type of adapter may result in
malfunction, overheating, and/or fire.
Refer to Chapter 8 for the adapter specified.
• To prevent malfunctions and/or fire, use the AC adapter in a well-ventilated location. To ensure
proper heat radiation and to prevent overheating, never cover or place any object on the adapter.
• To prevent malfunctions, always turn off the power switch (press it to the "{" position) of the
product before attaching the AC adapter.
3
Page 6
Safety Precautions
WARNING
6. Heat from the light source (when using the ECLIPSE 50i)
The lamp and surrounding areas (including the lamphouse) will become very hot during and
immediately after a period of illumination.
• Risk of burns. Never touch the lamp or surrounding areas during or immediately after a period of
illumination
• Always attach the lamphouse cover when using the product.
• Make sure the lamp and surrounding areas have cooled sufficiently before attempting to replace the
lamp
• To avoid risk of fire, do not place fabric, paper or highly flammable volatile materials such as
gasoline, petroleum benzine, paint thinner or alcohol near the lamphouse while the lamp is lit or
during a period of around thirty minutes after the lamp has been turned off.
7. Mercury lamps and xenon lamps (when the J-FL 50i55i Epi-fluorescence attachment is attached)
The mercury lamp (or xenon lamp) used for J-FL 50i55i Epi-fluorescence attachment requires special
care during handling. For safe and correct use of this system, carefully read the warnings below. Keep
in mind all potential hazards. Additionally, carefully read the manual for the super high-pressure
mercury lamp power supply (or high-intensity light source) and the manual (if provided) from the lamp
manufacturer, then follow the instructions given therein.
Hazards of mercury lamps and xenon lamps
1) When lit, mercury (and xenon) lamps radiate ultraviolet light that can damage the eyes and skin.
Direct viewing of the light may result in blindness.
2) The lamps contain sealed gas under very high pressure, pressure that increases when the lamp is on.
If the lamp is scratched, fouled, subjected to high external pressure or physical impact, or used
beyond its service life, the sealed gas may escape or the lamp may burst, resulting in gas inhalation,
injury from glass, or other injury.
3) When the lamp is lit, the lamp and surroundings will become extremely hot. Touching the lamp with
bare hands may result in burns; flammable materials placed near the lamp may ignite.
4) Using the wrong lamp type may result in accidents, including bursting of the lamp.
Safety is a top design priority for Nikon products. The preceding hazards should pose no danger as
long as the user observes all of the warnings and cautions given in the manuals, and uses the system
only for its intended purpose.
However, failure to heed the warnings and cautions given in the manuals, subjecting the system to
shock or impact, or attempting to disassemble the system may result in accidents and injury. Make
sure you are familiar with and adhere to all warnings and cautions.
8. Always turn off the lamp when changing filter cubes
(when the J-FL 50i55i Epi-fluorescence attachment is attached to the product).
When changing filter cubes, always turn off the light source of the Epi-fl attachment. Leaving the lamp
on may result in ultraviolet exposure.
9. Hazardous sample
This product is intended primarily for microscopic observations and image capture of cells and tissue
set on glass slides.
Check to determine whether a sample is hazardous before handling.
Handle hazardous samples according to the standard procedure specified for your laboratory. If the
sample is potentially infectious, wear rubber gloves and avoid touching samples. If contact occurs
between a sample and the product, decontaminate the contaminated portion according to the standard
procedure specified for your laboratory.
4
Page 7
Safety Precautions
1. Isolate the products from the power source during assembly, connection/disconnection of cords,
CAUTION
lamp replacement, and maintenance.
To prevent electric shock and/or malfunctions, always turn off the power switch(es) of the product
(press to the "{" position) and unplug the power cord from the wall outlet before assembly, connecting
or disconnecting of cords, lamp replacement, and cleaning of the product and the objective.
2. Lamp replacement precautions (when using the ECLIPSE 50i)
To avoid burns, wait at least 30 minutes after the lamp is turned off to give it sufficient time to cool.
To avoid electric shock or malfunctions, never attempt to replace the lamp without first turning off the
power switches for the product and the peripheral devices (press them to the "{" position) and
unplugging the power cord from the wall outlet.
Make sure the lamphouse cover is securely fitted to the lamphouse after lamp replacement. Never turn
on the lamp while the lamphouse cover is open. Do not break up used lamps; instead, dispose of them
as special industrial waste or as specified by local regulations.
3. Use the specified lamp (when using the ECLIPSE 50i).
The product's built-in power source is used for the halogen lamp that is a light source for the diascopic
illumination. A halogen lamp up to 6V-30W can be lit. Always use the specified halogen lamp. Using an
unspecified lamp may cause malfunctions.
Specified lamp: 6V-30W (PHILIPS 5761)
4. Avoid contact with water.
Never allow water to come into contact with the product, and keep the product away from liquids.
Splashing water onto the product may cause a short, resulting in malfunction or abnormal heating. If
water is splashed onto the product, immediately turn off the power switch (press to the "{" position)
and remove the power cord from the receptacle. Then wipe off moisture with a dry cloth or something
similar. If water enters the product, do not use; in this case, contact your nearest Nikon representative.
5. Do not place any object on top of the product.
Do not place any object on top of the product or cover it with a cloth or the like. The system
temperature will rise, resulting in malfunctions.
5
Page 8

Safety Precautions
6. Cautions on assembling, installing, and carrying the product
CAUTION
• Take care to avoid pinching your fingers or hands during product assembly.
• Scratches or fouling such as fingerprints on optical components (such as lens and filters) will
degrade microscope images. Be careful to avoid scratches or direct contact with the lens and filters.
• The main unit weighs about 9 kg. Grasp the main unit by the handle on the back of the product and
the recess at the base on the opposite side from the handle.
• Remove all attachments (if mounted) from the microscope before carrying the microscope.
• Do not install the product in a locker or cabinet.
7. Operating, transporting, and storage conditions
The product must be operated, transported, or stored under the following conditions. Using or storing
the product in hot, humid locations may result in mildew formation or condensation on lenses, imparing
performance or generating malfunction.
• Operating conditions: temperature (0 to 40°C), humidity (85% RH max., no condensation)
• Transporting/storage conditions: temperature (-20 to +60°C), humidity (90% RH max., no
condensation)
8. Use the product with the wiring cover (when using the ECLIPSE 55i).
Do not remove the wiring cover except when assembling the product or replacing batteries. Using the
system without the wiring cover may cause a short, resulting in abnormal heat.
9. Remove any covers from the product before switching on.
Do not use the product while covered with a cloth, etc., as this will result in abnormal heat and fire
hazards.
10. Caution concerning long, sustained observations
To relieve fatigue resulting from long observation sessions, limit continuous observations to one hour.
Take at least 10- to 15-minute breaks between observation sessions. Adjust the layout of other
equipment and the height of your chair.
11. Disposal of the product
To avoid biohazard risks, dispose of the product as contaminated equipment according to the standard
procedure specified for your laboratory.
6
Page 9
Safety Precautions
12. Rechargeable battery (when using the ECLIPSE 55i)
CAUTION
Use the Nikon EN-EL1 Li-ion rechargeable battery for the ECLIPSE 55i. Do not use any other type of
battery. Although the EN-EL1 is designed for use with Nikon digital cameras, it can also be used for the
ECLIPSE 55i.
Take the following precautions when handling the battery:
• Do not expose to open flames or excessive heat.
The battery may become hot, leak, or burst.
• Do not short circuit or disassemble.
The battery may become hot, leak, or burst.
• Always use the charger specified (e.g., MH-53).
If a different charger is used, the battery may become hot, leak, or burst.
• The EN-EL1 is designed specifically for Nikon digital cameras and the ECLIPSE 55i. Do not use it for
any other equipment.
The battery may become hot or leak.
• Do not carry or store with metallic objects such as necklaces or hairpins.
The battery may become hot, leak, or burst.
• Do not expose to direct sunlight, or leave inside a sun-heated car with all windows shut.
The battery may become hot, leak, or burst.
• Do not drop or expose to strong impact.
The battery may become hot, leak, or burst.
• Keep out of reach of small children.
The battery can be swallowed by small children. If swallowed, consult a physician immediately.
• Do not immerse in water or allow to become wet.
The battery may become hot or leak.
• Do not use if unusual features, such as discoloration or deformation, are present.
The battery may become hot or leak.
• Do not exceed the charging period, even if battery is not fully charged.
The battery may become hot or leak.
• Insulate the contacts with tape, etc., when disposing of the battery.
The battery may become hot, burst, or ignite on contact with other metals.
• Observe local waste disposal regulations upon disposal.
• Please read the instruction manuals supplied with the EN-EL1 and the battery charger.
7
Page 10

Notes on handling the product
1. Handle the product gently.
This product is a precision optical instrument and requires gentle handling. Avoid subjecting it to sudden
impact and shocks.
Even relatively minor impacts are capable of affecting the precision of the objective.
Notes on handling the product
2. Weak electromagnetic waves
The product emits weak electromagnetic waves. Do not install the product near precision electronic
devices to avoid degrading their performance. If the TV or radio reception is affected, move the TV or
radio farther from the product.
3. Scratches, dirt, and foreign particles on the lens
Scratches or fouling such as fingerprints on optical components (such as lens and filters) will degrade
microscope images. If these parts become dirty, clean them as described in chapter "7. Care and
maintenance" at the end of this manual.
4. Dirt on the lamps (when using the ECLIPSE 50i)
Never touch the lamp with bare hands. Dirt or fingerprints on the lamp will result in uneven illumination
and reduce the service life of the lamp. Always wear gloves when handling lamps.
5. Installation location
This product is a precision instrument. Use or storage in inappropriate environments may result in
malfunctions or poor performance. Consider the following factors when selecting an installation location:
• Select a vibration-free location. Install the product on a level surface.
• Install the product at least 10 cm away from walls.
• Choose a location less exposed to hazards in the event of collisions, earthquakes, or other potential
disasters. To keep the product from falling, use strong rope or other means if necessary to secure it
to the working desk or to another heavy, stable item.
• Avoid locations exposed to direct sunlight, locations immediately under room lights, and other bright
locations.
• Avoid locations with excessive dust.
• To avoid splashes, do not use the product near water.
• Make sure the ambient temperature is 0 to 40°C and humidity is 85% or less. Installing the product
in hot, humid locations may result in mildew formation or condensation, impairing performance or
generating malfunctions.
• Storage conditions for transportation are as follows: temperature (-20°C to +60°C), humidity (90%
RH max., no condensation)
• Do not install the product in a locker or cabinet.
• Select a layout that allows easy removal of the power cord from the product's AC inlet in the event of
an emergency.
• Room lights just above the product may enter the objective as extraneous light. If possible, switch
off room lights directly above the product when making observations.
• Do not use on a desk mat or the like.
8
Page 11

Notes on handling the product
6. Focusing knobs
• Never turn the focus knobs on the left and right sides of the product in opposite directions at the
same time. Doing so may damage the product.
• Turning the coarse focus knob past its farthest point will damage the product. Never use undue force
when turning the knob.
7. Protect the ports from dust and extraneous light (when the trinocular eyepiece tube or the C-TE
ergonomic binocular tube is attached).
To keep out extraneous light and dust, always attach the supplied cap to any port not currently in use.
8. Handling of filters (when J-FL 50i55i Epi-fluorescence attachment is attached to the product)
• Interference filters (especially excitation filters, which are exposed to strong light) degrade over time.
Replace them after the appropriate number of hours.
• Filter characteristics may alter if the filter is exposed to high humidity. To prevent changes in or
degradation of filter characteristics, avoid using or storing the filters under conditions of high humidity
or high temperature. Avoid subjecting filters to rapid temperature changes. When a filter is not in use,
store in a desiccator or hermetically sealed container with a drying agent.
• The filters in the nine types of filter cubes listed below offer sharp, high-resolution waveform
characteristics superior to normal filters. However, due to their sophisticated coatings, they must be
handled with special care. In particular, take care to avoid abrasion from cleaning. (Follow the
procedure described in section "1. Filter and lens cleaning" of chapter "7. Care and Maintenance.")
Single-band filter cubes: DAPI, FITC, TxRed, GFP
Multi-band filter cubes: F-R, F-T, D-F, D-F-R, D-F-T
9
Page 12
Abbreviations Used in This Manual
Abbreviations Used in This Manual
The product names and abbreviations used in this manual are given below.
The manual uses the following abbreviations:
Name of device
Microscope ECLIPSE 50i
Microscope ECLIPSE 55i
C-ER Eye Level Riser
C-TE Ergonomic Binocular Tube
C-TEP DSC Port for Ergonomic Binocular Tube
J-FL 50i55i Epi-Fluorescence Attachment
J-CY Cytodiagnostic Unit
C-HS Hand switch
DS Camera Head DS-5M
DS Camera Control Unit DS-L1
50i
55i
Eye Level Riser
Ergonomic Binocular Tube
DSC Port
Epi-fl Attachment
Cytodiagnostic Unit
Hand Switch
Camera Head
DS-L1
Abbreviation
DS Camera Cable
Super High Pressure Mercury Lamp Power Supply
Super High Pressure Mercury Lamphouse
Camera Cable
Mercury Lamp Power Supply
Mercury Lamphouse
10
Page 13

Contents
Contents
Introduction ....................................................................................................... 1
Warning/Caution symbols used in this manual ..................................................................1
Meaning of symbols used on the product ......................................................................... 2
Safety Precautions .............................................................................................. 3
WARNING.................................................................................................... 3
CAUTION..................................................................................................... 5
Notes on handling the product .............................................................................. 8
Abbreviations Used in This Manual........................................................................10
Chapter 1 Part Names.........................................................................................14
1.1 Names of Main Components ............................................................................... 14
1.2 Names of Parts Used to Make Adjustments........................................................... 15
1.2.1 Right view ................................................................................................ 15
1.2.2 Left view .................................................................................................. 16
1.2.3 Rear view (50i).......................................................................................... 17
1.2.4 Rear view (55i).......................................................................................... 18
1.2.5 Ergonomic binocular tube with camera attached ............................................ 19
1.2.6 With cytodiagnostic unit attached ................................................................ 20
1.2.7 With Epi-fl attachment mounted .................................................................. 21
Chapter 2 Microscopy ......................................................................................... 22
2.1 Bright-Field Microscopy ...................................................................................... 22
2.2 Microscopy with Cytodiagnostic Unit Attached ....................................................... 26
2.3 Microscopy with Epi-fl Attachment Mounted .......................................................... 29
2.4 Photomicroscopy ............................................................................................... 32
Chapter 3 Individual Operations ...........................................................................34
3.1 Power ON/OFF .................................................................................................. 35
3.1.1 Microscope................................................................................................ 35
3.1.2 Cytodiagnostic unit .................................................................................... 35
3.1.3 Light source of the Epi-fl attachment (mercury lamp)..................................... 35
3.2 Brightness Adjustment....................................................................................... 36
3.2.1 Adjustment using the brightness control knob ............................................... 36
3.2.2 Adjustment using the preset switch ............................................................. 37
3.2.3 Adjustment with the ND filter IN/OUT lever (for 50i) ...................................... 37
3.2.4 Automatic adjustment after magnification change
(only for a 55i when a cytodiagnostic unit is attached) ................................... 38
3.2.5 Adjustment with the ND filter of the Epi-fl attachment.................................... 38
3.2.6 Transmitted image in fluorescence observation.............................................. 38
3.2.7 Camera adjustment (adjusting the brightness of the image on the monitor)...... 39
3.3 Optical Path Switching ....................................................................................... 39
3.3.1 Optical path distribution ............................................................................. 39
3.3.2 Disabling the clicking of the optical path switching lever ................................. 39
3.4 Vertical Stage Motion......................................................................................... 40
3.4.1 Prohibited actions ...................................................................................... 40
3.4.2 Knob rotation direction and stage motion direction ........................................ 40
3.4.3 Number of knob turns and distance of stage travel ........................................ 40
11
Page 14

Contents
3.4.4 Adjusting the rotating torque of the coarse focus knob ................................... 41
3.4.5 How to refocus .......................................................................................... 41
3.5 XY Stage Motion ............................................................................................... 42
3.5.1 Prohibited action........................................................................................ 42
3.5.2 Knob rotation direction and stage motion direction ........................................ 42
3.5.3 Adjusting the knob heights ......................................................................... 42
3.5.4 Adjusting the knob rotation torque............................................................... 42
3.6 Diopter Adjustment ........................................................................................... 43
3.7 Interpupillary Adjustment .................................................................................. 44
3.8 Adjusting the Observation Position ...................................................................... 44
3.9 Adjusting the Condenser Position ........................................................................ 45
3.10 Adjusting the Aperture Diaphragm ...................................................................... 46
3.10.1 Adjusting the aperture diaphragm opening using the condenser scale .............. 46
3.10.2 Adjusting the aperture diaphragm opening using the centering telescope
(optional) ................................................................................................. 46
3.11 Selecting a Condenser ....................................................................................... 47
3.12 Adjusting the Field Diaphragm ............................................................................ 47
3.13 Oil Immersion Operation .................................................................................... 48
3.14 Water Immersion .............................................................................................. 49
3.15 Using the Cytodiagnostic Unit ............................................................................. 50
3.15.1 Magnification switching............................................................................... 50
3.15.2 Marking specimens .................................................................................... 50
3.16 Fluorescence Observation................................................................................... 51
3.16.1 Warning.................................................................................................... 51
3.16.2 Shutter of the Epi-fl attachment .................................................................. 51
3.16.3 Light shielding plate of the Epi-fl attachment................................................. 51
3.16.4 Field diaphragm of the Epi-fl attachment ...................................................... 51
3.16.5 Switching excitation methods ...................................................................... 52
3.16.6 Inserting and removing the filter cubes ........................................................ 52
3.16.7 ND filters of the Epi-fl attachment................................................................ 53
3.17 Selecting Fluorescent Filters ............................................................................... 54
3.17.1 Selecting excitation filters (EX filters)........................................................... 55
3.17.2 Selection of barrier filter (BA filter) .............................................................. 55
3.17.3 Replacing excitation and barrier filters .......................................................... 57
3.17.4 Filter cube internal spacers ......................................................................... 57
3.18 Image Capture ................................................................................................. 58
3.18.1 Adjusting light intensity .............................................................................. 58
3.18.2 Adjusting the condenser ............................................................................. 58
3.18.3 Confirming the photomicrographic range ...................................................... 58
3.18.4 Confirming focus ....................................................................................... 58
3.18.5 Making adjustments to keep out extraneous light .......................................... 59
3.18.6 Anti-vibration measures.............................................................................. 59
3.18.7 Fluorescence photomicrography................................................................... 59
Chapter 4 Assembly ...........................................................................................60
Chapter 5 Replacing Consumables ........................................................................71
5.1 Replacing the lamp (for the 50i).......................................................................... 71
5.2 Recharging the battery (for the 55i) .................................................................... 72
12
Page 15

Contents
5.3 Refilling Cytodiagnostic Unit Ink .......................................................................... 73
Chapter 6 Troubleshooting...................................................................................74
6.1 Optical............................................................................................................. 74
6.2 Operational ...................................................................................................... 75
6.3 Electrical .......................................................................................................... 75
Chapter 7 Care and Maintenance..........................................................................76
7.1 Lens Cleaning ................................................................................................... 76
7.2 Cleaning the Product ......................................................................................... 76
7.3 Disinfecting the Product ..................................................................................... 76
7.4 Storage............................................................................................................ 77
7.5 Periodic Inspections (fee charged)....................................................................... 77
Chapter 8 Technical Specifications ........................................................................78
8.1 Specifications ................................................................................................... 78
13
Page 16
y)
1
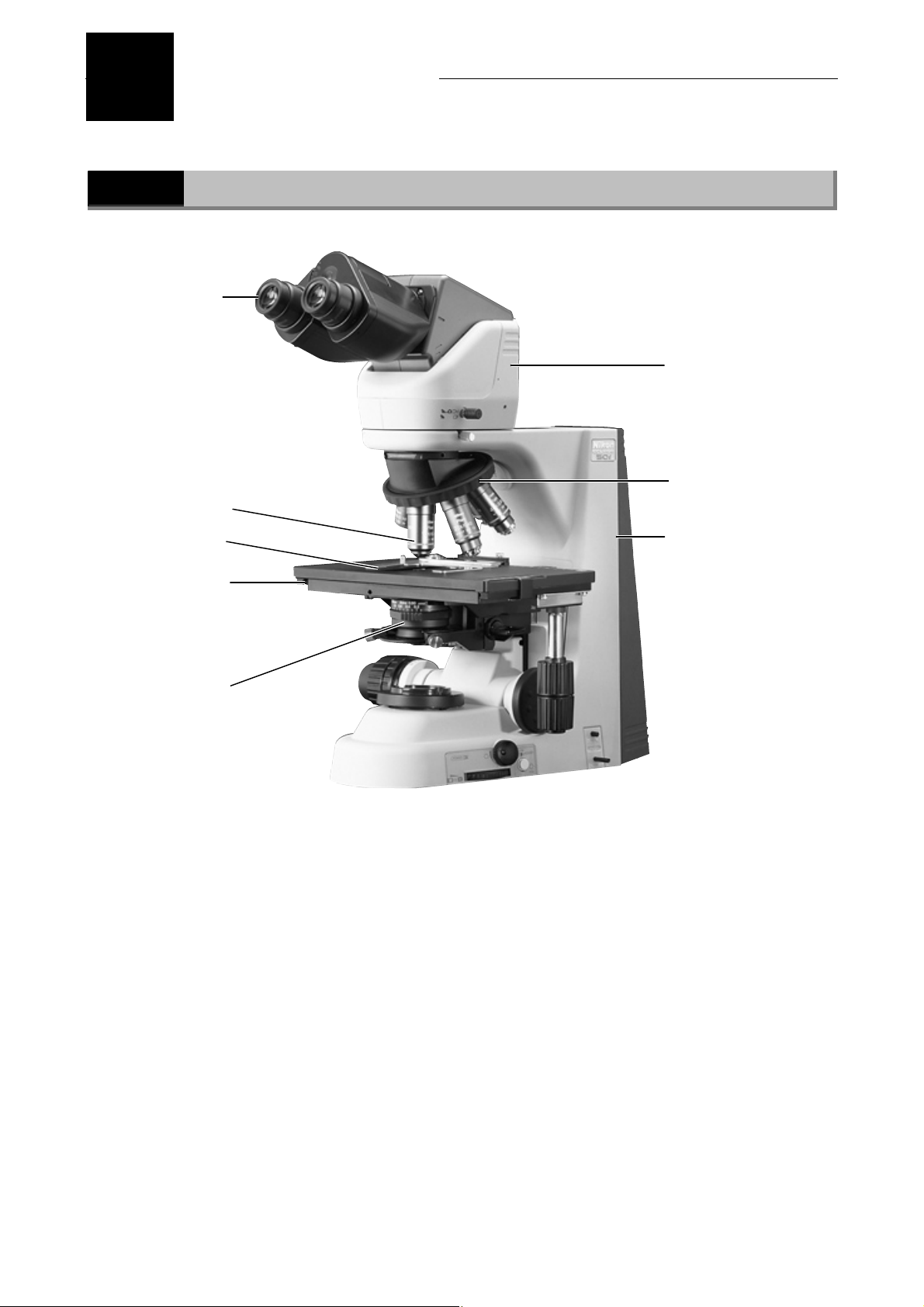
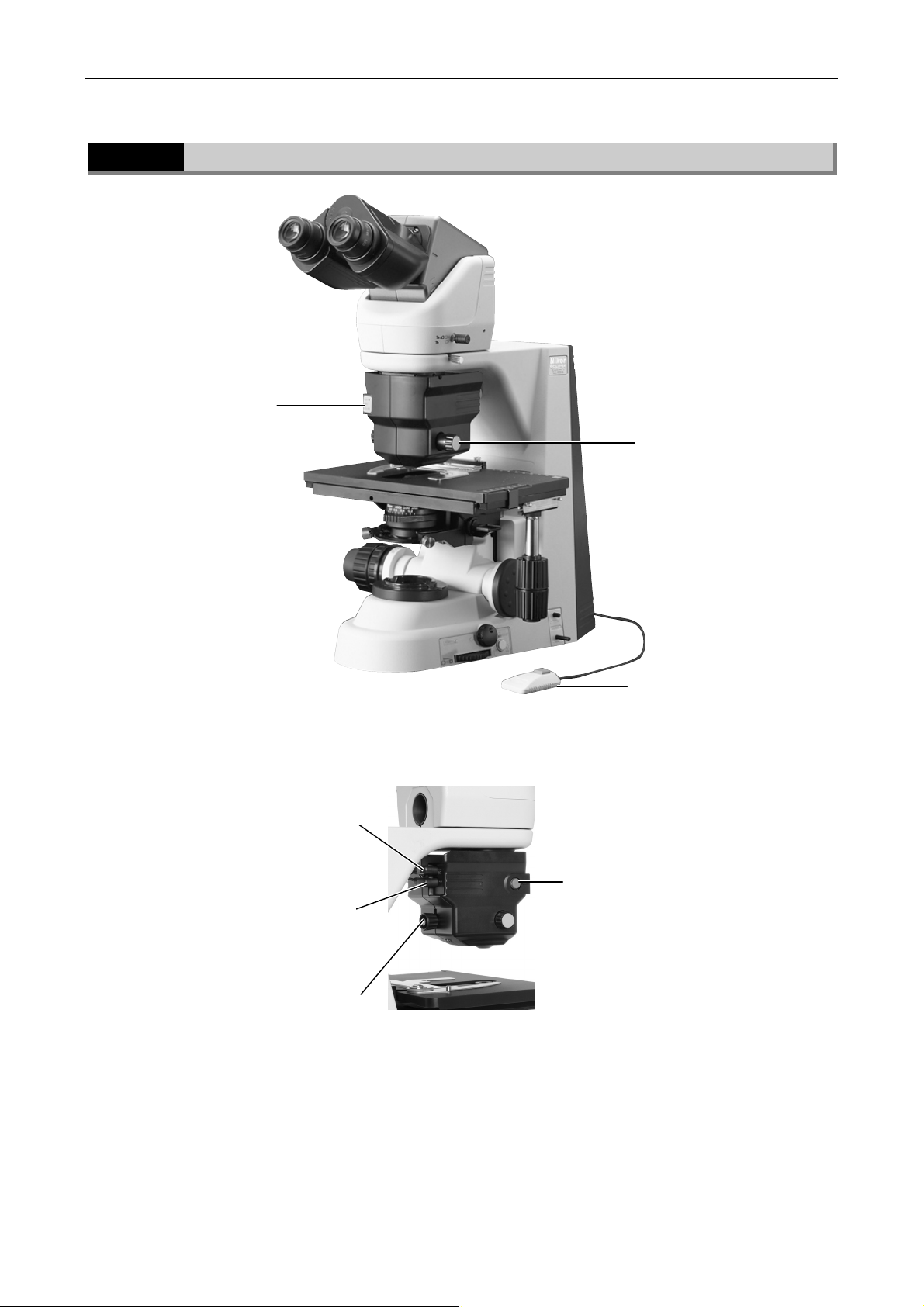
Part Names
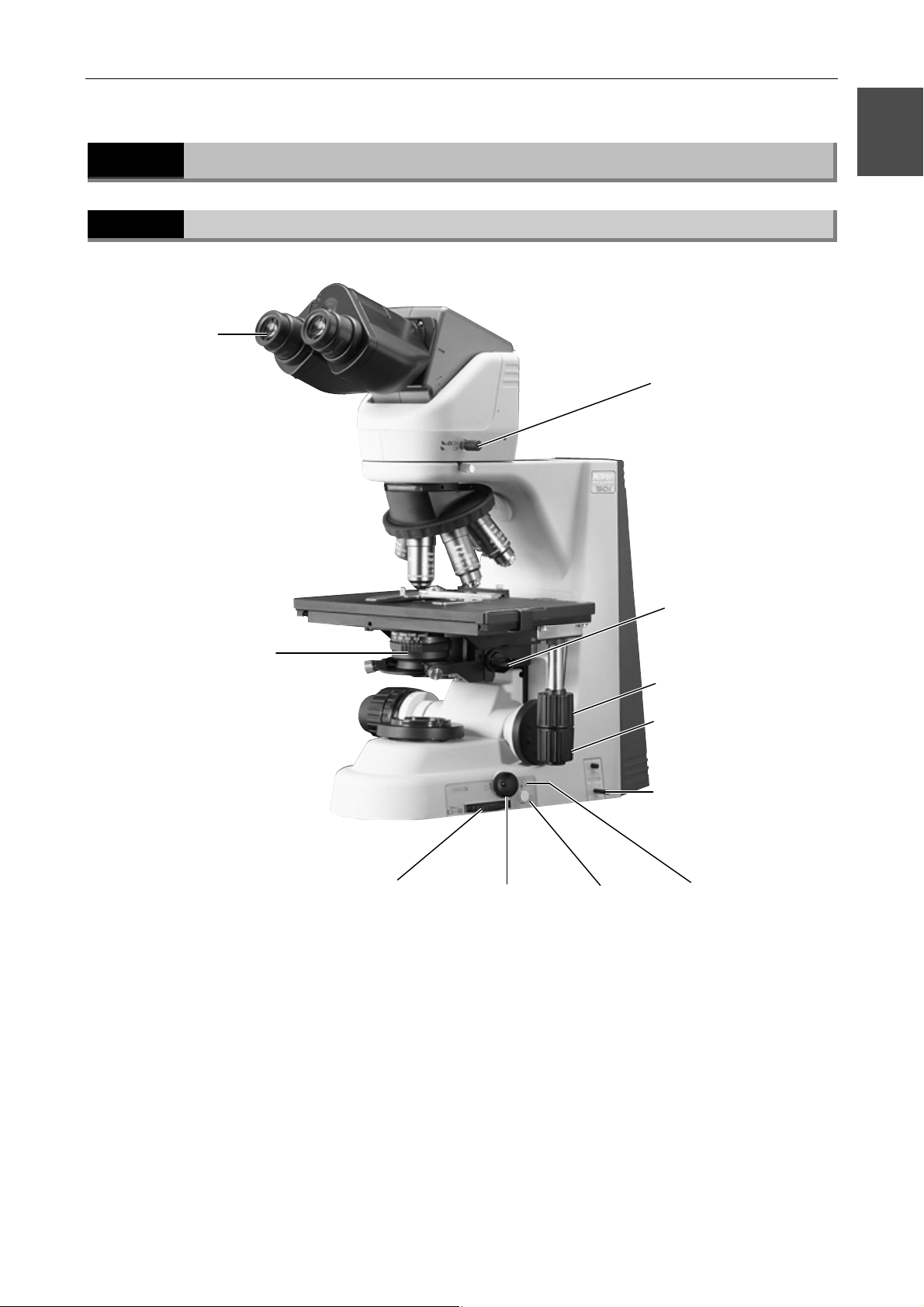
1.1
Specimen holder
Names of Main Components
Eyepiece
Eyepiece tube
Revolving nosepiece
Objective
Microscope (main bod
Stage
Condenser
14
Page 17
Chapter 1 Part Names
t
g
1.2 Names of Parts Used to Make Adjustments
1.2
1.2.1
Names of Parts Used to Make Adjustments
Right view
1
Diopter adjustmen
rin
Aperture diaphragm
knob
Optical path
switching lever
Refocusing lever
Y stage knob
X stage knob
50i:
ND filter IN/OUT lever
55i:
Color compensating filter
IN/OUT lever
Field diaphragm
knob
Brightness
control knob
Preset
switch
Preset brightness
control knob
15
Page 18

Chapter 1 Part Names
1.2 Names of Parts Used to Make Adjustments
1.2.2
Condenser focus knob
Coarse focus torque
Coarse focus knob
Left view
adjustment knob
Fine focus knob
Power switch
Condenser centering
screws
16
Page 19
Chapter 1 Part Names
1.2 Names of Parts Used to Make Adjustments
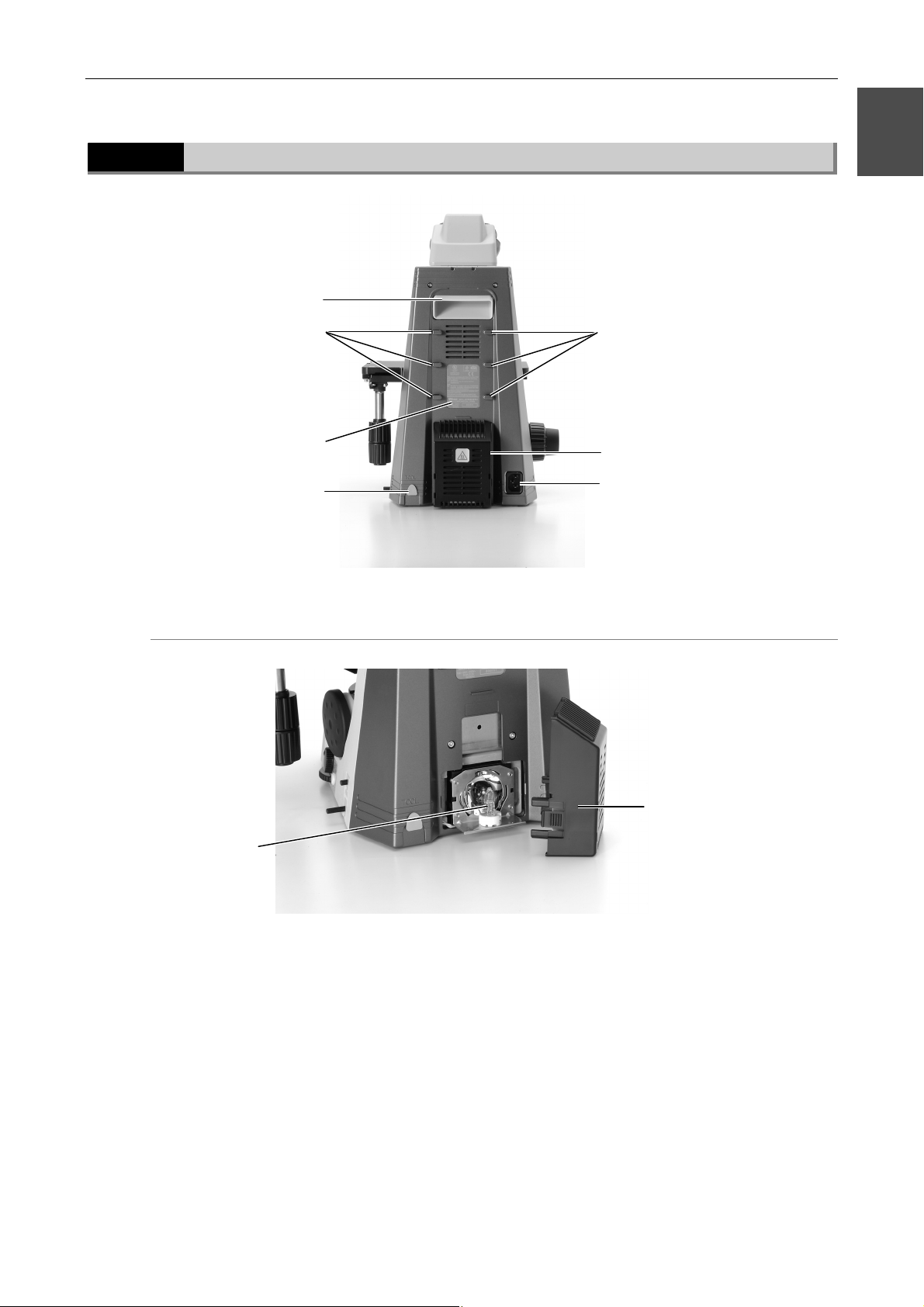
1.2.3
Rear view (50i)
Handle
1
Wiring guides
Input voltage indication
Tool
50i with lamphouse cover open
Wiring guides
Lamphouse
AC inlet
Lamphouse cover
Halogen lamp
17
Page 20
Chapter 1 Part Names
(
g
)
(
)
t
rcy
t
1.2 Names of Parts Used to Make Adjustments
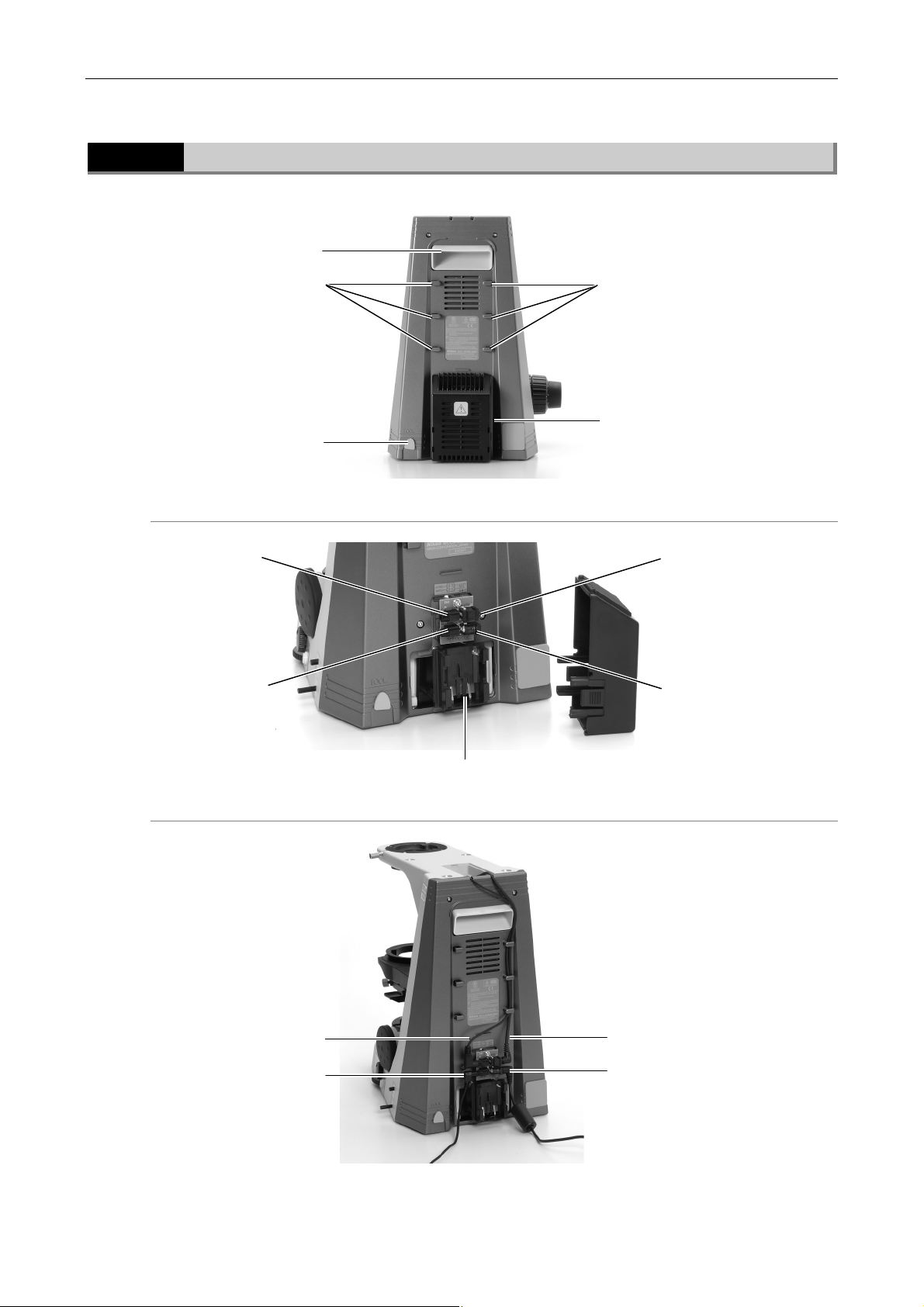
1.2.4
Rear view (55i)
Handle
Wire guides
Tool
Wire guides
Wiring cover
55i with wiring cover open
Connector J4
for cytodiagnostic
unit si
nal cable
Connector J2
(for cytodiagnostic unit
power supply cable)
Connector J3
for cytodiagnostic
unit hand switch
Battery holder
Connector J1
(for AC adapter)
55i with cables connected
Cytodiagnostic uni
signal cable
Cable fo
todiagnostic uni
hand switch
Cytodiagnostic unit
power supply cable
AC adapter cable
18
Page 21
Chapter 1 Part Names
1.2 Names of Parts Used to Make Adjustments
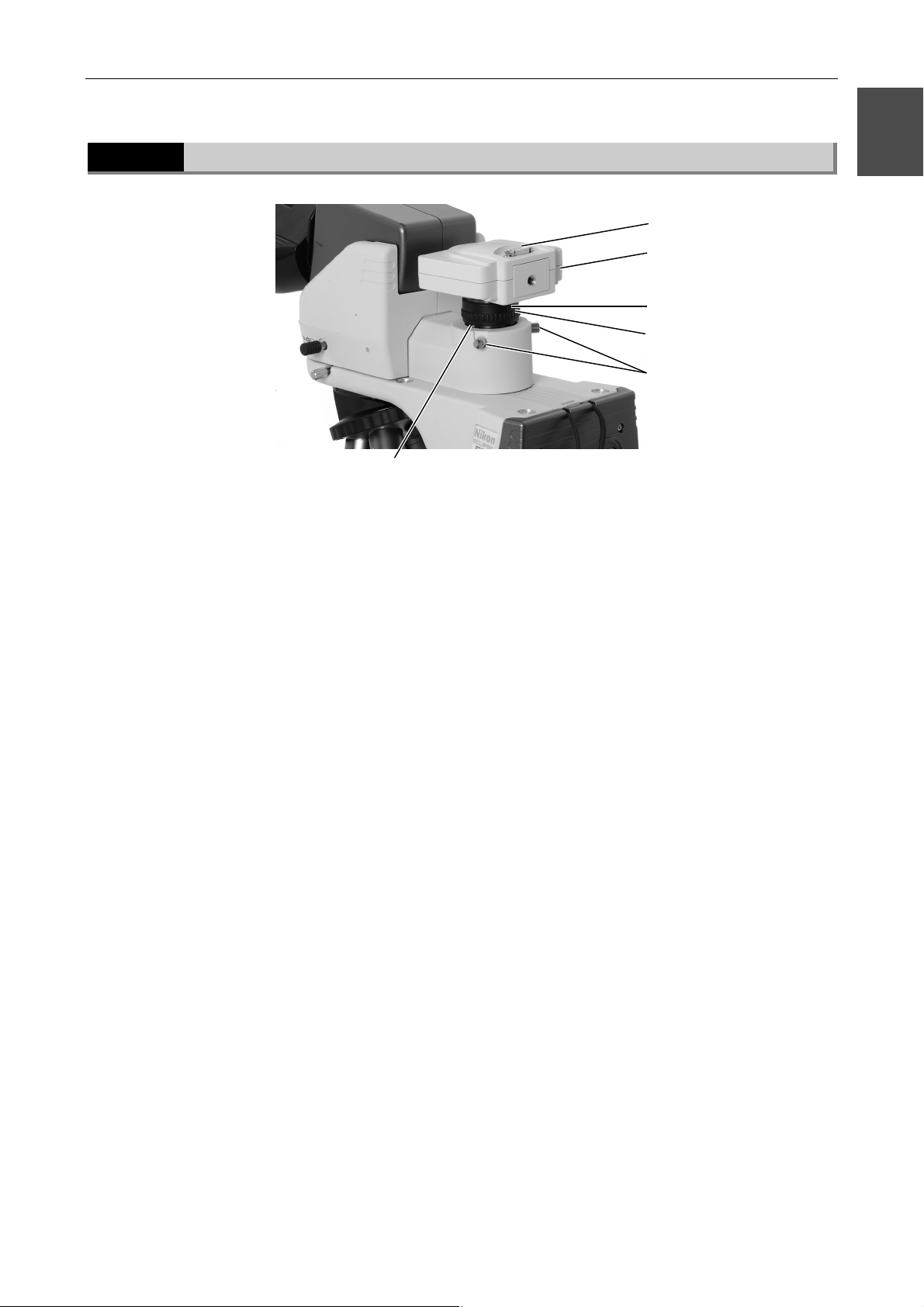
1.2.5
Ergonomic binocular tube with camera attached
Camera cable connector
Camera head
C mount
Camera fine focus
adjustment ring
Camera centering screws
1
Attachment guide fixing screw
19
Page 22
Chapter 1 Part Names
r
1.2 Names of Parts Used to Make Adjustments
1.2.6
Magnification indicato
With cytodiagnostic unit attached
Object marker knob
Hand switch
Side and rear views of cytodiagnostic unit
AC adapter cable (thick)
J-CY/PS Power supply cable (thick)
Hand switch cable (thin)
J-CY/SG Signal cable (thin)
Ink cartridge
50i:
55i:
Cytodiagnostic unit power switch
50i:
55i:
20
Page 23
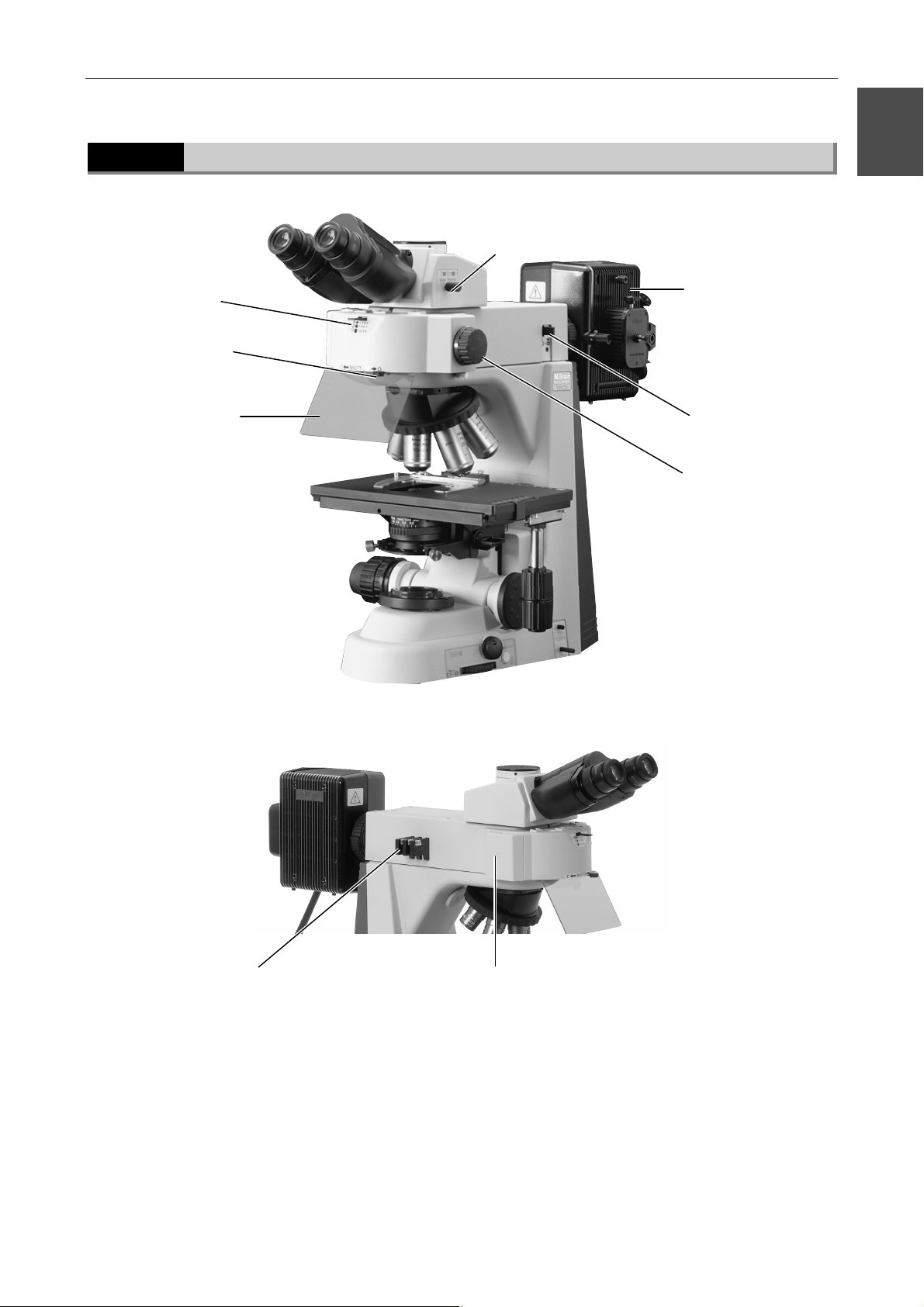
Chapter 1 Part Names
1.2 Names of Parts Used to Make Adjustments
1.2.7
With Epi-Fl attachment mounted
Optical path switching lever
1
Filter cube motion
restricting lever
Light shielding plate
Mercury lamphouse
Shutter
Field diaphragm lever
Filter cube switching
knob
(Mercury lamp requires a separate mercury lamp power supply.)
ND filters
Filter cube replacement cover
21
Page 24

p
p
2
Microscopy
2.1
1
Bright-Field Microscopy
The ECLIPSE 55i with the low magnification objective may result in uneven illumination in the
field of view.
(If the cytodiagnostic unit is attached, refer to the directions in the following section entitled “2.2
Microscopy with Cytodiagnostic Unit Attached.”)
Turn on power.
⇒ P.35
Press the power switch to
the “|” position.
Raise the condenser to the
2
uppermost position.
Fully open the field diaphragm and
3
Raise the condenser using the
condenser focus knob.
aperture diaphragm.
Fully open the
erture diaphragm
a
using the aperture
diaphragm knob.
Fully open the field
diaphragm using
the field dia
knob.
hragm
22
Page 25

Chapter 2 Microscopy
2.1 Bright-Field Microscopy
Set the 10x objective into the
4
optical path.
Select
the 10x
objective.
Set a specimen and move the
5
portion to be viewed into the
optical path.
⇒ P.42
Set a specimen
and secure in
place using the
specimen holder.
2
Move the portion to be viewed into the
optical path using the XY stage knobs.
Focus on the specimen.
6
⇒ P.40
Focus on the specimen using
the coarse and fine focus knobs.
23
Page 26

Chapter 2 Microscopy
2.1 Bright-Field Microscopy
Adjust the diopter and the
7
interpupillary distance.
⇒ P.43
⇒ P.44
Focus and center the condenser.
8
⇒ P.45
Focus the condenser
using the condenser
focus knob.
Switch to the desired objective
9
Center the condenser
using the condenser
centering screws.
and view the specimen.
Select the
Adjust the field diaphragm and
desired
objective.
aperture diaphragm each time you
change objectives.
⇒ P.46
⇒ P.47
Aperture diaphragm
knob
Field diaphragm
knob
24
Page 27

Chapter 2 Microscopy
2.1 Bright-Field Microscopy
10
Turn off power after completing
observation.
2
Press the power switch to
the “◯” position.
25
Page 28

Chapter 2 Microscopy
p
p
2.2 Microscopy with Cytodiagnostic Unit Attached
2.2
1
Microscopy with Cytodiagnostic Unit Attached
Turn on power.
⇒ P.35
Insert the cytodiagnostic
unit power switch.
Raise the condenser to the
2
Press the switch to
the “|” position.
uppermost position.
Raise the condenser using
the condenser focus knob.
Fully open the field diaphragm and
3
aperture diaphragm.
Fully open the
erture diaphragm
a
using the aperture
diaphragm knob.
Fully open the field
diaphragm using
the field dia
knob.
hragm
26
Page 29

Chapter 2 Microscopy
g
g
2.2 Microscopy with Cytodiagnostic Unit Attached
Set a specimen and move the
4
portion to be viewed into the
optical path.
⇒ P.42
Focus on the specimen.
5
Set a specimen
and secure in
place using the
specimen holder.
Move the portion to be viewed into the
optical path using the XY stage knobs.
2
⇒ P.40
Focus on the specimen using
the coarse and fine focus knobs.
Adjust the diopter and
6
interpupillary distance.
⇒ P.43
⇒ P.44
Focus and center the condenser.
7
⇒ P.45
Focus the
condenser using
the condenser
focus knob.
Center the
condenser usin
condenser centerin
screws.
the
27
Page 30

Chapter 2 Microscopy
2.2 Microscopy with Cytodiagnostic Unit Attached
Switch magnification using the
8
hand switch.
Adjust the field diaphragm and
aperture diaphragm for optimal
image quality.
⇒ P.50
⇒ P.46
⇒ P.47
Contrast may be reduced when viewing certain
specimens at 40× magnification. If this happens,
reduce magnification to 10× and stop down the
field diaphragm as far as possible. This will
minimize contrast loss.
To mark the specimen, follow the
9
procedure given below:
(1) Holding the object marker knob with both
hands, push it from the right toward the left,
then turn it toward the back to extend the
marker.
(2) Gently press down the entire object marker
knob to apply a mark.
(3) Turn the object marker knob toward the front
to retract the marker.
⇒ P.50
(4) Release both hands from the object marker
knob.
Aperture
diaphragm
knob
Object marker
Field
diaphragm
knob
(3) Turn the knob
to the front to
retract the
marker.
Select the
magnification using
the hand switch.
(2) Gently press down
(1) Push the knob
from the right
to the left, then
turn it to the
back.
the knob to mark
the specimen.
10
Turn off all power switches after
completing observations.
28
Press the cytodiagnostic unit
power switch to return the switch
to the extended position.
Press the power switch
to the “◯” position.
Page 31

Chapter 2 Microscopy
2.3 Microscopy with Epi-fl Attachment Mounted
2.3
1
2
Microscopy with Epi-fl Attachment Mounted
Before microscopy
• Check the cumulative operating hours of the mercury lamp. Replace the lamp if
its cumulative operating hours exceed the average service life.
• Use non-fluorescent slide glass.
• Use non-fluorescent immersion oil.
• To keep the specimen color from fading, keep the shutter closed when not
performing microscopy.
Perform steps 1 through 10 in “2.1 Bright-Field Microscopy.”
Turn off the microscope power
2
switch.
Press the power switch
to the “◯” position.
Close the shutter and block the
3
light emitted by the mercury lamp.
⇒ P.51
Insert the desired excitation filter
4
Close
the shutter.
cube into the optical path.
⇒ P.52
Select a cube using the filter
cube switching knob.
29
Page 32

Chapter 2 Microscopy
2.3 Microscopy with Epi-fl Attachment Mounted
Fully open the field diaphragm of
5
the Epi-fl attachment.
⇒ P.51
Turn on the mercury lamp, then
6
Fully open the field diaphragm.
open the shutter and center the
lamp. (Refer to the operating
manual for the light source.)
Set the 10x objective into the
7
optical path.
Select the
10x objective.
Set a specimen and move the
8
portion to be viewed into the
optical path.
⇒ P.42
Focus on the specimen.
9
Set a specimen
and secure in
place using the
specimen
holder.
Move the portion to be viewed into the
optical path using the XY stage knobs.
⇒ P.40
Focus on the specimen using
the coarse and fine focus knobs.
30
Page 33

Chapter 2 Microscopy
2.3 Microscopy with Epi-fl Attachment Mounted
10
11
Switch to the desired objective
Use the ND filters to adjust brightness.
and view the specimen.
• Refocus.
• Use the ND filters of the Epi-fl attachment to
adjust brightness.
• Adjust the field diaphragm so that it extends
slightly beyond the field of view.
• When using an oil immersion type objective,
apply immersion oil between the specimen
and the objective.
Select the desired objective.
2
⇒ P.51
⇒ P.48
To return to bright-field
microscopy.
• Close the shutter of the Epi-fl attachment and
block the light emitted by the mercury lamp.
Close the
shutter.
12
• Turn on the microscope power switch to turn
on the diascopic light source.
• Turn the filter cube switching knob and move
the position without a filter cube into the
optical path.
Press the switch to
the “|” position.
Turn off all power switches after
completing observations.
Press the power switch to
the “◯” position.
31
Page 34

Chapter 2 Microscopy
(A)
(B)Adj
2.4 Photomicroscopy
2.4
1
2
Photomicroscopy
For detailed discussions of the camera, photomicroscopic software, and PC, refer to the
operating manuals provided with the respective products. The following instructions assume a
DS camera head DS-5M and DS-L1 camera control unit.
Adjust the microscope for proper image observation.
See the directions given in sections from “2.1 Bright-Field Microscopy” to “2.3 Epi-fluorescence
Microscopy.”
Adjust the camera head attaching position until the image is
displayed properly.
(1) Adjustment based on stage motion
direction
Loosen the attachment guide fixing screw on
the C mount and adjust the camera position
so that moving the stage left-right moves the
image on the monitor in the opposite
direction. After making the appropriate
adjustments, tighten the screws firmly.
(2) Focus adjustment
If the image viewed through the eyepiece
appears to be in focus but the image on the
monitor is out of focus, turn the camera fine
focus adjustment ring on the C mount until
the image on the monitor is in focus.
Note that such out of focus situations can also
indicate incorrect diopter adjustment. Make
sure you have made diopter adjustments as
well. (Refer to P.43.)
(3) Centering the camera
Turn the right and left camera centering
screws to align the image seen through the
eyepiece with the image on the monitor.
Make camera settings.
3
For detailed discussion, refer to the operating manual provided with the camera.
When using the DS-L1, you must choose and enter at least the following information:
• Folder for data storage.
• Name of file to be saved. (You can select “Auto.”)
Attachment guide
fixing screw
usting (A) and (B)
Camera
fine focus
adjustment ring
Camera centering screws
• File format and file size.
• Date and destination of data
32
Page 35

Chapter 2 Microscopy
2.4 Photomicroscopy
Select the camera scene mode suitable for the microscopy
4
method.
Set the camera white balance.
5
To adjust white balance, press the WB button while capturing an image of a clear section of a
specimen slide. (For fluorescent photomicrography, adjust white balance under normal lighting
conditions before shooting.)
Capture and save images.
6
Position the specimen.
Refocus.
Adjust image brightness using the camera exposure compensation function.
Check the image using the Freeze button.
If the image is acceptable, press the CAPT. button to save the image.
2
(The operating procedure differs if DF/FL scene mode is selected. For detailed discussion, refer
to the operating manual provided with the camera.)
33
Page 36

3
Item Title Operating sections
3.1 Power ON/OFF Power switch, battery, AC adapter
3.2 Brightness Adjustment Brightness control knob, preset switch, ND filter, color
3.3 Optical Path Switching Optical path switching knob
3.4 Vertical Stage Motion Coarse/fine adjustment knobs, coarse torque adjustment
3.5 XY Stage Motion X knob, Y knob, XY knob torque adjustment screws
3.6 Diopter Adjustment Diopter adjustment rings
3.7 Interpupillary Adjustment Eyepiece sleeve
3.8 Adjusting the Observation Position Ergonomic binocular tube
3.9 Adjusting the Condenser Position Condenser focus knob, condenser centering screws
3.10 Adjusting the Aperture Diaphragm Condenser aperture diaphragm, objective
Individual Operations
compensating filter
ring, refocusing lever
3.11 Selecting a Condenser Condenser
3.12 Adjusting the Field Diaphragm Field diaphragm knob
3.13 Oil Immersion Operation Oil immersion objectives, oil immersion condensers
3.14 Water Immersion Water immersion objectives, water immersion condensers
3.15 Using the Cytodiagnostic Unit Cytodiagnostic unit, hand switch
3.16 Fluorescence Observation Epi-fl attachment
3.17 Selecting Fluorescent Filters Filter cube
3.18 Image Capture Camera
34
Page 37

Chapter 3 Individual Operations
3.1 Power ON/OFF
3.1
3.1.1
3.1.2
Power ON/OFF
Microscope
To turn on the microscope, press the power switch
to the “|” position.
To turn off the microscope, press the power switch
to the “◯” position.
Cytodiagnostic unit
Depress the power switch to turn on the
cytodiagnostic unit.
Turning on the unit starts initialization and sets
the magnification of the cytodiagnostic unit to
the 10x setting.
3
Power switch
Cytodiagnostic
unit power
switch
The “10x” magnification indicator lights.
Press the power switch. The switch pops out, and
the cytodiagnostic unit is switched off.
The magnification indicator turns off.
3.1.3
Light source of the Epi-fl attachment (mercury lamp)
Refer to the operating manual provided with the mercury lamp power supply.
Always observe all warnings and precautions described in the manual.
Magnification
indicator
35
Page 38

Chapter 3 Individual Operations
t
3.2 Brightness Adjustment
3.2
Transmitted
image
Brightness Adjustment
Image brightness can be adjusted by the following methods:
Method Operating controls Explanation
Brightness control knob 3.2.1 Adjusting lamp voltage
(The 50i is subject to shifts
in color temperature.)
ND filter
attachment/detachment
(for 50i only)
(for 55i when a
cytodiagnostic unit is
attached)
Automatic adjustment after
magnification change
ND filter Attaching/removing ND filters of the
Erasure of transmitted
image
Preset switch
Preset brightness control knob
ND filter IN/OUT lever 3.2.3
Hand switch
(Do not set the brightness control
knob to the maximum position.
Make sure the preset switch is not
depressed.)
Epi-fl attachment
Microscope power switch 3.2.6
3.2.2
3.2.4
3.2.5 Epi-fl image
(Monitor image) Camera adjustment Application software for camera
control: Display mode, exposure
mode, exposure compensation,
camera gain adjustment, etc.
3.2.1
Adjustment using the brightness control knob
With the preset switch in the out position, rotate
the brightness control knob. (The brightness
control knob is disabled if the preset switch is
depressed.)
Brightness control
knob
Clockwise rotation Becomes brighter
Counterclockwise
rotation
Image brightness
Becomes darker
Brightness
control knob
For 50i
3.2.7
Preset switch in ou
position
Adjusting brightness with the brightness control knob will affect the lamp color temperature and
alter the color balance of the image. If accurate color reproduction is critical, set the brightness
control knob to a midpoint setting and use the ND filters to make brightness adjustments.
36
Page 39

Chapter 3 Individual Operations
T
3.2 Brightness Adjustment
3.2.2
How to use the preset brightness control knob
For 55i when a cytodiagnostic unit is attached
3.2.3
Adjustment using the preset switch
Push in the preset switch to enable the brightness
level (lamp voltage) previously set with the preset
brightness control knob.
Toggle the preset switch – i.e., return it to the out
position – to enable the brightness level (lamp
voltage) previously set with the preset brightness
control knob.
Push in the preset switch to set it to the depressed position.
While viewing the actual image, turn the knob with a precision screwdriver until the desired
brightness is achieved.
Setting the preset switch to the depressed position enables the brightness level set with the
preset brightness control knob.
To adjust brightness automatically after switching magnification, make sure the preset switch is
in the out position.
Preset switch
Preset brightness
control knob
Adjustment with the ND filter IN/OUT lever (for 50i)
Pushing in the ND filter IN/OUT lever moves the ND
filter (light intensity adjustment filter) into the
optical path and reduces brightness. The color
balance of the image remains unaffected.
3
he ND filter enters optical path.
37
Page 40

Chapter 3 Individual Operations
3.2 Brightness Adjustment
3.2.4
Automatic adjustment after magnification change
(only for a 55i when a cytodiagnostic unit is attached)
If the cytodiagnostic unit is attached to a 55i,
pressing the hand switch to change magnification
will also activate brightness adjustment.
However, the brightness control knob must not be
at the maximum setting, and the preset switch
must not be in the depressed position for this
automatic adjustment function to activate.
3.2.5
Adjustment with the ND filters of the Epi-fl attachment
Pushing in the ND filter attach/detach lever moves
the ND filter into the optical path and darkens the
fluorescent image.
ND filters are used to adjust light intensity. Higher
filter numbers correspond to lower transmission
rates (i.e., darker images). ND filters do not affect
color balance. (The table on P.53 shows the
brightness levels achieved by different
combinations of the three filters.)
ND filter
Hand switch
ND4: Reduces light intensity to 1/4.
ND8: Reduces light intensity to 1/8.
ND16: Reduces light intensity to 1/16.
3.2.6
Transmitted image in fluorescence observation
For fluorescence observations, turn off the microscope power switch to cancel the transmitted
image.
Bright ambient lights will make it more difficult to view the image. We recommend keeping the
room dark during fluorescence observations.
38
Page 41

Chapter 3 Individual Operations
3.3 Optical Path Switching
3.2.7
Camera adjustment
(adjusting the brightness of the image on the monitor)
When observing images captured by the camera and displayed on the monitor, you can adjust
brightness by varying camera adjustment parameters, such as display mode, exposure mode,
metering mode, exposure compensation, and image level adjustment.
For detailed discussion, refer to the operating manual provided with the camera or camera
control software.
3.3
3.3.1
Optical Path Switching
Optical path distribution
With the ergonomic binocular tube or trinocular
eyepiece tube, the optical path switching lever
allows distribution of light to the binocular section
and camera port.
Position of optical path
switching lever
Optical path
switching lever
Optical path distribution (%)
Binocular section Camera port
3
Pushed in 100 0 Ergonomic binocular
tube
Trinocular eyepiece
tube T
Extended by two notches 0 100
tube F
3.3.2
Disabling the clicking of the optical path switching lever
The trinocular eyepiece tubes T and F have a “NO
CLICK” switch on their tube attaching surfaces.
Slide this switch in the direction of the arrow with
the tip of a pointed tool to disable clicking for the
optical path switching lever. Set the switch to this
position if you need to eliminate the slight
vibrations resulting from the clicking action.
Extended 50 50
Pushed in 100 0
Extended by one notch 20 80
Pushed in 100 0 Trinocular eyepiece
Extended 0 100
39
Page 42

Chapter 3 Individual Operations
3.4 Vertical Stage Motion
3.4
3.4.1
3.4.2
3.4.3
Vertical Stage Motion
Prohibited actions
Avoid the following actions, which can cause equipment malfunctions.
• Rotating the right and left coarse/fine focus knobs in opposite directions.
• Rotating the coarse focus knob past the stopper.
Knob rotation direction and stage motion direction
Turn the coarse or fine focus knob to raise or lower
the stage and to adjust image focus.
The coarse focus knob is located on either the right
side or the left side. A fine focus knob is provided
on both sides.
To lower the stage Turn the knob toward
the front.
To raise the stage Turn the knob toward
the back.
Fine focus knob
Number of knob turns and distance of stage travel
No. of knob turns Distance of stage travel (vertical direction)
Coarse focus knob
Coarse focus knob 1 turn Approx. 13.8 mm
Fine focus knob 1 turn Approx. 0.1 mm
Fine focus knob 1 graduation on scale
The vertical motion range (coarse/fine focus stroke) of the stage is from 2 mm above the focal
point (reference position) to approximately 28 mm below the focal point.
1 µm
40
Page 43

Chapter 3 Individual Operations
3.4 Vertical Stage Motion
3.4.4
3.4.5
Adjusting the rotating torque of the coarse focus knob
Adjust the rotation torque of the coarse focus knob
(rotation resistance) by turning the torque
adjustment ring (TORQUE) located at the base of
the coarse focus knob. If the torque is too low, the
stage may descend under its own weight.
When turned in the
direction of arrow
When turned in the
direction opposite to
arrow
Makes knob harder to
turn.
Makes knob easier to
turn.
Coarse focus torque
adjustment knob
How to refocus
The entire stage can be lowered by setting a finger
on the refocusing lever or top surface of the stage
and pushing down. When the finger is released, the
stage slowly returns to its original position.
If the stage is lowered to the lowest position, it will
be locked in that position.
Push down once again to disengage the lock,
allowing the stage to slowly return to the original
position.
This function is useful when replacing specimens.
Be sure to lower the stage slowly.
3
Refocusing
lever
41
Page 44

Chapter 3 Individual Operations
3.5 XY Stage Motion
3.5
3.5.1
3.5.2
3.5.3
3.5.4
XY Stage Motion
Prohibited action
Avoid the following actions, which can cause equipment malfunctions.
• Moving the stage to the left and right by holding the top surface of the stage
directly.
Knob rotation direction and stage motion direction
To move the stage in the X or Y direction, rotate
the stage X knob or stage Y knob.
Stage Y knob
Adjusting the knob heights
The heights (positions) of the X knob and Y knob can be changed. Hold the knob and move it
along its vertical axis to the desired height.
Adjusting the knob rotation torque
When the X knob and Y knob are moved to the top
and bottom positions, the torque adjustment
screws can be found between the knobs.
X direction
Stage X knob
Y direction
Turning the torque adjustment screw to move them
closer towards the respective knobs increases
rotational torque.
(To increase rotational torque, turn the adjustment
screw counterclockwise and clockwise, as viewed
from above, for the Y knob and X knob,
respectively.)
Avoid loosening these screws excessively. If they
are too loose, the top surface of the stage may
move, even at a very light touch.
Y knob torque
adjustment
screw
X knob torque
adjustment
screw
42
Page 45

Chapter 3 Individual Operations
3.6 Diopter Adjustment
3.6
Diopter Adjustment
Diopter adjustment compensates for differences in visual acuity between the right and left eyes,
improving binocular observation. It also minimizes focal deviations when switching objectives.
Adjust diopter settings for both eyepieces.
(1) Turn the diopter adjustment ring of each
eyepiece and align the end face of the diopter
adjustment ring with the line. (This is the
diopter adjustment reference position.)
(2) Perform steps 1) to 10) in “2.1 Bright-Field
Microscopy” to focus on the specimen with
the 10x objective.
(3) Set the 40x objective in the optical path.
Using the coarse/fine focus knobs, focus on
the specimen.
(4) Set the 4x or 10x objective in the optical
path.
(5) Focus on the specimen using the diopter
adjustment rings instead of the coarse/fine
focus knobs. When making focus
adjustments, be sure to look through the
right eyepiece with your right eye and the left
eyepiece with your left eye.
Reference position for diopter adjustment
Line
Set the 40x
objective in
the optical
path.
3
(6) Perform steps (3) through (5) twice.
Use this for focal adjustment.
Set the
magnification to
10x and observe
with the right eye.
Use this for focal
adjustment.
Observe with
the left eye.
Use this for focal adjustment.
43
Page 46

Chapter 3 Individual Operations
3.7 Interpupillary Adjustment
3.7
3.8
Interpupillary Adjustment
Interpupillary adjustment improves the ease of
binocular observation.
Perform steps 1) to 10) in “2.1 Bright-Field
Microscopy” and focus on the specimen using the
10x objective. Then, move the eyepiece sleeve until
the fields of view for the right and left eyes
coincide.
Adjusting the Observation Position
The ergonomic binocular tube makes it possible to
extend and tilt the binocular section. Adjust the
position of the binocular section for the most
comfortable viewing.
Converge until
the right and left
fields of view
coincide.
Field of view
44
Page 47

Chapter 3 Individual Operations
yep
p
3.9 Adjusting the Condenser Position
3.9
Adjusting the Condenser Position
Adjust the condenser position (focusing and centering) so that the light passing through the
condenser forms an image at the correct location (center of the optical path) on the specimen
surface.
(1) Perform steps 1) to 10) in “2.1 Bright-Field
Microscopy” to focus on the specimen using
the 10x objective.
(2) Stop down the field diaphragm to the
minimum setting.
(3) Turn the condenser focus knob to form the
field diaphragm image on the specimen
surface.
(4) Turn the condenser centering screws so that
the field diaphragm image is positioned in the
center of the field of view.
(5) Set the 40x objective in the optical path. Turn
the coarse/fine focus knobs and focus on the
specimen.
Turn the field diaphragm knob and
stop down the field diaphragm to its
minimum setting.
3
(6) Turn the condenser focus knob to form the
field diaphragm image on the specimen
surface.
(7) Adjust the condenser centering screws until
the field diaphragm is at the center of the
eyepiece field of view. This is easiest if you
set the field diaphragm aperture to slightly
smaller than the eyepiece field of view.
Condenser focus knob
Condenser centering
screws
Field diaphragm
iece field of view
E
Field dia
Eyepiece field of view
hragm
45
Page 48

Chapter 3 Individual Operations
3.10 Adjusting the Aperture Diaphragm
3.10
3.10.1
3.10.2
Adjusting the Aperture Diaphragm
The setting of the aperture diaphragm affects
optical image resolution, contrast, depth of field,
and brightness. Turning the condenser aperture
diaphragm ring (or aperture diaphragm knob)
changes the size of the aperture diaphragm.
A small aperture diaphragm opening reduces
resolution and brightness but increases contrast
and depth of focus. A large aperture diaphragm
size increases resolution and brightness but
reduces contrast and depth of focus. These
characteristics involve inherent tradeoffs and
cannot be optimized independently. Generally,
aperture settings that are 70 to 80% of the
numerical aperture of the objective will provide
satisfactory images with suitable contrast.
Since an excessively small aperture diaphragm
opening will degrade image resolution, we do not
recommend setting the aperture diaphragm to less
than 60% of the objective's numerical aperture.
The numerical aperture is indicated on
the side of the objective.
Aperture diaphragm knob
Adjusting the aperture diaphragm opening using the condenser
scale
Since the condenser scale indicates the numerical aperture, adjust the aperture diaphragm ring
according to the scale.
(Normally, the index on the aperture diaphragm ring should align with a scale line that
corresponds to 70 to 80% of the numerical aperture of the objective.)
Adjusting the aperture diaphragm opening using the centering
telescope (optional)
Remove one eyepiece and attach the centering telescope in place using the optional adapter.
Turn the aperture diaphragm ring to stop down to the minimum aperture. While holding down
the flange of the centering telescope, turn the eyepiece of the centering telescope and focus on
the aperture diaphragm.
Turn the aperture diaphragm ring to adjust the aperture. (Normally, the aperture diaphragm
should be adjusted to around 70 to 80% of the size of the field of view.)
After the adjustment, remove the centering telescope and adapter and reattach the eyepiece.
46
Page 49

Chapter 3 Individual Operations
3.11 Selecting a Condenser
3.11
Objective
magnification
1x x x x x
2x x x x
4x x
10x to 100x
Selecting a Condenser
Condenser (: Optimum, {: Suitable, x: Not suitable)
Achromatic/aplanat
condenser
Note 1: The entire field of view may not be covered if a UW eyepiece is attached.
Note 2: Indoor lighting and light from other sources reflected from the surface of the
condenser lens may enter the field of view. If this happens, dim the indoor lighting
or find some way to keep strong extraneous light from striking the stage.
Note 3: Swing out the top lens before use.
Depending on the type of objective, the indicated numerical aperture of the objective may not be
achieved.
For example, when an objective with an N.A. of 1.4 is used, the maximum aperture of the
swing-out condenser or the Abbe condenser will be only about 65% of the objective's N.A., even
when the condenser aperture diaphragm is wide open.
Swing-out
condenser
Note 3
{
{
Achromat
condenser
Note 1
{
{
Abbe
condenser
Note 1
{
{
Low-power
condenser
Note 2
x
1-100x
condenser
Note 3
{
Note 3
3
3.12
Refer to the condenser operating manual for information on phase contrast condenser.
Adjusting the Field Diaphragm
The field diaphragm controls the amount of
illumination falling on the area of the specimen
being viewed. Turning the field diaphragm knob
changes the size of the field diaphragm. For normal
observations, the size of the diaphragm should be
slightly wider than the boundary of the field of
view. Illuminating a broader area than necessary
will result in stray light entering the field of view,
generating flare and reducing image contrast.
Appropriate field diaphragm settings are
particularly important for photomicrography and
digital image capturing. In general, good results
will be obtained by stopping down the field
diaphragm to settings slightly wider than the area
to be reproduced within the photo frame or monitor
display.
Field diaphragm knob
47
Page 50

Chapter 3 Individual Operations
3.13 Oil Immersion Operation
3.13
Oil Immersion Operation
Objectives marked “Oil” are oil-immersion
objectives. Objectives of this type are used with
immersion oil applied between the specimen and
the tip of the objective.
For maximum performance, oil-immersion
objectives with numerical apertures of 1.0 or higher
should be combined with oil-immersion
achromatic/aplanat condensers. Oil-immersion
condensers are used by applying oil between the
specimen and the condenser.
Any bubbles in the immersion oil will degrade image quality. Be careful to prevent bubbles from
forming. To check for air bubbles, fully open the field diaphragm and aperture diaphragm,
remove the eyepiece, and examine the exit pupil (bright round section) of the objective inside
the eyepiece tube. If it is difficult to ascertain the presence of bubbles, attach a centering
telescope (optional) with the adapter (optional), then look for air bubbles while turning the
eyepiece section of the centering telescope to adjust focus. If you detect bubbles, remove them
by one of the following methods:
• Turn the revolving nosepiece slightly to move the oil-immersed objective back and forth
once or twice. (In the case of the condenser, gently turn the condenser focus knob to
move the condenser up and down slightly.)
• Add more oil.
• Remove the oil and apply new oil.
Use as little oil as possible (just enough to fill the space between the tip of the objective and the
specimen, or between the tip of the condenser and the specimen). Too much oil will result in
excess oil flowing onto the stage and around the condenser.
Any oil remaining on the oil-immersion objective or adhering to the dry-type objective will
noticeably degrade image quality. After use, thoroughly wipe off all oil, and make sure that no oil
remains on the tips of other objectives. Additionally, carefully wipe off oil from the condenser.
Use petroleum benzine to wipe off immersion oil. For optimum results, we recommend following
up petroleum benzine with absolute alcohol (ethyl or methyl alcohol).
If petroleum benzine is unavailable, use methyl alcohol alone. When using just methyl alcohol,
note that surfaces will need to be wiped repeatedly to ensure complete removal of immersion oil.
Usually, three or four times should be sufficient to clean the lens.
CAUTION
When using petroleum benzine or absolute alcohol, always follow the
instructions provided by the manufacturer. These liquids are highly
flammable and must be kept away from flames and sparks.
48
Page 51

Chapter 3 Individual Operations
3.14 Water Immersion
3.14
Water Immersion
Objectives marked “WI” or “W” are water-immersion objectives. These objectives are used with
immersion water (distilled water or physiological saline) applied between the specimen and the
tip of the objective. Microscopy procedures are the same as for oil-immersion objectives.
Since water evaporates readily, monitor the immersion water during observation. Avoid using
too much water, since excess water will flow onto the stage and around the condenser,
promoting corrosion.
After use, wipe off water from the tip of the objective and condenser, then follow up by wiping
with absolute alcohol.
If you observe water stains, apply a small amount of neutral detergent and wipe gently, then
follow up with absolute alcohol.
3
49
Page 52

Chapter 3 Individual Operations
3.15 Using the Cytodiagnostic Unit
3.15
3.15.1
Using the Cytodiagnostic Unit
Attaching a cytodiagnostic unit on the microscope allows users to switch magnification using the
hand switch and to mark the specimen.
Magnification switching
Pressing the hand switch toggles magnification
between 10x and 40x.
When a cytodiagnostic unit is attached on the 55i,
light intensity will vary with changes in
magnification. (But note that light intensity does
not vary if the brightness control knob is set to the
maximum setting, or if the preset switch is in the
depressed position.)
Hand switch
3.15.2
Marking specimens
Holding the object marker knob with both hands,
push it from the right toward the left, then turn it
toward the back to extend the marker.
Gently press down the entire object marker knob to
apply a circular mark.
Turn the object marker knob toward the front to
retract the marker.
Add ink if ink markings are thin.
(Refer to P.72.)
Object marker
(3) Turn the knob
to the front to
retract the
marker.
(1) Push the
knob from
the right to
the left, then
turn it to the
back.
(2) Gently press the
knob to mark the
specimen.
50
Page 53

Chapter 3 Individual Operations
3.16 Fluorescence Observation
3.16
3.16.1
3.16.2
3.16.3
3.16.4
Fluorescence Observation
Warning
The mercury lamp (or xenon lamp) used with the Epi-fl attachment requires careful
handling. Be sure to read the warnings described in the beginning of this manual and
in the operating manual provided by the manufacturers of the mercury lamp power
supply (or high-intensity light source) and lamp. Observe all the warnings and
precautions described in those documents.
Shutter of the Epi-fl attachment
The shutter blocks illumination. When suspending
microscopy, close the shutter to prevent fading of
specimen colors. (Set the shutter lever to the C
position to move the shutter into the optical path
and block light.) To protect important specimens,
make it a habit to use the shutter whenever
appropriate.
When pausing Epi-fluorescent microscopy to
perform microscopy using diascopic light, move the
shutter into the optical path to block the
Epi-fluorescent light.
Shutter
lever
Light shielding plate
Light shielding plate of the Epi-fl attachment
The light shielding plate protects the observer's eyes from reflected ultraviolet light, which is
originally emitted through the objective, from the specimen. To remove the plate, loosen the
clamp screw and pull it forward.
Field diaphragm of the Epi-fl attachment
The field diaphragm is used to restrict the illumination to the area of the specimen being viewed.
Operating the field diaphragm lever changes the size of the field diaphragm. For normal
observations, stop down the diaphragm so that the aperture boundaries are just outside (or
inside) the field of view. Illuminating an area broader than necessary will result in stray light
entering the field of view, generating flare, reducing image contrast, and expanding the area of
the specimen subject to fading.
Field diaphragm lever
3
Appropriate field diaphragm settings are particularly important for photomicrography and digital
image capturing. In general, good results will be obtained by stopping down the field diaphragm
to slightly wider than the area to be reproduced within the photo frame or monitor display.
51
Page 54

Chapter 3 Individual Operations
3.16 Fluorescence Observation
3.16.5
Pulled all the way to the front Free (switching possible)
Pushed in one increment
(notch)
Pushed in two increments
(notches)
3.16.6
Switching excitation methods
Four filter cubes can be inserted into the Epi-fl
attachment. Move the desired cube into the optical
path by turning the filter cube switching knob on
the right side of the attachment.
For bright-field observations, leave one cube
position empty, and move this empty position into
the optical path.
Use the fluorescent motion restricting lever located
at the upper front section to limit cube switching
operations.
Lever position Filter cube switching
Switching between positions 1 and 2, or between positions 3 and 4.
(The position at which the lever is pulled determines whether
switching is for positions 1 and 2 or positions 3 and 4.)
Lock (filter cubes cannot be switched)
Inserting and removing the filter cubes
WARNING
Always turn off the mercury lamp
before inserting or removing the filter
cubes.
Filter cube switching knob
Filter motion
restricting
lever
(1) Remove the cover on the left-hand side of the
Epi-fl attachment.
(2) Insert the filter cubes.
(3) Replace the cover.
(Refer to P.67 for details.)
Cover
52
Page 55

Chapter 3 Individual Operations
3.16 Fluorescence Observation
3.16.7
ND filters of the Epi-fl attachment
Push in the ND filter attach/detach lever to move
ND filters into the optical path and darken the
fluorescent image.
ND filters are used to adjust light intensity. Higher
filter numbers correspond to lower transmission
rates (i.e., darker images). ND filters do not affect
color balance.
ND4: Reduces light intensity to 1/4.
ND8: Reduces light intensity to 1/8.
ND16: Reduces light intensity to 1/16.
As shown below, you can combine these three
filters to achieve various levels of brightness.
Brightness ND4 ND8 ND16
1 - - -
○
1/4
- -
ND filter attach/detach lever
3
○
○
○
○
-
○
○
○
○
-
-
1/8 -
1/16 - -
○
○
○
(-: Outside optical path, ○: In optical path)
1/32
1/64
1/128 -
1/512
53
Page 56

Chapter 3 Individual Operations
)
3.17 Selecting Fluorescent Filters
3.17
Selecting Fluorescent Filters
A filter cube is comprised of the following three
optical components: an excitation filter (EX filter),
a barrier filter (BA filter), and a dichroic mirror
(DM). Select a filter cube with the appropriate
combination of optical components for the
specimen characteristics, the fluorescent pigment,
and the purpose intended. Keep in mind the
following:
• Different combinations of excitation and barrier
filters may be selected for the same excitation
method.
• Other types of excitation filters, barrier filters,
and dichroic mirrors can be purchased
separately.
• Excitation filters are exposed to strong light
during operations and tend to age rapidly.
Replace the excitation filter at frequent
intervals.
CAUTION
When using the UV2A or UV2B, be sure to remove the spacer inside by
unscrewing the ring retaining the excitation filter. These filters cannot be used
until the spacer inside the ring has been removed.
(Refer to 3.17.4 for details.)
Excitation
filter
Dichroic mirror (inside the cube
Barrier filter
54
Page 57

Chapter 3 Individual Operations
g
3.17 Selecting Fluorescent Filters
3.17.1
Selecting excitation filters (EX filters)
Excitation filters allow selective transmission of
light (excitation light) in the waveband required for
fluorescent light emissions from the specimen,
blocking light of all other wavelengths. The range
of wavelengths allowed to pass through a filter is
referred to as bandwidth.
The range of the bandwidth of the excitation filter
determines the brightness of the fluorescent image,
the generation of self-fluorescence (fluorescence
resulting from substances other than fluorescent
pigments), and degree of fading. The broader the
bandwidth, the greater the amount of excitation
light irradiated onto the specimen, increasing
brightness. However, this also increases the
amount of self-fluorescence and causes faster color
fading. Narrow bandwidth reduces the amount of
excitation light striking the specimen and causes
the image to appear darker, but reduces
self-fluorescence and fading. For specimens with
pronounced self-fluorescence, use excitation filters
with a narrow bandwidth (note that this will make
the fluorescent image darker).
EX filter
Spectral
transmission
0
Bandwidth
Wavelen
th
3
Excitation filters are exposed to strong light during
operations and tend to age rapidly. Replace the
filter at intervals determined by usage.
Narrow EX filter bandwidth Wide
Brightness of fluorescent image Dark Bright
Generation of self-fluorescence Low High
Degree of color fading Low High
3.17.2
Selection of barrier filter (BA filter)
The barrier filter allows only fluorescent light emitted by the specimen to pass, blocking
excitation light. This allows viewing of a fluorescent image without excess illumination (dark
background).
There are two types of barrier filters: LP filters block all light below a certain wavelength but
pass all light of longer wavelengths. BP filters pass only light of a certain waveband, blocking all
other light. Use the filter type appropriate for your intended purpose.
55
Page 58

Chapter 3 Individual Operations
g
g
(
)
3.17 Selecting Fluorescent Filters
LP filter (long-pass filter)
LP filters block all light below a certain wavelength
but pass all light of longer wavelengths. This border
wavelength is called a cut-on wavelength.
(1) For specimens dyed with a fluorescent
pigment in which the fluorescent waveband
and excitation waveband (light that the
specimen absorbs in order to emit fluorescent
light) are very close, select a barrier filter
with the shortest cut-on wavelength
permitted by performance requirements for
efficient fluorescent microscopy. If the cut-on
wavelength is long, excitation light and
fluorescent light will be entirely distinct,
tending to darken the background of
fluorescent images. However, recent
advances in filter performance have resulted
in increased use of filters of short cut-on
wavelengths.
(2) For multiple-dye specimens, use an LP filter
for microscopy of fluorescent images of all
dyes. Note that a combination involving an
ordinary dichroic mirror, an excitation filter,
and an LP-filter-type barrier filter will be
incapable of exciting dyes that emit
long-wavelength fluorescent light – for
example, TRITC in the case of FITC and
TRITC. This will result in very dark TRITC
fluorescent images. For such cases, we
recommend using multiband filters.
BP filter (bandpass filter)
The bandpass filter passes only light of a certain
wavelength, blocking all other wavelengths.
BP filters are used for microscopy of fluorescent
images involving a specific dye in multiple-dye
specimens. (For example, in a double-dye
specimen of FITC and TRITC, the BA520-560 filter
enables microscopy of just the FITC fluorescent
image.)
However, BP filters will not indicate
self-fluorescence, if any (because the fluorescent
image in the above combination is green only). LP
filters are better suited for making fine distinctions
in self-fluorescence based on slight color
differences.
Waveband
emitted by
FITC
Spectral
transmission
Both the FITC and TRITC ima
Waveband
emitted by
FITC
Spectral
transmission
Only the FITC fluorescent image is visible.
LP520
Wavelen
es are visible.
BA520-560
Wavelength
Waveband
emitted by
TRITC
th
BP type
Waveband
emitted by
TRITC
56
Page 59

Chapter 3 Individual Operations
T
p
3.17 Selecting Fluorescent Filters
3.17.3
3.17.4
Replacing excitation and barrier filters
Excitation and barrier filters can be removed from
the filter cube and replaced. (Dichroic mirrors
cannot be dismounted from the filter cube.)
Excitation filters are screw-in filters.
Barrier filters are slide-in filters. Align the
projection on the barrier filter with the groove on
the filter cube and turn clockwise approximately 30
degrees to secure in place.
Filter cube internal spacers
The filter cubes listed below cannot be inserted
directly into the Epi-fl attachment. Instead, as
described below, an internal spacer must first be
removed or reversed.
(1) Place the filter cube on the work surface with
the excitation filter facing up.
Excitation
filter
Filter cube
urn about 30° to secure in place.
Barrier filter
3
(2) Unscrew the ring retaining the excitation
filter.
(Be careful to avoid dropping the filter.)
(3) Remove the spacer inside the ring.
(4) Remove or reverse the spacer as appropriate
for the particular filter cube type. The actions
suitable for various filter cubes are given
below.
(5) Reattach the ring.
- Filter cubes requiring spacer removal
• UV-2A
• UV-2B
- Filter cubes requiring spacer reversal
• DAPI
• FITC
• GFP-L
• GFP-B
• TRITC
• Tx-Red
S
acer
Ring
57
Page 60

Chapter 3 Individual Operations
3.18 Image Capture
3.18
3.18.1
3.18.2
Image Capture
Images can be captured by attaching a camera head to the ergonomic binocular tube or
trinocular eyepiece tube.
For more detailed discussion of this topic, refer to the operating manual provided with the
camera head or camera control software.
Proper adjustment of light intensity and focus on the microscope side are important for obtaining
clear images. Listed below are key considerations in capturing clear images.
Adjusting light intensity
Lamp voltage: When the 50i is used in applications for which accurate color reproduction is
critical, set the brightness control knob at a midpoint and use ND filters to make
brightness adjustments.
Filter: Place a commercially available color compensation filter on the filter holder at the
microscope base, as necessary.
Adjusting the condenser
• Always focus and center the condenser.
3.18.3
3.18.4
• Center the annular diaphragm for phase contrast microscopy.
• For normal operations, set the diaphragm aperture to 70 to 80% of the N.A. of the
objective.
Confirming the photomicrographic range
The image on the monitor represents the photomicrographic range.
Confirming focus
Check focus by viewing through the eyepiece and viewing the monitor. If the focal positions for
the two images differ, adjust the focal position adjustment screw at the camera port.
58
Page 61

Chapter 3 Individual Operations
3.18 Image Capture
3.18.5
3.18.6
3.18.7
Making adjustments to keep out extraneous light
Field diaphragm: Stop down the diaphragm to a setting just slightly wider than the area shown
on the monitor.
Eyepiece: Cover the eyepiece with a cloth.
3
Anti-vibration measures
If the exposure is less than 1/8 of a second, reduce light intensity with ND filters to make
exposures longer than 1/8 of a second. (If accurate color reproduction is not important, you can
use the brightness control knob to reduce light intensity.)
Fluorescence photomicrography
The fluorescence of fluorescent specimens may fade during exposure. To prevent this, do the
following:
(1) Select a brighter optical combination.
Even if the overall magnification is the same on the monitor, the combination of
objective and camera zoom can result in significant variations in exposure time. We
recommend increasing the magnification with the objective rather than the zoom.
(Generally, the aperture of the objective increases with magnification. The larger the
numerical aperture, the brighter the resulting image.)
(2) Adjusting the excitation light
Excessively bright excitation light will accelerate specimen fading while making it more
difficult to acquire suitable fluorescent images. Use ND filters to adjust brightness.
(3) Specimen
Photomicrography of faded specimen sections requires prolonged exposure times and
results in poor color reproduction and low-quality images. Move the specimen to obtain
images from a fresh section of the specimen previously unexposed to excitation light.
For best results, use the differential interference contrast or phase contrast methods to
select a specimen section for photomicrography, and then switch to the fluorescent
method to capture images.
(4) Supersensitive TV camera (other than DS-5M)
Under certain conditions, it may help to place an IR (infrared ray) blocking filter in
front of the supersensitive TV camera sensor. Experiment with the IR blocking filter to
determine its characteristics.
59
Page 62

(2)
(1)
4
Assembly
Checking the Input Voltage
1
Check the input voltage indicated on the back of the
microscope. Use the microscope only if this indication
matches the power supply voltage for the area in which
the microscope will be used.
CAUTION
If the voltage indication and supply voltage
differ, do not attempt to use the
microscope. Contact your nearest Nikon
representative to seek advice.
Input voltage
indication
Wiring at the Rear and Installing the Battery (for the 55i)
2
(1) Confirm that the power switch of the microscope is turned off.
(2) Remove the rear wiring cover.
(3) Connect the appropriate cables.
Cytodiagnostic
unit
Hand switch
Battery holder
55i rear
AC adapter
55i rear
Connector J1: INPUT 12V DC (+ -C • - – )
Connector J2: J-CY/PS
Connector J3: SWITCH (when using cytodiagnostic unit)
Connector J4: J-CY/SG
Cytodiagnostic unit rear
Connector A: INPUT 12V DC (+ -C • - – )
Connector B: SWITCH
60
Page 63

Chapter 4 Assembly
y
Cables provided with the cytodiagnostic unit
(4) Use a fully recharged battery (when using the 55i on
(1) J-CY/PS cable
(2) J-CY/SG cable (both ends are identical)
battery power).
Insert the battery into the battery holder.
If the 55i is running on battery power, there is no
need to connect the AC adapter to the J1 connector
(INPUT 12V DC) at the rear of the 55i.
Cytodiagnostic unit
connector
55i connector
4
CAUTION
Always use the specified battery type
(EN-EL1).
Batter
Battery holder
61
Page 64

Chapter 4 Assembly
(5) Replace the rear wiring cover.
Engage the cover hook in the slot on the rear of the
unit in the direction indicated by the arrow in the
diagram.
CAUTION
The rear wiring cover should always
be in position, to protect the LED light
source and battery holder from
impact, etc.
Slot for cover hook
Lamphouse cover
Lamp
Lamphouse cover
Cover hook
62
Page 65

Chapter 4 Assembly
Wiring of the Cytodiagnostic Unit
3
This procedure is required for the 55i.
This procedure is optional for the 50i.
(1) Remove the cover at the top of the
microscope arm.
(2) For the 55i, insert the two cables for the
cytodiagnostic unit from the top of the arm
and lead them out through the opening at the
bottom of the arm.
For the 50i, insert the AC adapter cable and
the hand switch cable from the top of the arm
and lead them out through the opening at the
bottom of the arm.
(3) Secure the two cables in place using the
guide at the back of the microscope as shown
to the right.
(4) Replace the cover
Insert the cables into to the space
exposed by the removed cover
For the 50i:
AC adapter and hand switch cable
For the 55i:
Signal cable (thin) (J-CY/SG) and power
cable (thick) (J-CY/PS)
4
Wiring for the 55i
63
Page 66

Chapter 4 Assembly
Attaching a Stage
4
(1) Turn the coarse focus handle to remove the
cushioning material from the substage
section.
(2) Turn the coarse focus handle until the
elevating section is brought to the lowermost
position.
(3) Place the stage on the substage and fix into
place with the tool stored in the back of the
microscope.
(4) Place a specimen holder on the stage and
tighten screws.
Stage fixing screw
Specimen holder fixing screw
Attaching a Condenser
5
(1) Turn the coarse focus handle until the
elevating section is raised to the uppermost
position.
(2) Turn the condenser focus knob until the
substage is brought to the lowermost
position.
(3) Insert a condenser and adjust so that it faces
toward the front. Secure in place with the tool
stored in the back of the microscope.
(4) Turn the condenser focus knob until the
substage is raised to the uppermost position.
Condenser focus knob
Condenser fixing screw
64
Page 67

Chapter 4 Assembly
Mounting an Epi-fl Attachment (when using an Epi-fl
6
attachment)
(1) Place an Epi-fl attachment on the microscope
arm.
(2) Secure in place with a clamp screw.
(3) Fix the Epi-fl attachment clamp bolts using
the hex wrench provided with the attachment.
(4) Attach a mercury lamphouse to the bayonet
mount on the back. (For more information,
refer to the instruction manual provided with
the mercury lamp power supply.)
1) Attach a collector lens to the lamphouse.
2) Turn the bayonet mount clockwise as far as
it will go.
3) Insert the lamphouse into position.
4) Turn the bayonet mount back to its original
position to lock the lamphouse.
5) Attach a mercury lamp.
6) Connect the mercury lamphouse to the
mercury lamp power supply.
Clamp screw used to fix
the Epi-fl attachment
Epi-fl
attachment
clamp bolts
Epi-fl attachment mounted in place
Mercury lamphouse attached in place
Bayonet mount
4
Attaching an Eyepiece Tube
7
(1) Place an eyepiece tube on the microscope arm (or Epi-fl attachment).
(2) Secure in place with a clamp screw. (For the Epi-fl attachment, secure in place with a
fixing screw using the tool stored in the back of the microscope.)
Fixing screw
Clamp screw
Trinocular eyepiece tube attached on
the Epi-fl attachment
65
Page 68

Chapter 4 Assembly
Attaching an Eyepiece
8
Make sure the notch on the eyepiece side and the
protrusion of the eyepiece sleeve are aligned.
Insert the eyepiece.
Attaching a Revolving Nosepiece (or Cytodiagnostic Unit)
9
Protrusion
When attached correctly in place
Notch
9-1 Attaching a Revolving Nosepiece
(1) Lift the revolving nosepiece from a position
just forward of the point directly below the
fitting part and slide toward the back to
attach. (Continue sliding the revolving
nosepiece until its front position is aligned
with that of the fitting part.)
Revolving nosepiece fixing screw
(2) Secure in place with the tool stored in the
back of the microscope.
9-2 Attaching a Cytodiagnostic Unit
(1) Insert the separately packed ink cartridge to
the hole at the rear of the cytodiagnostic unit.
(2) Lift the cytodiagnostic unit from slightly
forward of the point directly below the fitting
part and slide toward the back to attach.
(Continue sliding the cytodiagnostic unit until
its front position is aligned with that of the
fitting part.)
(3) Secure in place with the tool stored in the
back of the microscope.
Ink cartridge
Cytodiagnostic unit fixing screw
66
Page 69

Chapter 4 Assembly
(4) Check to confirm that the cytodiagnostic unit
power switch is off.
(5) Firmly insert two cables leading out from the
bottom of the arm into the connectors on the
back of the cytodiagnostic unit.
(6) If the cables are slack and prone to obstruct
operations, secure them with a wire-binding
band provided with the cytodiagnostic unit.
For the 50i: AC adapter cable (thick)
For the 55i: Power cable (thick)
4
For the 50i: Hand switch cable (thin)
For the 55i: Signal cable (thin)
Binding band
10
Attaching Objectives
(when a revolving nosepiece is attached)
Screw objectives into the revolving nosepiece. When attaching the objective in this way, make
sure that the magnification of the objective increases when the revolving nosepiece is turned
clockwise (clockwise when viewed from above the eyepiece).
67
Page 70

Chapter 4 Assembly
11
Inserting Filter Cubes and Light Shield (when the Epi-fl
attachment is mounted)
(1) Remove the cover on the front left of the
Epi-fl attachment.
(2) Insert the filter cube.
The filter cubes listed below cannot be
inserted directly into the Epi-fl attachment
filter bay. The internal spacer must be
removed or reversed, as described on P. 57.
• UV-2A
• UV-2B
• DAPI
• FITC
• GFP-L
• GFP-B
• TRITC
• Tx-Red
Filter cube bay
Filter cube
(3) Insert a nameplate into the position with the
same address as the one indicated on the
filter cube select knob on the right side of the
microscope.
(4) Turn the filter cube select knob and insert a
filter cube into the remaining open bay.
(5) Replace the cover.
Spacer
Nameplate
Filter cube select knob
For clarity, the eyepiece tube is not shown here.
Address indication
68
Page 71

Chapter 4 Assembly
(6) Attach a light shield to the front bottom of the
12
Epi-fl attachment with the tool stored in the
back of the microscope.
Light shield
Light shield attached in place
Attaching a Camera (when using a camera)
12-1 When attaching to the ergonomic binocular tube:
4
(1) To attach a camera head, screw it into the C
mount on the DSC port.
(2) Remove the rear cover of the ergonomic
binocular tube and insert the DSC port.
(3) Secure the DSC port into place with the tool
stored in the back of the microscope.
(4) Attach the camera cable to the camera head.
(Adjust the camera head position before using
the camera. Refer to 2.4 Photomicroscopy.)
Camera head
DSC port
Camera cable connector
Camera fine
focus adjusting
ring
12-2 When attaching to the trinocular eyepiece tube
(1) Attach the camera head to the trinocular eyepiece tube using the DSC adapter.
(2) Attach the camera cable to the camera head.
(Adjust the camera head position before using the camera.)
Attachment guide
fixing screw
DSC port
fixing screw
Camera centering
screws
69
Page 72

Chapter 4 Assembly
13
Attaching the Power Cord
13-1 For the 50i:
(1) Check to confirm that the microscope power switch is off.
(2) Insert one end of the power cord into the AC inlet at the back of the microscope.
(3) Insert the other end of the power cord into a wall outlet.
Power switch for
the microscope
13-2 When using the cytodiagnostic unit with the 50i
(1) Check to confirm that the cytodiagnostic unit
(2) Connect one end of the power cord to the AC
AC inlet
power switch is off.
adapter connecting to the cytodiagnostic unit.
Use only the specified AC adapter and power
cord.
(3) Insert the other end of the power cord into a
wall outlet.
13-3 When not using the battery with the 55i:
(1) Check to confirm that the microscope power
switch is off.
(2) Connect one end of the power cord to the AC
adapter leading out from the wiring section on
the back of the microscope.
Use only the specified AC adapter and power
cord.
(3) Insert the other end of the power cord into a
wall outlet.
Microscope assembly is now complete.
AC adapter
Power cord
70
Page 73

5
Replacing Consumables
5.1
(1) Remove the lamphouse cover on the back of
(2) Replace with a new lamp.
Replacing the lamp (for the 50i)
CAUTION
• Be careful to avoid burns:
Wait until the lamp and nearby parts have cooled before attempting to
replace the lamp.
• Be careful to avoid electrical shock:
Turn off the power switch and unplug the power cord from the outlet.
• Be careful to avoid abnormal heat generation:
Use only the lamp specified.
• Be careful to avoid actions that might reduce lamp service life:
Avoid touching the bare lamp bulb with bare hands. Soiling will reduce the
service life of the lamp.
5
the microscope. Remove the old lamp.
Avoid touching the bare lamp bulb with your
bare hands.
Use only the lamp specified (PHILIPS 5761).
(3) Replace the cover.
Engage the cover hook in the slot on the rear
of the unit in the direction indicated by the
arrow in the diagram.
CAUTION
The lamphouse cover must be attached. Failure to replace the lamphouse cover
may result in burns or fire from the heat generated by the lamp.
Lamp
Slot for cover hook
Lamphouse cover
Lamphouse cover
Cover hook
71
Page 74

Chapter 5 Replacing Consumables
y
5.2 Recharging the battery (for the 55i)
5.2
Recharging the battery (for the 55i)
(1) Remove the wiring cover on the back of the
CAUTION
• When storing or carrying the battery removed from the microscope, be sure
to attach the electrode cover (included with the battery). Shorting the battery
terminals may result in various problems, including liquid leakage, heat
generation, or burst.
• Use only the battery charger specified for use with the EN-EL1.
• Before storing the battery for extended periods, fully recharge, then fully
discharge the battery. (This should be done at least once a year.)
• Remove the battery from the microscope or battery charger when not in use.
If the battery remains attached, it may overdischarge and become unusable,
since a trace current flows even when power is turned off.
• Store the battery in a cool place when not in use.
・ Ideally, store in locations with ambient temperatures around 15°C to 25°C
and low humidity.
・ Avoid unusually cold or hot locations.
• Recharge the battery at room temperature (5°C to 35°C).
• To avoid degrading battery performance, do not recharge the battery until it
has been in use for some time and is at least partially discharged.
• Battery temperature may increase somewhat immediately after recharging.
This does not indicate a problem, and battery performance will not be
affected.
• If battery life declines significantly, even after a full recharge, the battery
needs to be replaced. Please purchase a new rechargeable battery (EN-EL1).
• If the battery terminals are soiled, wipe them clean with a dry cloth.
microscope, then remove the battery from
the battery holder.
(2) Connect the battery to the designated battery
charger and plug the power cord of the
battery charger into a wall outlet.
(3) Reinstall the charged battery in the battery
holder.
(4) Replace the wiring cover.
CAUTION
The wiring cover must be attached to protect the LED light source and the
battery mount from impact shock.
72
Battery holder
Battery charger
Wiring cover
Batter
Page 75

Chapter 5 Replacing Consumables
5.3 Refilling Cytodiagnostic Unit Ink
5.3
Refilling Cytodiagnostic Unit Ink
CAUTION
• Use only the specified refill ink (J-CY
refill ink).
• Never attempt to disassemble the ink
cartridge.
• Avoid touching the object marker
knob while the ink cartridge is
removed.
(1) Remove the ink cartridge from the rear of the
cytodiagnostic unit.
Ink cartridge
Object marker
knob
5
(2) Apply a few drops of ink to the red portion at
the tip. (Overfilling may result in ink
leakage.)
(3) Once the ink is fully absorbed, place a piece
of paper over the tip to absorb any excess
ink.
(4) Replace the ink cartridge.
Ink cartridge
73
Page 76

6
Troubleshooting
If the product does not function properly, take appropriate action as described below. If the
problem is still not resolved after referring to "Troubleshooting," please contact your nearest Nikon
representative
6.1
Problem Possible causes Remedy
Field of view vignetts.
Illumination is uneven
across the field of view.
Field of view is not
visible.
Dirt or dust is present in
the field of view.
Image quality is poor.
Contrast is poor.
Resolution is poor.
Optical
Parts are attached incorrectly.
Movable parts are not switched
correctly.
Field diaphragm image is not focused
on the specimen surface.
Field diaphragm is stopped down too
far.
Dirt or dust is present on the lens or
container.
Dirt or dust is present on the lens or
container.
Field diaphragm image is not focused
on the specimen surface.
Dirt or dust is present on the lens or
container.
Objective correction ring does not
match the thickness of the
container’s bottom plate.
Field diaphragm image is not focused
on the specimen surface.
Confirm that parts (nosepiece, condenser,
etc.) are correctly attached.
Move parts (e.g., optical path switching
lever, nosepiece, filter cube switching
knob) until a click is felt.
Focus and center the condenser.
Open the field diaphragm slightly wider
than the field of view.
Clean off the dirt, and use a clean
container.
Clean off the dirt, and use a clean
container.
Focus and center the condenser.
Clean off the dirt, and use a clean
container.
Adjust the correction ring.
Focus and center the condenser.
Revolving nosepiece is not attached
Focus is uneven.
Image flows.
Image is tinged yellow. Lamp voltage is too low (for the 50i).
Field of view is too
bright.
correctly, or has not been fully
rotated to the click stop position.
Specimen is tilted relative to the
stage surface.
Revolving nosepiece is not attached
correctly, or has not been fully
rotated to the click stop position.
There is no ND filter in the optical
path.
Lamp voltage is too high (for the 50i).
74
Attach it correctly and rotate to the click
stop position.
Correctly reposition the specimen on the
stage.
Attach it correctly and rotate to the click
stop position.
Increase the voltage with the brightness
control knob.
Place ND filters in the optical path.
Reduce the voltage with the brightness
control knob.
Page 77

Chapter 6 Troubleshooting
6.2 Operational
Problem Possible causes Remedy
Field of view is too dark.
6.2
Problem Possible causes Remedy
Image is not in focus
although the objective is
raised to the highest
position.
Images in left and right
eyepieces are not
coincident.
Eyes are fatigued.
Operational
Condenser aperture diaphragm is
stopped down too far.
Field diaphragm image is not focused
on the specimen surface.
Optical path switching lever is not set
to the 100% eyepiece.
Stage is attached incorrectly. Attach it correctly.
Interpupillary adjustment has not
been performed.
Diopter adjustment has not been
performed.
Brightness is inadequate.
This should normally be set to 70 to 80%
of the objective N.A.
Focus and center the condenser.
Switch to the 100% eyepiece.
Make adjustments.
Make adjustments.
Use the brightness control knob or ND
filters to adjust brightness.
6
6.3
Problem Possible causes Remedy
There is no power even
though the power switch
is on.
Lamp does not light. Lamp has burned out. Replace the lamp with a specified one.
Lamp burns out quickly. Lamp type is incorrect. Replace the lamp with a specified one.
Power does not turn on,
even though the
cytodiagnostic unit (if
used) power switch is
on.
Magnification of the
cytodiagnostic unit (if
used) does not change
even if the hand switch
is depressed.
Illumination brightness
is unaltered even if
magnification is changed
(when cytodiagnostic
unit is attached to the
55i).
Electrical
Power cord is not connected, or is
connected improperly.
Cables are not connected, or are
connected improperly.
The 55i (if used) power switch is not
set to on.
Hand switch cable or signal cable
(55i) is not connected, or is
connected improperly.
Signal cable is not connected, or is
connected improperly.
Brightness control knob is set to
maximum.
Preset switch is depressed.
Connect it properly.
Connect cables properly, and push them in
as far as possible (see p. 60).
Turn on the 55i power switch (press "|").
Connect the cable properly and push it in
as far as possible (see p. 60).
Connect the signal cable properly and push
it in as far as possible (see p. 60).
Set the brightness control knob to a level
below maximum.
Press the preset switch so that the switch
comes out.
75
Page 78

7
Care and Maintenance
7.1
Lens Cleaning
Keep the lens free of dust, fingerprints, etc. Dirt on the lenses or filters will affect image quality.
If any of the lenses become dirty, clean them by the procedure given below.
• Brush away dust with a soft brush or wipe away gently with gauze.
• If fingerprints or grease gets on a lens, moisten a piece of soft, clean cotton cloth, lens
tissue, or gauze with absolute alcohol (ethyl or methyl alcohol) and wipe.
• Use petroleum benzine only to remove immersion oil from the objective. For optimum
results, we recommend following up petroleum benzine with absolute alcohol (ethyl or
methyl alcohol). If petroleum benzine is unavailable, use methyl alcohol alone. When
using just methyl alcohol, note that surfaces will need to be wiped repeatedly to ensure
complete removal of immersion oil. Usually, three or four times should be sufficient to
clean the lens.
• Never use petroleum benzine to clean the entrance lens at the bottom of the eyepiece
tube or prism surface of the eyepiece tube.
• Absolute alcohol and petroleum benzine are highly flammable. Be careful when handling
these materials, particularly around open flames or when turning the power switch on or
off.
• Follow the instructions provided by the manufacturer when using absolute alcohol.
7.2
7.3
Cleaning the Product
• We recommend using a silicon cloth to clean the product.
• For stubborn dirt, dampen a piece of gauze with dilute neutral detergent and wipe gently.
• Use of organic solvents on plastic parts may result in discoloration.
Disinfecting the Product
• For routine disinfection of the product, we recommend using 70% medical alcohol.
• If contact occurs between a sample and the product, determine whether the sample is
hazardous. If the sample is hazardous, follow the standard procedures for your
laboratory.
• Use of organic solvents on plastic parts may result in discoloration.
76
Page 79

Chapter 7 Cleaning and Maintenance
7.4 Storage
7.4
7.5
Storage
• Store the product in a dry location where mold is unlikely to form.
• Store the objectives and eyepieces in a dry box or similar container with a drying agent.
• Place the vinyl cover over the product to protect it from dust.
• Switch off the microscope (press the switch to the "O" position) and wait for the
lamphouse to cool before covering the product with the vinyl cover.
Periodic Inspections (fee charged)
To maintain the peak performance of the product, we recommend periodic inspections. Contact
your nearest Nikon representative for more information. (Parts and service charges apply for this
service.)
7
77
Page 80

8
Technical Specifications
8.1
Specifications
Nikon Microscope ECLIPSE 50i
Model ECLIPSE 50i
Optical system Infinity-corrected CF optical system
Objective: CFI60
Objectives: Field number 22 (with ergonomic binocular tube/binocular
tube/cytodiagnostic unit), 25 (with trinocular eyepiece tube T/F)
Nosepiece: Sextuple
Focus up/down motion Drive system: Manual coarse/fine motion
(calibration markings for fine motion: 1 µm/marking)
Stroke: 2 mm upward, 28 mm downward
With one-touch refocusing mechanism
Lamp ratings 6V/30W halogen lamp
Lamp type Halogen lamp (PHILIPS 5761)
Average lamp life 100 hours
Input ratings
Power cord
Operating conditions
Transport/storage
conditions
External dimensions and
weight (main unit)
100-120V AC / 230V AC (±10%, 50/60Hz, 0.9A / 0.5A)
•
When used in 100-120V areas:
UL-listed detachable power cord set (3 conductor grounding Type SVT, AWG 18,
3 m long maximum, rated at 125V AC minimum)
•
When used in 220-240V areas:
EU/EN-approved 3-conductor power cord set (3 conductor grounding Type
H05VV-F, 3 m long maximum, rated at 250V AC minimum)
Temperature: 0 to 40°C
Humidity: 85% RH max. (no condensation)
Altitude: 2000 m max.
Degree of pollution: Degree 2
Installation: Category II
Protection class: Class I
Indoor use only
Temperature: -20 to 60°C
Humidity: 90% RH max. (no condensation)
Ex tern al dimensi ons: 18 4 (W) x 358 (H) x 383 (D) mm (excluding projections)
Weight: Approx. 9 kg
78
Page 81

Chapter 8 Technical Specifications
8.1 Specifications
•
Safety standards
UL-listed product (UL61010A-1)
•
Meets FCC Part 15B Class A requirements.
This equipment has been tested and found to comply with the limits for a Class A
digital device, pursuant to Part 15 of the FCC Rules.
These limits are designed to provide reasonable protection against harmful
interference when the equipment is operated in a commercial environment.
This equipment generates, uses, and can radiate radio frequency energy and, if not
installed and used in accordance with the instruction manual, may cause harmful
interference to radio communications.
Operation of this equipment in a residential area is likely to cause harmful
interference in which case the user will be required to correct the interference at his
own expense.
•
This Class A digital apparatus complies with Canadian ICES-003.
Cet appareil numérique de la classe A est conforme à la norme NMB-003 du
Canada.
•
Complies with Australian EMI (AS/NZS2064 Group1 Class B).
CE Marking
•
Meets EU IVDD (In vitro diagnostic medical device Directive) requirements. (GM-
approved: in vitro diagnostic medical device)
•
Meets EU Low Voltage Directive requirements.
•
Meets EU EMC Directive (EN61326) requirements.
•
UL-listed and GS-approved certification were obtained for the following
combination: 50i, cytodiagnostic unit, hand switch, and specified adapter.
8
79
Page 82

Chapter 8 Technical Specifications
8.1 Specifications
Nikon Microscope ECLIPSE 55i
Model ECLIPSE 55i
Optical system Infinity-corrected CF optical system
Objective: CFI60
Objectives: Field number 22 (with ergonomic binocular tube/binocular
tube/cytodiagnostic unit), 25 (with trinocular eyepiece tube T/F)
Nosepiece: Sextuple
Focus up/down motion Drive system: Manual coarse/fine motion
(calibration markings for fine motion: 1 µm/marking)
Stroke: 2 mm upward, 28 mm downward
With one-touch refocusing mechanism
Light source for
transmitted illumination
Input voltage 12V DC (provided from the AC adapter)
Power supply AC adapter
Specified AC adapter Manufacturer: ILAN ELECTRONICS LTD.
AC adapter power cord
Specified battery (Li-ion
rechargeable battery)
White LED
Model: F1650K
Rated input voltage: 100-240V AC, 1.2A max. (when inputting 115V AC),
50/60Hz
Rated output voltage: 12V DC, 3.5A max.
Other: UL-listed product, GS-approved, CE-certified
•
When used in 100-120V areas:
UL-listed detachable power cord set (3 conductor grounding Type SVT, AWG 18,
3 m long maximum, rated at 125V AC minimum)
•
When used in 220-240V areas:
EU/EN-approved 3-conductor power cord set (3 conductor grounding Type
H05VV-F, 3 m long maximum, rated at 250V AC minimum)
Manufacturer: Nikon
Code: EN-EL1
Operating conditions
Rating: 7.4V DC
Specified battery charger: MH-53 or similar
Temperature: 0 to 40°C
Humidity: 85% RH max. (no condensation)
Altitude: 2000 m max.
Degree of pollution: Degree 2
Installation: Category II
Protection class: Class I
Indoor use only
80
Page 83

Chapter 8 Technical Specifications
8.1 Specifications
Transport/storage
conditions
External dimensions and
weight (main unit)
Safety standards
Temperature: -20 to 60°C
Humidity: 90% RH max. (no condensation)
Ex ternal d imen sions : 184 (W) x 358 (H) x 383 (D) mm (excluding projections)
Weight: Approx. 8.5 kg
•
UL-listed product (UL61010A-1)
•
Meets FCC Part 15B Class A requirements.
This equipment has been tested and found to comply with the limits for a Class A
digital device, pursuant to Part 15 of the FCC Rules.
These limits are designed to provide reasonable protection against harmful
interference when the equipment is operated in a commercial environment.
This equipment generates, uses, and can radiate radio frequency energy and, if not
installed and used in accordance with the instruction manual, may cause harmful
interference to radio communications.
Operation of this equipment in a residential area is likely to cause harmful
interference in which case the user will be required to correct the interference at his
own expense.
•
This Class A digital apparatus complies with Canadian ICES-003.
Cet appareil numérique de la classe A est conforme à la norme NMB-003 du
Canada.
•
Complies with Australian EMI (AS/NZS2064 Group1 Class B).
CE Marking
•
Meets EU IVDD (In vitro diagnostic medical device Directive) requirements.
(GM-approved: in vitro diagnostic medical device)
•
Meets EU Low Voltage Directive requirements.
•
Meets EU EMC Directive (EN61326) requirements.
•
UL-listed and GS-approved certification were obtained for the following
combination: 55i, cytodiagnostic unit, hand switch, specified adapter, and
specified battery.
8
81
Page 84

Chapter 8 Technical Specifications
8.1 Specifications
J-FL 50i55i Epi-fluorescence Attachment for Nikon Microscopes
Model J-FL 50i55i Epi-fluorescence Attachment
Optical system Infinity-corrected CF optical system
Variable intermediate magnification: 1X
Fluorescent turret Manual quadruple turret
Field diaphragm
ND filter Manual, 3 filters (curved type filters ND4, ND8, ND16)
Shutter Manual (front-operated)
Compatible lamp
housing
Operating conditions
Transport/storage
conditions
Weight Approx. 2 kg
Manual (φ1 to 20 mm)
Hg, Xe, centered halogen (incompatible with precentered type)
Temperature: 0 to 40°C
Humidity: 85% RH max. (no condensation)
Altitude: 2000 m max.
Degree of pollution: Degree 2
Installation: Category II
Protection class: Class I
Indoor use only
Temperature: -20 to 60°C
Humidity: 90% RH max. (no condensation)
82
Page 85

Chapter 8 Technical Specifications
8.1 Specifications
J-CY Cytodiagnostic Unit for Nikon Microscopes
Model J-CY Cytodiagnostic Unit
Optical system Infinity-corrected CF optical system
Objective: 20X
Variable magnification lens: 2X, 0.5X
Magnification
adjustment system
Marker section Quick stamp system (Ink: J-CY refill ink)
Input voltage 12V DC, 1.5A
Power supply AC adapter
Specified AC adapter Manufacturer: ILAN ELECTRONICS LTD.
AC adapter power cord
Quick magnification changeover by rotary solenoid
Operated by C-HS hand switch
(Power may be supplied directly from the ECLIPSE 55i [through the
cytodiagnostic-unit power supply connector] using the cable set for the 55i &
cytodiagnostic unit.)
Model: F1650K
Rated input voltage: 100-240V AC, 1.2A max. (when inputting 115V AC),
50/60Hz
Rated output voltage: 12V DC, 3.5A max.
Other: UL-listed product, GS-approved, CE-certified
•
When used in 100-120V areas:
UL-listed detachable power cord set (3 conductor grounding Type SVT, AWG 18,
3 m long maximum, rated at 125V AC minimum)
•
When used in 220-240V areas:
EU/EN-approved 3-conductor power cord set (3 conductor grounding Type
H05VV-F, 3 m long maximum, rated at 250V AC minimum)
8
Operating conditions
Transport/storage
conditions
Weight Approx. 1 kg
Temperature: 0 to 40°C
Humidity: 85% RH max. (no condensation)
Altitude: 2000 m max.
Degree of pollution: Degree 2
Installation: Category II
Indoor use only
Temperature: -20 to 60°C
Humidity: 90% RH max. (no condensation)
83
 Loading...
Loading...