Melag Vacuklav 41-B, Vacuklav 43-B User manual

Technical Manual
Autoclave
Vacuklav®41-B
Vacuklav®43-B
For Users and Service personnel
Please read the accompanying User Manual before you start operation of the autoclave. The instructions contain important safety precautions. Make sure to keep the
Technical Manual together with the User Manual near the autoclave. The instructions
are part of the product.

Foreword
This manual has been created for the autoclaves Vacuklav®41-B and Vacuklav®43-B. They are identical except
for their chamber depth and device depth.
The device name "autoclave" is used to designate the steam sterilizers Vacuklav®41-B and Vacuklav®43-B.
You also receive a User Manual with the autoclave. It contains important safety instructions and information
which you need to operate the autoclave. Read the User Manual completely through in proper sequence before
beginning operation with the autoclave.
This Technical Manual includes declarations of conformity, suitability statements and recommendations, instructions for setting up, installing and initial start-up of the autoclave including installation record, extended technical
information on the software and hardware and other technical data. The Technical Manual is meant for interested persons and service personnel.
Technical Manual Vacuklav®41-B, Vacuklav®43-B
MELAG Medical Technology Berlin
Valid for Vacuklav®41-B, Vacuklav®43-B
as of software version 2.4x
1st Edition February 2008
Responsible for the contents: Engineering Department
MELAG Medical Technology
Geneststraße 7-10
10829 Berlin
Germany
E-Mail: info@melag.de
www.melag.de
© 2008-2009 MELAG Berlin
Document: Melag_Vacuklav_41B_43B_TechnicalManual_v3.doc/Revision: 3 – 09/1793
Subject to technical changes

Technical Manual Vacuklav®41-B and Vacuklav® 43-B
CONTENTS
Foreword.......................................................................................................................................................... 2
Device views.........................................................................................................................................................8
Device views Flex-Display................................................................................................................................... 9
Installation and setting up................................................................................................................................... 10
Removal from the packaging............................................................................................................................10
Space requirements......................................................................................................................................... 11
Installation surface............................................................................................................................................ 11
Electrical connection......................................................................................................................................... 11
One-way drain.................................................................................................................................................. 11
Supply with → feed water.................................................................................................................................12
Installation material........................................................................................................................................... 12
Installation examples........................................................................................................................................ 13
Record of installation and setting up.................................................................................................................16
Please send to MELAG....................................................................................................................................16
Device and installation data.............................................................................................................................. 16
Executed work.................................................................................................................................................. 17
Contrast setting................................................................................................................................................ 19
Counter readings.............................................................................................................................................. 19
Program modifications......................................................................................................................................19
Electromagnetic compatibility............................................................................................................................ 20
Electromagnetic environment........................................................................................................................... 20
Recommended protective distances................................................................................................................21
Technical tables...................................................................................................................................................22
Precision and drift behaviour – Sensors ..........................................................................................................22
Precision and drift behaviour –
Measuring chain............................................................................................................................................... 22
Precision and drift after 1 year.......................................................................................................................... 22
Precision and drift after 5 years........................................................................................................................22
Nominal value tolerances................................................................................................................................. 23
Pressure-Time-Charts......................................................................................................................................24
Quality of the → feed water.............................................................................................................................. 26
Frequently asked questions (FAQ) about the software.................................................................................... 27
Checklist for software update.............................................................................................................................42

Technical Manual Vacuklav®41-B and Vacuklav®43-B
Konformitätserklärung im Sinne der EG Richtlinie über Medizinprodukte 93/42/EWG
Declaration of conformity
Vacuklav®41-B
Vacuklav®43-B
In accordance with the EC- guidelines for medical devices 93/42/EWG
Manufacturer: MELAG oHG
Address: Geneststraße 9 – 10
Country: Germany
Product: Steam sterilizer (autoclave)
Type of device: Vacuklav®41-B/Vacuklav®43-B
Classification: Class 2a
10829 Berlin
We herewith declare that the above designated product conforms to the following
guideline:
Appendix I of the EC-guidelines for medical devices 93/42/EWG.
Notified body: DEKRA Certification Services GmbH
Handwerkstraße 15
70565 Stuttgart
No. of registration: 50199-Z2-00
The cited medical device is designated with the CE sign since 25-06-2004.
Berlin, 12-03-2008
..............................................
General Management
Qual ity – Made in Germa ny
Evidence Based Sterilization www.melag.de
2

Technical Manual Vacuklav®41-B and Vacuklav®43-B
Konformitätserklärung im Sinne der EN 13060 für Dampf-Klein-Sterilisatoren („Bescheinigung“)
Declaration of conformity
Vacuklav®41-B
Vacuklav®43-B
Declaration of conformity according to EN 13060 for small steam sterilizers
Manufacturer: MELAG oHG
Address: Geneststraße 9 - 10
Country: Germany
Product: Steam sterilizer (autoclave)
Type of device: Vacuklav®41-B/Vacuklav®43-B
Classification: Class 2a
10829 Berlin
We herewith declare that the above designated product conforms to the general requirements set forth in the EN13060 Standard and has successfully passed the
Type tests according to EN 13060
for fulfilling requirements on a device with
„Class B“ – Sterilization program
(B-method)
Berlin, 12-03-2008
..............................................
General Management
Quali ty – Made in Germa ny
Evidence Based Sterilization www.melag.de
3

Technical Manual Vacuklav®41-B and Vacuklav®43-B
Eignungsbeleg nach den Empfehlungen der KHI am RKI (vom April 2006)
Certificate of Suitability
Vacuklav®41-B
Vacuklav®43-B
According to the recommendations of the Commission for Hospital Hygiene and In-
fection Prevention at the Robert-Koch Institute (of April 2006)
Manufacturer: MELAG oHG
Address: Geneststraße 9 - 10
Country: Germany
Product: Steam sterilizer (autoclave)
Type of device: Vacuklav®41-B/ Vacuklav®43-B
Classification: Class 2a
10829 Berlin
We herewith declare that the above designated sterilizer is suited for sterilization of
▪ Solid instruments (wrapped and unwrapped)
▪ Porous goods (wrapped and unwrapped)
▪ Hollow bodies-instruments Type A (wrapped and un-
wrapped)
▪ Hollow bodies-instruments Type B (wrapped and unwrapped)
Instructions on load quantities and loading variants are set forth in the User Manual
and must be observed.
Be sure to observe the manufacturer's instructions for medical devices intended for
sterilization according to DIN ISO 17664.
Berlin, 12-03-2008
..............................................
General Management
Quali ty – Made in Germa ny
4

Technical Manual Vacuklav®41-B and Vacuklav®43-B
Evidence Based Sterilization www.melag.de
5

Technical Manual Vacuklav®41-B und Vacuklav®43-B
Geeigneter Prüfkörper nach den Empfehlungen der KHI am RKI (vom April 2006)
Suitable test body
Vacuklav®41-B
Vacuklav®43-B
According to the recommendations of the Commission for Hospital Hygiene and In-
fection Prevention at the Robert-Koch Institute (of April 2006)
Manufacturer: MELAG oHG
Address: Geneststraße 9 - 10
10829 Berlin
Country: Germany
Product: Steam sterilizer (autoclave)
Type of device: Vacuklav®41-B/Vacuklav®43-B
Classification: Class 2a
We herewith declare that the following test system is suited for testing the above
cited sterilizer:
Product: Helix-Test body according to EN 867-5
Type designation: MELAcontrol
Berlin, 12-03-2008
..............................................
General Management
®
Quali ty – Made in Germa ny
Evidence Based Sterilization www.melag.de
6
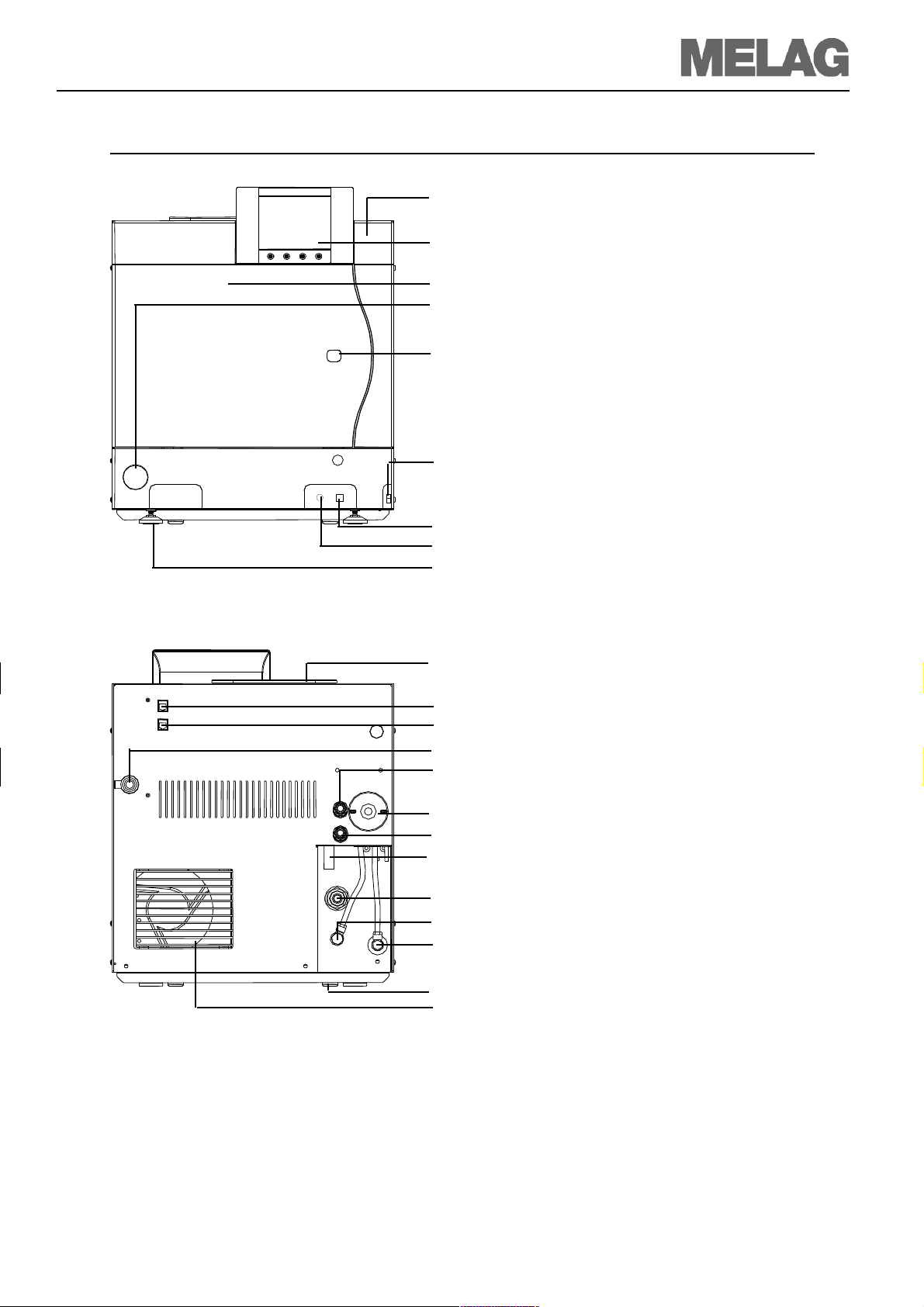
Technical Manual Vacuklav®41-B and Vacuklav® 43-B
Device views
Front side
(1)
Protective cap for slot of the →CF card
(2)
Control panel
(3)
Door (pivot opens to the left)
(4)
Protective cap for manometer to display the pressure of
the →double jacket steam-generator
(5)
Cap for emergency opening of the door
(7)
Mains switch (concealed, accessible from the side)
Back side
Fig. 1:Front- and rear view
(8)
Ethernet-1-Data connection*
(9)
Reset button overheating protection*
(10)
Front foot of the unit (adjustable)
*behind covering
(11)
Tank filler cap
(12)
Ethernet-2 data connection
(13)
Ethernet-3 data connection (can be retrofitted)
(14)
Power cable
(15)
Safety valve chamber
(16)
Sterile filter
(17)
Safety valve double jacket
(18)
Emergency overflow
(19)
One-way drain
(20)
Connection pressure discharge
(21)
Purified feed water inlet (for MELAdem®, swivelling
threaded fitting for hose ∅8x1, alternatively straight)
(22)
Back foot of the unit
(23)
Cooler
7

Technical Manual Vacuklav®41-B und Vacuklav®43-B
Device views Flex-Display
Front side
(1)
Protective cap for slot of the →CF card
(2)
Control panel
(3)
Door (pivot opens to the left)
(4)
Protective cap for manometer to display the pressure of
the →double jacket steam-generator
(5)
Cap for emergency opening of the door
(6)
Back side
(7)
Mains switch (concealed, accessible from the side)
(8)
Ethernet-1-Data connection*
(9)
Reset button overheating protection*
(10)
Front foot of the unit (adjustable)
*behind covering
(11)
Tank filler cap
(12)
Ethernet-2 data connection
(13)
Ethernet-3 data connection (can be retrofitted)
(14)
Power cable
(15)
Safety valve chamber
(16)
Sterile filter
(17)
Safety valve double jacket
(18)
Emergency overflow
(19)
(20)
One-way drain
(21)
Connection pressure discharge
(22)
Purified feed water inlet (for MELAdem®, swivelling
threaded fitting for hose ∅8x1, alternatively straight)
(23)
Back foot of the unit
(24)
Cooler
8
Technical Manual Vacuklav®41-B and Vacuklav® 43-B
Installation and setting up
Please read the User Manual of the autoclave and the water treatment
Warning
Warning
unit before setting up the autoclave.
If the autoclave has already been put into operation, please observe the
following before setting up again in case of any eventual transport:
■ To transport the autoclave, for instance if the practice moves to a new
location, wait until the pressure indicator on the manometer display
shows null bar after the autoclave is switched off. Be sure to check
the pressure indicator on the manometer before moving the autoclave
■ To transport the unit over longer distances, an →authorised person
must prepare the autoclave beforehand according to instructions and
completely empty the →double jacket steam generator. In order to do
so, you can use the Draining program in the SPECIAL menu.
Non-compliance can lead to severe burns and damages to the autoclave
as well as malfunctions.
The autoclave sterilizes on the basis of the →fractionated vacuum. A
membraneous pump is used to create the especially deep vacuum. Thus
the autoclave can be immediately put into operation without additional installation work, apart from providing the necessary power supply. For the
optional connection of the one-way drain and an external water treatment
unit please follow the water main and general information for the proper installation which must be observed when setting up the device.
Removal from the packaging
Unpack autoclave Lift the autoclave out of the carton with the carrying strap.
Remove carrying strap To remove the carrying strap, unscrew the four screws on each side of the
housing. Retighten these screws firmly without the washers. Keep the carrying strap and the washers.
After switching on After switching on the autoclave and before initial start-up, open the door
and remove the trays and the accessories from the chamber.
To do this you must go into door mode:
▪ Press the Door button on the Welcome start screen; afterwards the
door can be opened.
After 5 seconds of inactivity, the display automatically switches back
Notice!
to the main menu. The autoclave then begins to suck in the feed water needed for the sterilization, which triggers an error message!
9
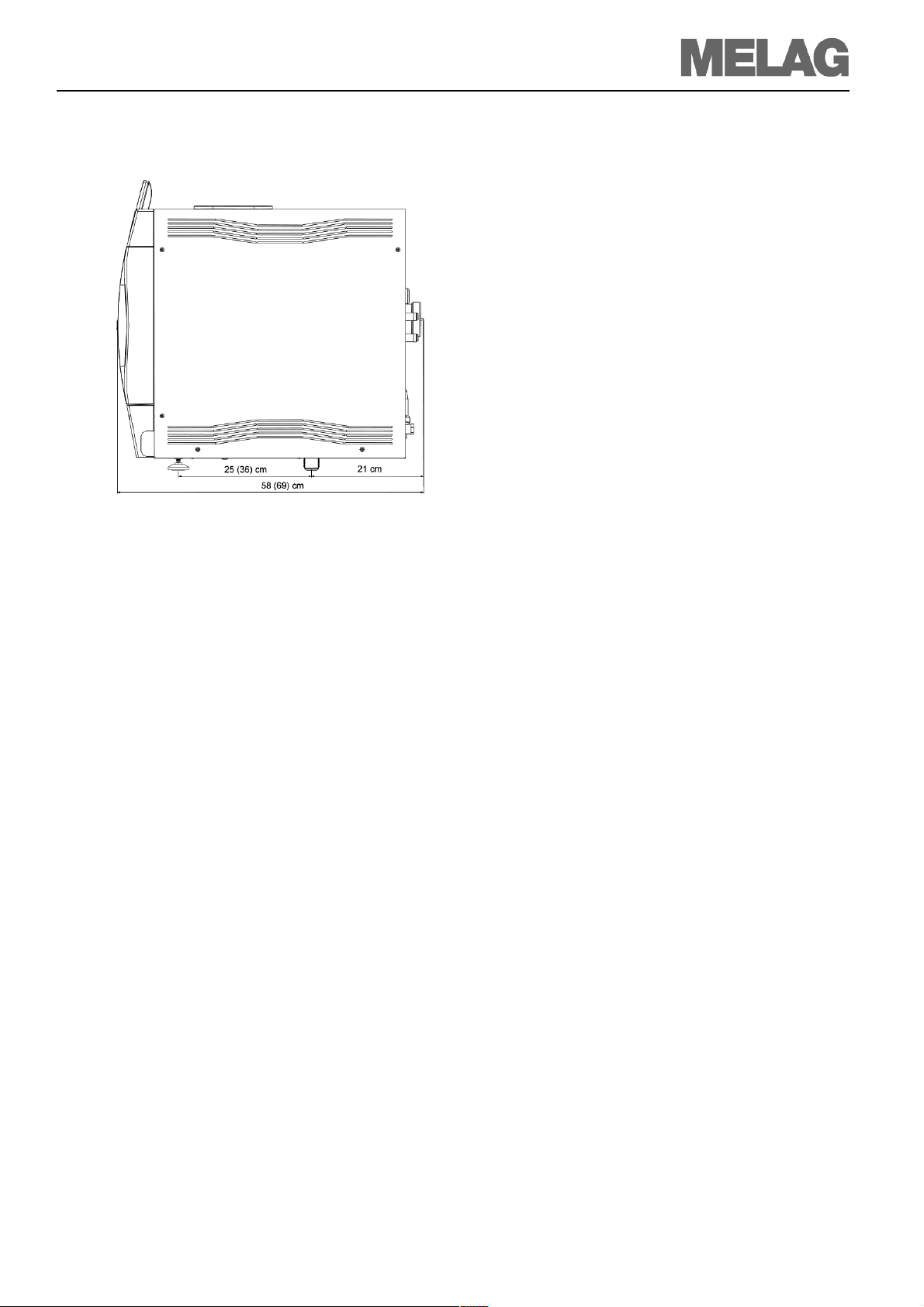
Installation and setting up
Fig. 2: Dimensions Vacuklav®41-B/43-B
Space requirements
The space requirements for the autoclave corresponds
to its dimensions plus at least 5 cm on the side and 10
cm at the back side. The autoclave should be freely accessible from above, for easy filling of the storage tank
and to ensure proper ventilation.
The autoclave works with a cooler located at the rear of
the device.
The function and service life can be impaired if the heat
dissipation over the cooler is restricted.
Therefore we do not recommend incorporating the autoclave; this is only possible if sufficient air circulation is
ensured.
Dimensions in parentheses: Vacuklav®43-B
For →Feed water supply
In case of external feed water supply there is additionally required space
for the water treatment unit MELAdem40 or MELAdem47.
Installation surface
Flat and level Place the autoclave on a flat and level surface.
Load capacity The Vacuklav®41-B weighs without load and without feed water 59 kg.
The Vacuklav®43-B weighs without load and without feed water 66 kg.
Electrical connection
Power socket 230 V, 50 Hz, connected load 3400 VA, separate fuse protection 16, pro-
tection from leakage current 30 mA
Power cable The power cable is 1.35 m long.
Log printer
MELAprint®42
If you want to connect a log printer to the autoclave, you need another
socket for its power supply.
One-way drain
Wall drain or siphon drain A wall drain, nominal DN40 or a siphon drain (sink drain) is required for
the one-way drain.
Waste water hose A 1 m wastewater hose (MELAG Art. No. 39180) can be ordered from
MELAG to directly connect the autoclave to the waste water, i.e. the pressure discharge goes directly into the siphon of the domestic water supply.
One-way overflow hose on
the siphon
If you close the one-way overflow hose of the autoclave at the siphon of
the domestic water supply, you need a 2 m wastewater hose (Art. No.
36585) which you can also order from MELAG.
The drain must be located at least 30 cm beneath the autoclave and be installed dip-free with continuous descent.
10
Installation and setting up
Feed water one-way system Since the contamination of feed water in autoclaves using a water circula-
Provide feed water supply
from internal storage tank
Quality of
feed water
Notice!
Supply with → feed water
tion system regularly leads to early damages to the autoclaves and instruments, the autoclave works in the gentle yet effective one-way water system. This means that it uses fresh purified feed water for every sterilization
run. The autoclave gets the feed water either from a water storage container which the practice team has refilled e.g. with distilled water from the
MELAdest®65. Or it gets the feed water fully automatically from the water
treatment unit MELAdem®40 or MELAdem47.
The quality of the →distilled or demineralized feed water for the steam
generation must be at least VDE 0510.
Before the initial start-up of the autoclave, the right chamber of the internal
storage tank of the autoclave must be filled with feed water up to the maximum marking.
At the initial start-up of the autoclave, the unit needs to be filled once with
2.5 L to fill the double jacket. For this reason we recommend you to refill
the right chamber of the internal storage tank afterwards immediately.
Installation material
Additional material, that can be ordered MELAG Art.
No.
Water connection-Set MELAdem®40 09033
Water connection-Set MELAdem®47 09034
Water connection-Set consisting of (contained in Art. No. 09033 and 09034: 25655
Feed water-inlet fixtures for hose ∅6x1 (replaces pipe fitting for hose ∅8x1)
Hollow screw for feed water inlet fixtures 53430
2x Cu seal 1/8“ for Art. No. 53461 42360
2x Cu seal 1/4" for Art. No. 53471 (replace by twisting Art. No. 53471) 36060
Pressure discharge fitting (1/4"-fast screw connection for hose (∅8x1 (screw on at the device)
One-way drain hose PTFE-pipe ∅8/6 –cut to size
One-way drain hose (overflow hose, 2m) 36585
Surface-mounted siphon with double hose nozzle 37410
Double hose nozzle with anti-flooding flap for connection to existing sink siphon 37400
Water tap 3/4“ (with safeguard combination) 37310
additional tap with return flow inhibitor and pipe aerator
(to attach to an available angle valve)
Leak monitor (water stop) with shut-off valve and probe 01056
Safety combination, consisting of return flow inhibitor and pipe aerator according to EN 1717 49600
Table 1: Installation material
21140
53471
39180
58130
11
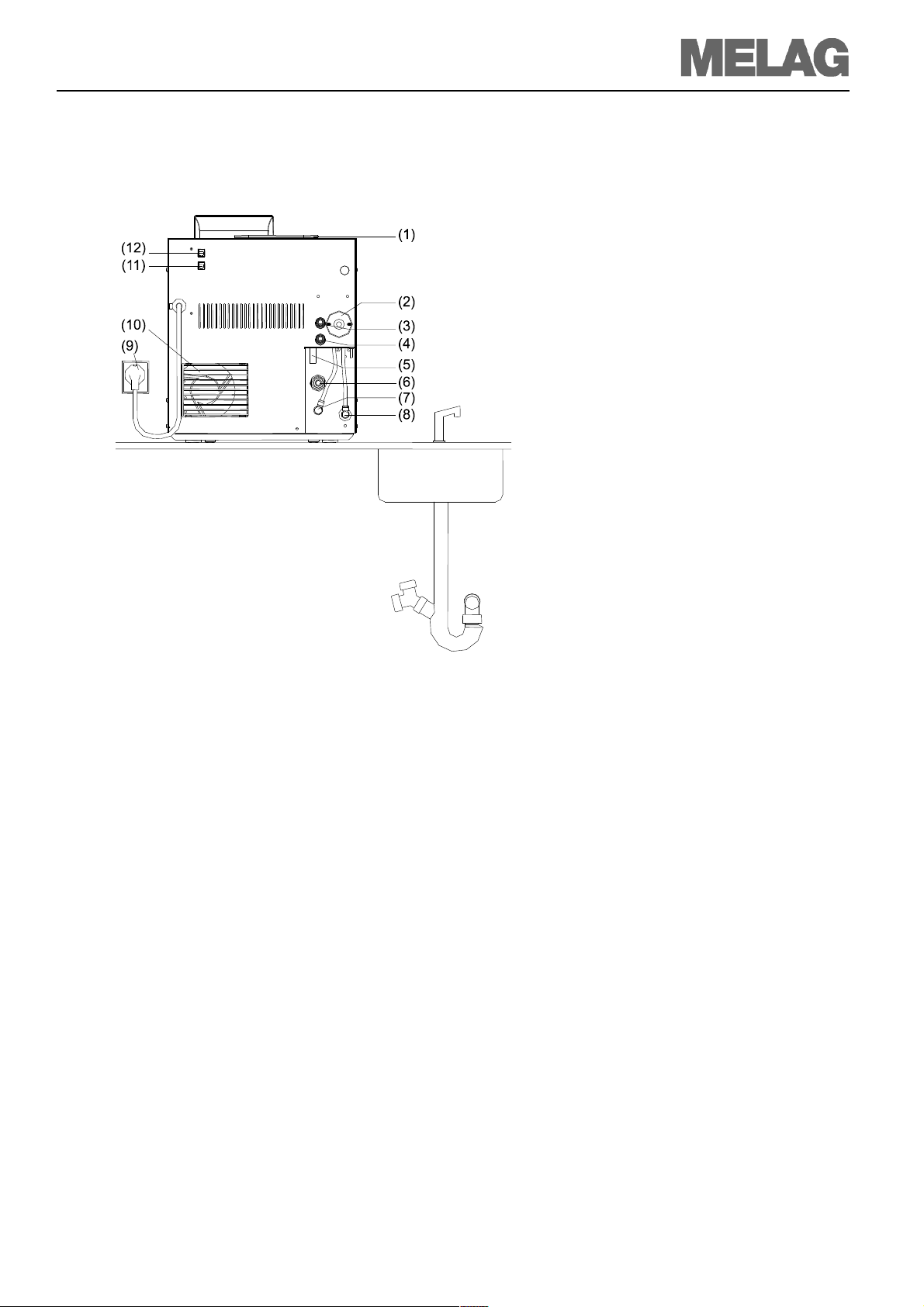
Installation and setting up
Example 1 – Standard scope of delivery Direct of →feed water from the internal
Installation examples
storage tank
Wastewater is collected in the wastewater chamber of the storage tank
No water connection necessary
The autoclave is supplied with feed water
directly over a hose from the internal storage tank. Thus no additional water connection is required aside from the power supply line. An integrated float-actuated level
switch in the device notifies a lack of feed
water. A program can be started only after
water is refilled.
The used feed water (wastewater) is collected in the wastewater chamber of the internal storage tank and later drained off
manually. A float-actuated level switch in
the wastewater chamber also notifies that
the wastewater chamber is full.
Fig. 3: Direct feed water supply from the internal storage tank
(1) Tank filler cap
(2) Sterile filter
(3) Safety valve chamber
(4) Safety valve double jacket
(5) Emergency overflow
(6) One-way drain
(7) Connection pressure discharge
(8) Feed water inlet fitting
(9) Power cable
(10) Cooler
(11) Ethernet-3-Data connection (can be retrofitted)
(12) Ethernet-2-Data connection
Example 2 – MELAdem®40 Supply of →feed water from the Ion
exchanger MELAdem®40
Pressure discharge is directly connected with siphon
12
 Loading...
Loading...