Melag Euroklav 23-29 Service manual

Technical Manual
Autoclave
Euroklav®23 VS+
®
Euroklav
as of software version V4.06
29 VS+
For users and personnel
Please read the accompanying Technical Description before you start operation of
the autoclave. The instructions contain important safety precautions. Make sure to
keep the Technical Manual together with the Technical Description near the
autoclave. The instructions are part of the product.

Foreword
This manual has been created for the autoclaves Vacuklav®23 VS+ and Vacuklav®29 VS+. They are identical
except for their chamber depth and device depth.
The device name "autoclave" is used to designate the steam sterilizers Vacuklav
You also receive a Technical Description with the autoclave. It contains important safety instructions and
information which you need to operate the autoclave. Read the Technical Description completely through in
proper sequence before beginning operation with the autoclave.
This Technical Manual includes declarations of conformity, suitability statements and recommendations,
instructions for setting up, installing and initial start-up of the autoclave including installation record, extended
technical information on the software and hardware and other technical data. The Technical Manual is meant for
interested persons and service personnel.
®
23 VS+ and Vacuklav®29 VS+.
Technical Manual Euroklav®23 VS+, Euroklav®29 VS+
MELAG Medical Technology Berlin
Valid for Euroklav
as of software version 4.06
1st Edition October 2007
Responsible for the contents: Engineering Department
MELAG Medical Technology
Document: TH_2_GB_23VS+_29VS+.doc/Revision: 1 – 07/1064
Subject to technical changes
®
23 VS+, Euroklav®29 VS+
Geneststraße 9-10
10829 Berlin
Germany
E-mail: info@melag.de
www.melag.de
© 2007 MELAG Berlin

Technical Manual Euroklav®23 VS+ and Euroklav®29 VS+
CONTENTS
Foreword ...................................................................................................................................................... II
Declaration of conformity according to the EC-guide lines for medical devices 93/42/EWG......................... 4
Device views.......................................................................................................................................................... 5
Installation and setting up.................................................................................................................................... 6
Removal from the packaging........................................................................................................................ 6
Space requirements ..................................................................................................................................... 6
Installation surface ....................................................................................................................................... 6
Electrical connections................................................................................................................................... 7
One-way drain.............................................................................................................................................. 7
Supply with feed water ................................................................................................................................. 7
Installation material ...................................................................................................................................... 7
Installation examples.................................................................................................................................... 8
Record of installation and setting up ................................................................................................................ 12
Device and installation data ....................................................................................................................... 12
Executed work............................................................................................................................................ 13
Program modifications................................................................................................................................ 14
Electromagnetic compatibility............................................................................................................................ 15
Electromagnetic environment..................................................................................................................... 15
Recommended protective distances .......................................................................................................... 16
Technical tables................................................................................................................................................... 17
Nominal value tolerances ........................................................................................................................... 17
Pressure-Time-Charts ................................................................................................................................ 17
Quality of the feed water ............................................................................................................................ 19
Brief instructions................................................................................................................................................. 20
Emergency door opening in case of power failure ..................................................................................... 20
Replace device fuses ................................................................................................................................. 21
Program overview : MAIN menu......................................................................................................................... 22
Program overview: SETUP menu: Function...................................................................................................... 23

Technical Manual Euroklav®23 VS+ and Euroklav®29 VS+
Declaration of conformity according to the EC-guide line s for medical devices 93/ 42/EWG
Declaration of conformity
Euroklav®23 VS+
Euroklav
In accordance with the EC-guidelines for medical devices 93/42/EWG
Manufacturer: MELAG oHG
Address: Geneststraße 9 – 10
10829 Berlin
Country: Germany
Product: Steam sterilizer (autoclave)
Type of classification: Euroklav®23 VS+/ Euroklav®29 VS+
Classification: Class 2a
®
29 VS+
We herewith declare that the above designated product conforms to the following
guideline:
Appendix I of the EC-guidelines for medical devices 93/42/EWG.
Notified body: DEKRA Certification Services GmbH
Handwerkstraße 15
70565 Stuttgart
No. of registration: 50199-Z3-00
The cited medical device is designated with the CE sign since 19-10-2007.
Berlin, 19-10-2007
……………………………………
General Management
Evidence Based Sterilization
4
Quality – Made in Germany
www.melag.de
Technical Manual Euroklav®23 VS+ and Euroklav®29 VS+
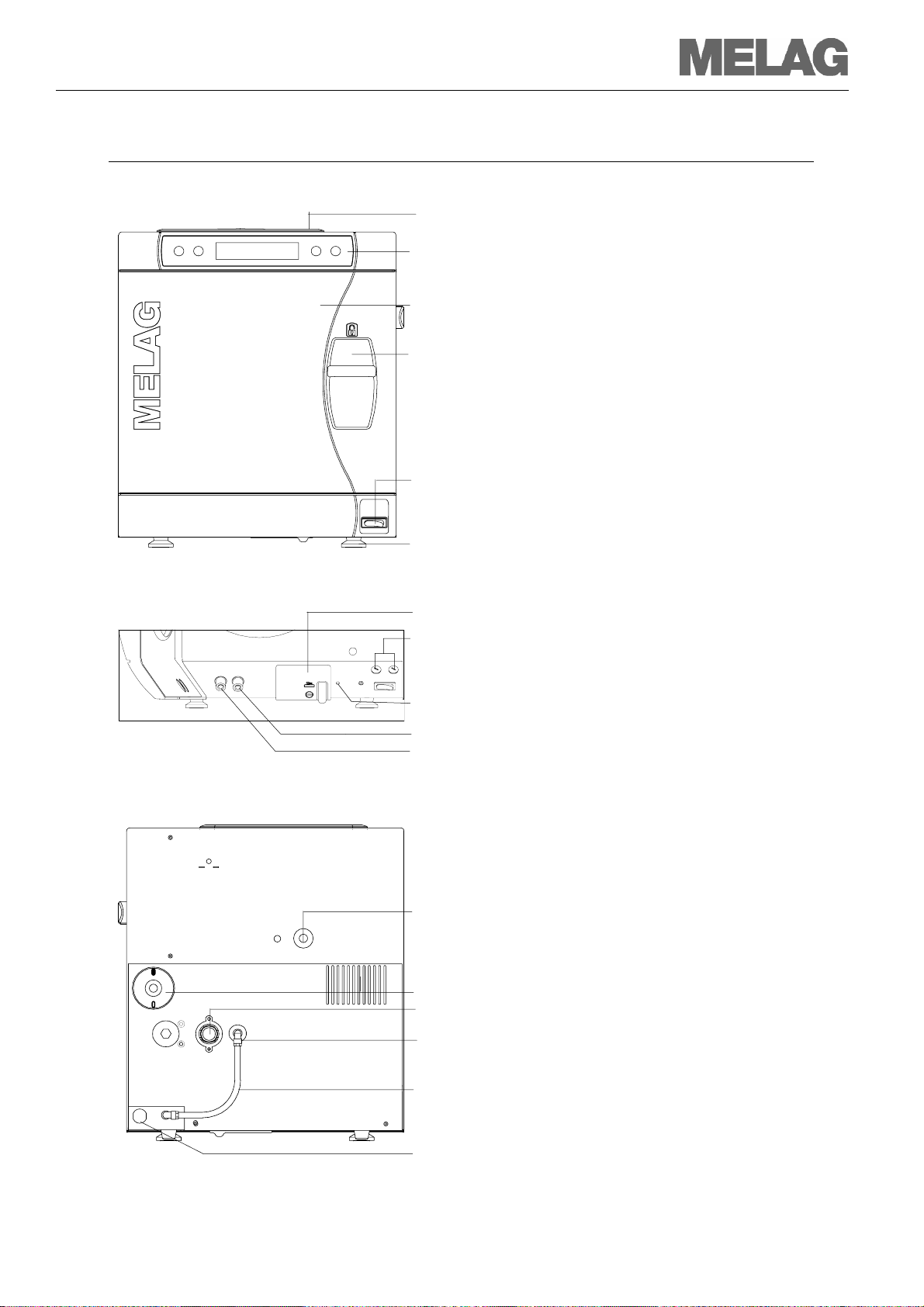
Device views
Front side
(1) Tank cap
Front side below with opened door
(7) Serial data and printer connection (RS232)
Back side
Figure 1: Device views
(2)
(3)
(4)
(5)
(6)
(8)
(9)
(10)
(11)
(12)
(13)
(14)
(15)
(16)
(17)
Operating and display field
Door (pivots opens to the left)
Sliding closure grip
Mains switch
Front of the unit (adjustable)
Device fuses – 2x 16A/ gRl
Reset button for overheating protection
Connection for emptying the storage tank – feed water
Connection for emptying the storage tank – waste water
*hidden behind the white cover
Safety valve
Sterile filter
One-way drain
Purified feed water inlet via internal storage tank
Hose bridge for internal feed water supply
Power cable
5
Technical Manual Euroklav®23 VS+ and Euroklav®29 VS+
y
Installation and setting up
Warning!
The autoclave sterilizes on the basis of the →pre-vacuum method
Please read the Technical Description of the autoclave and the
water treatment unit before setting up the autoclave.
combined with the fractionated flow method. A piston pump used to create
the vacuum combines the advantages of great efficacy with extreme
robustness in continuous operation. Thus the autoclave can be
immediately put into operation without additional installation work, apart
from providing the necessary power supply. For the optional connection of
the one-way drain and an external water treatment unit please follow the
water main and general information for the proper installation which must
be observed when setting up the device.
Removal from the packaging
Unpack autoclave
Remove carrying strap
After switching on
Lift the autoclave out of the carton with the carrying strap.
To remove the carrying strap, unscrew the four screws on each side of the
housing. Retighten these screws firmly without the washers. Keep the
carrying strap and the washers.
After switching on the autoclave and before initial start-up, open the door
and remove the trays and the accessories from the chamber.
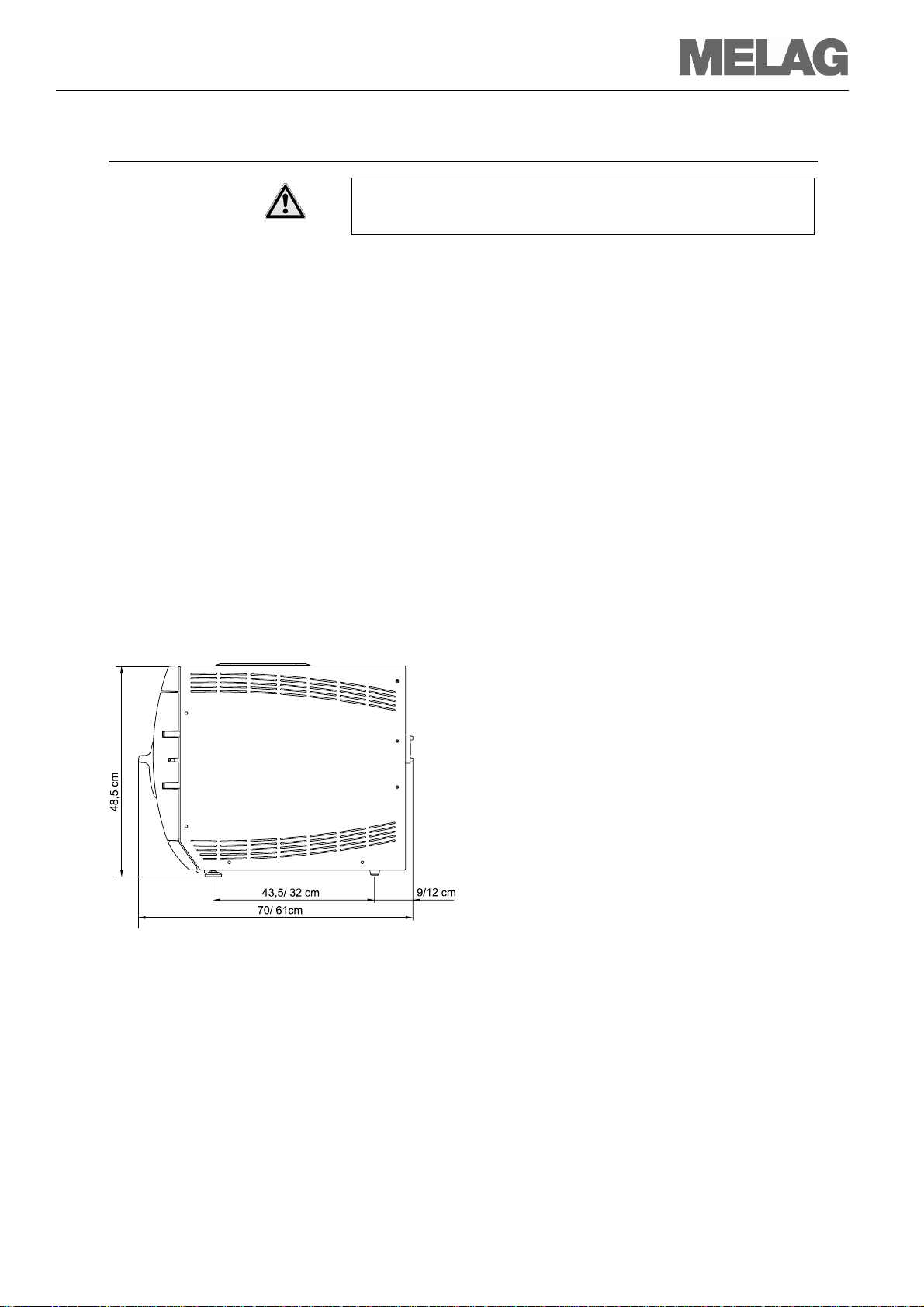
Space requirements
The space requirements for the autoclave
corresponds to its dimensions plus at least 10 cm on
both sides.
The autoclave should be freely accessible from
above, or easy filling of the storage tank and to
ensure proper ventilation.
The autoclave is not suitable for incorporation
into existing furnishings.
Dimensions of Euroklav
Figure 2: Dimensions Euroklav®23 VS+/ Euroklav®29 VS+
For →Feed water suppl
In case of external feed water supply there is additionally required space
for the water treatment unit MELAdem
Installation surface
®
23 VS+/Euroklav®29 VS+
®
40 or MELAdem
®
47.
Flat and level
Load capacity
6
Place the autoclave on a flat and level surface.
The Euroklav®23 VS+ weighs without load and without feed water 45 kg.
The Euroklav
®
29 VS+ weighs without load and without feed water 42 kg
Installation and setting up
r
Electrical connections
Power socket
connected load
Power cable
Log printer
MELAprint
220V-240V, 50/ 60Hz; separate fuse protection 16 A,
protection from leakage current 30 mA
as of software version 4.07 2600 W for Euroklav®23 VS+
software version V4.06 3000 W for Euroklav®23 VS+
The power cable is 1.35 m long.
If you want to connect a log printer to the autoclave, you need another
®
42
socket for its power supply.
One-way drain
Wall drain or
siphon drain
Wastewater hose
A wall drain, nominal DN40 or a siphon drain (sink drain) is required for the
one-way drain.
A wastewater hose with a length of 2 metres (MELAG Art. No. 36585) can
be ordered from MELAG to connect the autoclave to the waste water.
The drain must be located at least 30 cm beneath the autoclave and be
installed dip-free with continuous descent.
Supply with feed water
2100 W for Euroklav
2400 W for Euroklav
®
29 VS+
®
29 VS+
Feed water One-way and
Circulatory-
flow system
Quality of
feed wate
The autoclave works both according to the feed water one-way system as
well as the feed water circulatory flow system. In the one-way system, it
uses fresh purified feed water for every sterilization run. In the feed water
circulatory flow system, the autoclave works in a more water-conserving
manner, since the feed water is used for several sterilization runs.
provided the instruments have been carefully prepared, that means
cleaned and rinsed (e.g. in a thermal disinfector).
The autoclave gets the feed water either from the internal storage tank,
which the practice team has refilled e.g. with distilled water from the
MELAdest
treatment unit MELAdem
The Quality of the →distilled or demineralized feed water for the steam
generation must be at least VDE 0510.
®
65. Or it gets the feed water fully automatically from the water
®
40 or MELAdem
®
47.
Installation material
Additional material, that can be ordered MELAG Art. No.
1 One-way drain hose, 2 m 36585
1 Surface mounted siphon with double hoze nozzle 37410
1 Double hose nozzle with anti-flooding flap for connection to an available siphon drain 37400
1 Water tap 3/4“ with safety combination 37310
1 Additional water tap with return flow inhibitor and pipe aerator (to attach to an available angle
valve)
1 Leak monitor (water stop) with shut-off valve and probe 01056
1 Feedwater filter MELAdem
1 Safeguard combination, consisting of return flow inhibitor and pipe aerator according to EN
1717
®
48240
58130
48660
7
 Loading...
Loading...