Page 1

xPONENT for MAGPIX
Software User Manual IVD
Page 2

©
Luminex Corporation, 2011. All rights reserved. No part of this publication may be
reproduced, transmitted, transcribed, or translated into any language or computer language,
in any form or by any means without prior express, written consent of Luminex Corporation.
LUMINEX CORPORATION
12212 Technology Boulevard
Austin, Texas 78727-6115
U.S.A.
Voice: (512) 219-8020
Fax: (512) 219-5195
xPONENT for MAGPIX Software User Manual IVD
PN 89-00002-00-273 Rev B
January 2011
Luminex® Corporation (Luminex) reserves the right to modify its products and services at any
time. This guide is subject to change without notice. Although prepared to ensure accuracy,
Luminex assumes no liability for errors or omissions, or for any damages resulting from the
application or use of this information.
The following are trademarks of Luminex Corporation: Luminex®, xMAP®, xTAG®,
xPONENT®, Luminex® 100™, Luminex® 100 IS®,
Luminex® 200™, Luminex® SD™, Luminex
XYP™, MagPix®, MAGPLEX® Microspheres, Microplex® Microspheres, LabMAP, xTAG
®
®
Microspheres, LumAvidin®, SeroMAP™ Microspheres, xMAP® FLEXMIR®, xMAP® FLEXMIR
v2, xTAG® Kit for Factor V,II and MTHFR 677/1298, xTAG® Kit for CYP2C19, xTAG® Kit for
CYP2D6, xTAG® Kit for CYP2C9+VKORC1, xTAG® Respiratory Viral Panel, xMAP® Flock
Monitor™, xMAP® NeoPlex4™, xMAP® Pneumo14, xTAG® Ashkenazi Jewish Panel, xTAG
®
Cystic Fibrosis Kit.
All other trademarks, including ProClin®, Cheminert®, Windows® Pentium® and Dell® are
trademarks of their respective companies.
®
i
Page 3

Standard Terms and Conditions for Use of Instrument
Product
By opening the packaging containing this product ("Product") or by using such Product in any
manner, you are consenting and agreeing to be bound by the following terms and conditions.
You are also agreeing that the following terms and conditions constitute a legally valid and
binding contract that is enforceable against you. If you do not agree to all of the terms and
conditions set forth below, you must promptly return the Product for a full refund prior to using
them in any manner.
1. Acceptance
ALL SALES ARE SUBJECT TO AND EXPRESSLY CONDITIONED UPON THE TERMS
AND CONDITIONS CONTAINED HEREIN, AND UPON BUYER'S ASSENT THERETO. NO
VARIATION OF THESE TERMS AND CONDITIONS SHALL BE BINDING UPON LUMINEX
CORPORATION ("LUMINEX") UNLESS AGREED TO IN WRITING AND SIGNED BY AN
AUTHORIZED REPRESENTATIVE OF LUMINEX. For purposes of this agreement, "Seller"
shall mean either Luminex, if the Product is purchased directly from Luminex, or a Luminex
authorized reseller. Buyer, by accepting the Product shall be deemed to have assented to the
terms and conditions set forth herein, notwithstanding any terms contained in any prior or
later communications from Buyer and whether or not Seller shall specifically or expressly
object to any such terms.
2. Warranties
THIS WARRANTY IS APPLICABLE FOR PARTS AND SERVICE FOR LUMINEX
INSTRUMENTS PURCHASED DIRECTLLY FROM LUMINEX TO BUYER AND ONLY TO
THE EXTENT SUCH INSTRUMENTS ARE LOCATED IN NORTH AMERICA AND THE
COUNTRIES THAT COMPRISE THE EUROPEAN UNION. LUMINEX MAKES NO
WARRANTY, EITHER EXPRESS OR IMPLIED, WITH RESPECT TO PRODUCTS SOLD,
DISTRIBUTED, LOCATED OR USED OUTSIDE OF NORTH AMERICA OR THE
COUNTRIES COMPRISING THE EUROPEAN UNION. PRODUCTS SOLD OUTSIDE OF
NORTH AMERICA OR THE COUNTRIES COMPRISING THE EUROPEAN UNION ARE
SOLD ONLY ON AN "AS IS, WHERE IS" BASIS. NOTWITHSTANDING THE FOREGOING,
LUMINEX SHALL PROVIDE BUYER A WARRANTY ON FIELD SERVICE PARTS
PROCURED FROM LUMINEX FOR MAINTENANCE OF LUMINEX INSTRUMENTS IN ALL
COUNTRIES IN THE WORLD AND PER THE TERMS AND CONDITIONS HEREIN. TO
THE EXTENT THAT THE FOREGOING DISCLAIMERS ARE INVALID OR
UNENFORCEABLE UNDER THE LAWS OF ANY JURISDICTION, THE WARRANTY,
DISCLAIMER, LIMITATION OF LIABILITY AND OTHER PROVISIONS SET FORTH BELOW
SHALL THEREUPON BE EFFECTIVE TO THE FULLEST EXTENT PERMITTED BY
APPLICABLE LAW.
Notwithstanding Buyer's acceptance thereof, if Product is purchased directly from Luminex,
Luminex warrants that for a period of twelve (12) months from date of delivery that the
Product shall conform in all material respects with the Product Specifications provided by
Luminex with the Product. The warranty provided herein specifically excludes any software or
xPONENT for MAGPIX
ii
Page 4

hardware not provided by Luminex. If Product is purchased from a Luminex authorized
reseller, any warranty obligations shall be provided in writing directly by such Luminex
authorized reseller to Buyer. THIS WARRANTY IS EXCLUSIVE AND LUMINEX MAKES
NO OTHER WARRANTY, EXPRESS OR IMPLIED, INCLUDING WITHOUT LIMITATION
ANY IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR
PURPOSE. Seller's warranties made in connection with this sale shall not be effective if
Seller has determined, in its sole discretion, that Buyer has misused the Product in any
manner, has failed to use the Product in accordance with industry standards or practices or
has failed to use the Product in accordance with instructions, if any, furnished by Seller.
BUYER'S EXCLUSIVE REMEDY WITH RESPECT TO PRODUCT PROVED TO SELLER'S
SATISFACTION TO BE DEFECTIVE OR NONCONFORMING SHALL BE REPAIR OR
REPLACEMENT OF SUCH PRODUCTS WITHOUT CHARGE OR REFUND OF THE
PURCHASE PRICE, IN SELLER'S SOLE DISCRETION, UPON THE RETURN OF SUCH
PRODUCTS IN ACCORDANCE WITH SELLER'S INSTRUCTIONS BELOW. NEITHER
SELLER NOR LUMINEX SHALL IN ANY EVENT BE LIABLE FOR INCIDENTAL,
CONSEQUENTIAL OR SPECIAL DAMAGES OF ANY KIND RESULTING FROM ANY USE
OR FAILURE OF THE PRODUCT, EVEN IF SELLER OR LUMINEX HAS BEEN ADVISED
OF THE POSSIBILITY OF SUCH DAMAGE INCLUDING, WITHOUT LIMITATION,
LIABILITY FOR LOSS OF WORK IN PROGRESS, DOWN TIME, LOSS OF REVENUE OR
PROFITS, FAILURE TO REALIZE SAVINGS, LOSS OF PRODUCTS OF BUYER OR
OTHER USE OR ANY LIABILITY OF BUYER TO A THIRD PARTY ON ACCOUNT OF
SUCH LOSS, OR FOR ANY LABOR OR ANY OTHER EXPENSE, DAMAGE OR LOSS
OCCASIONED BY SUCH PRODUCT INCLUDING PERSONAL INJURY OR PROPERTY
DAMAGE UNLESS SUCH PERSONAL INJURY OR PROPERTY DAMAGE IS CAUSED BY
SELLER'S GROSS NEGLIGENCE.
In the event that Product is located outside of North America or the European Union and fails
to conform to the warranty set forth herein, during the warranty period: (i) Buyer shall notify
Luminex in a timely manner in writing that such Product failed to conform and shall furnish a
detailed explanation of any alleged nonconformity; (ii) Buyer at it's expense will contract
either Luminex or a Luminex trained service engineer to assess the issue and identify the
defective FS-PART; and (ii) at Luminex's option and election, Buyer shall either return such
nonconforming Product to Luminex's manufacturing facility or destroy such Product and
provide Luminex with written certification of destruction. In the event that a FS-PART is
returned to Luminex's manufacturing facility, Luminex may analyze such FS-PART for
defects. In the event that Luminex determines that such FS-PART is not defective, the FSPART shall be shipped to Buyer then Buyer shall be responsible for the payment for such FSPART and related shipping charges. Furthermore, in the event that Luminex determines that
such FS-PART is defective then Luminex shall be responsible for the payment for such FSPART and related shipping charges. Except as expressly provided herein, Buyer shall not
have the right to return a Product to Luminex without Luminex's prior written consent.
3. Buyer's Use of Product
Buyer shall not use this Product for any commercial purpose, including without limitation
performance of testing services, unless expressly agreed to in writing by Luminex or as
specifically authorized by Luminex through a Luminex distributor. Buyer agrees that no rights
or licenses under Luminex's patents shall be implied from the sale of the Product, except as
expressly provided herein or as specifically agreed to in writing by Luminex, and Buyer does
not receive any right under Luminex's patent rights hereunder. Buyer acknowledges and
agrees that the Product are sold and licensed only for use with Luminex's laser based
fluorescent analytical test instrumentation. Buyer further acknowledges that, unless otherwise
iii
Page 5

indicated on the Product label, the Product has not received approval from the United States
Food and Drug Administration or other federal, state or local regulatory agencies and have
not been tested by Seller or Luminex for safety or efficacy in food, drug, medical device,
cosmetic, commercial or any other use, unless otherwise stated in Seller's technical
specifications or material data sheets furnished to Buyer. Buyer expressly represents and
warrants to Seller that Buyer will use the Product in accordance with the Product label, if
applicable, and will properly test and use any Product in accordance with the practices of a
reasonable person who is an expert in the field and in strict compliance with the United
States Food and Drug Administration and all applicable domestic and international laws and
regulations, now and hereinafter enacted.
BUYER HEREBY GRANTS TO LUMINEX A NONEXCLUSIVE, WORLDWIDE,
UNRESTRICTED, ROYALTY-FREE, FULLY PAID-UP LICENSE, WITH THE RIGHT TO
GRANT AND AUTHORIZE SUBLICENSES, UNDER ANY AND ALL PATENT RIGHTS IN
INVENTIONS COMPRISING MODIFICATIONS, EXTENSIONS, OR ENHANCEMENTS
MADE BY BUYER TO THE PRODUCT OR TO THE MANUFACTURE OR USE OF THE
PRODUCT ("IMPROVEMENT PATENTS"), TO MAKE, HAVE MADE, USE, IMPORT,
OFFER FOR SALE OR SELL ANY AND ALL PRODUCT; EXPLOIT ANY AND ALL
METHODS OR PROCESSES; AND OTHERWISE EXPLOIT IMPROVEMENT PATENTS
FOR ALL PURPOSES. NOTWITHSTANDING THE FOREGOING, "IMPROVEMENT
PATENTS" SPECIFICALLY EXCLUDES PATENT CLAIMS CONCEIVED AND REDUCED
TO PRACTICE BY BUYER CONSISTING OF METHODS OF SAMPLE PREPARATION,
METHODS OF CONJUGATING PRODUCT TO ANALYTES, THE COMPOSITION OF
MATTER OF THE SPECIFIC CHEMISTRIES OF THE ASSAYS DEVELOPED BY BUYER
AND METHODS OF PERFORMING THE ASSAYS (I.E., THE PROTOCOL FOR THE
ASSAY).
Buyer has the responsibility and hereby expressly assumes the risk to verify the hazards and
to conduct any further research necessary to learn the hazards involved in using the Product.
Buyer also has the duty to warn Buyer's customers, employees, agents, assigns, officers,
successors and any auxiliary or third party personnel (such as freight handlers, etc.) of any
and all risks involved in using or handling the Product. Buyer agrees to comply with
instructions, if any, furnished by Seller or Luminex relating to the use of the Product and not
misuse the Product in any manner. Buyer shall not reverse engineer, decompile, disassemble
or modify the Product. Buyer acknowledges that Luminex retains ownership of all patents,
trademarks, trade secrets and other proprietary rights relating to or residing in the Product
and Buyer receives no rights to such intellectual property rights by virtue of its purchase of
Product other than as expressly set forth herein. Buyer shall have no right to use any
trademarks owned or licensed to Luminex without the express written permission of Luminex.
4. Buyer's Representations, Release and Indemnity
Buyer represents and warrants that it shall use the Product in accordance with Paragraph 2,
"Buyer's Use of Product," and that any such use of Product will not violate any law,
regulation, judicial order or injunction. Buyer agrees to release, discharge, disclaim and
renounce any and all claims, demands, actions, causes of action and/or suits in law or equity,
now existing or hereafter arising, whether known or unknown, against Seller and Luminex,
and their respective officers, directors, employees, agents, successors and assigns
(collectively the "Released Parties"), with respect to the use of the Product. Buyer agrees to
indemnify and hold harmless the Released Parties from and against any suits, losses, claims,
demands, liabilities, costs and expenses (including attorney, accounting, expert witness, and
consulting fees) that any of the Released Parties may sustain or incur as a result of any claim
against such Released Party based upon negligence, breach of warranty, strict liability in tort,
xPONENT for MAGPIX
iv
Page 6

contract or any other theory of law or equity arising out of, directly or indirectly, the use of the
Product or by reason of Buyer's failure to perform its obligations contained herein. Buyer shall
fully cooperate with the Released Parties in the investigation and determination of the cause
of any accident involving the Product which results in personal injury or property damage and
shall make available to the Released Parties all statements, reports, recordings and tests
made by Buyer or made available to Buyer by others.
5. Patent Disclaimer
Neither Seller nor Luminex warrants that the use or sale of the Product will not infringe the
claims of any United States or other patents covering the product itself or the use thereof in
combination with other products or in the operation of any process.
v
Page 7

End-User License Agreement (EULA) for Luminex
Software
This Luminex End-User License Agreement (“EULA”) is a legal agreement between you
(either an individual or a single entity, also referred herein as “you”) the end-user and
Luminex Corporation (“Luminex”) regarding the use of the xPONENT software product
provided to you above, which includes computer SOFTWARE and online or electronic
documentation and may include associated media and printed materials (if any)
(“SOFTWARE”). The terms also apply to any updates, supplements, web content or internetbased services, such as remote access.
BY USING THE SOFTWARE, YOU ACCEPT THESE TERMS. IF YOU DO NOT ACCEPT
THEM, DO NOT USE THE SOFTWARE. INSTEAD, RETURN IT TO LUMINEX OR THE
LUMINEX AUTHORIZED THIRD PARTY FROM WHICH YOU PURCHASED THE
SOFTWARE FOR A REFUND OR CREDIT. IF YOU COMPLY WITH THESE LICENSE
TERMS, YOU HAVE THE RIGHTS TO USE THE SOFTWARE AS SPECIFICALLY SET
FORTH BELOW.
1. OVERVIEW. The SOFTWARE is protected by copyright laws and international copyright
treaties, as well as other intellectual property laws and treaties. The SOFTWARE is
licensed, not sold.
2. ADDITIONAL LICENSING REQUIREMENTS AND/OR USE RIGHTS.
Trial and Conversion. Some or all of the SOFTWARE may be licensed on a trial basis.
a.
Your rights to use trial SOFTWARE are limited to the trial period. The trial SOFTWARE
and length of the trial period are set forth during the activation process. The
SOFTWARE may be used for evaluation purposes only during the trial period and not
for any commercial use, including without limitation to any diagnostic use. You may
have the option to convert your trial rights to perpetual rights. Conversion options will
be presented to you at the expiration of your trial period.
b. Activation. You can activate the SOFTWARE by obtaining a license key provided by
Luminex Technical Support at support@luminexcorp.com or 1-877-785-2323 or
1-512-381-4397.
c. Branding. You may only add additional branding or other graphics to SOFTWARE with
Luminex’s express written consent.
d. Upgrades. You may only obtain updates or upgrades for the SOFTWARE from
Luminex Technical Support at orders@luminexcorp.com or authorized resellers. For
more information on obtaining updates from authorized resellers, see http://
www.luminexcorp.com.
®
xPONENT for MAGPIX
vi
Page 8

3. GRANT OF LICENSE. Subject to the terms and conditions of this EULA, Luminex hereby
grants to you a nonexclusive, nontransferable, nonassignable license (without right to
sublicense) under Luminex’s copyrights and trade secrets to use the SOFTWARE on a
single computer running with a single unit of a specific model of Luminex instrument, as
such model is identified on the packaging included with the SOFTWARE. You may make
one (1) copy of the SOFTWARE for backup or archival purposes only. You may also
install the SOFTWARE on up to three (3) additional computers for purposes of
performing ancillary tasks (i.e. preparing templates/protocols, performing further analysis
or re-running previous data), provided such computers are at a single location and are
NOT connected with a Luminex instrument. In addition, You may purchase the right to
use the SOFTWARE on additional computers, as agreed to in writing with Luminex or its
authorized reseller, for purposes of performing ancillary tasks (i.e. preparing templates/
protocols, performing further analysis or re-running previous data), provided such
computers are at a single location and are NOT connected with a Luminex instrument.
Although no rights or licenses under any of Luminex's patents are granted by or shall be
implied from the license of the SOFTWARE or the sale of Luminex instrumentation to
you, the purchaser, you may obtain a license under Luminex’s patents, if any, to use this
unit of Luminex instrumentation with fluorescently labeled microsphere beads authorized
by Luminex by purchasing such beads from Luminex or an authorized Luminex reseller.
4. RESTRICTIONS
• SOFTWARE must only be installed and operated on a single computer running with a
Luminex instrument, as set forth above.
You may not use this SOFTWARE for any commercial purpose, including in the
•
performance of testing services, unless expressly agreed to in writing by Luminex or as
authorized in writing by Luminex through an authorized reseller of the SOFTWARE.
• You may only use the SOFTWARE with microspheres manufactured by Luminex or
with kits developed, manufactured and distributed by licensees authorized in writing by
Luminex.
• You must maintain all proprietary notices on all copies of the SOFTWARE.
• You may not distribute copies of the SOFTWARE to third parties.
• You may not reverse-engineer, decompile, disassemble, or otherwise attempt to derive
source code from the SOFTWARE.
• You may not copy (other than one backup or archival copy), distribute, sublicense,
rent, lease, transfer or grant any rights in or to all or any portion of the SOFTWARE.
• You must comply with all applicable laws regarding the use of the SOFTWARE.
• You may not modify or prepare derivative works of the SOFTWARE, including
modifying any branding or graphics.
• You may not use the SOFTWARE in a computer-based service business or publicly
display visual output of the SOFTWARE.
• You may not transmit the SOFTWARE over a network, by telephone, or electronically
by any means.
vii
Page 9

5. TERM AND TERMINATION. Your rights under this EULA are effective until termination.
You may terminate this EULA at any time by destroying the SOFTWARE, including all
computer programs and documentation, and erasing any copies residing on your
computer equipment. Luminex may terminate this EULA upon thirty (30) days written
notice to you. Your rights under this EULA automatically terminate without further action
on the part of Luminex if you do not comply with any of the terms or conditions of this
EULA. Upon any termination of this EULA, you agree to destroy the SOFTWARE and
erase any copies residing on your computer equipment.
6. RIGHTS IN SOFTWARE. All rights and title in and to the SOFTWARE and any copies
thereof are owned by Luminex or its suppliers. This EULA is not a sale and does not
transfer to you any title or ownership interest in or to the SOFTWARE or any patent,
copyright, trade secret, trade name, trademark or other intellectual property right therein.
You shall not remove, alter, or obscure any proprietary notices contained on or within the
SOFTWARE and shall reproduce such notices on any back-up copy of the SOFTWARE.
All title and intellectual property rights in and to the content which may be accessed
through use of the SOFTWARE is the property of the respective content owner and may
be protected by applicable copyright or other intellectual property laws and treaties. This
EULA grants you no rights to use such content.
7. EXPORT RESTRICTIONS. You agree that you will not export or re-export the
SOFTWARE to any country, person, entity, or end-user subject to U.S.A. export
restrictions. You hereby warrant no state or federal agency has suspended, revoked, or
denied your export privileges.
NO WARRANTY. THE SOFTWARE IS LICENSED “AS IS.” ANY USE OF THE
8.
SOFTWARE IS AT YOUR OWN RISK. THE SOFTWARE IS PROVIDED FOR USE
ONLY WITH LUMINEX PRODUCTS. TO THE MAXIMUM EXTENT PERMITTED BY
APPLICABLE LAW, LUMINEX AND ITS SUPPLIERS DISCLAIM ALL WARRANTIES,
EITHER EXPRESS OR IMPLIED, INCLUDING, BUT NOT LIMITED TO, IMPLIED
WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE,
AND NONINFRINGEMENT.
9. LIMITATION OF LIABILITY. IN NO EVENT SHALL LUMINEX OR ITS SUPPLIERS BE
LIABLE FOR ANY SPECIAL, INCIDENTAL, INDIRECT, OR CONSEQUENTIAL
DAMAGES WHATSOEVER (INCLUDING, WITHOUT LIMITATION, DAMAGES FOR
LOSS OF BUSINESS PROFITS, BUSINESS INTERRUPTION, LOSS OF BUSINESS
INFORMATION, OR ANY OTHER PECUNIARY LOSS) ARISING OUT OF THE USE OF
OR INABILITY TO USE THE SOFTWARE, EVEN IF LUMINEX HAS BEEN ADVISED OF
THE POSSIBILITY OF SUCH DAMAGES.
10. MISCELLANEOUS. This EULA is governed by the laws of the State of Texas, U.S.A.,
without reference to conflicts of laws principles. You shall not assign or sublicense or
otherwise transfer the rights or license granted hereunder, by agreement or by operation
of law, without the prior written consent of Luminex, and all assignments in violation of
this prohibition shall be null and void. This EULA is the complete and exclusive
agreement of Luminex and you and supersedes all other communications, oral or written,
relating to the subject matter hereof. No change to this EULA shall be valid unless in
writing and signed by the party against whom enforcement is sought. The waiver or
failure of Luminex or you to exercise in any respect any right or rights provided for herein
shall not be deemed a waiver of any further right hereunder. If any provision of this EULA
is held unenforceable, the remainder of this EULA will continue in full force and effect.
xPONENT for MAGPIX
viii
Page 10

Table of Contents
Chapter 1 Introduction ........................................................................................................1
Software Packages ......................................................................................................................................1
About This Manual .......................................................................................................................................2
Warnings, Notes and Symbols ....................................................................................................................2
General Guidelines ......................................................................................................................................2
Biological Samples ................................................................................................................................3
Bead (Microsphere) Handling
Bead Concentration ...............................................................................................................................4
MAGPIX Technology .............................................................................................................................4
Repetitive MagPlex Bead Measurements .............................................................................................5
Classification and Reporter Fluorochromes ..........................................................................................6
Fluidics 1 and Fluidics 2 ........................................................................................................................6
Sample Volume .....................................................................................................................................6
Plates ....................................................................................................................................................7
Touch Screen ........................................................................................................................................7
................................................................................................................3
Chapter 2 Application Administrator Tasks .....................................................................9
System Setup ..............................................................................................................................................9
Viewing System Status
Application Settings .............................................................................................................................10
LIS Settings .........................................................................................................................................11
External Analysis Program Settings ....................................................................................................11
Arranging Main Navigation Buttons .....................................................................................................13
Maintenance Options ..........................................................................................................................13
Group Setup Tab .................................................................................................................................13
Setting Up Group Permissions ............................................................................................................16
User Setup .................................................................................................................................................16
User Setup Tab ...................................................................................................................................17
Global User Settings ...........................................................................................................................17
Create User Account Screen ...............................................................................................................18
Batch Options Tab ...............................................................................................................................19
Alert Options Tab ................................................................................................................................21
CSV Options Tab ................................................................................................................................24
Archive Options Tab ............................................................................................................................26
Licensing Tab ......................................................................................................................................28
Schedule Tab ......................................................................................................................................29
Report Options Tab .............................................................................................................................29
........................................................................................................................10
Chapter 3 Using xPONENT ...............................................................................................31
Starting xPONENT .....................................................................................................................................31
Initial Startup ..............................................................................................................................................31
Logging In to xPONENT ............................................................................................................................31
Logging Off of and Exiting xPONENT ........................................................................................................32
ix
Page 11

Using Online Help ......................................................................................................................................32
Screen elements ........................................................................................................................................32
System Monitor ..........................................................................................................................................34
Home Page ................................................................................................................................................35
Daily Activities .....................................................................................................................................36
Adjusting the Sample Probe Height ...........................................................................................................37
System Initialization ...................................................................................................................................38
Adding or Importing CAL and VER Kit Information ............................................................................. 39
Setting Up the System Initialization Routine ....................................................................................... 39
Running System Initialization ..............................................................................................................40
Exporting CAL or VER Kits
Deleting CAL and VER Kit Information ................................................................................................41
Creating Calibration and Verification Reports .....................................................................................41
Setting Up Batches ....................................................................................................................................41
Batches Page ............................................................................................................................................ 41
Using the Batches Page ......................................................................................................................43
Create a New Batch from an existing Protocol ....................................................................................43
Create a New Batch from a new Protocol ...........................................................................................44
Create a New Multi-Batch ................................................................................................................... 45
Running a Batch ..................................................................................................................................50
Importing a Batch ................................................................................................................................50
Exporting a Batch ................................................................................................................................50
Delete Batch ........................................................................................................................................50
Editing a Batch ....................................................................................................................................51
Settings Tab .......................................................................................................................................51
Analytes Tab ....................................................................................................................................... 53
Protocols Tab ......................................................................................................................................56
Stds and Ctrls Tab ...............................................................................................................................57
Managing Sample Lists ............................................................................................................................. 59
Create Sample Tab .............................................................................................................................61
Performing Analysis ...................................................................................................................................63
Current Batch Tab ...............................................................................................................................64
Select Replay Mode ............................................................................................................................68
Analyzing a Saved Batch .................................................................................................................... 69
Results Page ............................................................................................................................................. 72
Results Tab .........................................................................................................................................72
Settings Tab ........................................................................................................................................74
Log Tab ...............................................................................................................................................77
Sample Details Tab .............................................................................................................................78
LIS Results Tab ...................................................................................................................................79
Reports Tab .........................................................................................................................................80
Using Protocols, Lots, and Kits ..................................................................................................................82
Creating an Allele Call Protocol ...........................................................................................................82
Importing a Protocol ............................................................................................................................83
Adding a New Lot for Protocol .............................................................................................................83
Deleting a Protocol ..............................................................................................................................83
Exporting a Protocol ............................................................................................................................84
Lots and Kits ........................................................................................................................................84
.................................................................................................................40
Chapter 4 Performing System Maintenance ...................................................................87
Initial Startup ..............................................................................................................................................87
Adjusting the Sample Probe Height .................................................................................................... 87
xPONENT for MAGPIX
x
Page 12

Revive After Storage Routine ..............................................................................................................89
Calibration/Verification Routine ...........................................................................................................90
Daily Activities ............................................................................................................................................91
Defining the System Initialization Routine ...........................................................................................91
Running the System Initialization Routine ...........................................................................................91
Auto Maint Tab ..........................................................................................................................................92
Lot Management Tab ..........................................................................................................................94
Performing Individual Maintenance Commands ........................................................................................95
Cmds and Routines Tab ......................................................................................................................95
Probe and Heater Tab .............................................................................................................................101
System Info Tab .......................................................................................................................................102
System Status Tab ............................................................................................................................104
Support Utility Tab ...................................................................................................................................106
Sending a Support File ......................................................................................................................106
Shutting Down the Analyzer ....................................................................................................................107
Contacting Technical Support ..................................................................................................................107
Viewing the Luminex Website ..................................................................................................................107
Glossary ...........................................................................................................................109
Table of Contents
xi
Page 13

xPONENT for MAGPIX
xii
Page 14

Chapter 1: Introduction
Luminex® xPONENT® for MAGPIX® (xPONENT 4.1) IVD software was developed to
improve workflow and efficiency in the laboratory. Designed for ease-of-use, xPONENT
enables both new and advanced users to set up and run assays in a minimal amount of time.
This manual describes the features and functions of xPONENT for MAGPIX. To ensure that
you have the most up-to-date version of this manual, visit http://www.luminexcorp.com/
support/tech_manuals.html. Versions of this manual in languages other than English are
available on the Luminex website.
Software Packages
Multiple levels of user access can be licensed for xPONENT.
• Basic - Allows instrument control.
• Secure - Includes all of the Basic functions as well as administrator-controlled user
permission levels.
Additional features for which you can obtain a license:
• 21 CFR Part 11 - Includes all of the Secure package features as well as the option to
require electronic signatures to perform certain tasks. (Electronic signatures are listed in
the system log.)
• Automation - Includes the ability to communicate with external hardware.
• Remote Web Monitoring - Enables you to view alerts and system status using a
webpage.
• LIS - Enables the system to communicate with an external Laboratory Information System
(LIS) database. The LIS package enables you to export and import patient result data in
ASTM file format.
You must have an instrument control license to operate the instrument.
For more information about purchasing upgraded packages, or to obtain specific package
documentation, contact your vendor.
1
Page 15
About This Manual
The conventions in this manual assume a basic familiarity with computers and a knowledge
of Microsoft® Windows® software. Commands are often available through more than one
method, for example, from the toolbar and from menus that appear when right-clicking an
area of the screen. However, for ease of use, the procedures in this help describe only one
method for accessing commands.
Warnings, Notes and Symbols
The following informational notes and warnings are used in this manual.
NOTE: This message is used to provide general helpful information.
CAUTION: A caution advises users that failure to take or avoid a specific
action can result in loss of data.
WARNING: A warning advises users that failure to take or avoid a specific
action can result in physical harm to the user or the hardware.
You may encounter the following symbols while using xPONENT for MAGPIX. These
represent warnings, conditions, identifications, and important information.
General Guidelines
Warning, Biological Hazard Heat/Hot Surface Warning
General Warning
Caution, Risk of Danger
Manufacturer Reference Manual
In Vitro Diagnostic
European Representative
xPONENT for MAGPIX
2
Page 16

CAUTION: Modifying or deleting xPONENT system files may cause
degradation of system performance. You can repair modified or
deleted xPONENT system files by uninstalling and re-installing
the xPONENT software. Luminex recommends that you contact
Technical Support before uninstalling and re-installing xPONENT
CAUTION: Using unauthorized third-party software with xPONENT software
may result in corruption or failure of the xPONENT software. Use
third-party software at your own risk.
CAUTION: If you are using a screen saver on the PC on which xPONENT is
installed, xPONENT prevents it from activating. A dialog box
displays each time xPONENT is launched, recommending that
the screen saver and any power management settings be turned
off.
CAUTION: This system contains electrical and mechanical components that,
if handled improperly, are potentially harmful. In addition,
biological hazards may be present while operating the system.
Luminex recommends that you adhere to standard laboratory
safety practices. The protection provided by the equipment may
be impaired or the warranty voided if Luminex MAGPIX is used
in a manner not specified by the instructions or by Luminex
Corporation. Refer to the Luminex® MAGPIX™ Installation and
Hardware User Manual for detailed safety information.
Biological Samples
CAUTION: Human and animal samples may contain biohazardous
infectious agents. Where exposure to potentially biohazardous
material—including aerosol—exists, follow appropriate biosafety
procedures and use personal protective equipment such as
gloves, gowns, laboratory coats, face shields, or mask and eye
protection. Use ventilation devices. Observe all local, state, and
federal biohazard handling regulations when disposing of
biohazardous waste material.
Dilute concentrated biological samples, such as plasma or serum, at least 1:5 with reagents
as part of assay setup or as a final dilution step to reduce the chance of system clogs. If you
are running a MagPlex® kit, follow the dilution instructions in the kit’s product insert.
Bead (Microsphere) Handling
MagPlex beads come in various configurations. To reduce foaming and surface precipitation,
avoid agitating the beads until you are ready to vortex and use them. The beads will settle
and must be resuspended by vortexing before use. In addition:
• Multiple pipetting from the original container may affect bead concentrations.
• Protect MagPlex beads from light at all times to prevent photobleaching. Photobleaching
effects are cumulative. To maintain the integrity of the beads, minimize their exposure to
light during your development and manufacturing phases.
• Store MagPlex beads at 2°- 8°C.
Introduction
3
Page 17

NOTE: Refer to the product information sheet that accompanies your IVD
assay for additional information.
CAUTION: Do not use strong organic solvents with MAGPIX. For
information about specific compatibility, visit the Luminex
Technical Support website at http://www.luminexcorp.com/
support/faqs.html.
Safety Precautions
WARNING: All samples should be regarded as potentially contaminated and
treated as infectious. Samples should be handled at the
Biosafety Level 2, as recommended for any potentially
infectious human serum or blood specimen in the Center for
Disease Control/National Institutes of Health Manual, “Biosafety
in Microbiological and Biomedical Laboratories,” 1984.
WARNING: Although beads do not contain hazardous or carcinogenic
components at toxic levels, they may be toxic if swallowed. In
addition, contact with acids liberates toxic gases. If beads come
into contact with skin, wash immediately with copious amounts
of water. In case of an accident, seek medical advice
immediately and show the product label or container to your
medical provider. A material safety data sheet is available upon
request.
WARNING: The Drive Fluid and the solution in which MagPlex beads are
stored contain ProClin®, which may cause an allergic reaction.
Use personal protective equipment, including gloves and safety
glasses. Check your assay's package insert for assay
component information.
Bead Concentration
Follow the instructions in the
MAGPIX Technology
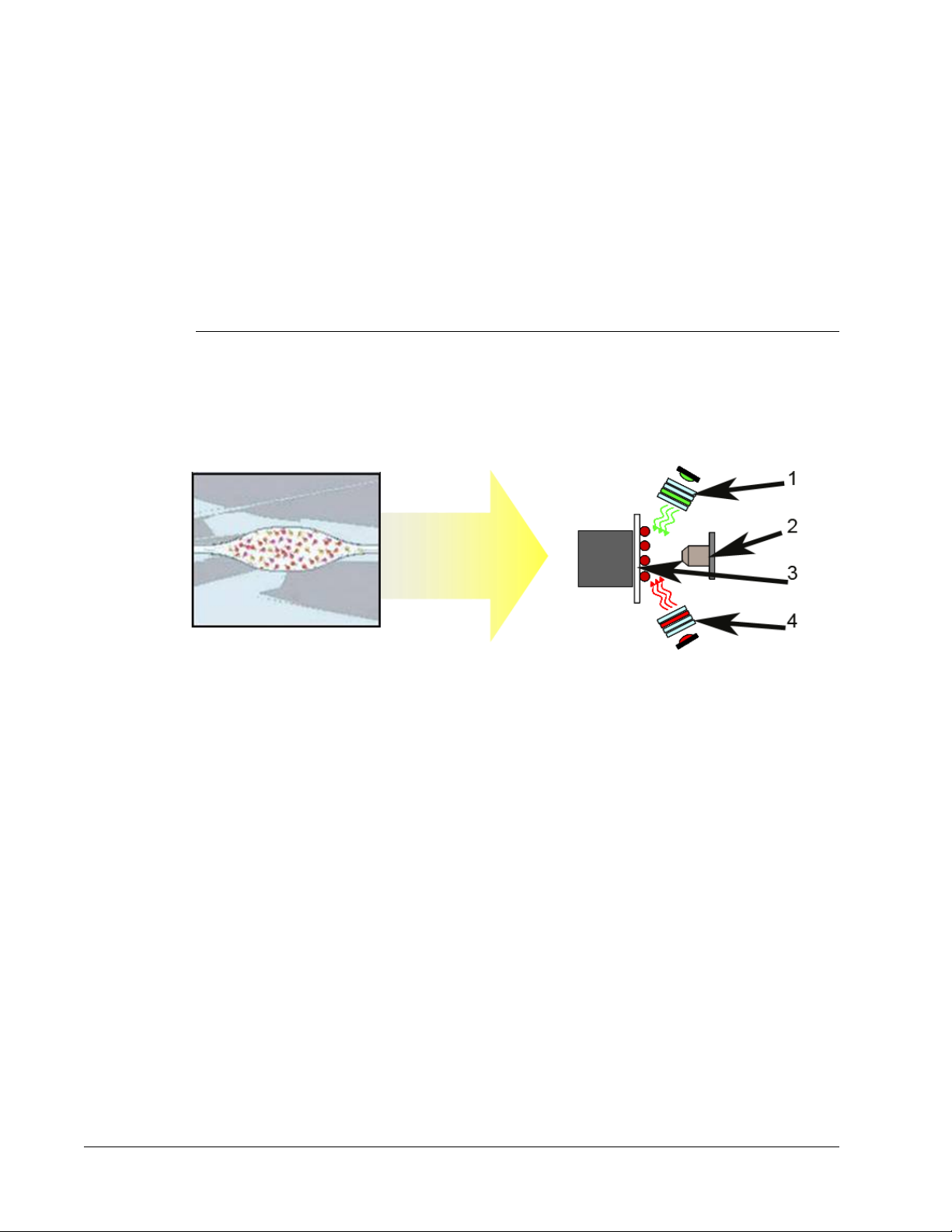
The MAGPIX system operates by using magnetic beads (microspheres) that are coated with
a reagent specific to a particular bioassay, enabling the capture and detection of specific
analytes from a sample. The sample mixture is aspirated by the sample probe and conveyed
via Drive Fluid into the camera chamber, where the beads are pulled down into a monolayer
by the magnet, and immobilized, and imaged. Within the chamber, beads are exposed to a
red LED and a green LED, which excite both the internal dyes that identify each bead’s color
signature and the reporter fluorescence from the surface of the beads. The red LED is
responsible for classifying the beads. The CL1 and CL2 filters function to categorize the
beads based on color signature and place them properly on the bead map as well as throw
out any doublets that may exist.The green LED with the RP1 filter produces the reporter
fluorescence which identifies the analytes captured during the assay. The beads are then
flushed to the waste container, clearing room for the next sample.
Calibration is important to ensure that the optical system functions effectively and that
different Luminex MAGPIX systems report similar results. Calibrating the MAGPIX system
IVD kit’s product insert and use the provided software protocol.
xPONENT for MAGPIX
4
Page 18
normalizes the settings for the classification channels (CL1 and CL2) and the reporter
channel (RP1). Use the Luminex MAGPIX Calibration Kit to accomplish this.
Following calibration, use the Luminex MAGPIX Performance Verification Kit to check all of
the optical channels in the system for correct calibration. It is essential to verify every time
you calibrate. If there is a problem with optical integrity or fluidics, MAGPIX may pass
calibration but fail performance verification. The Luminex MAGPIX Performance Verification
Kit includes reagents to verify the calibration and optical integrity for the Luminex MAGPIX
system as well as reagents to verify the fluidics channels using observations of bead count
and well-to-well carryover.
FIGURE 1.
Interrogation of MagPlex® Beads by the Red and Green LEDs
1. Green LED 2. Camera
3. Chamber 4. Red LED
Repetitive MagPlex Bead Measurements
In a MagPlex assay, the reporter signal is the result of the assay. Due to small bead size,
MagPlex bead suspension exhibits near solution-phase reaction kinetics. This means that
each set of beads used for a particular assay shows a statistically even distribution of
reporter molecules bound to the surface of each bead. The fluorescence signal of reporter
molecules bound to the surface of each bead set is measured and used to determine the
result of each assay in a multiplex. During data acquisition, numerous beads of each set are
analyzed and the median statistic is computed for that set by xPONENT. The more beads of
a set measured, the more confidence that can be given for that particular measurement.
Luminex recommends that you use R-Phycoerythrin as your reporter fluorophore.
Follow the instructions in the IVD kit’s product insert and use the software protocol provided.
Introduction
5
Page 19

Classification and Reporter Fluorochromes
MagPix beads in the calibration kit are used to autofocus the camera and calibrate the CL1,
CL2, and RP1 channels. The beads in the verification kit are a mix of 6 different regions that
cover the range of the 50-plex map. Both calibration and verification beads are triple-dyed,
and the fluorescence signal of these dyes enables classification of each bead set.
TABLE 1.
MAGPIX Active Bead Regions (by Region)
Region Region Region
MC10012 MC10013 MC10014
MC10015 MC10018 MC10019
MC10020 MC10021 MC10022
MC10025 MC10026 MC10027
MC10028 MC10029 MC10030
MC10033 MC10034 MC10035
MC10036 MC10037 MC10038
MC10039 MC10042 MC10043
MC10044 MC10045 MC10046
MC10047 MC10048 MC10051
MC10052 MC10053 MC10054
MC10055 MC10056 MC10057
MC10061 MC10062 MC10063
MC10064 MC10065 MC10066
Fluidics 1 and Fluidics 2
Although it undergoes a wash step in between wells, the probe can be susceptible to carryover from well-to-well. Fluidics 1 contains one bead set. Fluidics 2 contains a buffer solution
and a different control bead. The function of this maintenance procedure is to measure how
much (as a percentage) of the first bead set in Fluidics 1 is found in the well where Fluidics 2
has been loaded.
Sample Volume
Sample volumes range in size from 20 µL to 200 µL. Ensure that approximately 25 µL more
than the sample volume remains in the well after aspiration. This amount may vary
depending on the type of plate used. Your sample volume must be large enough to prevent
aspirating air into the fluid line when acquiring sample, and small enough to prevent spill-over
xPONENT for MAGPIX
6
MC10067 MC10072 MC10073
MC10074 MC10075 MC10076
MC10077 MC10078
Page 20

when the analyzer flushes the sample lines after sample acquisition and expels
approximately 75 µL of sample back into the well.
Examples
• If you use a sample volume of 50 µL and aspirate 50 µL, you will acquire air bubbles.
• If you use a sample volume of 200 µL and a standard sample pickup of 50 µL, the well will
Follow the instructions in the IVD kit’s product insert and use the software protocol provided.
Do not dilute MagPix Calibration or Verification beads, or the Fluidics 1 and Fluidics 2 beads.
Plates
Follow the xMAP-based IVD kit instructions for use. Follow these guidelines when choosing
plates:
overflow when the analyzer washes the sample lines after acquisition and expels fluid back
into the well, because the amount of fluid expelled back into the well is approximately 75
µL.
CAUTION: Sample volume is critical to the proper functioning of your
MAGPIX instrument. Aspirating too few beads can result in
insufficient bead count or insignificant data results. Aspirating too
many beads can result in saturation of the chamber and prevent
proper bead classification, which may also result in low bead
counts or inconclusive data.
• When using uncovered plates, use black opaque plates to reduce photobleaching.
For heated assays, use CoStar® Thermowell® 96-well, thin-wall polycarbonate, model P
•
plates.
• For unheated assays, use a 96-well plate with an overall height no greater than 0.75
inches (19 mm).
WARNING: The heater plate of the MAGPIX plate carrier may be hot. Do
See the recommended consumables list on the Luminex website at http://
www.luminexcorp.com/support/recommendedmaterials/index.html for more information.
Touch Screen
A touch screen option is available with xPONENT for MAGPIX.
not touch the heater plate.
Introduction
7
Page 21

xPONENT for MAGPIX
8
Page 22

Chapter 2: Application Administrator
Tasks
System Setup
If you have a secure version of xPONENT for MAGPIX, you must have xPONENT
administrative privileges to perform System Setup tasks. If you do not have a secure version
of xPONENT for MAGPIX, all users may be able to perform system setup tasks. To view
administrative options, click Admin
on the navigation bar.
9
Page 23
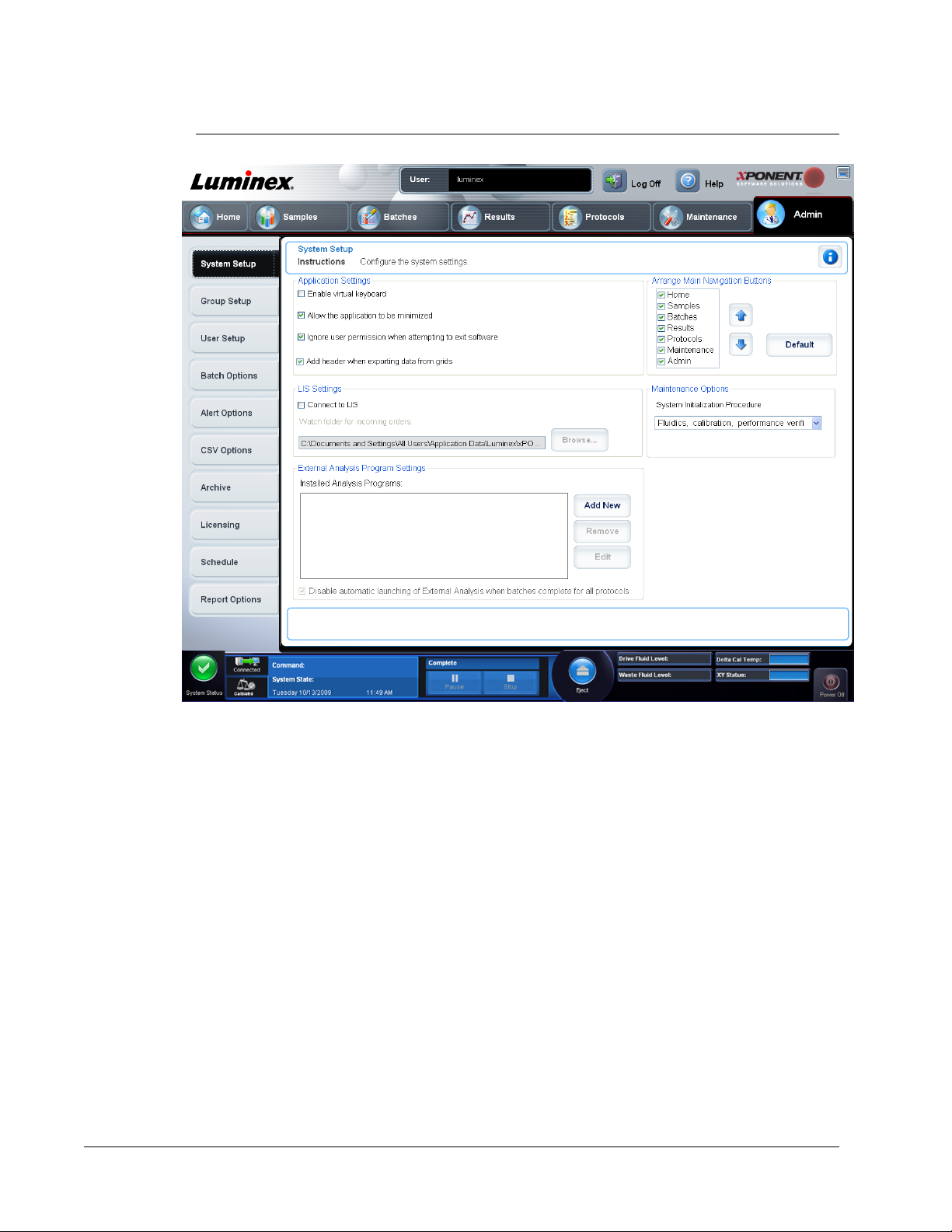
FIGURE 2.
System Setup Tab
Viewing System Status
The System Monitor displays at the bottom of all xPONENT windows. It displays the
physical state of the Luminex system. Values are reported directly from the Luminex system.
Application Settings
These settings change graphical user interface (GUI) preferences.
Enable Virtual Keyboard -
Allow the application to be minimized - Enables you to minimize xPONENT so that you
can access the desktop. If this check box is not selected, you cannot minimize xPONENT.
Ignore user permission when attempting to exit software software regardless of whether or not they have been granted this permission in the User
Setup tab
Add header when exporting data from grids -
Use Application Settings - Defines how the software runs and displays.
Select the options you want to enable in the software and click Save.
xPONENT for MAGPIX
10
Enables the touchscreen virtual keyboard.
Enables users to exit the
Adds a header row when exporting the data.
Page 24

LIS Settings
You must have the LIS version of the software to perform this task.
To connect to the LIS, check Connect to LIS. In the Watch folder for incoming orders box,
browse to the location where xPONENT should watch for incoming orders.
Select the options you want to enable in the software and click Save.
External Analysis Program Settings
Use this option if you are using a program other than xPONENT to analyze collected data.
This tab contains the following fields:
Installed Analysis Programs - Lists the analysis programs currently installed.
Browse - Opens another dialog box to select the file location for the third-party analysis
program. The selected location appears in the Path
box.
Command Line Parameters - Type the command line parameters for the parameters you
want xPONENT to use with the external analysis program. If the information is supplied with
the external analysis program documentation, use that information. Otherwise, you can type
the following default parameters, built into xPONENT, in any order:
• #c - Output.csv, full file path
• #p - Protocol name
• #b - Batch name
• #u - Logged in user name
box, and the name displays in the Name
To keep the default command line settings, leave Command Line Parameters blank.
Set Default - Sets the chosen analysis program as the default analysis program for
xPONENT.
OK - Saves the chosen settings and exits the dialog box.
Cancel - Cancels changes and exits the dialog box.
Remove - Removes the selected program from the Installed Analysis Programs list.
Edit - Opens the New External Analysis Program dialog box where you can edit the
settings for the selected program.
Disable Automatic launching of External Third Party Analysis when batches complete
for all protocols - Disables the automatic launch of the third-party analysis program
automatically after batch acquisition.
Application Administrator Tasks
11
Page 25
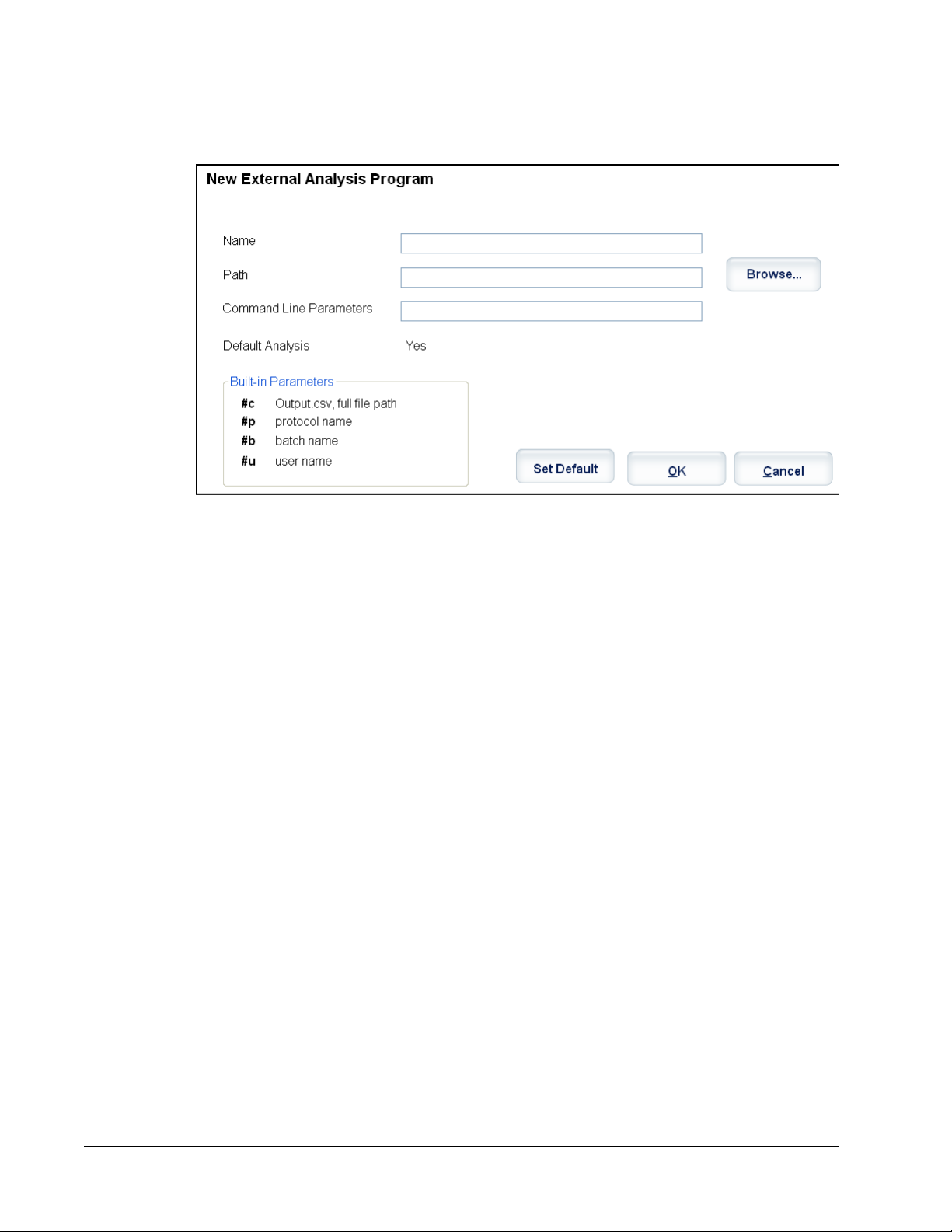
FIGURE 3.
New External Analysis Program Dialog Box
Adding an External Analysis Program
1. If the program is on an external media source such as a CD or flash drive, insert the
media into the appropriate drive on the PC.
2. Click Add New to open the
3. Type a name for the external analysis program.
4. Click Browse to navigate to the .exe file for that program. Double-click the file name.
5. Type the command line parameter for the parameters you want xPONENT to use with
the external analysis program. If the information is supplied with the external analysis
program documentation, use that information. Otherwise, you can type the following
default parameters built into xPONENT, in any order:
• #c - Output.csv, full file path.
• #p - Protocol name.
• #b - Batch name.
• #u - Logged in user name.
To keep the default command line settings, leave Command Line Parameters blank.
6. Click Set Default if you want to make this program the default analysis program, then
click OK to close the dialog box.
7. Click OK if you want to add the program as an installed analysis program, but not as the
default analysis program.
8. Click Save.
New External Analysis Program dialog box.
xPONENT for MAGPIX
12
Page 26

Editing an Analysis Program
1. In the Installed Analysis Programs list, click the program you want to edit.
2.
Click Edit. The Edit External Analysis Program dialog box opens.
3. Edit the Name, Path, or Command Line Parameters, or make this the default analysis
program if there are two or more programs installed. The default analysis program name
displays in bold text.
Removing an Analysis Program
To remove an analysis program from the Installed Analysis Programs list:
1. In the Installed Analysis Programs list, select the program you want to uninstall.
2. Click Remove. To prevent the external analysis program from starting automatically,
select Disable automatic launching of External Analysis when batches complete for
all protocols.
Arranging Main Navigation Buttons
Use this section to arrange the main pages at the top of the xPONENT screen.
NOTE: The Home page, and in some instances the Admin page, cannot be
moved.
To arrange the main navigation buttons:
1. Select or clear the check boxes by each page name to hide or display the page.
2. Click a page name and use the up and down arrows to change the order in which the
pages display, from left to right.
3. Click Save.
4. Click Default if you want to restore the main navigation.
Maintenance Options
Run one of the System Initialization procedures as part of your daily startup routine.
Luminex recommends that you verify daily and calibrate weekly. You should also verify and
calibrate if any of the following occurs:
• The delta calibration temperature exceeds ± 5° C.
• You move the instrument.
• You experience sample acquisition problems.
Available system initialization procedures are:
• Fluidics preparation, calibration, performance verification
• Fluidics preparation, performance verification
Group Setup Tab
This tab is accessible only in the Security or 21 CFR Part 11 packages. Use this tab to assign
permissions to different groups of users. If you have the 21 CFR Part 11 package, you can
require an electronic signature in order to perform selected tasks.
Application Administrator Tasks
13
Page 27
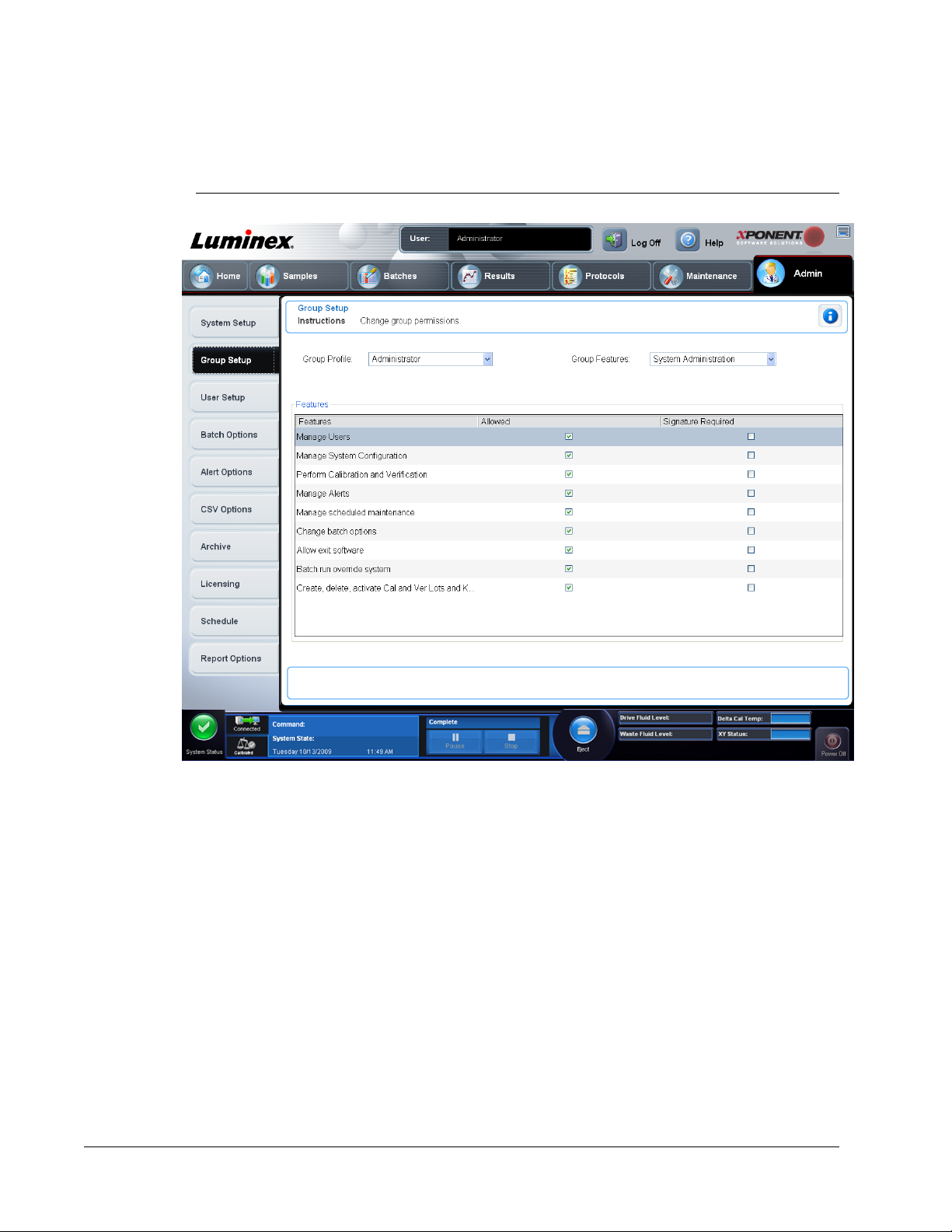
NOTE: The 21 CFR Part 11 package also provides full access to the Secure
Package functionality.
FIGURE 4.
Group Setup Tab
Users are assigned to groups. These users then have permissions granted to their group.
This tab contains the following:
Group Profile - The following user groups are predefined:
• Administrator
• Supervisor
• Service
• Technician2
• Technician1
• Reviewer
The user will belong to the group you select.
xPONENT for MAGPIX
14
NOTE: Assign permissions directly to an individual by assigning the user to
a specific Group Profile on the User Setup tab.
Page 28

Group Features - The Group Features
a category from the list, the Features section displays the individual tasks that are a part of
that category. The following categories are available
• System Administration
• Batch Management
• Protocol Management
• Lot and Std/Ctrl Kit management
• Import and export data
• Archiving
The Allowed check box next to the desired permission in the Features section enables the
selected group to perform that task. The Signature Required check box next to the desired
permission requires a digital signature whenever a user in the selected group performs that
task.
Clear the Allowed check box and select Signature Required to require the electronic
signature of another user whose account is configured to allow the action. When you do this,
the current user cannot complete the action without this electronic signature.
The following permissions are available for these groups:
System Administration
• System Administration Manage Users (add, edit, or delete users)
• Manage System Configuration
• Perform Calibration and Verification
• Manage Alerts
• Manage scheduled maintenance
• Change batch and CSV options
• Allow exit software
• Batch run override system
• Create, delete, activate CAL and VER Lots and Kits
list contains permission categories. When you select
Batch Management
• Create Batch
• Edit Batch
• Delete Batch
• Run Batch
• Validate and Invalidate Results
• Approve Batch
• Reanalyze Results
• Save Batch after changing results
• Change Formula
• Reacquire errored wells for partial batch
• View Processed Batch Results
Application Administrator Tasks
15
Page 29

• Export Processed Batch Results
• Change Sample Load Volume During Run
Protocol Management
Delete Protocol
Lot and Std/Ctrl Kit management
• Create Std/Ctrl Kit and Lots
• Edit Std/Ctrl Kit and Lots
• Delete Std/Ctrl Kit and Lots
Import and Export Data
• Export Batch, Protocol, Kit or Lot Files
• Import Batch, Protocol, Kit or Lot Files
Archiving
• Backup/Restore
• Import/Archive
When you or any user perform an action that requires an electronic signature, the Electronic
Signature dialog box opens. The user ID autopopulates. Type your password and any
comments. Click OK to complete the electronic signature, or Cancel to cancel the signature.
Setting Up Group Permissions
1. In the Group Profile list, click the group profile you want to set up.
2.
3. In the Features section, select the Allowed check box next to the desired permission to
4. Click Save.
User Setup
The User Setup page includes the following information:
Global User Settings - Define global user settings.
User Setup Tab - used to create or edit a user’s credentials, view a list of users, and remove
a user from the system,
Create New User
List of Users - View and Edit
In the Group Features list, click which group features you want to set for the group
profile you selected.
enable the selected group to perform that task. If you are using the 21 CFR Part 11
package, enable the Signature Required check box next to the desired permission to
require a digital signature whenever a user in the selected group performs that task.
These tasks are tracked in the System Log.
NOTE: You must have xPONENT administrative privileges and be using
either the 21 CFR Part 11 or Secure package of the software to
perform user setup tasks.
xPONENT for MAGPIX
16
Page 30
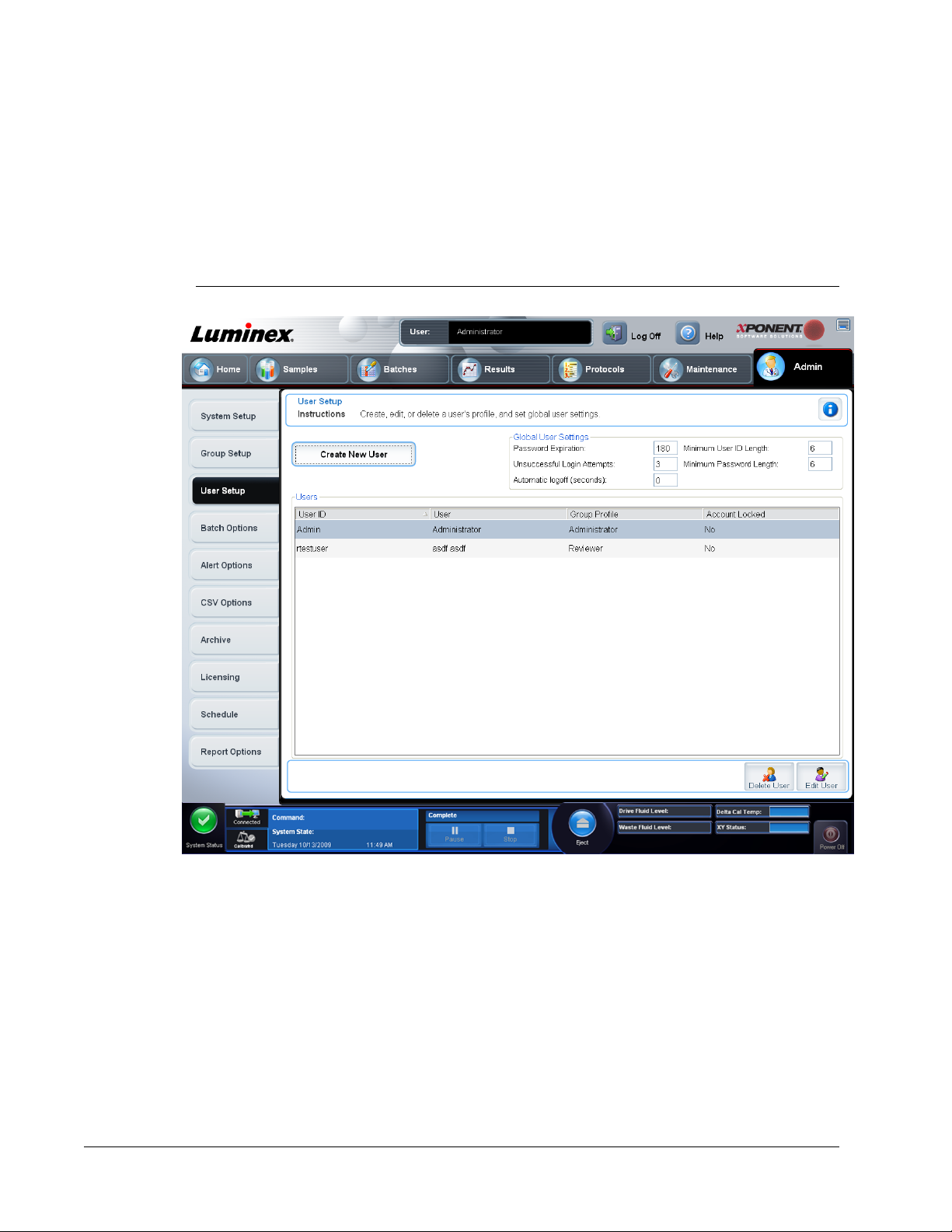
Buttons to delete or edit settings for a selected user.
User Setup Tab
Use this tab to create or edit a user account, remove a user from the system, or view a list of
authorized users, along with their profile details. You must be using either the 21 CFR Part 11
or Secure package of the software to perform user setup tasks.
FIGURE 5.
User Setup Tab
This tab contains the following.
Create New User - Click to open the Create User Account screen.
Global User Settings - Settings common to all users.
Users - A list of users in the system. To edit information about a user, select the User ID and
click Edit. To delete a user, select the User ID and click Delete.
Global User Settings
Use this tab to define global user settings as well as view and edit the accounts of current
users.
Application Administrator Tasks
17
Page 31
Defining Global User Settings
1. In the Global User Settings section, type a length of time (in days) for the Password
Expiration
2. Set the number of allowed Unsuccessful Login Attempts. After this number of failed
attempts to log in, the user’s account is locked.
3. Set how many seconds pass before an Automatic Logoff is initiated.
4. Type the Minimum User ID Length. The default minimum User ID length is six
characters.
5. Type the Minimum Password Length. The default minimum password length is six
characters.
6. Click Save.
. The default is 180 days.
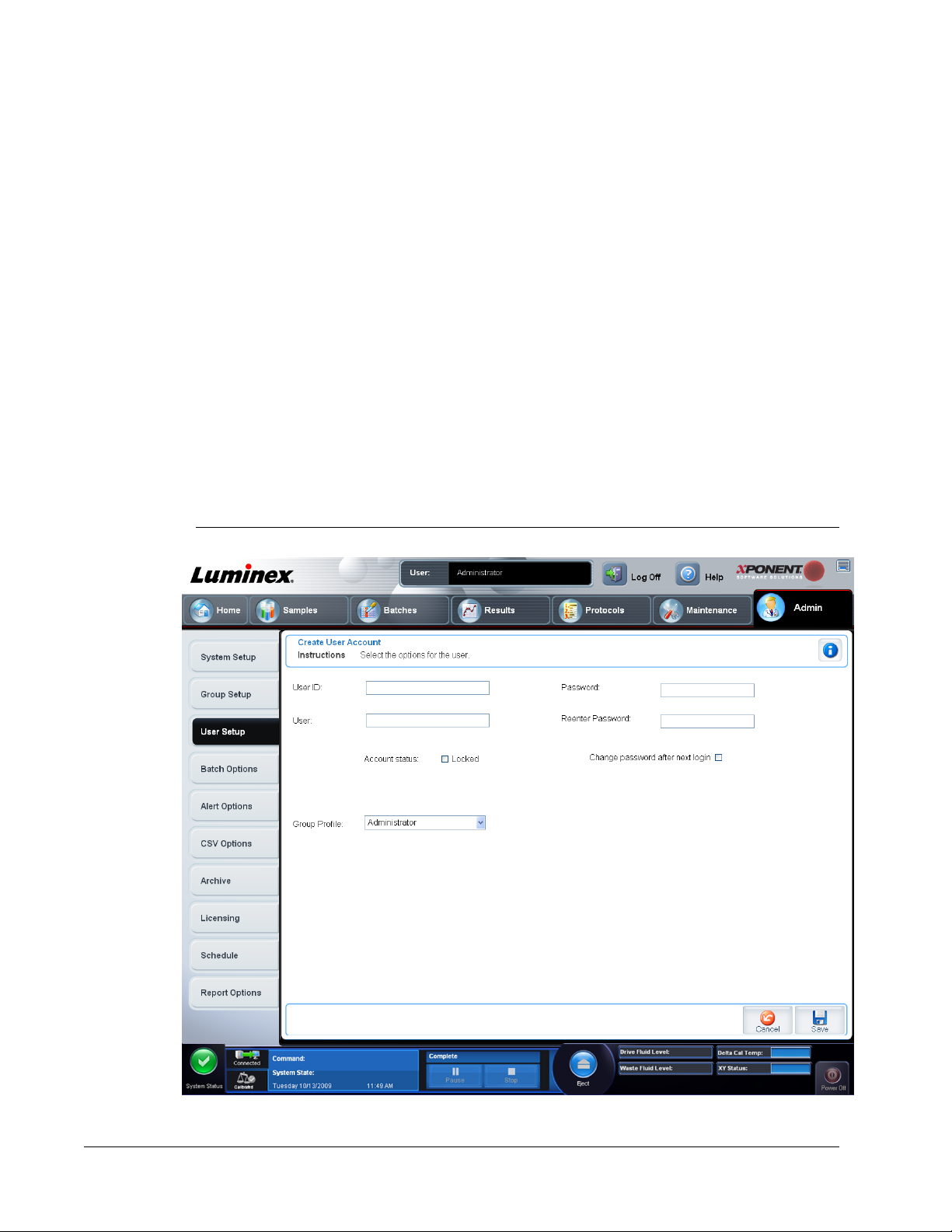
Create User Account Screen
Use this screen to create and set options for a new user. Any user with access to this tab can
assign rights to any and all users.
FIGURE 6.
Create User Account Screen
xPONENT for MAGPIX
18
Page 32

Creating a New User
1. Click Create New User. The
2. Type the user ID in the User ID box. The user ID is not case-sensitive. You can change
the required number of characters required for a user ID on the User Setup tab. Once
you create and delete a user ID, you cannot use that user ID again.
3. Type the user’s name in the User box.
4. Select the Account Status check box to lock the account, or clear this check box to
unlock the account.
NOTE: If a user account is locked, the administrator is notified at each log in.
5. Type a password for the user in the Password box, then re-type it in the Reenter
Password box. If you want the user to change the password on first login, select Change
password after first login. The required length for passwords is set on the Group
tab.
Setup
6. In the Group Profile list, select the role for the user you are creating.
7. Click Save or cancel to return to User Setup without saving.
Batch Options Tab
If you are using the 21 CFR Part 11 or Secure package, you must have administrative
privileges to set batch options. Use this tab to set options for acquisition and batch analysis.
Create User Account screen opens.
Application Administrator Tasks
19
Page 33

FIGURE 7.
Batch Options Tab
Batch Options. This section displays the following options:
• Allow running a batch with expired reagents - Allows batches to run with expired
reagents.
• Allow running a batch if XY temperature is not in range - Allows batches to run if the
XY temperature is not in range.
• Allow running a batch if the instrument is not calibrated or verified - Allows batches to
run if the instrument is not calibrated.
• Prompt for reacquiring of the last errored wells for an aborted partial batch - Allows
users to choose to reacquire aborted wells when resuming a stopped/partial batch or to
start with the next unacquired well.
Batch Settings This section displays the following options:
• Calibration expiration days - Type the desired number of days a system calibration
remains active before expiring. The default number of days is seven.
• Analysis display decimal places - Type the number of digits the system displays. The
default number of digits is three.
xPONENT for MAGPIX
20
Page 34

• Minimum bead count for obtaining results - Type any whole number from 0 to 1000000.
This is the minimum bead count that must be observed by the instrument for a particular
analyte before this data is used in statistical calculations and is displayed on the graphs
and results table. This number varies based on assay. When set to a number greater than
0, the analyzer does not display data for bead sets not generating events equal to or less
than this value. Type 0 to display all events. The default setting is 1.
• Allow batches to be run or saved without lot number, expiration or manufacturer Allows saving lots without normal required information.
• Run Routine after batch complete - Select this option to specify a particular routine to
run after a batch is finished. If you select this option, then select a routine from the Routine
to run after batch completes list.
• Run Rinse before batch starts - This options performs a Rinse at the last selected
location on the Cmds and Routines page for the Rinse command. Ensure that you have
sufficient room in the reservoir to perform this Rinse.
Batch Thresholds
Low bead count detection - Selecting either Error
warning if the number of successive wells in the Well Count box are run without reaching
the number of beads in the Bead Count box. Click in either box to type a number other
than the default. Warning pause pauses the batch to allow the user to change the load
volume and then resume the batch.
Cancel - Cancels the changes.
Save - Saves the changes.
Alert Options Tab
To view this tab, click the Alert Options tab on the Admin page. Use this tab to set options
for alerts about various system events.
or Warning stops the batch or logs a
Application Administrator Tasks
21
Page 35

FIGURE 8.
Alert Options Tab
Alert Options - Offers three check boxes for each alert - Dialog, Email, and Sound. This
section displays the following events:
• Locked User - The user's account is locked and must be unlocked by an administrator.
• Batch Complete - The batch run is complete.
• Heater In Range - The plate heater is warmed to the designated range.
• Scheduled Maintenance - A scheduled maintenance routine is now due.
• Low Bead Count Detected - A bead count below the accepted range is encountered.
• Driver Fluid Low/Waste Full - The Drive Fluid level is running low and may need to be
replaced soon, or the Waste fluid is full and needs to be emptied.
• Routine Complete - The currently running routine is complete.
• Delta Cal Temp Exceeded Tolerance During Batch - The delta cal temperature fell
outside the designated range during the running of the batch.
Select the appropriate check boxes to display a pop-up message, send an email, or play a
sound to alert you when the event occurs. All check boxes are selected by default. Cancel
and Save buttons display if you change a setting.
Speakers Attached/Speakers Not Attached - Enables sounds to be played through
speakers. Click this button if you have speakers attached to your system. The button title will
alternate between attached/not attached status when you click it.
xPONENT for MAGPIX
22
Page 36

Setup Email - Opens the Setup Email Dialog
box
FIGURE 9.
Setup Email Dialog Box
Enable or disable email notification by selecting or clearing the Email Active check box.
Contact your network administrator to determine the information needed in the Mail Server
Host, From Email Address, From Email Password, and Mail Server Port boxes, and if
you need to select the Enable SSL (Secure Sockets Layer) check box
The Test button tests the email settings. Select Apply to initiate the settings you’ve selected,
or click OK to use the settings and exit the dialog box. Cancel exits the dialog box without
saving the settings.
Setting Notification Options for System Events
1. Select Dialog if you want a dialog box to open for a specific event.
2. Select Email if you want to send an email notification for a specific event.
3. Select Sound if you want a sound to play for a specific event.
Application Administrator Tasks
23
Page 37

4. If you choose to send an email notification for a specific event, and the email address is
not already set up, click Setup Email at the bottom of the page to open the Setup Email
dialog box.
• Turn email notifications on or off by checking or clearing the Email Active check box at
the bottom of the screen.
Contact your system administrator to determine the correct information for the Mail
•
Server Host, From Email Address, From Email Password, and Mail Server Port
boxes, and whether you need to check the Enable SSL (Secure Sockets Layer) check
box.
• Type addresses to which you want to send alert notifications in the Email Addresses
box; separate address with a comma (,).
• Click Test to send a test email to the addresses you have typed.
5. Click OK to apply any changes and close the dialog box or Apply to apply any changes
but remain in the Setup Email dialog box.
CSV Options Tab
To view this tab, click the CSV Options tab on the Admin page. You must have
administrative privileges to perform this task if you are using the 21 CFR Part 11 or Secure
package. Use this tab to define what the CSV file will contain and where it will be stored.
xPONENT for MAGPIX
24
Page 38

FIGURE 10.
CSV Options Tab
Automatically export results CSV file when batch is complete - Automatically export
results CSV file when batch is complete - Automatically exports the CSV file when the system
finishes analyzing the batch. This allows running programs on exported data without having
to manually start the export.
Automatically export batch when batch is complete - Exports batch information
automatically when a batch has completed.
Maximum number of data columns in CSV file - Sets the number of columns in your CSV
output file.
Use US regionalization format only - Exports data only in US regionalization format.
Include Advanced Statistics - Exports advanced statistics in the CSV file.
CSV Export Folder and Automatically Exported Batch File - Displays the path and
location where the CSV file or automatically exported batch file will be exported. Click Browse
to change the file export location.
Automatically convert the raw run files to CSV format for each well in the batch Automatically converts raw run files to CSV format for each well in the batch. This option
creates a CSV-formatted file for the raw bead data of each well.
Test Sort Order - Defines a method to sort the test data. The options are By Analyte Name,
By Region ID, or By Setup Order.
Application Administrator Tasks
25
Page 39

Cancel - Cancels changes.
Save - Saves changes.
Setting CSV Options
1. In the CSV and Batch Export Options section, select the options you want to apply to
the CSV file. If you select Maximum number of data columns in CSV file, type a
maximum number of columns.
In the CSV File Export Folder and Automatically Exported Batch Folder box, type the
2.
location to which you want the file sent, or click Browse to navigate to the folder.
3. In the Test Sort Order box, select the method that you want to use to sort the tests in the
protocols.
Archive Options Tab
To view this tab, click the Archive Options tab on the Admin page. Use this tab to set
options for backing up, exporting, and restoring files.
FIGURE 11.
Archive Options Tab
Archive. Use this section to choose file types to archive destinations for the archive file. This
section contains the following
xPONENT for MAGPIX
26
Page 40

• File Type - Choose from Protocols, Batches, Std/Ctrl Kits, Lots, Patient Samples, or
Log. The selected information appears in the list below the Archive Folder path.
• Keep data after archive - Retains data instead of deleting after you archive it.
• Exclude Raw Data Files - Select this option if you have selected Batches in the File Type
list to exclude files that contain only raw data.
• Browse- Chooses the network or local PC destination folder for the exported archive file.
The selected file displays in the Archive file export folder box.
NOTE: Files are restored from the path displayed in the Archive file export
folder box. Click Browse to select the file path if necessary.
The list below the Archive Folder path displays all the files of the type selected in the File
Type list. This list displays information for each file.
Current Storage Utilized
amount of space left, in the database and on the hard disk.
Backup System - nables backups and restores for xPONENT.
• Backup - Backs up the entire system (Click View Backup Log to view information about
the backup).
• Restore - Restores all information in the archive file to the system. This will overwrite all
information.
• View Backup Log - Displays a log of system backups.
- Displays the amount of space currently in use, as well as the
Import - Select a file type to import in the File Type list and click Import to import all files of
that type from the archive. The imported files display in the list below the Archive Folder
path.
Archive - Select a file type in the File Type list, and then select a file in the list below the
Archive Folder path and click Archive to archive the file. If you have selected Keep data
after archive, the file will still display in the list, otherwise it will no longer be displayed.
Archiving a File
1. In the File Type drop-down list, select the file type you want to archive. File type options
are Protocols, Batches, Std/Ctrl Kits, Lots, Patient Samples, and Log.
2. Select the files you want to archive in the list below the Archive Folder box (this list
displays all files of the selected file type in the internal database).
3. In the Archive Folder box, verify the location to which you want to archive the file(s). To
change the location, click Browse, then navigate to the new location and click OK. Note:
If you change the default archive location, ensure that the Archive Folder box reflects
that same location when you import those archived files.
4. Select Keep data after archive if you want to keep the file in the list after archiving rather
than deleting it.
Application Administrator Tasks
27
Page 41

5. To exclude raw data files from the archive (suggested if you are archiving Batches) select
Exclude raw data files.
6. Click Archive. The command executes. When complete, an Archive Successful
box opens. To delete a partial batch, open the Admin page, then click the Archive tab.
Clear the Keep data after archive box. Click Archive to delete the partial batch.
NOTE: To delete a partial batch, open the Admin page, then click the
Archive tab. Clear the Keep data after archive box. Click Archive
to delete the partial batch.
dialog
Backing Up xPONENT Data and Settings
1. Click Backup. A Warning
2. Click OK to continue with the backup. A progress bar displays.
NOTE: The backup file outputs by default to C:\Documents and Settings\All
Users\Documents\Backup\xPONENT.zip.
3. When the backup is complete, a System Backup dialog box opens.
dialog box displays.
Adding a Previously Archived File Back into the Database
1. In the
2. Click Browse and navigate to the archived file location if you previously changed the
3. Click Import. The Import Complete dialog box opens after the files have imported. All
4. Click OK
File Type list, click the file type you want to import.
location in the Archive Folder box.
archived files of the type selected in the File Type list will now display in the list below the
Archive Folder box, and be available for use with xPONENT.
Restoring the System Using a Backup File
1. Click Restore. A Warning dialog box displays.
CAUTION: If you click Restore, all data and settings are overwritten
2. Click OK to continue restoring the system. The File dialog box opens. The most recent
backup file displays in the File Name box. To use a different backup file, click Browse to
navigate to a different file.
3. Click OK to start the restore. A progress bar displays, followed by a Warning dialog box
stating that xPONENT needs to be restarted.
4. Click OK to restart xPONENT. (You must restart xPONENT after the restore task is
complete.)
Licensing Tab
Contact Luminex Technical Support to upgrade xPONENT for MAGPIX or to obtain a new
license.
xPONENT for MAGPIX
28
NOTE: You must restart the computer for the new license to take effect.
Page 42

Schedule Tab
Use the Schedule tab to enable or change the recurrence of scheduled maintenance
reminders.
FIGURE 12.
Schedule Tab
• Select the time of day that you want to receive alerts in the Alert Time list.
• Select how often you want to receive reminders in the Recurrence list.
• Enable or disable the reminders by selecting or clearing the Enabled check box.
Report Options Tab
Use this tab to add your company information and logo to reports.
Application Administrator Tasks
29
Page 43
FIGURE 13.
Report Options Tab
• Type a company name in the Company box, and any additional information in the Info:
box.
• Click Import Logo and then Open after navigating to the desired file to import a graphic for
the report header.
• Click Clear Logo to remove the logo.
• Save saves all changes.
xPONENT for MAGPIX
30
NOTE: Use a logo file with 920 x 125 pixels. To make the logo to appear to
the right of your company name, include 120 pixels of white space to
the left of the logo, in the graphic file; otherwise, the logo may appear
behind the company information.
Page 44

Chapter 3: Using xPONENT
Starting xPONENT
Perform the following steps if xPONENT does not launch automatically after starting you turn
on the computer.
• On the PC desktop, click the Luminex xPONENT icon, or click Start >
Luminex > xPONENT > Luminex xPONENT
• If you have a trial license, click OK in the dialog box that opens.
• If this is the first time you have started the software, the User License Agreement may
display. Read the license agreement. Select I accept the terms of this license
agreement, then click OK.
Initial Startup
When you turn on the system for the first time, perform the following procedures.
1. Adjust the Sample Probe Height
2. Revive After Storage (Luminex) Routine
3. Calibration/Verification Routine
Logging In to xPONENT
If your version of xPONENT is licensed for 21 CFR Part 11 and/or Security, an application
administrator must set up user IDs (and passwords, if required). If you are not using a version
with 21 CFR Part 11 and/or security, users can log in with any user name or with no user
name.
Programs >
CAUTION: Use of this software by untrained personnel can result in
inaccurate data and test results. Personnel who will use
31
Page 45

xPONENT must read this manual thoroughly before operating
the software.
1. On the System Login tab, type your user ID.
2. If you are using a secure version of the software, type your password. The Home
opens.
Logging Off of and Exiting xPONENT
1. Click Log Off.
2. Click OK in the Confirm Logout dialog box. If you do not want to exit the software, stop
here. To exit all of the way out, continue with step 3.
3. Click Exit.
4. In the Do you want to exit the software now? dialog box, click Yes.
Using Online Help
English-language help is available at all times while you are using xPONENT. To display
online help for the page or tab in which you are currently working, click the blue “i” icon at the
upper-right of the xPONENT window. This opens a help window with information specific to
page
that page or tab.
To display system-level help, click the blue question mark at the top of the xPONENT
window, then click Contents and Index. The online help opens, where you can navigate to
any available topic.
To display quick start information, click the blue question mark at the top of the xPONENT
window, then click Quick Start. This displays information about the basic steps to start the
system.
To display software information, click the blue question mark at the top of the xPONENT
window, then click About Luminex xPONENT. The xPONENT information dialog box opens,
displaying the software version information.
Screen elements
This section details the screen elements and the common terms used in this manual to
describe them.
xPONENT for MAGPIX
32
Page 46

FIGURE 14.
Screen Elements
Page
The main elements at the top of the window are Pages. Click a page to go to that page in the
software. All of the pages except the Home page, and in some cases the Admin page, can
be moved or deleted.
Tab
The elements on the left side of the window are Tabs. Click a tab to display information and
tasks on that tab. Some tabs are numbered and must be completed sequentially.
Right-click Menus
Certain sections of the software such as tables, lists, and text boxes have right-click option
menus. Menus are different depending upon the item you right-clicked.
• Print All
• Print Selection - Prints only the selected section or cell
• Import - Imports a file
• Export - Opens a File Dialog dialog box. Click Browse to select a location, file name, and
file type (TXT or CSV) for the export. This exports all data from the right-clicked item
- Prints all sections or cells of the item
1. Page 2. Tab 3. Right-Click Menu
• Cut - Cuts the selected data
Using xPONENT
33
Page 47

• Copy All - Copies all data
• Copy - Copies only selected data
• Paste
• Delete - Erases text or data from the selection
- Pastes previously copied text or data into the box
System Monitor
The System Monitor displays at the bottom of all xPONENT windows. It displays the
physical state of the Luminex system. Values are reported directly from the Luminex system.
FIGURE 15.
System StatusButton - This button has two functions: When clicked, it opens the system
log. It also displays the current status of the system. If there are no warnings or errors, the
System Status button is green with a check mark. If there is a warning or other important
user notification, the button is yellow with an X.
Connection Status or Disconnected). To ensure the analyzer connects to the PC, turn on the analyzer before
you start xPONENT.
Command Display - Displays the following:
System Monitor
1. System Status Button 2. Connection Status
3. Command Display 4. Progress Bar and Buttons
5. Eject XY Button 6. Drive Fluid Level
7. Waste Fluid Level 8. Delta Cal Temperature
9. XY Status 10. Power Off Button
Displays the status of the analyzer’s connection to the PC (Connected
• The command currently running.
• The system state (i.e. running, idle, etc.).
• Date and time.
A yellow Check Calibration button is visible in the command display if the calibration or
verification command has failed, when any verification is not current, or when the calibration
or verification was performed prior to the calibration expiration setting.
Progress - Displays a bar graph showing the progress of the current command or routine; if
the command or routine is finished, it displays a full progress bar and the command status as
Complete.
xPONENT for MAGPIX
34
Page 48

Pause - Pauses the system after the current command completes. Pause does not stop the
system in the middle of running a command. You cannot run another command while the
system is paused.
Stop - Stops the system, regardless of command status.
Eject - Ejects the plate. Once the plate is ejected, the
Retract retracts the plate, and the Retract button changes back to Eject.
Temp - Displays the difference in temperature between the current reading and the reading
when the system was calibrated, in degrees Celsius. If the temperature is out of tolerance,
this shows a high or low arrow. When clicked, it opens the Auto Maint tab.
XY Status - Displays the current location of the command, and the temperature of the plate
heating block in degrees Celsius. When clicked, it opens the Probe & Heater tab
Drive Fluid Level - The Drive Fluid liquid level sensor warns you when there is enough Drive
Fluid in the container to run one plate or less. The system stops if the container is empty.
Waste Fluid Level - The waste fluid container liquid level sensor warns you when the waste
fluid container is almost full. The system stops if the tank is full.
Home Page
The Home page displays a welcome message, batch creation buttons, Daily Activities
shortcuts, and the Installed Protocols list.
Eject button changes to Retract.
Using xPONENT
35
Page 49

FIGURE 16.
Home Page
Daily Activities
System Initialization - Perform a system initialization routine.
Shutdown -
Probe and Heater
Drive Fluid Lot -
fluid container was shipped. This information is optional.
Create a New Batch from the highlighted Protocol below - Creates a new batch using a
selected protocol from the Installed Protocols list. This button displays the same fields as
the Create a new batch from existing Protocol button on the Batches page.
Scroll - Use the up and down arrows to scroll through the list of installed protocols.
Sys Info - Opens the System Info tab of the Maintenance page. The System Information
page displays licensing information, the instrument serial number, the date of the last CAL
Calibration, VER Verification, Fluidics tests, and other important information.
Reports - Opens the Reports tab of the Results page.
Return to the Home page at any time by clicking Home at the top of the screen.
xPONENT for MAGPIX
36
Perform a shutdown routine.
- Adjust the probe height or plate heater.
Enter the Drive Fluid lot number, which is printed on the box in which the
Page 50

Adjusting the Sample Probe Height
Adjust the sample probe height to ensure that the probe drops far enough into the well to
acquire sample.
NOTE: Ensure that there is no liquid in the wells or reservoirs before
adjusting the sample probe height.
1. On the Home page, click Probe and Heater under Daily Activities. The
Heater tab opens.
2. Based on the type of plate you are using, place alignment disks or an alignment sphere
from the Height Alignment Kit in the well, as specified below:
• Filter-bottom plate - two (2) 5.08mm disks
• Mylar-bottom plate - two (2) 5.08mm disks
• Conical (V-shaped) plate - one (1) sphere
If you are using a standard 96-well plate, you do not need to use any of the disks or
spheres in the Height Alignment Kit.
3. Ensure that the well location is selected on the plate image. Luminex recommends that
you use well D6 (this is the center of a standard 96-well plate). A green pin marks the
selected well.
4. Click Eject to eject the plate holder.
5. Place a strip well in the off-plate reagent block.
6. In the Strip Wells section, click D1.
7. Verify that the reservoir is empty.
8. In the Reservoir section, click well RB1.
9. Verify that the plate is not warped. Warped plates can lead to incorrect probe height
adjustment.
10. Place the plate on the plate holder with well A1 in the marked position.
11. Click Retract to retract the plate holder.
12. Type a name for the plate in the Plate Name box.
13. Click Auto Adjust Height. The probe automatically adjusts itself to the locations you
selected.
14. Click Eject to eject the plate holder. If you used alignment disks or spheres, remove them
from the plate.
15. Click Save to save the probe height settings for the plate and the well.
Probe &
NOTE: When you calibrate and save the probe height settings for all three
areas under a plate name, all areas retain the calibration.
CAUTION: Ensure that the probe height is set correctly before calibrating
the system.
Using xPONENT
37
Page 51

CAUTION: Correct sample probe height is critical to successful sample
acquisition and calibration. Problems with the sample probe can
lead to fluid leaks and inhibit sample acquisition. To adjust the
height of the sample probe, follow the steps above.
FIGURE 17.
Sample Probe Height Adjustment
1. Well D6 2. Well D1 3. Well RB1
System Initialization
xPONENT for MAGPIX contains a pre-defined startup routine to prepare the analyzer for data
acquisition. This section describes calibration and performance verification of the system.
Calibrator MagPix beads are used to normalize the settings for the reporter channel and
classification channels. Verification MagPix beads are used to verify calibration and optical
integrity of the system. Fluidics beads are used to assess well-to-well carryover.
Once calibrated, the values remain until you recalibrate. You can track system calibration and
verification results through the Calibration report. Target value information for calibration and
verification beads is available on the Luminex website at http://www.luminexcorp.com/
support/calibration/index.html.
xPONENT for MAGPIX
38
Page 52

Calibrate your system at least once a week using the Calibration/Verification button on the
Auto Maint tab of the Maintenance
• The delta calibration temperature exceeds ± 5° C.
• You move the instrument.
• You experience sample acquisition problems.
Verify the system daily using the Performance Verification button on the Auto Maint tab of
the Maintenance page. Refer to your assay kit instructions for additional calibration
frequency requirements.
Before you can calibrate the system, you must import MagPix calibrator and verification bead
lot information. This information is available on the CD that accompanies the Performance
Verification Kit and Calibration Kit, and is also available on the Luminex website at http://
www.luminexcorp.com/support/calibration/index.html.
NOTE: Do not move the system waste line while calibrating the system.
page. In addition, recalibrate the system if:
Adding or Importing CAL and VER Kit Information
You can add CAL and VER Kit information from the Home page or from the Maintenance
page.
To add or import CAL and VER kit information from the Home
1.
On the Home page, click System Initialization.
a.
b. Click Import Kit at the bottom right side of the window. The Import Calibration or
Performance Kit dialog box opens.
c. Locate the MagPix calibrator or verifier kit information file and click Open.
2. To add or import kits from the Maintenance page:
page:
a. Open the Maintenance page, then open the Lot Management tab.
b. Click Import Kit. The Import Calibration or Performance Kit dialog box opens.
c. Select the MagPix calibrator or verification information file you want to import, then
click Open.
Setting Up the System Initialization Routine
You can set up the system initialization routine to include calibration and verification under
the Admin page, System Setup tab, Maintenance Options section.
Using xPONENT
39
Page 53
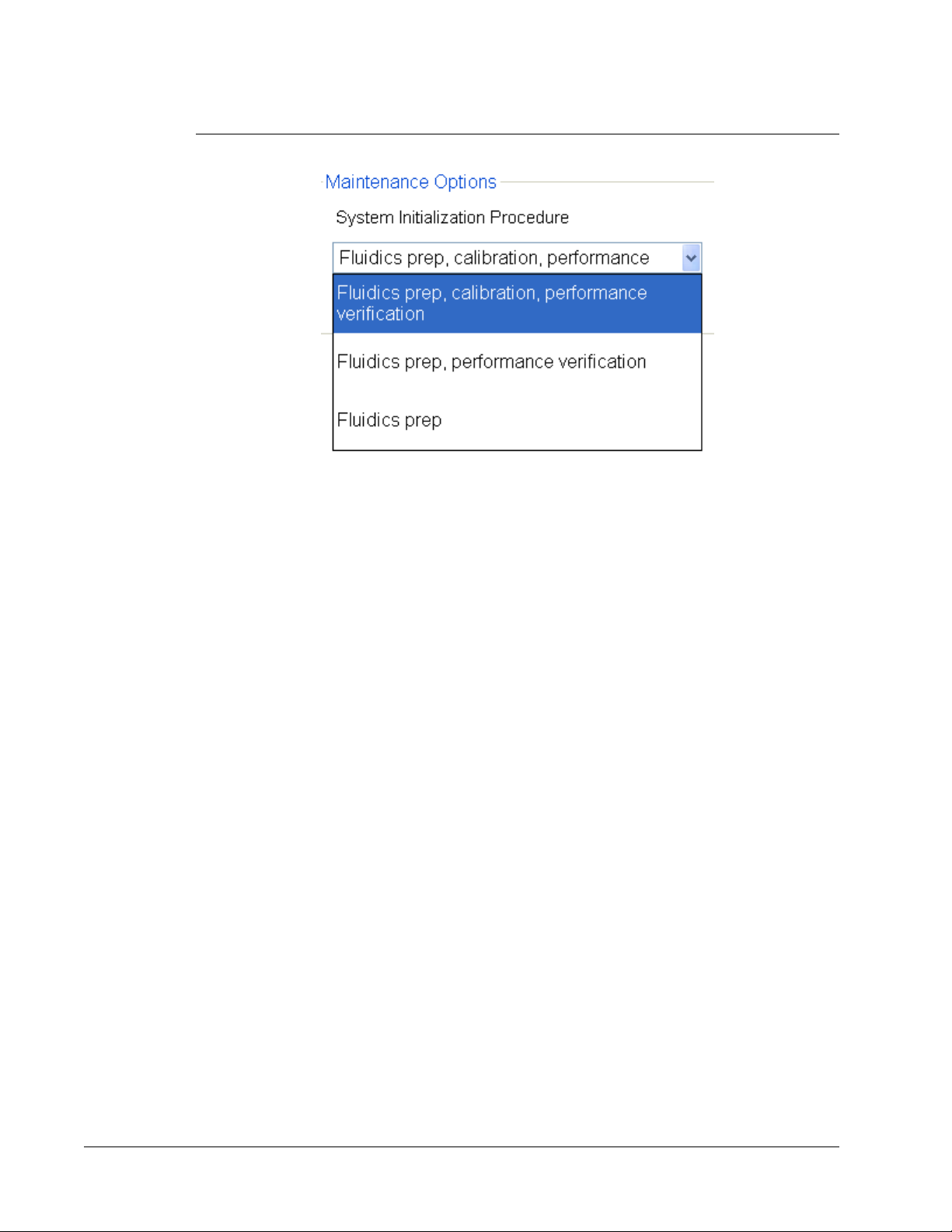
FIGURE 18.
Select System Initialization Routine
Running System Initialization
1. On the Home
2. Select the Calibration Kit active (current) lot number from the list.
3. Select the Performance Verification Kit active (current) lot number from the list.
4. Vortex the calibration and verification beads for twenty seconds. Do not dilute the
reagents.
5. Click Eject on the Status Bar.
6. Place the off-plate reagent block on the heater plate.
7. Add at least 5 drops of the reagents to the wells, as indicated in the plate image or
according to the kit instructions.
8. Click Retract.
9. Click Run.
Automated maintenance options are also available on the Maintenance page, Auto Maint
tab.
page, click System Initialization under Daily Activities.
Exporting CAL or VER Kits
1. Open the Maintenance page, then open the Lot Management tab.
2. In the Active Reagents section, select the kit you want to export from the Calibration
Kit or Performance Verification Kit lists.
3. Click Export.
4. In the Export Calibrator or Verification Lot dialog box, select an export location for the
kit.
5. Type the kit name for the exported kit into the File Name box.
6. Click Save. The dialog box closes.
xPONENT for MAGPIX
40
Page 54

Deleting CAL and VER Kit Information
1. Open the Maintenance
2. In the Active Reagents section, select the kit you want to delete from the Calibration Kit
or Performance Verification Kit lists.
3. Click Delete Kit.
CAUTION: There is no confirmation dialog box when you delete a kit.
page, then open the Lot Management tab.
Creating Calibration and Verification Reports
Open the
1.
2. In the Report drop-down list, select Calibration and Verification Reports.
3. In the Type drop-down list, select ALL, CAL, VER, or Fluidics.
4. Type a Start date and a Through date for the date range you want to view.
5. Click Generate to display the report.
6. Use the left or right Page arrows to navigate to the different report pages.
7. Click Print to print the report, or Save to save the report.
8. Click New Report to generate another report.
Results
page, then open the Reports tab.
Setting Up Batches
Batches consist of protocols and samples for acquisition and can span more than one plate.
Protocols contain predefined commands that must be included in every batch acquisition.
You can group batches together as a multi-batch. Multi-batches can consist of any number of
batches that have been set up from different protocols and are processed consecutively.
Multi-batches cannot be run on multiple plates.
NOTE: When setting up a batch, if the number of samples exceeds the
number of wells in one microtiter plate, you can add additional plates
in the Add and Change Plate
are identified on the bottom of the plate image as Plate a of b, where
is the plate number and b
a
Batches Page
Options on the Batches tab on the Batches page are:
• Create a New Batch from an existing Protocol
• Create a New Multi-Batch
Depending on your selection, this page displays the following tabs:
secondary window. Additional plates
is the total number of plates.
Using xPONENT
41
Page 55
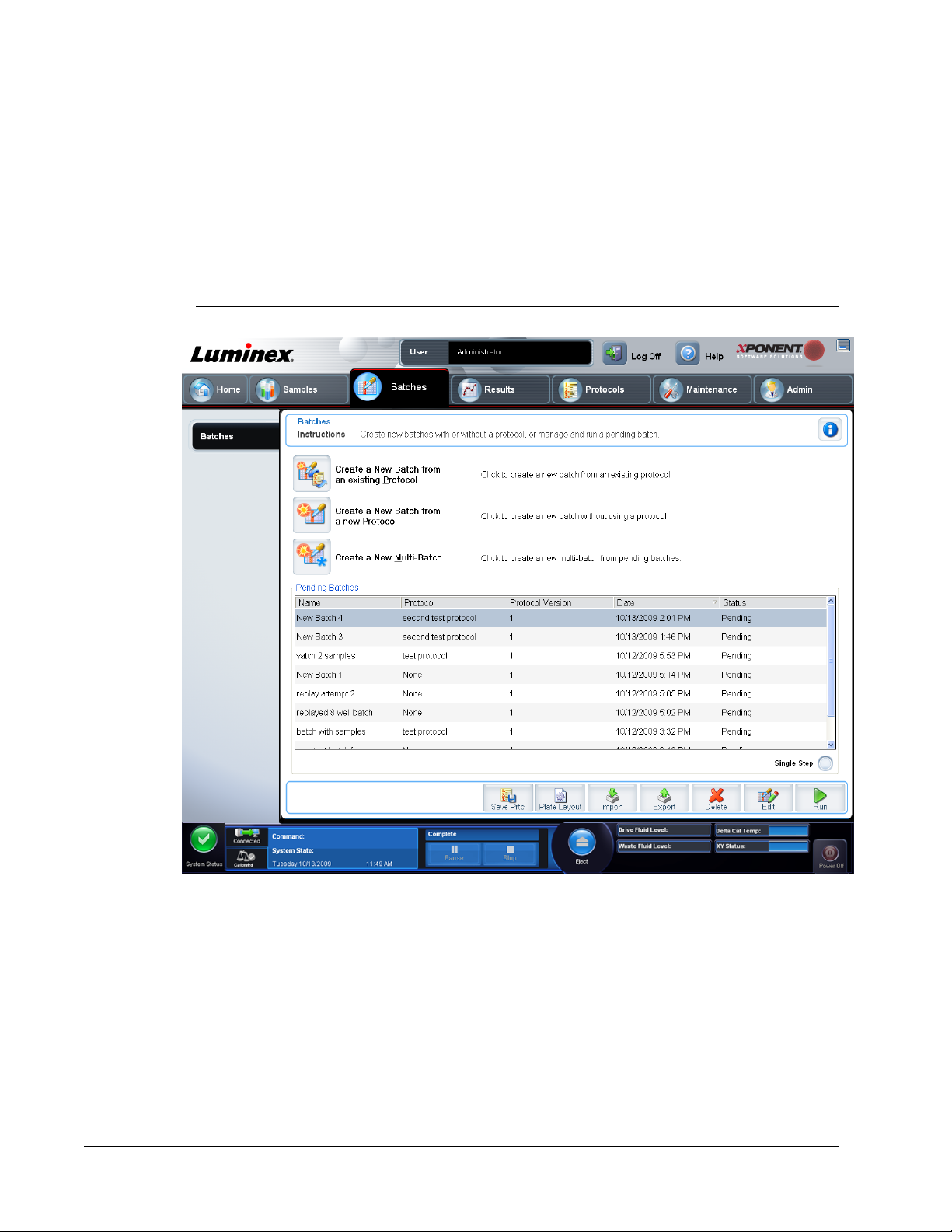
• Protocols - Displays when Create a New Batch from an existing Protocol is clicked.
• Stds & Ctrls - Displays when Create a New Batch from an existing Protocol is clicked.
• Plate Layout - Displays when Create a New Batch from an existing Protocol is clicked.
• New MultiBatch - Displays when Create a New Multi-Batch is clicked.
NOTE: These tabs (except New MultiBatch) are sequential. You must
complete each screen in a specific order.
FIGURE 19.
Batches Page
The following fields and actions are available on the Batches tab:
The Pending Batches list displays the name of the protocol used with the batch, the protocol
version, date, and status for each pending batch. The following buttons only appear if the
pending batches have data:
Single Step - Instructs the system to acquire one well and then pause. This ensures that the
system is working correctly before you run an entire batch.
Save Prtcl. - Saves protocol and/or assay information for a standard/control.
Plate Layout - Opens the Report dialog box, which includes the Batch Plate Layout
Report. Confirm that the plate layout conforms to your specific assay instructions.
Import - Imports a batch.
xPONENT for MAGPIX
42
Page 56

Export - Exports a batch
Delete - Deletes a batch.
Edit - Edits a batch.
Run - Runs a batch.
Using the Batches Page
1. Open the Batches page.
Click one of the following:
2.
• Create a New Batch from an Existing Protocol
• Create a New Multi-Batch
3. Type the batch name in the Batch Name box.
4. Type an optional description of the batch in the Enter Optional Description box.
5. If you are creating a batch from an existing protocol, select the protocol in the list. Click
Next. If the protocol uses standards and/or controls, the Stds & Ctrls tab displays.
6. The Plate Layout tab appears. View the details of the active reagents, apply different
assay standards/controls, or manually enter new information. Confirm that the plate
layout conforms to your specific assay instructions. Click Next.
7. On the Plate Layout tab, assign well commands for this batch.
8. Click Run Batch to begin batch acquisition, or click Save to save batch information to the
Pending Batch list to be run at a later time.
NOTE: If the batch spans more than one plate, the tray ejects automatically
when all defined wells have been acquired. A dialog box displays
prompting you to insert the next plate.
Create a New Batch from an existing Protocol
This option creates a new batch using a selected protocol from the Installed Protocols list.
When you click this option, the following tabs appear:
1. Protocols
2. Stds & Ctrls
3. Plate Layout - Confirm that the plate layout conforms to your specific assay instructions.
These tabs are numbered because you must complete the steps on each tab sequentially.
For example, you must complete the Protocols tab before you can access theStds & Ctrls
tab.
Installed Protocols - Displays a list of protocols. The list contains the following information
about each protocol:
• Name
• Version
• Manufacturer
• Date
Using xPONENT
43
Page 57

NOTE: Luminex recommends that you analyze the manufacturer’s assay kit
controls with each batch.
To create a new batch from an existing protocol:
1. Read the instructions provided with the assay kit you are using.
2. Open the
3. Click Create a New Batch from an existing Protocol.
4. Type the batch name in the Batch Name box.
5. If you want a description for the batch, type it in the Enter Optional Description box.
• Click the existing protocol that you want to use. If the protocol uses standards and/or
controls, you can view the active reagents. If the selected protocol uses standards/
controls, the next tab that appears is the Stds & Ctrls tab. You can view details about
the active reagents, apply different standards/controls, or manually add new
information on this tab.
• If the selected protocol does not use standards/controls, the next tab that appears is
the Plate Layout tab. You can assign well commands for this batch on this tab.
Confirm that the plate layout conforms to your specific assay instructions.
6. Click Next.
7. Click Run Batch to begin batch acquisition, or click Save to save the batch information
as a pending batch. Pending batches can be run at any time.
Batches page.
NOTE: If the batch spans more than one plate, the tray ejects automatically
when all defined wells have been acquired. A dialog box displays
prompting you to insert the next plate.
Create a New Batch from a new Protocol
Click this option to create a new batch from a new protocol. This option enables you to create
a protocol while your are creating the batch.
To create a new batch from a new protocol:
1.
Open the Batches page.
2.
Click Create a New Batch from a new protocol to open the Settings tab.
3. Type a name in the Name box.
4. Type a description in the Description box.
5. Define the settings in the Acquisition Settings section.
If you select Qualitative, Qualitative, or Allele Call from the Analysis Type list, you can
select Analyze results while acquiring samples to view real-time analysis. If you select
None as your analysis setting, you cannot analyze results using xPONENT.
6. Click Next. The Analytes tab opens. Click the analytes of interest in the numbered grid.
Information about the analytes appears in a list on the right side of the grid. Name the
analytes.
7. To change the Default Analysis, click Change. The Analysis Settings dialog box
opens.
8. Select the analysis method from the Method list. Click OK to change the default analysis
for analytes to be selected. Click Apply to All Analytes to apply the selection to all
analytes. The Analysis dialog box closes.
xPONENT for MAGPIX
44
Page 58

9. Type a unit of measurement in the Units box.
10. Type the desired bead count for each analyte in the Count box.
If you click Apply All, this applies to all analytes.
11.
12. To change individual units or counts, change them in the analyte table.
13. Type the desired bead count for each analyte in the Count box.
14. Click Next. The Stds & Ctrls tab opens if you selected an analysis type other than None.
• If you are using an assay standard/control kit, click Apply Std/Ctrl Kit. In the Select
Std/Ctrl Kit dialog box, click the kit from the list and click OK. Applying a kit only works
for kits already installed, but you can also manually type the information.
• If you are not using a kit, type the appropriate information in the Standard Information
and Control Information sections. The number of standards and/or controls in these
sections is defined on the Settings tab in the Analysis Settings section. If your batch
uses controls, enter the appropriate values for Expected Values. Click Low Value
from the Show list, and enter the low value for each analyte. Click High Value from the
Show list, and enter the high value for each analyte. Reagent information is not
required for a custom batch unless you want to use the analysis feature.
15. Click Next. The Plate Layout tab opens.
• To add well commands, click the appropriate wells and mark them as unknown,
standard, control, background, or wash. You can also delete commands that you’ve
added, and change the starting location on the plate. If you want to run in replicate,
change the Replicate Count to the appropriate number and the Grouping to your
preferred grouping method.
• As you add commands to your plate, they appear in the Command Sequence list.
Here you can give each of your wells an ID. You can also import an ID list and move
your commands up and down to change the order in which they will be acquired.
16. Click Single Step to acquire the first well, then pause acquisition.
17. Click Run Batch to start acquisition, or Save to save the batch for a later time. You can
also save the protocol and/or standard and control information by clicking Save Prtcl.
NOTE: If the batch spans more than one plate, the tray ejects automatically
when all defined wells have been acquired. A dialog box displays
prompting you to insert the next plate.
Create a New Multi-Batch
Use this button to add or remove batches to the multi-batch set up and to run a multi-batch.
A multi-batch is a set of batches that you want to process consecutively. You add batches to
the multi-batch from pending batches in your database. You can also create a new batch to
add to the database for the multi-batch. You can include as many batches as you need. The
software does not limit you to a certain number of batches per multi-batch. This feature
enables you to conserve plates.
You must ensure the batches fit on one plate. After you add each batch, the software
automatically adds the next batch to the first well of the next column or row (depending on the
plate direction) as long as space remains on the plate. You can also select a well first, which
places the next batch in your chosen location. If space limitations create an overlap, an error
message appears. Results for each batch are saved as individual batch files.
Using xPONENT
45
Page 59
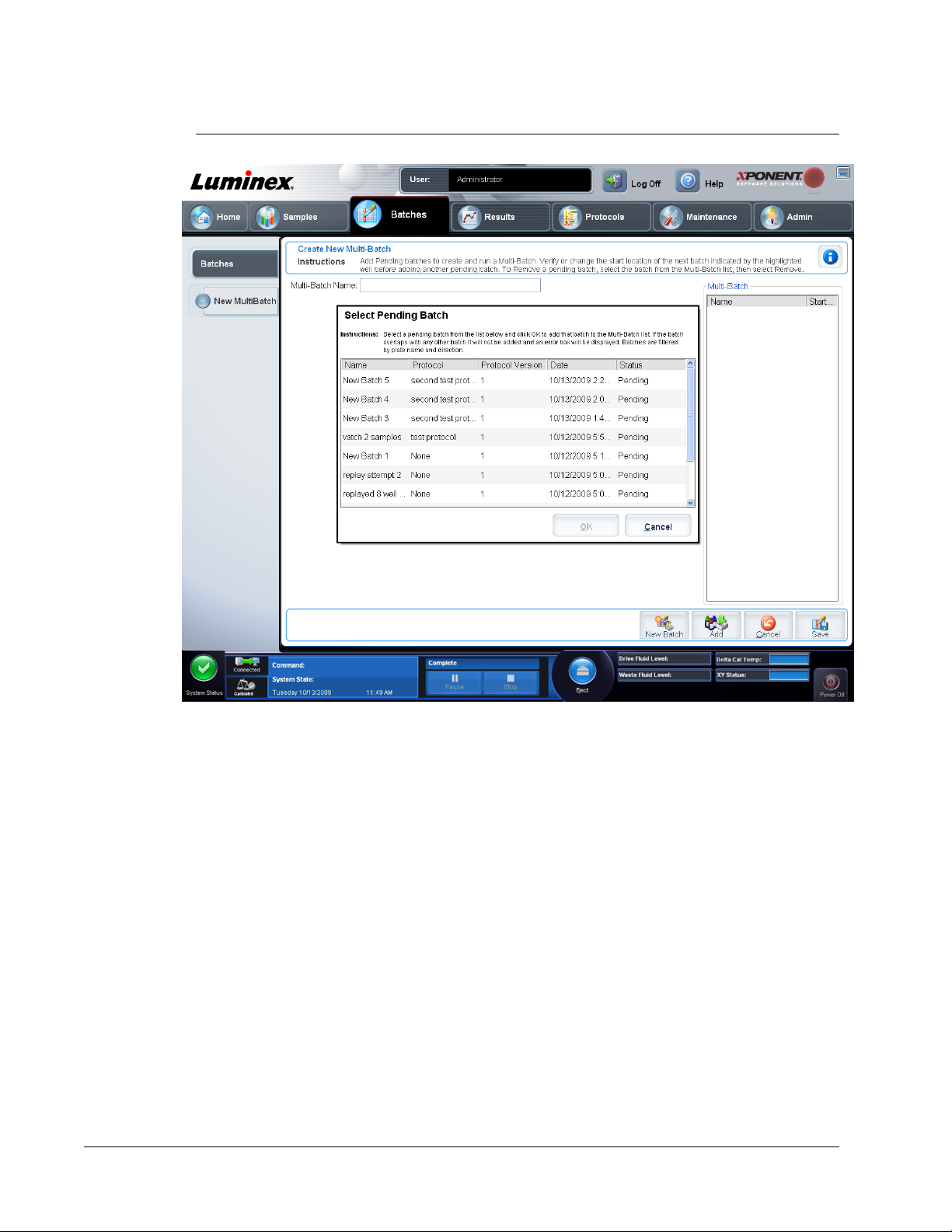
FIGURE 20.
New Multibatch Tab
This page contains the following:
Multi-Batch Name - Use this field to create a name for new multi-batches.
Select Pending Batch - Contains a list of all pending batches. This list includes name,
protocol, protocol version, date, and status information for each pending batch. Select the
batch you want to add to the plate. Click OK. A plate layout diagram automatically populates
the wells for the batch. Confirm that the plate layout conforms to your specific assay
instructions. Click Add to open this box again and add additional batches.
To create a new Multi-Batch:
1. Open the Batches page.
2. Click Create a New Multi-Batch. The New MultiBatch tab and Select Pending Batch
dialog box open simultaneously.
3. In the Select Pending Batch list, select the batch to add. Click OK.
4. Click Add to open the Select Pending Batchdialog box. Select the new batch that
appears and click OK. Repeat for all batches to be run.
5. Click New Batch to open the Select Protocol for New Batch tab or click the Protocols
tab. Follow the steps for creating a new batch from a new or existing protocol.
xPONENT for MAGPIX
46
Page 60

6. Click Save to return to the New Multibatch tab.
7. Click Run
to run the multi-batch.
FIGURE 21.
Select Pending Batch Dialog Box
Using xPONENT
47
Page 61

FIGURE 22.
New MultiBatch Tab with Plate Layout
xPONENT for MAGPIX
48
Page 62
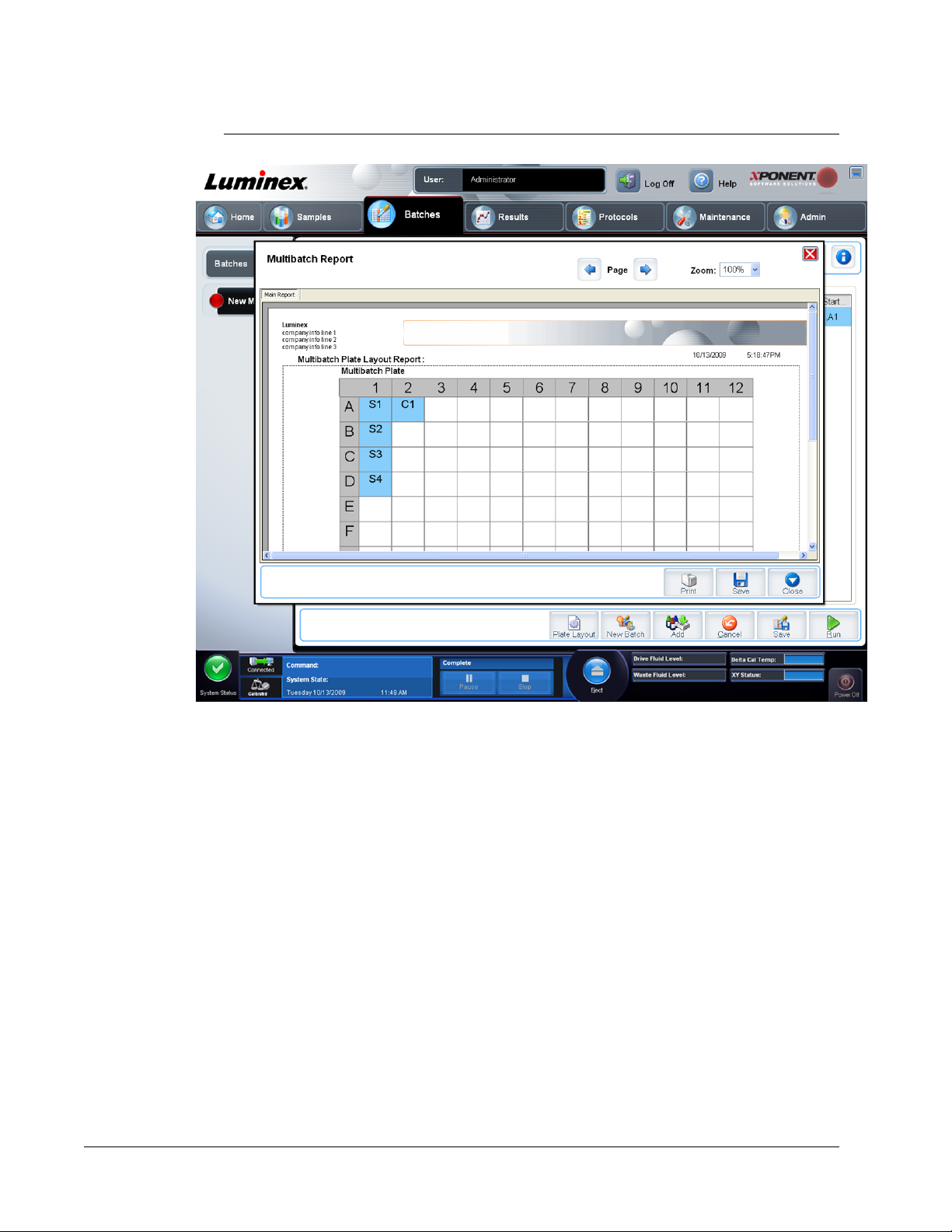
FIGURE 23.
Multibatch Report Dialog Box
Save Multi-batch
After creating a multi-batch, you can save it to the Select Pending Batch list. When saved to
this list, the protocol appears as “Multibatch.”
Batches saved to a multi-batch cannot be edited or deleted unless they are removed from the
multi-batch. However, you can edit the multi-batch itself. To remove a batch from a multibatch, click a well in the plate layout, and click Remove.
To save a multi-batch:
1. Create a new multi-batch.
2. Select a pending batch.
3.
Type the name for the multi-batch in the Multi-Batch Name field.
4. Click Save. You are returned to the Batches page, and the multi-batch is added to the
pending batches list.
Using xPONENT
49
Page 63

Running a Batch
To run a batch, click Run Batch to open the Batches page. Select the pending batch that
you want to run, then click Run.
NOTE: If the batch spans more than one plate, the tray ejects automatically
when all defined wells have been acquired. A dialog box displays
prompting you to insert the next plate.
Importing a Batch
You only need to import batches to the system once. You must type lot information for the
standard and control reagents as specified in the protocol. This lot information is used for
every batch set up using the protocol, until it is changed.
To import a batch:
1.
Open the Batches
2. Click Import. The Import Batch dialog box opens. Batch files are MDF files.
3. Click Browse to open the Select File dialog box. Navigate to the batch file you want to
import, then click Open.
4. Click OK in the Import Batch dialog box. The batch displays in the Pending Batches
list.
page.
Exporting a Batch
1. Open the Batches page.
2. In the Pending Batches section, click the batch you want to export, then click Export.
The Export Batch dialog box opens.
NOTE: You can export batches, but not multi-batches.
3. Click Browse. The Select File dialog box opens.
4.
Navigate to the location to which you want to save the file, then click Save
5.
Click OK in the Export Batch dialog box.
NOTE: When exporting a large batch and including the LXB files, the export
process may take ten minutes or more.
Delete Batch
You can only delete unprocessed batches. Batches are deleted from the Open Batch list and
moved to the Open Incomplete Batch list.
To delete a batch:
.
xPONENT for MAGPIX
50
Page 64

1. Open the Batches page.
2. In the Pending Batches
The Delete Pending Batch dialog box opens.
3. Click Yes.
NOTE: Batches saved to a multi-batch cannot be edited or deleted unless
they are removed from the multi-batch. However, you can edit the
multi-batch itself. To remove a batch from a multi-batch, click a well
in the plate layout, then click Remove.
NOTE: To delete a partial batch, open the Admin page, then click the
Archive tab. Clear the Keep data after archive box. Click Archive
to delete the partial batch.
Editing a Batch
section, click the batch you want to delete, then click Delete.
Open the
1.
2. Click the batch you want to edit, then click Edit. The Protocol tab opens.
3. Edit the information as needed on the Protocol, Std & Ctrls, and Plate Layout tabs. For
the Plate Layout tab, confirm that the plate layout conforms to your specific assay
instructions.
4. Click Save on the Plate Layout tab.
NOTE: Batches saved to a multi-batch cannot be edited or deleted unless
Settings Tab
Use this tab to name your new batch and configure acquisition settings. For existing batches,
you can view the acquisition parameters of the selected saved batch and print the batch
settings report.
Batches
they are removed from the multi-batch. However, you can edit the
multi-batch itself. To remove a batch from a multi-batch, click on a
well in the plate layout, and click Remove.
page.
Using xPONENT
51
Page 65

FIGURE 24.
Settings Tab
This tab contains the following:
Name and description boxes - Type the name and description in the appropriate box.
Acquisition Settings: -
• Volume - This is the volume the instrument will aspirate into the system for analysis.
• XY heater - Select to enable the XY heater. In the box type the desired value in degrees
Celsius. The temperature range is 35 to 60° C.
• Plate name - The name assigned to the plate during sample probe height adjustment.
• Sample wash - This automatically washes each sample within the instrument.
Analysis Settings - Displays the analysis type to be used for the batch.
xPONENT for MAGPIX
52
CAUTION: Acquiring data before the heater has reached the proper
temperature can compromise test results.
Page 66

• Analysis Type - Use this list to choose from the following analysis types:
• None - No analysis. Select if you have your own data post-processing program and
want to obtain only fluorescent intensity results. You cannot apply standards or controls
when you select None. You cannot analyze acquisitions with this setting.
• Qualitative - Qualitative analysis determines results as either positive or negative,
reactive or non-reactive. The software is flexible in defining custom result ranges, such
as negative, low positive, or high positive. Determinations are based on a single
standard. For qualitative analysis the Luminex software uses a specific algorithm: .
• Quantitative - Determines the sample concentrations from standard curves using
regression
and Logistic 5P. Type the desired values for standards and controls in the Number of
Standards and Number of Controls boxes. Select either Fit of All Standards or Mean
of Replicates for the calculation of the curve fit.
• Analyze results while acquiring samples - The software allows real-time viewing of the
results as the instrument analyzes samples. This feature is not available if you select None
as your analysis type.
• Number of Standards - Displays the number of standards for the protocol.
• Use External Analysis Program - This indicates the use of a third-party program to
analyze data.The Analysis Program list becomes active when this is selected. Applies
only to qualitative and quantitative analyses.
• Number of Controls - Displays the number of controls for the protocol.
• Analysis Program - Displays which program is used for data analysis.
• Fit of all Standards - The standard curve will be determined by using each individual
standard replicate when calculating the standard curve. For example, if you run duplicates
of a 7-point standard curve, the software will calculate the standard curve by using 14
points. Applies only to quantitative analysis.
• Mean of Replicates - The standard curve will be determined by averaging the individual
standard replicates when calculating the standard curve. For example, if your un duplicates
of a 7-point standard curve, the software calculates the standard curve by using 14 points.
Applies only to quantitative analysis.
• Cancel - Returns you to the main Batches tab.
method specified in the IVD assay protocol. methods , Linear, Logistic 4P,
Analytes Tab
Use this tab to select or edit analytes used in the batch or protocol.
Using xPONENT
53
Page 67

FIGURE 25.
Analytes Tab
This tab contains the following:
Analyte grid - A grid representing each analyte from 1 to 50. Select All selects all analytes,
and Deselect All deselects all analytes. Click a numbered analyte to select it; click the
analyte again to deselect it. You can also click and drag to select groups of analytes.
Selected analytes are red. Deselected analytes are gray. An analyte marked as an intra-well
normalization bead is blue.
Default Analysis - The default analysis changes based on the Analysis Type selected in
the Settings tab. You can change the analysis settings for all analytes by clicking Change if
this button is enabled in this tab. This displays the Analysis Settings dialog box.
xPONENT for MAGPIX
54
Page 68

FIGURE 26.
Analysis Settings Dialog Box
If you selected Quantitative on the Settings tab, the default analysis formula is 5P
Weighted. To change the default, select one of the following from the Method list:
• No Analysis
• Cubic Spline
• Linear Fit
• Logistic 4P
• Logistic 5P
If you selected Logistic 4P or Logistic 5P, select a weight type of either None or 1/y2. .
If you selected Qualitative on the Settings tab, the default analysis is Luminex Qualitative.
Change the default value by selecting either Luminex Qualitative or No Analysis.
Click Apply to All Analytes to apply your selection to all selected analytes. Click OK to
change the default analysis to the analysis you selected. Click Cancel to close the dialog box
without saving.
Units - Type the desired units for the analytes in this box.
Count - Type the desired bead count for the analytes by clicking in the Count box. If each
selected bead set does not acquire this number of events, a warning is added to the log that
not enough bead events were acquired.
Using xPONENT
55
Page 69

Apply All - Applies the information in the Units and Counts fields to all analytes.
You can also specify the minimum allowable bead count per well that xPONENT analyzes.
This excludes data from any beads carried over during acquisition.
Selected Analytes List - Selected analytes appear in a list on the right side of the analyte
grid. This list includes the following information
• Name - The name of the analyte. Click and type to rename the analyte.
• Analysis - To change the type of analysis for an analyte, click this field to open the
Analysis Settings
In the Analysis Settings dialog box:
• Select a method from the Method list.
• If necessary, select a weight type from the Weight Type list.
• Apply the analysis to all analytes in the list by clicking Apply to All Analytes.
• Select Mark as Intra-Well Normalization Bead to make the analyte an intra-well
normalization bead.
• Add a range to the analysis by clicking Add Range.
• Select Use Threshold Ranges to enable ranges for the analysis.
• Click Add Range to add a range.
• Type a Range Name, a Low Value, a High Value, and select Inclusive if you wish to
include the low and high values in the range. Click OK to exit the dialog box.
• Units - The unit of measurement you specified in the Unit box. Click this box to type a
value for the analyte.
• Count - Type the desired bead count for the analytes by clicking in the Count box. If each
selected bead set does not acquire this number of events, a warning is added to the log
that not enough bead events were acquired.
• Region - Refers to the specific analyte selected. This is a number between 12 and 78.
dialog box and select another analysis from the list.
Group - If you have selected Allele Call from the Analysis Type in the Settings tab, this
button displays. Click Group to group 2, 3, or 4 analytes for the group. Multiple groups can
be defined.
Cancel - Click Cancel to return to the Batches tab.
Back - Click Back to return to the Settings tab.
Next - Click to go to the next tab. If the Analysis Type selected in the Settings tab was
None or Allele, this takes you to the Plate Layout tab. If the Analysis Type selected was
Quantitative or Qualitative, this button takes you to the Stds & Ctrls tab.
Protocols Tab
Use this tab to name a batch, type a description, select a protocol, and view active reagents.
xPONENT for MAGPIX
56
Page 70

FIGURE 27.
Protocols Tab
This tab contains the following:
Batch Name/Description - Used to name and describe a batch.
Select a Protocol - Contains the protocol name, version, manufacturer, and creation date for
each protocol.
Active Reagents - Displays assay and control lots/kits associated with the selected protocol.
The Standard/Ctrls Kit Name - Lot# field displays the assay standard/control kit/lot name
and lot number currently associated with the selected protocol. The Standard Lots and
Controls Lots fields display any standard or control lots associated with the selected
protocol.
Cancel - Returns to the main Batches tab.
Next - If you have selected a protocol with no standards or controls (displayed as None in the
Active Reagents section), clicking Next continues to the Plate Layout tab. Confirm that the
plate layout conforms to your specific assay instructions. If you have selected a protocol with
standards and controls, clicking Next continues to the Stds & Ctrls tab.
Stds and Ctrls Tab
Use this tab to apply a kit or lot to the batch. Standards and controls are also accessible from
the Protocols page.
Using xPONENT
57
Page 71

FIGURE 28.
Stds & Ctrls Tab
This tab contains the following:
Apply Std/Ctrl Kit - Opens the Select Std/Ctrl Kit dialog box. The dialog box displays the
Std/Ctrl Kit Lot #, Std/Ctrl Kit Name, Expiration, and Manufacturer for the kit. Select a
Std/Ctrl kit from the list and then click OK to close the dialog box. The kit information will
display in the boxes to the right of the Apply Std/Ctrl Kit button. The selected kit must be
associated with the same analyte names. You can also type information by clicking in the
Name, Std/Ctrl Kit Lot #, Expiration, and Manufacturer boxes and typing the information.
Assay Standard Information - Displays the selected standard reagents in a list. The list
displays the Reagent, Name, Lot #, Expiration, Manufacturer, and expected concentration
value of each analyte.
• Apply Std Lot - Opens the Select Lot dialog box. Select a lot from the list and then click
OK to apply the lot.
• Apply Values - Applies a value across or down the Reagent, Name, Lot #, Expiration,
and Analyte fields. Type a value in these fields by double-clicking on them, and then using
one of the two Apply Values arrows to apply that value down or across the list of analytes.
xPONENT for MAGPIX
58
NOTE: The Dilution list and Apply Dilution button only appear if a
quantitative analysis has been selected.
Page 72

• Dilution - Contains the following dilution options:
• 1:2 - Halves the standard from each previous iteration.
• 1:10 (Log) - Computes a value of one-tenth of the standard from each previous iteration.
• 1/2 Log - Creates a 1:3.16 dilution, or half of each 1:10 (Log) from each previous
iteration.
Alternatively, type a number for your own dilution factor.
• Apply Dilution - Applies the dilution selected in the Dilution list.
NOTE: Click a column header to re-sort the display.
NOTE: Click the Reagent
highest number standard to standard number one. This is useful for
applying dilutions in which the last standard is the highest standard.
Assay Control Information - Lists the selected control reagents. The list displays the
Reagent, Name, Lot Number, Expiration, and Manufacturer. Existing control lot
information can be applied or new information can be typed manually.
• Apply Ctrl Lot - Opens the Select Lot dialog box. Select a lot from the list and then click
OK.
• Show Concentration - Expected, Low, and High set the expected, lowest, or highest
acceptable concentration of the analyte in the sample.
• Apply Values - Applies a value down or across the list of analytes.
Cancel - Returns to the Batches tab.
Back - Returns to the previous tab.
Next - opens the Plate Layout tab.
Managing Sample Lists
Use the Samples page to create new samples, view the details of a sample list, or to create
a new batch. Samples can also be added with a Lab Information System (LIS). To learn more
about how to use a LIS with xPONENT, see LIS Settings on page 11.
column header to re-sort the order from the
Using xPONENT
59
Page 73

FIGURE 29.
Samples Tab
This page contains the following tabs:
• Create Sample - Displays when the Create New Samples button is clicked.
• Edit Samples - Displays when the Details button is clicked.
These tabs are numbered because you must complete the steps on each tab sequentially.
For example, you must complete the Protocols tab before you can access the Stds & Ctrls
tab.
After you select a protocol, this tab displays the following:
Create New Samples - Opens the Create New Samples tab. This tab contains the following:
xPONENT for MAGPIX
60
WARNING: Human and animal samples may contain biohazardous
infectious agents. Where exposure to potentially biohazardous
material—including aerosol—exists, follow appropriate biosafety
procedures and use personal protective equipment such as
gloves, gowns, laboratory coats, face shields, or mask and eye
protection. Use ventilation devices. Observe all local, state, and
federal biohazard handling regulations when disposing of
biohazardous waste material.
Page 74

Protocol - Displays the protocol selected in the Samples tab. If xPONENT has an LIS
license enabled, any sample details provided by the LIS also appear in the Sample List
Version - Displays the version number of the protocol. This cannot be edited.
Sample - If you have the LIS-enabled version of the software and are currently connected to
the LIS, the sample list autopopulates when the LIS provides sample orders. You can only
view or run a sample list created in the LIS; you cannot edit it. If you do not have the LISenabled version of the software, use Create New Samples to create a new sample. After you
have typed and saved the sample information, it appears in the list to the left. The list
displays the samples you have already created. To reorder the sample’s acquisition location,
use the Move arrows.
The Delete, New, Edit, and Undo buttons display depending on the actions you take in the
Create Sample tab.
Delete - Deletes the selected sample.
New - Creates a new sample.
Edit - Edits a selected sample.
Undo - Reopens the Create Sample tab without saving any changes made using the Edit or
New buttons.
Save - Saves changes made to the sample list.
Close - Returns to the Samples tab.
Sample Lists - Contains a list of protocols, including the version number and the number of
samples associated with each protocol.
Details - Opens the Edit Samples tab to view or edit sample details for the selected protocol.
Create Batch - Opens the Protocols tab to create a batch.
.
Create Sample Tab
Click Create New Sample on the Sample page to display this tab. Use this tab to type and
view sample information.
Using xPONENT
61
Page 75
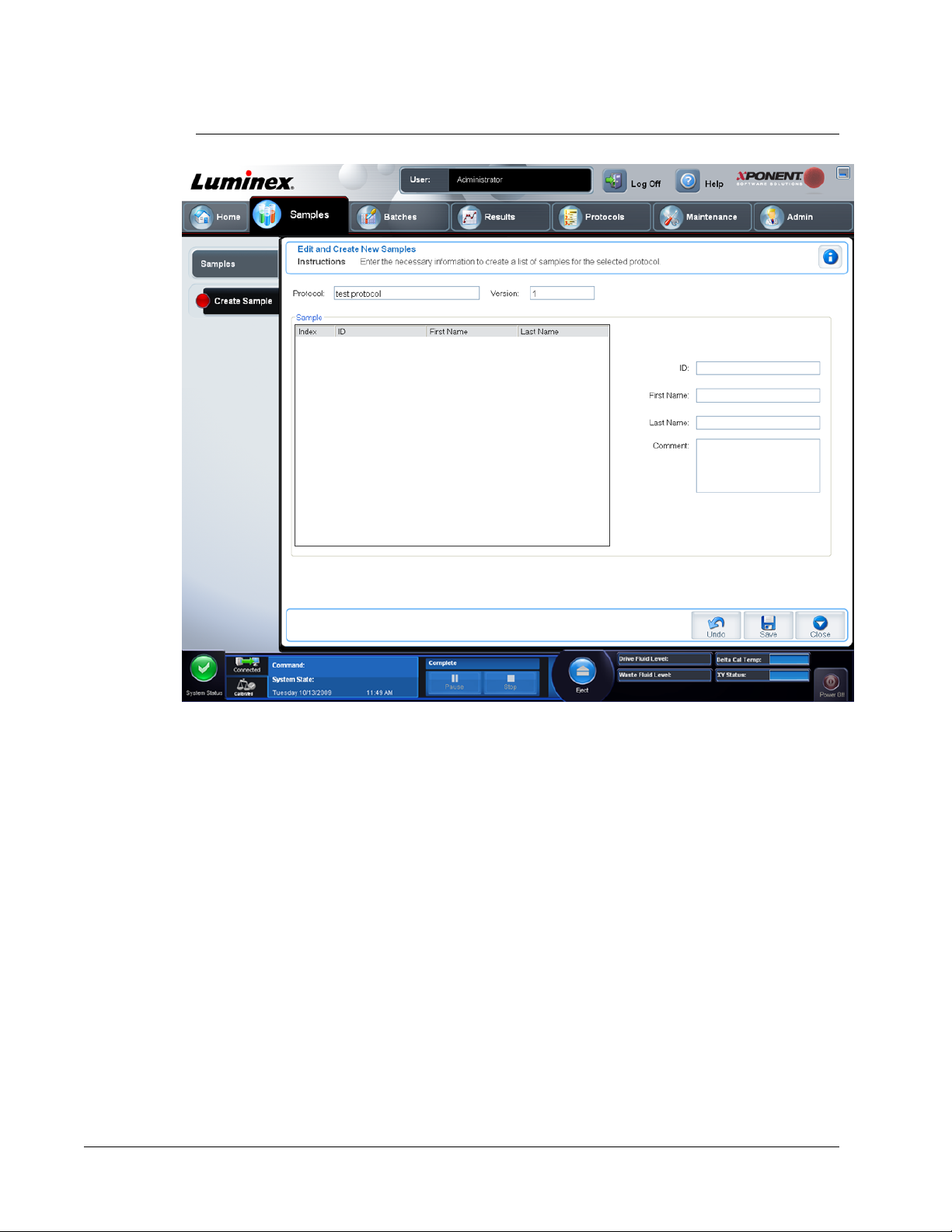
FIGURE 30.
Create Sample Tab
This tab contains the following:
Protocol - Displays the protocol selected in the Samples tab. If xPONENT has a LIS license
enabled, any sample details provided by the LIS also appear in the Sample
Version - Displays the protocol version number. This cannot be edited.
Sample - If you have the LIS-enabled version of the software and are currently connected to
the LIS, the sample list autopopulates when the LIS provides samples orders. You can only
view or run a sample list created in the LIS; you cannot edit it. Use Create New Samples to
create a new sample. After you have typed and saved the sample information, it appears in
the list to the left. This list displays the samples you have already created. To reorder the
sample’s acquisition location, use the move arrows.
The following Delete, New, Edit, and Undo buttons only display depending on actions taken
in the Create Sample tab.
Delete - Deletes a highlighted sample.
New - Creates a new sample.
Edit - Edits a highlighted sample.
Undo - Reopens the Create Sample tab without saving any changes made using the Edit or
New buttons.
xPONENT for MAGPIX
62
list.
Page 76

Save - Saves changes made to the Sample list.
Close - Returns to the Samples tab.
Creating a New Sample List
1. Open the Samples
2. In the Sample Lists section, select the protocol you are using for the sample list, then
click Create New Samples. The Create Sample tab opens.
3. In the ID box, type the sample ID.
4. Type a patient first name in the First name box if desired.
5. Type a patient last name in the Last name box if desired.
6. To add a comment about the sample, type it in the Comment box; this is optional.
7. Click Save to add the sample to the Sample list.
8. To add more samples, click New. Repeat step 3 through step 7 until you have added all
of the samples you want in your samples list.
9. Once you have added the all desired samples, click Close.
page.
Editing a Sample List
1. Open the Samples page.
2. In the Samples Lists section, choose the protocol you want to edit, then click Details.
The Edit Samples tab opens.
NOTE: If the number in the Number of Samples
samples attached to the protocol.
3. Click a sample, then use the Move
changing the order in which they will be acquired.
arrows to move it up or down in the sample list,
column is “0”, there are no
4. To add a new sample to the list, click New, then perform the following steps:
a. In the ID box, type the sample ID.
b. Type a patient first name in the First name box if desired
c. Type a patient last name in the Last name box if desired.
d. To add a comment about the sample, type it in the Comment box; this is optional.
e. Click Save to add the sample to the Sample list.
5. To edit an existing sample, click the sample, then click Edit.
6. Once you have completed editing the sample list, click Close.
Performing Analysis
If you are using third-party software to perform analysis, follow the data analysis instructions
provided in the IVD assay kit insert.
You may direct the system to acquire samples in replicate regardless of batch type. For
qualitative batches, qualitative results for replicates are averaged and the reported
interpretation is determined from this replicate average.
Using xPONENT
63
Page 77
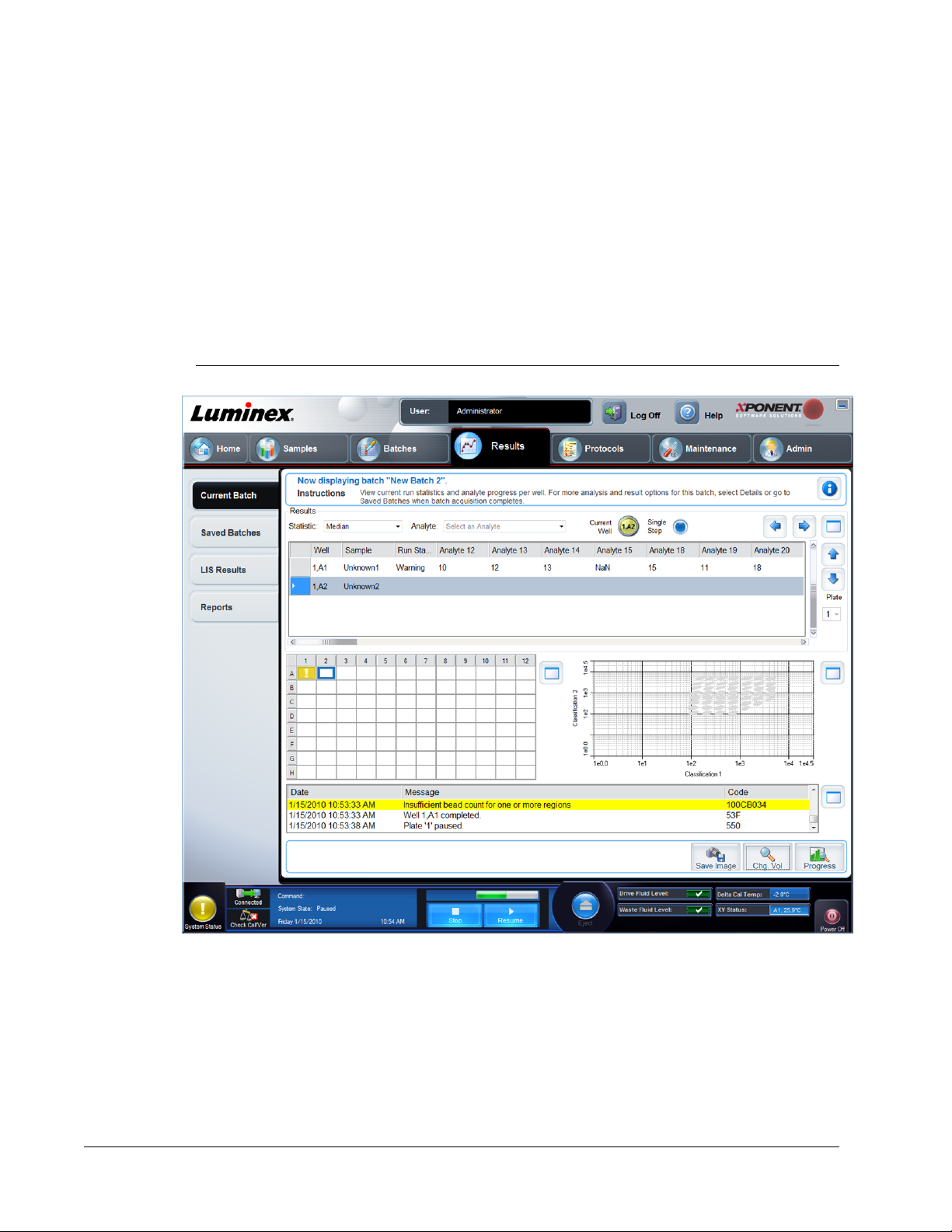
Current Batch Tab
To view the Current Batch tab, click the Results page. The Current Batch tab also displays
when you run a batch. Use this tab to view results, statistics, and log information related to
the current batch, and to perform statistical analysis on batch results. This tab offers real-time
monitoring of batch sampling during acquisition through a display of sample bead statistics
and analytes. The statistics available on this tab are intra-well statistics.
The statistics displayed change according to the analysis type selected . The buttons on this
tab change based on your user settings.
FIGURE 31.
Current Batch Tab
Fields and entries available on this tab are:
Statistic - Select the statistic you want to view from the drop-down list.
Analyte - Contains a list of analytes that were run in the batch. Select an analyte to view all
statistics for that analyte.
Current Well - Displays the statistics of the well currently being analyzed (this changes to
Displayed Well if you are viewing a batch using the Open button of the Saved Batches tab).
xPONENT for MAGPIX
64
Page 78

Single Step - Allows analysis of one well at a time. Select or clear this check box to turn this
function on or off.
Scroll Arrows - Use the up, down, left, and right arrow buttons to scroll through the table.
Maximize/Minimize - Use this toggle button to maximize and minimize the Plate Image, Dot
Plot, and List.
Plate - Select a plate from the drop-down list.
Save Image - Saves the information displayed on this page as an image file.
Progress - Click this button to display the Current Batch Run screen and to view real-time
batch processing results.
Analyze Current Batch
When you run a batch, the Current Batch tab opens. Here, you can view real-time analysis
of acquired analytes. Use this tab to view results, statistics, and log information related to the
current batch, and to perform statistical analysis on batch results. To view a single statistic
type for all analytes, click the statistic you want to view in the Statistics list. To view all
statistic types for a single analyte, click the analyte in the Analyte list. This tab offers realtime monitoring of batch sampling during acquisition through a display of sample bead
statistics and analytes. The statistics available on this tab are intra-well bead statistics. They
do not describe replicate well assay results.
Statistic
• Median - The point in a data set where there are as many values occurring above it as
there are below it.
• Test Result - The calculated analysis value for quantitative or qualitative assays derived
from standards with known values.
• Range - A semi-quantitative result for a particular numerical result falling between a
predefined set of values such as “Normal” or “Negative”.
• Net MFI (Median Background) - Net MFI median background.
• Mean - Average of all values in a set of results.
• % CV of microspheres - The measure of relative dispersion within the distribution.
% CV = 100 x Std Dev / Mean
• Standard Deviation - For calculating sample variability or dispersion, Luminex uses the
standard deviation formula.
• Peak - The value that is equal to the largest number of data points within the distribution.
For example, in data set {1,2,2,3,3,3,4,5}, 3 is the peak because it occurs the largest
number of times in the distribution list.
• Trimmed Count*
• Trimmed Mean*
- To view a particular statistic for analytes in a batch, select one of the following:
• Trimmed % CV of microspheres*
• Trimmed Standard Deviation*
• Trimmed Peak*
• % CV of Replicates - The measure of relative dispersion within the distribution of results
for replicate samples.
% CV = 100 X Std Dev / Mean
Using xPONENT
65
Page 79

• % Recovery - A measure of how accurately your observed results match your expected
results following regression analysis.
(Observed concentration) / (Expected concentration) x 100%
• Expected Result - The known or expected test result value for a standard or control.
• Control Range - Low - The lowest value for an assay control used to determine pass/fail
criteria for an assay.
• Control Range - High - The highest value for an assay control used to determine pass/fail
criteria for an assay.
• Normalized Net Median - For each analyte in a well the Normalized Net Median (NNM) =
(net median of analyte) / (net median of normalization bead)
• Units - The unit of measure for an analyte, for example, pg/mL.
*Trimmed statistics remove the lower and upper five percent of the extreme statistic values,
then use the remaining values for the Mean, Standard Deviation, or % CV calculations.
The statistics displayed change according to the analysis type selected.
Analyte - Contains a list of analytes run in the batch. Select an analyte to view all statistics
for that analyte.
Current Well - Displays the statistics of the well currently being displayed (this changes to
Displayed Well if viewing a batch using the Open button of the Saved Batches tab).
Single Step - Allows analysis one well at a time. Click to turn this function on or off.
Results
buttons to move through the table, or use the scroll bars. The Maximize/Minimize toggle icon
expands the batch table and returns it to the standard size.
Plate - A list of the available plates (if more than one).
- Displays statistics related to the batch. Use the up, down, left, and right arrow
CAUTION: If using multiple plates, ensure that plates are used in the proper
order. Failure to do so can result in inaccurate data and test
results.
Right-click inside the dot plot area to access the following options:
Display Mode - Select either Logarithmic or Linear display. The software defaults to
Logarithmic display. Click maximize for a magnified view of the dot plot.
Log - Displays a log of system processes. The log includes the following information:
• Date
• Message
• Code
Log entries indicating warnings are highlighted yellow. Errors are highlighted red. Other log
entries are not highlighted. Click the Maximize icon to maximize the log. The log expands
to fill the whole window. Click the Minimize icon to return to the standard size.
Save Image - Opens a Save As dialog box to save a screen capture.
Details - Opens the Results tab, enabling more analysis and results.
Progress - Click to display counts per region of the selected well. Analyte counts are
displayed in a bar graph as they are acquired. The scroll bar at the bottom of the Progress
display scrolls through the analyte list.
xPONENT for MAGPIX
66
Page 80

• Maximize - Maximizes the Progress display to fill the window.
• Zoom - Enable a closer look at the analyte progress.
• Save Image - Opens a Save As dialog box to save a screen capture.
• Default - Returns to the dot plot display.
Well Report - Displays a representation of the plate and the status of sample acquisition.
Wells will display one of three possible states:
• Yellow - Well acquired, with a problem (select the Log tab for more information).
• Green - Well acquired successfully.
- Well acquisition unsuccessful. The system may have stopped, depending on the
• Red
circumstances (select Log tab for more information).
Sample Lists - Contains a list of protocols, including the version number and the number of
samples associated with each protocol.
All Regions - Displays all regions of the batch.
All Regions - Displays all regions of the batch.
Default - Returns to the dot plot display.
Using xPONENT
67
Page 81

Select Replay Mode
To replay a batch, open the Results page, then the Saved Batches tab. Select the batch
that you want to replay and click Replay at the bottom of the screen. This opens the Select
Replay Mode dialog box. Select Replay batch or Recalculate data as described below.
The information that you must enter to replay or recalculate is the same.
A batch can be reprocessed multiple times.
NOTE: When replaying or recalculating large batches, the operation may
take 1 hour or more to complete. Batch replay cannot be stopped
while in progress. Allow adequate time for the operation to complete.
The operation is complete when all progress bars have disappeared.
NOTE: The initial batch data and output file always remain intact and
unchanged. Each time you replay or recalculate a batch, the system
handles it as if it is a new batch, creating a separate batch entry and
output file.
FIGURE 32.
Select Replay Mode Dialog Box
xPONENT for MAGPIX
68
Page 82

Analyzing a Saved Batch
Open the Results page, then open the Saved Batches tab. Select the batch name, then
click Open
• Click the Results tab to view statistical information about the batch.
• Click the Log tab to view a log for the activity that occurred during acquisition of the
selected batch.
• Click the Sample Details tab to view sample details for each sample in the batch. If you
are using the LIS package, click Transmit to transmit the data to the LIS.
Saved Batches Tab
Use this tab to open a batch that has been run and view its details, and export, approve, or
replay a batch.
. The Results, Log, and Sample Details tabs appear.
FIGURE 33.
Saved Batches Tab
Completed Batches - Displays a list of completed batches that includes the Name,
Protocol, Protocol Version, Date, Status, and User information, for each batch. Batches
that have not been run are not included.
Filter - Opens the Filter Setup dialog box.
Using xPONENT
69
Page 83

FIGURE 34.
This dialog box lets you choose the saved batch that you want to display in the Completed
Batches list, based on the options you select or clear in the following check boxes:
• Batch Name
• Protocol
• Batch Status
• Lot ID
• Kit ID
• Analyte
• Sample ID
• First Name
• Last Name
• User ID
• Date
Filter Setup Dialog Box
Reset - Clears all check boxes.
OK - Closes the dialog box and applies any changes you made.
Cancel - Closes the dialog box and cancels any changes you made.
Save Prtcl - Saves the protocol and kit information for the selected batch.
Plate Layout - Opens the Report dialog box, which includes the Batch Plate Layout
Report. Confirm that the plate layout conforms to your specific assay instructions.
Approve - Opens the Batch Approval Confirmation dialog box to approve the selected
batch. Only approved batches can be transmitted to the LIS. If your software is licensed for
LIS use, you can transmit batches to the LIS from the Sample Results tab. Once you have
approved a batch, the status of the batch changes to Approved in the Completed Batches
list.
Exp Results - Opens the Save As dialog box to choose an export destination for the CSV
file containing your results.
xPONENT for MAGPIX
70
Page 84

Import - Opens the Open dialog box to choose a batch file (.mdf) to import. Select Include
Raw Files (LXB) to import the raw data file as well.
Export - Opens the
Select Include Raw Files (LXB) to include the raw file in the export. Select Overwrite to
overwrite pre-existing files.
Open - Opens the Results tab. Use this tab to view the saved batch results for the selected
batch.
Export Batch dialog box to choose a destination for the batch file (.mdf).
Invalidate Standards and Controls
NOTE: It is possible to invalidate or remove a control in data analysis.
However, Luminex does not recommend invalidating controls.
For information on assay controls and guidelines regarding accepting or rejecting control
values, contact the assay kit manufacturer.
To invalidate standards and controls:
1. Open the Results
2. Open the Saved Batches tab.
3. Click the batch name, then click Open. The Results tab opens.
4. Click the square area next to left of the standard you wish to invalidate, then click
Invalidate. The whole row turns red.
page, then open the Saved Batches tab.
Viewing Batch Settings
1. Open the Results page, then open the Saved Batches tab.
2. Click Saved Batches, then click the batch for which you want to view details.
3. Click Open, then click the Settings tab.
4. Click the left and right Page arrows to view the pages of the batch settings report.
5. Click Save to open the Save As dialog box. Navigate to the location where you want to
save the batch settings report, and click Save.
Viewing Batch Logs
1. Open the Results page, then open the Saved Batches tab.
2. Click Saved Batches, then click the batch for which you want to view details.
3. Click Open. The Results tab opens.
4. Click Log to open the Log tab.
Viewing Sample Details
1. Open the Results page, then open the Saved Batches tab.
2. Click Saved Batches, then click the batch for which you want to view details.
3. Click Open, then click Sample Details. The Sample Details tab opens. If you are using
an LIS licensed package of the software, click Transmit to transmit sample details to the
LIS database. You can transmit either a single analyte per sample or the entire sample.
Using xPONENT
71
Page 85

Results Page
Once data is collected in a batch, observation and analysis take place in the Results page.
This page contains the following tabs:
• Current Batch tab
• Saved Batches tab
• Results tab
• Log tab
• Sample Details tab
• LIS Results
• Reports tab
The information that you can view and the actions you can perform differ depending on which
of these tabs you click.
Results Tab
Access this tab by clicking the Results page, then Saved Batches. The Results tab opens.
tab
xPONENT for MAGPIX
72
Page 86

FIGURE 35.
Results Tab
This tab displays the following:
• Results
• Statistic
• Analyte
• Displayed Well
• Well information (Well, Sample, Run Statistic; Analytes by number)
• Plate Layout image. Confirm that the plate layout conforms to your specific assay
instructions.
• Bead Map
The Results tab has the same Save Image and Progress buttons as the Current Batch
tab. The following buttons are added to the Results tab:
• Formula - Opens the Change Analysis dialog box with a list of analytes used in the batch.
Click an analyte to open the Analysis Settings dialog box from which you can select a
new analysis setting for the analyte.
• Approve - Opens the Batch Approval Confirmation dialog box, which contains the data
for analytes selected in the Results tab. Click Yes to approve the batch. The dialog box
confirms the approval.
Using xPONENT
73
Page 87

• Validate - Validates an entire selected row or cell in the Results table. Average rows or
cells cannot be selected. If you haven’t selected an item or the item you selected does not
need to be validated, a warning dialog box displays. Your xPONENT system administrator
must give you privileges to invalidate standards if you are using the Secure xPONENT
package.
• Invalidate - Invalidates an entire selected row or cell in the Results table. The selection
will turn red when invalidated. Select the same item and click Validate to remove the
invalidation status.
• Save - Saves the batch. This button only appears if a change was made to the batch.
• Analyze
clicked Invalidate on a portion of the data, or if you have clicked Validate on an
invalidated portion of the data. This will analyze the data again with the invalidated
standard removed.
• Close - Closes the batch and reopens the Saved Batches tab.
Click the Results page, then open the Saved Batches tab. Click the batch name, then click
Open. The Results Settings, Log, and Sample Details tabs appear.
• Click the Results tab to view statistical information about the batch.
• Click the Log tab to view a log for the activity that occurred during acquisition of the
selected batch.
Click the Sample Details tab to view sample details for each sample in the batch. If you are
using the LIS package, click Transmit to transmit the data to the LIS.
- Analyzes data that has been invalidated. This button only appears if you have
Settings Tab
Use this tab to view the acquisition parameters of the selected saved batch and print the
batch settings report.
xPONENT for MAGPIX
74
Page 88

FIGURE 36.
Settings Tab
Name and description boxes - Type the name and description in the appropriate box.
Acquisition Settings:
• Volume - This is the volume the instrument will aspirate into the system for analysis. Type
the desired sample volume in microliters. Use values from 10 to 200 µl. To avoid air intake,
add at least 25 µl to the sample well in addition to the sample size. The default value is 50
µl.
• XY heater - Select Enabled to enable the XY heater. In the box type the desired value in
degrees Celsius. The temperature range is 35 to 60° C.
CAUTION: Acquiring data before the heater has reached the proper
temperature can compromise test results.
• Plate name - The name assigned to the plate. You can select a different plate from the list.
• Sample wash - Select this option for assays without a final wash step prior to reading the
plate on the instrument. This automatically washes each sample within the instrument.
Final washes are required for proper analysis.
Using xPONENT
75
Page 89

Analysis Settings - Use this section to set the analysis type, set the number of standards
and controls, select an external analysis program, and choose whether to analyze results
while acquiring samples.
Analysis Type - Use this list to choose from the following analysis types:
• None - No analysis. Select if you have your own data post-processing program and
want to obtain only fluorescent intensity results. You cannot apply standards or controls
when you select None. You cannot analyze acquisitions with this setting.
• Qualitative - Qualitative analysis determines results as either positive or negative,
reactive or non-reactive. The software is flexible in defining custom result ranges, such
as negative, low positive, or high positive. Determinations are based on a single
standard. For qualitative analysis the Luminex software uses a specific algorithm, shown
below.
Where:
FI = Fluorescent Intensity
Ki = a “Quali” value entered in lot information to determine the value or the qualitative
assay standard.
The “Quali” value determines a cutoff or threshold. This, in conjunction with ranges using
the Lum Qual formula or an edited range specific for your assay, helps to determine
qualitative results for unknown samples.
Two predefined formulas using the algorithm are included in the system. You can use
them as-is or edit their range values to meet your requirements.
• Quantitative - Determines the sample concentrations from standard curves using
regression methods Cubic Spline, Linear, Logistic 4P, and Logistic 5P. Type the
desired values for standards and controls in the Number of Standards and Number of
Controls boxes. Select either Fit of All Standards or Mean of Replicates for the
calculation of the curve fit.
NOTE: Luminex recommends Fit of All Standards as the most accurate
calculation of the curve fit.
Based on a range of quantitative, numerical results, a threshold range can be applied to
a quantitative analysis, for example, high, low, saturated, and expected.
• Allele Call - Sets analysis for an allele call. Analytes must be put in groups of 2, 3, or 4
analytes.
• Min MFI Enabled - Select this box to enable a minimum MFI for the Allele Call analysis.
Type a value in this box to set the minimum MFI for analysis.
• Analyze results while acquiring samples - The software allows real-time viewing of the
results as the instrument analyzes samples. This feature is not available if you select None
as your analysis type.
• Number of Standards - Click to type the number of standards for the protocol. Applies
only to qualitative and quantitative analyses.
• UseExternal Analysis Program - Select this check box to use a third-party program to
analyze the data. The Analysis Program list becomes active when this is selected.
Applies only to qualitative and quantitative analyses.
• Number of Controls - Click to type the number of controls for the protocol. Applies only to
qualitative and quantitative analyses.
• Analysis Program - Use this list to select which program to use for data analysis.
xPONENT for MAGPIX
76
Page 90
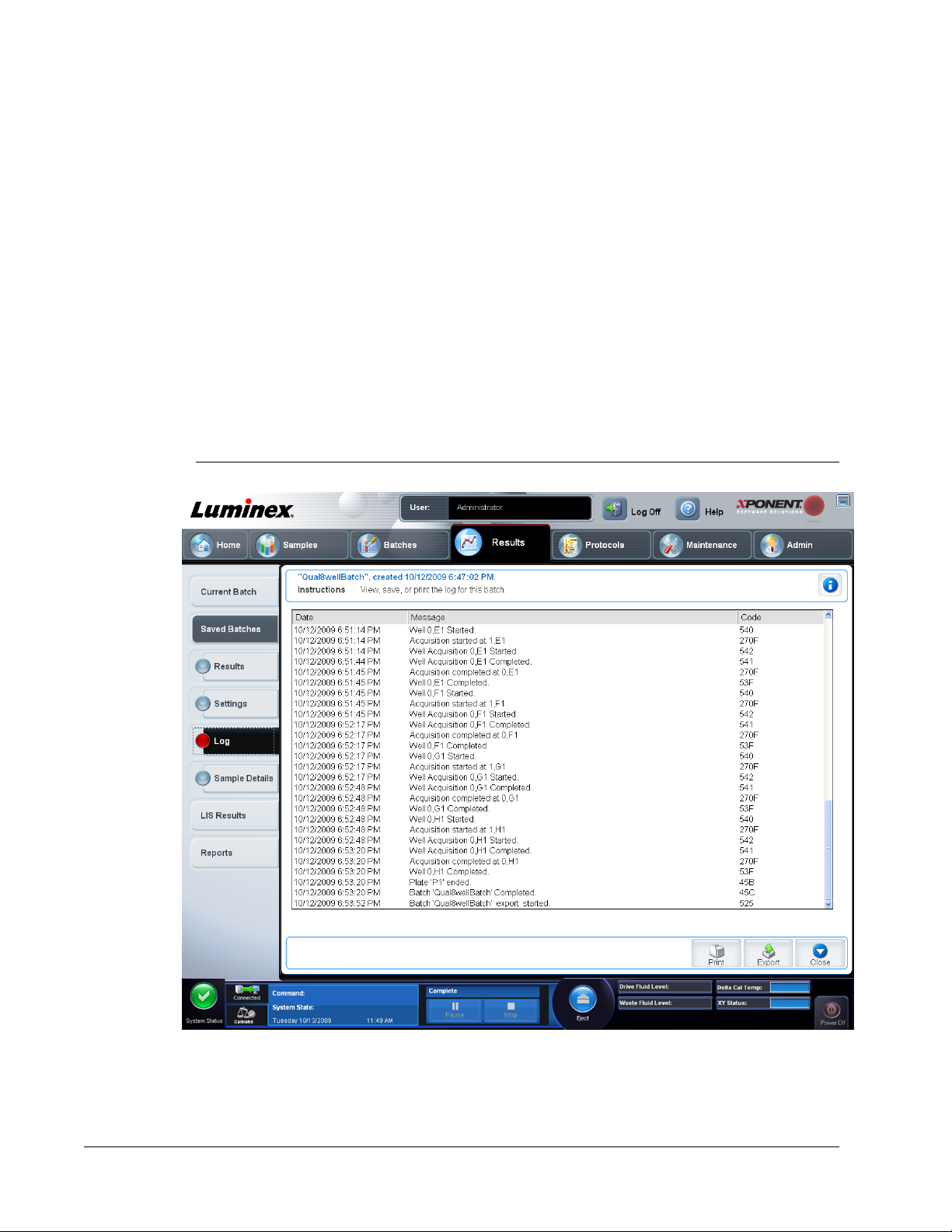
• Fit of all Standards - The standard curve will be determined by using each individual
standard replicate when calculating the standard curve. For example, if you run duplicates
of a seven-point standard curve, the software will calculate the standard curve by using 14
points. Applies only to quantitative analysis.
• Mean of Replicates - The standard curve will be determined by averaging the individual
standard replicates when calculating the standard curve. For example, if your un duplicates
of a seven-point standard curve, the software calculates the standard curve by using 14
points. Applies only to quantitative analysis.
Cancel- Returns you to the main Batches tab.
Next - Click to advance to the Analytes tab.
Log Tab
This tab displays a log of the activity that occurred during the acquisition of the selected
batch.
FIGURE 37.
Log Tab
This tab displays the following information about each activity:
Using xPONENT
77
Page 91

• Date
• Message
• Code
Log entries are displayed in yellow if a well was acquired with a warning. Log entries are
displayed in red if a problem occurred during acquisition.
- Prints the log.
Print
Export - Opens the Save As dialog box so that you can save the log file. Browse to a
location and click Save.
Close - Reopens the Saved Batches tab.
Sample Details Tab
Use this tab to view sample results.
FIGURE 38.
Sample Details Tab
Arrows - Scroll through the sample details.
Transmit - For systems configured for LIS transmission, select a single analyte or the entire
sample and click Transmit to send the results.
Close - Reopens the Saved Batches tab.
xPONENT for MAGPIX
78
Page 92

LIS Results Tab
This tab displays saved batches containing LIS samples.
FIGURE 39.
LIS Results Tab
Filter- Opens the Filter Setup dialog box.
Clear - Click to turn off the filter.
Completed Samples - Displays Name, Protocol, Sample Count, Date, Status, and User
information for each batch displayed in this list.
Transmit - Transmits a batch to a LIS if a licensed version of xPONENT is connected to one.
Details - Opens the Sample Details tab to view sample results.
To transmit a batch to an LIS:
1. Open the Results page, then open the LIS Results tab.
2. Select a batch, then click Open.
3. Click Yes to approve and transmit the batch to the LIS database.
Using xPONENT
79
Page 93
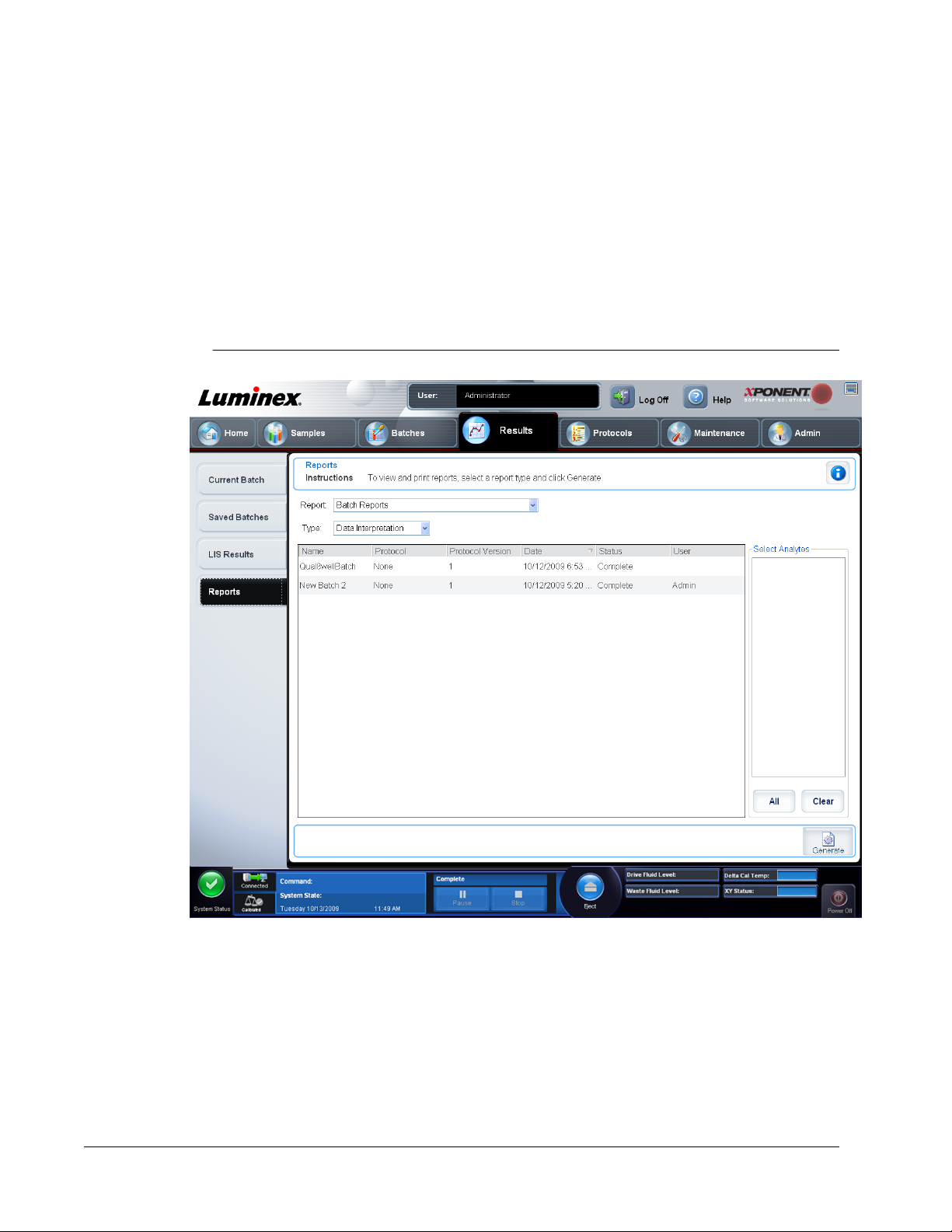
Reports Tab
Use this tab to view, generate, and print reports. xPONENT can format your batch or multibatch results in a variety of export formats and provide different types of information in three
different types of reports:
• Batch Reports
• Protocol Reports
•
System Log Reports
FIGURE 40.
Reports Tab
Report and Type lists - Choose a report from one of the following categories:
Batch Reports - A list of batches appears below the Type list. The list includes Name,
Protocol, Protocol Version, Date, Status, and User information. Choose from the following
reports:
• Data Interpretation
• Batch Settings
• Plate Layout
xPONENT for MAGPIX
80
Page 94

• Batch Audit
• Patient Report
If you choose Data Interpretation
displays a list of the analytes in the batch. Select the analytes you want to include in the
report. Select All to select all the analytes in the list. Select Clear to clear all the analytes
in the list.
A series of new buttons appears when you click Generate Report:
• Analyte arrows - This feature is directly below the Report list. Use the left and right
arrows to display information for individual analytes of those selected for the report.
• Page arrows - use the arrows to scroll through the pages being displayed.
• Save All - Click to open the Browse For Folder dialog box. Select a location to save the
file, and click OK. This file includes all selected analytes.
• Print All - Click to print the information for all the analytes in the report.
• Save - Click to open the Save As dialog box. Select a location and click Save. This saves
only the analyte information currently being viewed.
• Print - Click to print the analyte information currently being viewed.
• New Report - Click to return to the main Reports window.
NOTE: If running a data interpretation of an allele call batch report, be aware
that when choosing analytes from the Select Analytes list, selecting
one analyte will select all analytes in that group.
, a Select Analytes section appears on the right and
Protocol Reports - A list of protocols displays. Choose from the following reports:
• Protocol Settings
• Plate Layout
• Protocol Audit
Select a protocol and then click Generate to generate the report.
Calibration and Verification Reports - Displays a list of calibrations and verifications.
Choose from the following selections:
CALVER - Select a calibration component, a date from the Date list, and click Generate to
generate the report.
Performance Verification Reports
following:
• Condensed
• Detailed
Select a report type, a date range from the Date list, and click
report.
System Log Reports - Displays a list of system log reports. Choose from the following
selections:
• All
• Maintenance
• Security
• Warning and Errors
- Displays two report types. Choose either of the
Generate to generate the
Using xPONENT
81
Page 95

Select a system log report, a date from the Date list, and click Generate to generate the
report.
Advanced Reports - Enables a single option, User Report
Click Generate to generate the report.
NOTE: Install the printer before initiating the Print command.
To generate, view, and print a report:
1. Open the Results page, then open the Reports tab.
2. In the Report list, click the report you want to view.
3. In the Type list, select the report type you want to view.
4. Select the item for which you want to generate the report. If you are creating a batch
report, select the analyte to include in the report.
5. Click Generate. The report displays in the lower part of the Reports tab.
6. Click Print to print the report, or Save to save the report.
Using Protocols, Lots, and Kits
Protocols for IVD assay kits should be supplied by the IVD kit manufacturer.
, in the list.
Creating an Allele Call Protocol
This protocol contains no standards or controls. An allele call compares groups of two, three,
or four analytes to identify genotypes.
To create an allele call protocol:
1. Open the Protocols page, then open the Protocols tab. Click Create New Protocol.
The Protocol Settings tab opens.
2. In the Name box, type the name of the protocol.
3. In the Description box, type a description of the protocol.
4. In the Version box, type the version of the protocol.
5. In the Manufacturer box, type the manufacturer information for the protocol.
6. Define the settings in the Acquisition Settings section. For information about each of
these settings, #unique_117.
7. Define settings in the Analysis Settings section, selecting Allele Call as the analysis
type. For information about the settings in this section, #unique_117.
8. Click Next. The Analytes tab opens.
9. Click the desired analyte (bead ID) in the numbered grid. Information about the analyte
appears in a list on the right side of the grid. For an allele call analysis, you must select a
group of two, three, or four analytes.
10. Click Group to group the analytes for the allele call. The grouped analytes display in a list
to the right. Select more analytes and then click Group again if you want to add another
group to the analysis.
11. Click and type an analyte name in the Name column to the right of the analyte grid.
xPONENT for MAGPIX
82
Page 96

12. In the Count box, type the total desired bead count for each analyte. Click Apply All.
13. To set a bead count and the units for a single analyte, click in the Units
columns directly to the right of the analyte grid, and type a units value and bead count.
14. Click in the Call % column and type a value to set an individual analyte’s call percentage.
15. Click Next. The Plate Layout tab opens.
• To add well commands, highlight the appropriate wells and mark them as unknown,
standard, control, background, or wash. You can also delete commands that you’ve
added, and change the starting location on the plate. If you want to run in replicate,
change the Replicate Count to the appropriate number and the Grouping to your
preferred grouping method.
• Delete a command by clicking the well, and clicking Delete. The Delete Options
dialog box opens. Select Delete just the selected wells to delete a single well
command, or Delete all wells containing these samples to delete all wells with the
same command.
• As you add commands to your plate, they appear in the Command Sequence list.
Type a well ID in the ID field. Here, you can give each of your wells an ID. You can
also import a protocol file by clicking Import List.
• Move commands up or down in the sequence by highlighting a command and using
the Move Command arrows to move the command up or down in the list.
16. Click Save.
and Count
Importing a Protocol
1. Open the Protocols page, then open the Protocols tab. Click Import.
2. In the Open dialog box, navigate to the protocol file you want to import, then click Open.
3. The imported protocol displays in the Installed Protocols list.
Adding a New Lot for Protocol
1. Open the Protocols page, then open the Protocols tab. Click the protocol to which you
want to add a lot.
2. Open the Stds & Ctrls tab.
3. Click Create New Std/Ctrl Lots and select a protocol from the drop down list in the
Select Protocol dialog box, then click OK. The Std/Ctrl Details tab opens.
4. Click Apply Std/Ctrl Kit to associate a kit with the protocol. If you are not using a kit,
type the appropriate Standard and Controls information in the Assay Standard
Information and Assay Control Information sections.
5. Click Save.
Deleting a Protocol
1. Open the Protocols page, then open the Protocols tab.
2. Select a protocol.
3. Click Delete. The Delete Protocol dialog box opens.
4. Click Yes.
Using xPONENT
83
Page 97

Exporting a Protocol
1. Open the Protocols
2. Select a protocol.
3. Click Export. The Save as dialog box opens.
4. Select a location to export the file to, and click Save.
Lots and Kits
Assay kits include standards and/or controls. After you enter the assay kit information, it can
be used in multiple protocols. However, you should create separate kits specifically for use
with each protocol. For assay reagents specified in protocols, you can create new lots, edit lot
information, select pre-existing lots for reuse, import lots, and export lots.
Once a lot is used, changing or modifying it will prompt you for a new lot name.
Creating a Kit
To create a kit:
1. Open the Protocols page, then open the Protocols tab. Select the protocol that you
want to use for the kit, then click NewStd/Ctrl. The Std/Ctrl Details tab opens.
2. Click on the protocol that you want to use for the kit, then click New Std/Ctrl. The Std/
Ctrl Details tab opens.
3. Type the name of the kit in the Name box, the lot number in the Std/Ctrl Kit Lot# box,
the expiration date using MM/DD/YY format in the Expiration box, and the manufacturer
in the Manufacturer box.
4. Click Apply Std Lot if you want to apply a standard lot. The Select Lot dialog box opens.
Click a lot and select OK.
page, then open the Protocols tab.
5. Click Apply Ctrl Lot to apply a control lot. The Select Lot dialog box opens. Select a lot
and click OK.
6. Alternatively, type the appropriate information in the AssayStandard Information and
Assay Control Information sections. The number of standards and/or controls in these
sections is defined in the protocol. If your batch uses controls, select Expected, Low or
High from theShow Value options. Use the Apply Values arrows to apply values down
or across the range of analytes.
7. Click Save.
Creating Lot
To create lots, you must use a protocol that uses either Quantitative or Qualitative analysis
settings.
To create a lot:
1. Open the Protocols page, then open the Protocols tab. Click the Stds & Ctrls tab, then
click Create New Std/Ctrl Lots.
2. In the Select Protocol dialog box, select the protocol you want to use for this lot, then
click OK. The Std/Ctrl Details tab opens.
xPONENT for MAGPIX
84
Page 98

3. If the protocol uses standards, type the appropriate information for each standard in the
Assay Standard Information section. In each analyte column, type the expected
concentration for the analyte.
4. Alternatively, click Apply Std/Ctrl Kit and select a lot from the Select Lot
Click OK to apply the lot.
5. If your batch uses controls, select Expected, Low, or High from the Show Value
options. Use the Apply Values arrows to apply values down or across the range of
analytes.
6. Click Save.
dialog box.
Importing a Lot
1. Open the Protocols page, then open the Protocols tab. Click the Stds & Ctrls tab, and
then click Import.
2. In the Open dialog box, navigate to the file, then click Open.
Deleting a Lot
To delete a lot:
1. Open the Protocols page, then open the Protocols tab. Click the Stds & Ctrls tab.
2. In the Installed Kits And Lots section, click the lot you want to delete, then click Delete.
Exporting a Lot
NOTE: Lots and kits can only be exported if the protocol they were originally
created with exists within the system. If the protocol has been
deleted, the lot or kit cannot be exported.
To export a lot:
1. Open the Protocols
2. In the Installed Kits And Lots section, click the lot you want to export, then click Export.
The Save As dialog box opens.
3. Navigate to the location you want to export the file to, then click Save.
page, then open the Protocols tab. Click the Stds & Ctrls tab.
Editing a Lot
To edit a lot:
1. Open the Protocols page, then open the Protocols tab. Click the Stds & Ctrls tab.
2. In the Installed Kits And Lots section, select a lot and then click Edit. The Std/Ctrl
Details tab opens. Change the lot information as appropriate.
Using xPONENT
85
Page 99

xPONENT for MAGPIX
86
Page 100

Chapter 4: Performing System
Maintenance
Initial Startup
When you turn on the system for the first time, perform the following procedures.
1. Adjust the Sample Probe Height
2. Revive After Storage (Luminex) Routine
3. Calibration/Verification Routine
Adjusting the Sample Probe Height
Adjust the sample probe height to ensure that the probe drops far enough into the well to
acquire sample.
NOTE: Ensure that there is no liquid in the wells or reservoirs before
adjusting the sample probe height.
1. On the Home page, click Probe and Heater under Daily Activities. The Probe &
Heater tab opens.
2. Based on the type of plate you are using, place alignment disks or an alignment sphere
from the Height Alignment Kit in the well, as specified below:
• Filter-bottom plate - two (2) 5.08mm disks
•
Mylar-bottom plate - two (2) 5.08mm disks
• Conical (V-shaped) plate - one (1) sphere
If you are using a standard 96-well plate, you do not need to use any of the disks or
spheres in the Height Alignment Kit.
3. Ensure that the well location is selected on the plate image. Luminex recommends that
you use well D6 (this is the center of a standard 96-well plate). A green pin marks the
selected well.
4. Click Eject to eject the plate holder.
5. Place a strip well in the off-plate reagent block.
6. In the Strip Wells section, click D1.
87
 Loading...
Loading...