Page 1

LD-221С
ENGPOLUKR
Compressor Nebulizer LD
Instruction Manual
Inhalator kompresorowy LD
Instrukcja obsługi
Компресорний інгалятор LD
Інструкція з експлуатації
Page 2
ENG
2
#
12
"
13
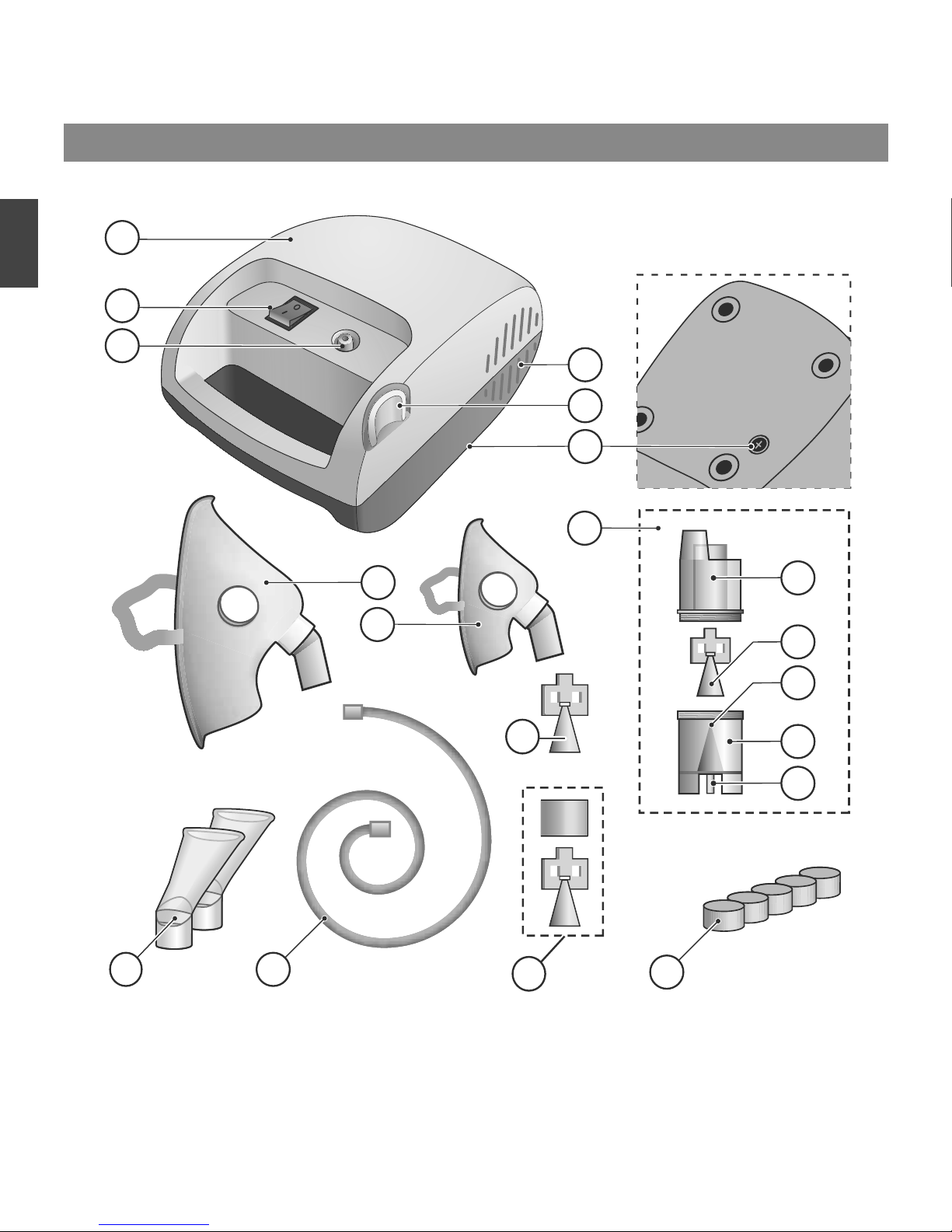
PARTS AND COMPONENTS
Page 3

ENG
3
DEVICE PARTS DESCRIPTION
№ position
on the
scheme
NAME DESCRIPTION
1 Compressor Nebulizer compressor unit for to create air pressure.
2 Power Switch Tumbler I/O – switch on/switch off power.
3 Connector of Compressor Fitting compressor for connecting an inhalation tube.
4 Intake Holes for air intake to cool the compressor.
5 Holder for nebulizer Holder for the nebulizer.
6 Socket for the Filter Position the air fi lter for nebulizer.
7 Nebulizer LD-N105 Chamber of the inhalation of aerosol solution.
7.1 Upper Part of Nebulizer Part of the aerosol chamber. Place connected to the coupling.
7.2 Baffl e “B” (blue) LD-N002 Inhalation baffl e (universal). Consumable material.
7.3 Nozzle Conical hole to create a thin jet of air.
7.4 Bottom of Nebulizer
Part of the aerosol chamber designed for inhalation solution with
a seat for the baffl e.
7.5 Connector of Nebulizer Connector for connectning the inhalation tube
8 Adult Mask LD-N041 Adult inhalation mask. Consumable material.
9 Сhild Mask LD-N040 Child inhalation mask. Consumable material.
10 Inhalation mouthpiece Inhalation mouthpiece. Consumable material.
11 InhalationTube LD-N051 Inhalation tube the length of 2 m. Consumable material.
12
Baffle «C» (red) LD-N003
Inhalation baffle (mainly the upper respiratory tract). Consumable
material.
13
Baffle «A» (yellow) LD-N001
inhalation baffle (mainly the lower respiratory tract). Consist of two
parts: cone and cylinder.
Consumable material.
14 Filter Inhaled Air fi lter for nebulizer. Consumable material.
Page 4

ENG
4
NEBULIZER THERAPY – WHAT IS IT?
NEBULIZER IS A DEVICE FOR FORMATION AND SPRAYING OF AEROSOL. THE WORD “NEBULIZER” IS
DERIVED FROM THE LATIN WORD “NEBULA” FOG, CLOUD AND WAS FIRST USED IN 1874 FOR A DEVICE
THAT TURNS A LIQUID SUBSTANCE INTO AN AEROSOL FOR MEDICAL PURPOSES. ONE OF THE FIRST
PORTABLE “AEROSOL APPARATUSES” WAS CREATED BY J. SALESGIRONS IN PARIS IN 1859. THE FIRST
NEBULIZERS WERE USED AS STEAM JET ENERGY SOURCES AND WERE APPLIED FOR INHALATION THE
VAPORS OF RESINS AND ANTISEPTICS BY TUBERCULOSIS PATIENTS. PRESENTLY, THE TERM “INHALER” IS
OFTEN USED INSTEAD OF “NEBULIZER”.
The purpose of the nebulizer therapy is to quickly deliver to the respiratory passages a therapeutic doze of
a preparation in aerosol form. Continuous supply of aerosol allows, within several minutes, creating high
concentration of a medicine in the upper and lower respiratory passages and lungs, with low probability
of any by-eff ects. Respectively, eff ective bronchodilation (bronchi expansion) is reached, and the need for
hospitalization is eliminated or the hospital stay is reduced.
Little Doctor Internatiоnаl (S) Pte. Ltd. off ers you to use inhaler LD-221C, whose distinctive features are
the possibility to use a wide range of medicines, low inhalation solution residual volume, and reliable and
simple use. We thank you for your choice.
GENERAL INFORMATION
Compressor nebulizer LD is designed for treating the diseases of respiratory passages and lungs by medicine solution aerosols in clinic and at home.
This Instruction Manual is designed to assist the user with safe and eff ective operation of the Compressor
Nebulizer LD.
Use this Device according to the rules described in this Manual. Operate the Device only as intended. Do
not use the Device for any other purposes. Read and understand the whole Instruction Manual.
Functionally, the device consists of an air compressor and nebulizer (aerosol formation chamber). The air
compressor, on/off power switch and air fi lter are united in one casing. From the air compressor, the compressed air is fed through a pipe to the nebulizer, where aerosol is formed. For cooling the compressor, air
is force-feed into the device’s casing.
SAFETY INFORMATION
To assure the correct use of the product, basic safety measures should always be followed including the warnings and cautions listed in this instruction manual.
WARNING
● For regime of medication shall follow the instructions of your physician or licensed healthcare practitioner.
● Do not cover the compressor with a blanket, towel, or any other type of cover during using. This
could result in the compressor overheating or malfunctioning.
● Do not use the device where the device may be exposed to fl ammable gas or vapors.
● Do not use mineral water in the nebulizer for nebulizing purposes.
● Always dispose of any remaining medication in the medication tank after each use. Use fresh
medication each time you use the device.
Page 5

ENG
5
● Do not leave the device or its parts where it will be exposed to extreme temperatures or changes
in humidity, such as leaving the device in a vehicle during warm or hot months, or where it will be
exposed to direct sunlight.
CAUTION
● Limit the use of the device to 20 minutes at a time, and wait 40 minutes before using the device again.
● Provide close supervision when this device is used by, on, or near infants, children or compro-
mised individuals.
● Do not insert any object into the compressor.
● Make sure that the air fi lter is clean. If the air fi lter has changed color or has not been used for
60 days, replace the fi lter.
● Make sure that the nebulizer kit is correctly assembled, the air fi lter is properly installed, and the
air tube is correctly connected to the compressor and the nebulizer kit. Air may leak from the air tube
during use if not securely connected.
● Inspect the compressor (main unit) and the nebulizer parts each time before using the device.
Make sure no parts are damaged, the nozzle and air tube are not blocked and the compressor
operates normally.
● Do not use the device if the air tube is bent.
● Do not block the air fi lter cover.
● Do not alter the baffl e, the nozzle in the medication tank or any part of the nebulizer kit.
● Do not add more than 10ml of medication to the medication tank.
● Do not operate the device at temperatures greater than 40˚C.
● Do not tilt the nebulizer kit so the angle of the kit is greater than 45˚. Medication may fl ow into the mouth.
● Do not shake the nebulizer kit while using the device.
● Do not subject the compressor, or any of the components to strong shocks, such as dropping on the fl oor.
● This device is approved for human use only.
● Do not disassemble or attempt to repair the device or components.
● Use the device only for its intended use as described in the instruction manual. Do not use
attachments not recommended by the manufacturer.
● Dispose of the device, components and optional accessories according to applicable local regulations. Unlawful disposal may cause environmental pollution.
● Make sure that the air tube is securely attached to the compressor (main unit) and nebulizing
parts, and does not come loose. Twist the air tube slightly when inserting it into the connectors to
avoid the tube disconnecting during use.
RISK OF ELECTRICAL SHOCK
● Do not use the compressor (main unit) and the power cord while they are wet.
● Do not plug or unplug the power cord into the electrical outlet with wet hands.
● Do not immerse the compressor (main unit) in water or other liquid.
● Do not spill water or other liquids on the compressor .These parts are not waterproof. If liquid
spills on these parts, please unplug the power cord and wipe off the liquid with gauze or other soft
absorbent material immediately.
● Do not use or store the device in humid locations or outdoors. Use the device within the operating temperature and humidity.
● Do not overload power outlets. Plug the device into the appropriate voltage outlet.
● Do not use extension cords. Plug the power cord directly into the electrical outlet.
Page 6

ENG
6
● Unplug the power cord from the electrical outlet after using the device. Never leave this product
unattended when plugged in.
● Unplug the power cord from the electrical outlet before cleaning the device.
● Completely read all of the instructions included the optional accessories before using them.
● Not to position the ME EQUIPMENT so that it is diffi cult to operate the disconnection device.
● The power switch is used to isolate the device from the supply mains.
● The direction of movement of the actuator of the supply mains switch is comply with IEC 60447.
MAINTENANCE AND STORAGE
● Keep the device out of the reach of unsupervised infants and children. The device may contain
small parts that can be swallowed.
● Do not leave the cleaning solution in the nebulizer parts. Rinse the nebulizer parts with clean hot
tap water after disinfecting.
● Wash the nebulizer parts after each use. Dry the parts immediately after washing.
● Do not store the air tube with moisture or medication remaining in the air tube. This could result
in infection as a result of bacteria.
● Store the device and the components in a clean, safe location.
● Do not carry or leave the nebulizer with medication in the medication tank.
● Do not place or attempt to dry the device, components or any of the nebulizer parts in a microwave oven.
● Do not wrap the power cord around the compressor (main unit).
The followings are maintenance and repair which can be taken by operator, or which must be operated by manufacturer or distributor.
SERVICE AND MAINTEMANCE RESPONSIBLE
Change the inhalation tube Operater
Change the applied part Operater
Change the air fi lter Operater
Clean the surface of the device Operater
Daily cleaning and disinfecting Operater
All components which need to be repaired or
changed by seperating the device
Authorized service center of distributor or
manufacturer
WARNING:
● Do not modify this equipment without authorizaiton of the manufacturer
● Do not service or maintenance the device while in use with the patient.
CARE AND DISPOSAL
●
Regularly clean the device and all accessories. It is recommended that all the accessories should
be wiped with a 3% solution of hydrogen peroxide with addition of 0.5% solution of a detergent (for
example, a laundry powder). After that, the nebulizer should be washed by a rich jet of water. The
mouthpieces and nose nozzles may be treated by boiling for 10 minutes or autoclaving at 150°С. After
the treatment, wipe dry all parts of the device with a soft cloth.
●
Regularly check the filter for dirt and replace it when needed. FILTER REPLACEMENT IS
RECOMMENDED AT LEAST ONCE A YEAR.
Page 7
ENG
7
●
Repair the Device only in authorized organizations.
● On expiration of the warranted service life apply from time to time to authorized repair orga-
nizations to check the technical condition of the Device. Dispose to time to authorized repair
organizations to check the technical condition of the Device. Dispose of the Device and its
components according to the application local regulations. No special requirements to disposal
of this Device are defi ned by the manufacturer.
USING THE DEVICE
Preparing for inhalation.
IMPORTANT: Before using the appliance for the fi rst time it is necessary to make
a full cleaning, as described in last paragraph «SAFETY INFORMATION».
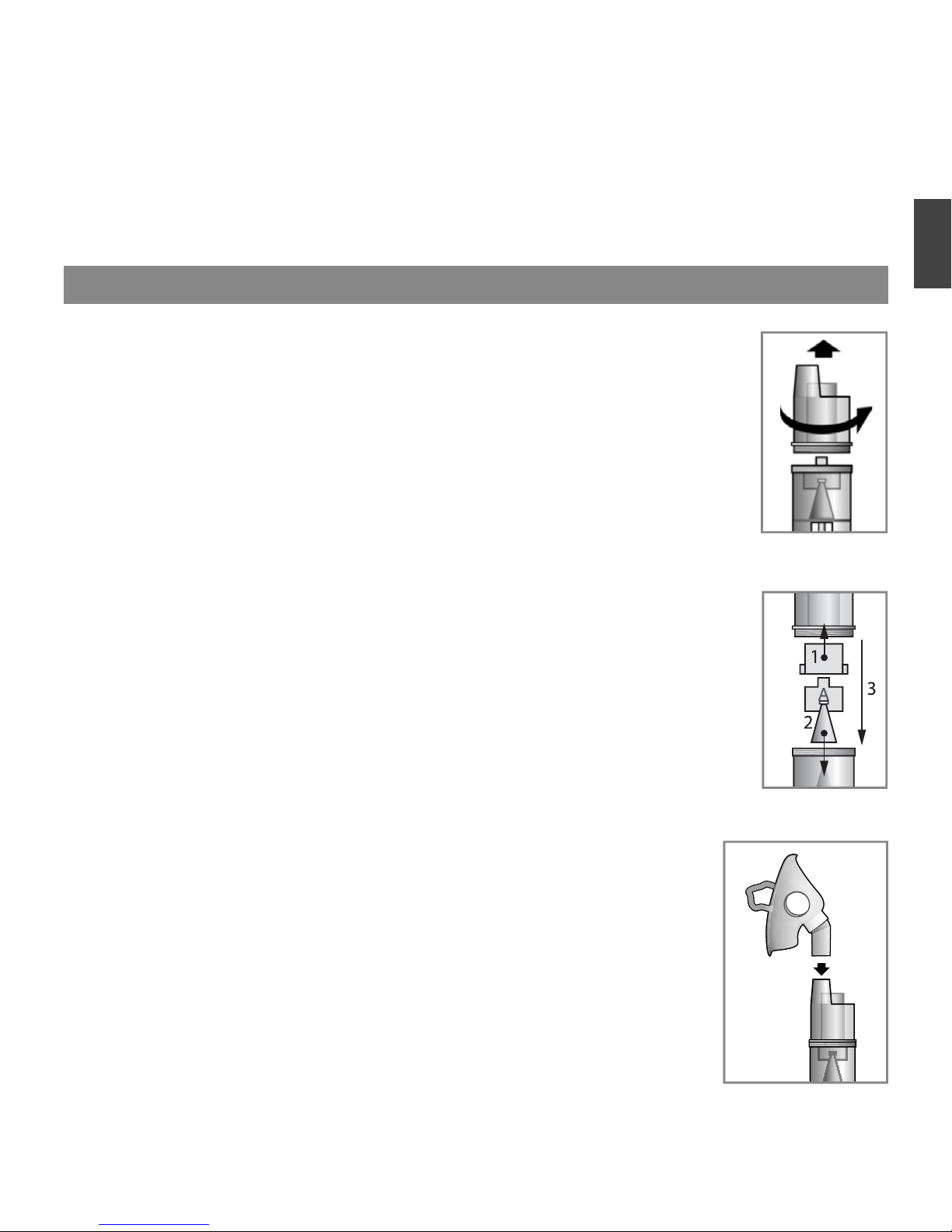
1.
Place the nebulizer in front of you on the table. Make sure the device is turned off
(power switch is in position «O»), and the power cord is not plugged into the mains.
2. Remove the top of the nebulizer by turning it counterclockwise (Fig.1).
3. Set the desired baffl e.
Factory installed baffl e inside the nebulizer is baffl e «B» (Blue), which is effective
to affect the entire respiratory tract.
For a more effective impact medicines on the upper respiratory tract, set, instead
of the blue baffl e, red baffl e «C».
For a more effective impact on the lower respiratory tract - baffl e «A» yellow
color, which consists of two parts (Fig. 2, fi gures indicate the order of assembly).
Graphics of the differential particle size distribution by mass for different nozzles
are shown in Fig. 4.
4. Fill the bottom of the nebulizer inhalation solution. The dosage should not exceed
the recommended by your doctor. The number of nebuliser solution is determined
by the scratches on the case. The maximum reservoir volume of 10 ml.
5. Attach the nebuliser at the top, turning it clockwise until it stops.
6.
Depending on the type of inhalation, using either a mouthpiece or
nozzle or mask.
The mask, mouthpiece or nose nozzle is connected directly to the upper
part of the nebulizer
(Fig.3).
Hold the nebulizer vertically.
IMPORTANT: Each patient is encouraged to use personal mouthpiece, a
mask and / or nozzle for the nose.
7. Plug the power cord to an electrical outlet.
8. Connect one end of the inhalation tube to the fi tting of compressor, and
others - to the fi tting of nebulizer.
9. Turn on the nebulizer, switching the power switch in position «I». NEBULIZER IS READY FOR INHALATION.
Fig. 3
Fig. 1
Fig. 2
Page 8
ENG
8
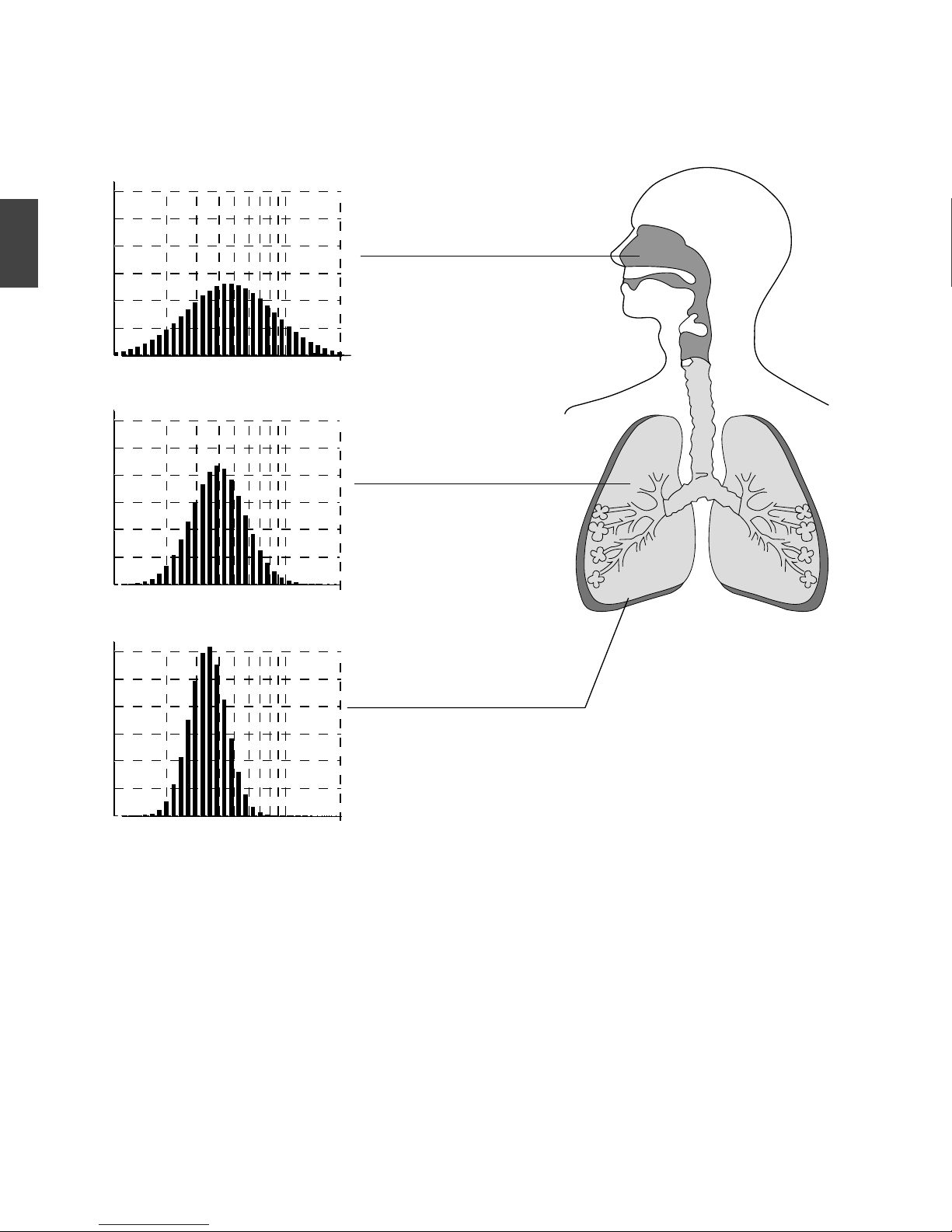
Depending on the type of baffl e, the particles of different sizes are distributed aerosols as follows:
QBSUJDMFTJ[FTNLN
UPUIFUPUBMNBTT
0
5
10
15
QBSUJDMFTJ[FTNLN
UPUIFUPUBMNBTT
0
5
10
15
QBSUJDMFTJ[FTNLN
UPUIFUPUBMNBTT
0
5
10
15
NBJOMZUIFVQQFS
SFTQJSBUPSZUSBDU
#BGGMF$SFE
#BGGMF#CMVF
#BGGMF"ZFMMPX
VOJWFSTBM
NBJOMZMPXFSSFTQJSBUPSZUSBDU
'PSFGGFDUJWFEFMJWFSZNFEJDJOFTUPBTQFDJGJDTJUF
PGSFTQJSBUPSZUSBDU OFFE UP VTFUIF BQQSPQSJBUF
CBGGMF
Performing the inhalation.
The length of one treatment session should not exceed 20 minutes. Consult your attending physician about
the length of the inhalation procedure.
You should always be calm and relaxed during the inhalation. Breathing should be slow and deep, so that the
preparation could fi ll the lungs well and reach the deep portions of bronchi.
Briefl y hold your breath, and then exhale slowly. Do not attempt to breathe too rapidly. Make pauses if you
feel that you need it.
Fig. 4
Page 9

ENG
9
Breath-actuated nebulizer.
The special design of the nebulizer in the form of chambers connected in a certain manner provides diff erent ways of air streams during inhaling and exhaling.
It allows obtaining the air stream with greatest aerosol concentration when
inhalation and reducing aerosol loss when exhaling. The eff ectiveness of inhalation using the breath-actuated nebulizer is increased signifi cantly.
Completing the inhalation.
When the inhalation solution is used up and the inhalation time recommended by the doctor has expired,
turn the device off by putting the tumbler in «O» position and unplug it. After inhalation, breathe fresh air
for some time for better treatment eff ect.
After each application of the device, the residual preparation should
be removed out of it. Clean and wash the device as described in last paragraph «SAFETY INFORMATION»
.
WARRANTY
The following LD product is covered by warranty for the period 36 months. The warranty does not
apply to the consumables (masks, mouthpieces, inhalation tubes etc.). The warranty liabilities are contained
in the warranty card given at the sale of this device to a purchaser. The addresses of organizations for warranty maintenance are given in the warranty card.
COMPLETENESS
№
NAME MODEL QUANTITY, pc.
1 Compressor — 1
2
Nebulizer (with
inhalation baffl e) LD-N105 1
3
Inhalation baffl e LD-N001
1
4
Inhalation baffl e LD-N003
1
5 Inhalation mouthpiece LD-N022 2
6 Adult inhalation mask LD -N041 1
7 Child inhalation mask LD -N040 1
8 Inhalation tube LD-N051 1
9 Inhalation fi lter LD-N055 5
10 Package — 1
11 Instruction Manual — 1
12 Warranty Card — 1
TECHNICAL SPECIFICATIONS
Model LD-221C
Type Compressor Nebulizer
Rating: AC 230V, 50Hz, 0.6A
Page 10

ENG
10
Extreme Pressure 205Kpa~400Kpa
Free Flow Range
≥10L/min
Nebulizing Pressure
70Kpa~190Kpa
Noise Level
≤ 65dB
Max capacity of nebulizer kit
10ml
Residual volume of inhalation solution, or below 0.5 ml
Nebulization
rate, approximately
Inhalation baffl e
«A» LD-N001
Inhalation baffl e
«B» LD-N002
Inhalation baffl e
«C» LD-N003
0.31 ml/min.
0.43 ml/min.
0.50 ml/min.
Particle size (ММАD)
Inhalation baffl e
«A» LD-N001
Inhalation baffl e
«B» LD-N002
Inhalation baffl e
«C» LD-N003
3.5 mkm
4.0 mkm
5.0 mkm
Max. operating time
20 minutes
Operation mode
20 minutes on, 40 minutes off
Operation Temperature and
humidity/ atmospheric pressure
10°C ~ 40°C,
95% and below/ 860~1060hPa
Transport and storage temperature
and humidity/ atmospheric pressure
-10°C ~ 40°C,
95% and below/ 500~1060hPa
Electric shock protection level
Type BF
Net Weight, no more 1255 g
Size (electronic block), mm 140 х 90 х 190
Pollution Degrees Degrees 2
Overvoltage Category Category II
High Altitudes (m) 2000m
Year of manufacture Year and month the manufacture is
given in the bottom of the unit body in
a serial number after symbol “A”
Key to symbols
IP protection level
Important: Read the manual
CE marking in conformity with
EC directive 93/42/EEC
Type BF Applied Part
CLASS II
Page 11

ENG
11
TROUBLESHOOTING TIPS
No power on device when the power switch is on:
Turn the power switch off . Plug the power plug into an electrical outlet. Turn the device on. •
No nebulization or low nebulization rate when the power is on:
Add the correct amount of prescribed medication to the medication cup. •
Make sure the nebulizer kit is correctly assembled and the inhalation accessory is correctly attached. •
Hold the nebulizer kit correctly. Do not tilt the nebulizer kit so the angle of the kit is greater than 45 •
degrees.
Make sure the air tube is correctly attached to the compressor and the nebulizer kit. •
Make sure the air tube is not folded, kinked or bent. Inspect the air tube for any damage. Replace the air tube •
if damaged.
The device is very hot:
Do not cover the compressor with any type of cover during use. Turn the device off . Wait 40 minutes before •
using the device again.
CERTIFICATION AND STATE REGISTRATION
This device manufacturing is certifi ed according to international standard ISO 13485. Device comply with
the requirements of European Directive MDD 93/42/ЕЕС, international standards EN 980, EN 1041, EN
1060-1, EN 1060-3, EN 10601-1-2, ISO 14971.
Complaints and requests should be addressed to:
Little Doctor Europe Sp. z o.o.
57G Zawila Street, 30-390, Krakow, Poland
Service phone: +48 12 2684748, 2684749
Manufactured under control:
Little Doctor International (S) Pte. Ltd., 35 Selegie Road #09-02 Parklane Shopping Mall, Singapore
188307, Singapore. Postal address: Yishun Central P.O. Box 9293 Singapore 917699.
Manufacturer:
Little Doctor Electronic (Nantong) Co., Ltd., No.8, Tongxing Road Economic & Technical
Development Area, Nantong 226010, Jiangsu, PEOPLE'S REPUBLIC OF CHINA
Distributor in Europe:
Little Doctor Europe Sp. z o.o.
57G Zawila Street, 30-390, Krakow, Poland
Sales Offi ce phone: +48 12 2684746, 12 2684747, fax: +48 12 268 47 53
E-mail: biuro@littledoctor.pl
www.LittleDoctor.pl
Authorized Representative in the EU:
Little Doctor Europe Sp. z o.o.
57G Zawila Street Krakow 30-390 Poland
Page 12
ENG
12
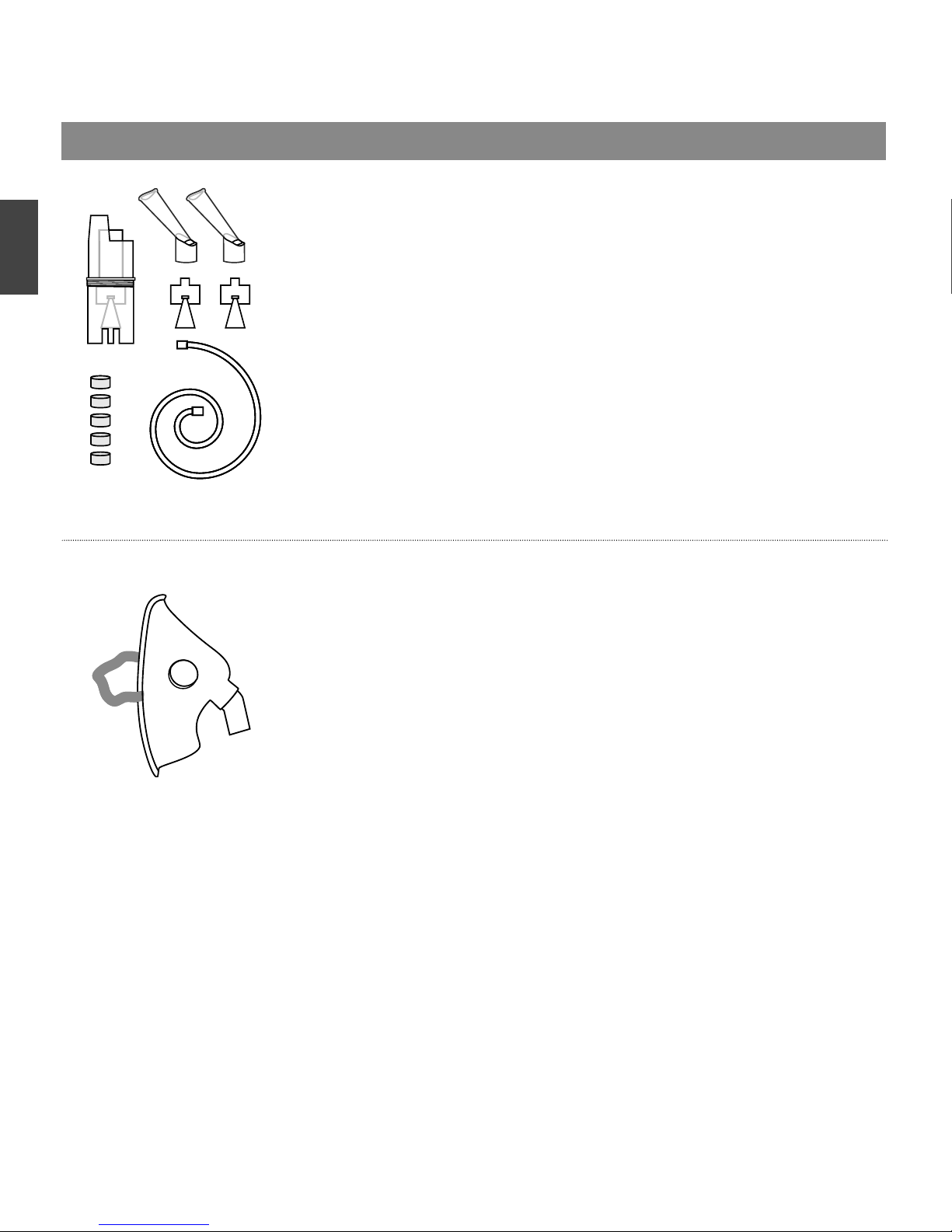
ACCESSORIES FOR NEBULIZER LD*
Inhalation set №1
•
Designed for use with compressor nebulizer LD
•
Complete set included:
- Nebulizer LD-N105 assembled – 1 pc.
- Inhalation mouthpiece LD-N022 – 2 pcs.
- Inhalation tube (2 м) LD-N051 – 1 pc.
- Inhalation fi lter LD-N055 – 5 pcs.
- Inhalation baffl e «A» LD-N001 – 1 pc.
- Inhalation baffl e «C» LD-N003 – 1 pc.
Adult inhalation mask LD-N041
•
Designed for use with compressor nebulizer and ultrasonic
nebulizer LD
•
Made of PVC.
•
For individual use.
•
Package quantity – 1 pc.
* Sold separately.
Page 13

ENG
13
Child inhalation mask LD-N040
•
Designed for use with compressor nebulizer and ultrasonic
nebulizer LD
•
Made of PVC.
•
For individual use.
•
Package quantity – 1 pc.
Inhalational mouthpiece LD-N022
•
Designed for use with compressor nebulizer LD
•
Made of plastic
•
For individual use
•
Package quantity – 10 pcs.
Page 14

POL
14
#
12
"
13
PODSTAWOWE CZĘŚCI URZĄDZENIA
Page 15

POL
15
FUNKCJE I CZĘŚCI URZĄDZENIA
NR NA
SCHEMACIE
NAZWA OPIS/FUNKCJA
1 Kompresor
Kompresor inhalatora służący do wytwarzania sprężonego
powietrza.
2 Przycisk Włącz/Wyłącz Przełącznik I/O – włączanie/wyłączanie zasilania elektrycznego.
3 Gniazdo kompresora
Gniazdo kompresora umożliwiające podłączenie przewodu
powietrza.
4 Wloty powietrza Otwory do poboru powietrza chłodzącego kompresor.
5 Narożne mocowanie Narożne mocowanie.
6 Gniazdo na fi ltr Miejsce instalacji fi ltra powietrza.
7 Nebulizator LD-N105 Komora, w której z roztworu do inhalacji wytwarzany jest aerozol.
7.1 Górna część nebulizatora Część komory aerozolowej. Miejsce podłączenia złączki.
7.2
Rozpylacz „B” (niebeski)
LD-N002
Rozpylacz do inhalatora (uniwersalny). Materiał eksploatacyjny.
7.3 Dysza
Stożkowaty otwór, umożliwiający powstanie delikatnego
strumienia powietrza.
7.4 Dolna część nebulizatora
Część komory aerozolowej na roztwór inhalacyjny (z oddzielonym
przegrodą pojemnikiem na roztwór).
7.5 Nasada nebulizatora Nasada do podłączenia przewodu powietrznego.
8
Maska dla dorosłych
LD-N041
Maska inhalacyjna dla dorosłych. Materiał eksploatacyjny.
9 Maska dziecięca LD-N040 Maska inhalacyjna dla dzieci. Materiał eksploatacyjny.
10 Ustnik Ustnik do inhalatora. Materiał eksploatacyjny.
11
Przewód powietrzny
LD-N051
Rurka inhalacyjna o długości 2 m. Materiał eksploatacyjny.
12
Rozpylacz «С»
(czerwony) LD-N003
Rozpylacz do inhalatora (nebulizacja górnych dróg oddechowych).
Materiał eksploatacyjny.
13
Rozpylacz «А» (żółty)
LD-N001
Rozpylacz do inhalatora (nebulizacja dolnych dróg oddechowych) składają-
cy się z dwóch części, tj. ze stożka i cylindra. Materiał eksploatacyjny.
14 Filtr Filtr powietrza do inhalatora. Materiał eksploatacyjny.
Page 16

POL
16
CZYM JEST TERAPIA AEROZOLOWA?
Nebulizator to urządzenie do wytwarzania i rozpylania aerozolu. Słowo „nebulizator” pochodzi od łacińskiego
„nebula” (mgła, chmura). Po raz pierwszy użyto go w 1874 roku w celu nazwania urządzenia przetwarzającego substancje płynne w aerozol do celów medycznych. Jeden z pierwszych przenośnych “aparatów aerozolowych” stworzył J. Sales-Girons w Paryżu w 1859 roku. Pierwsze nebulizatory wykorzystywały jako źródło
energii strumień pary. Stosowano je do inhalacji chorych na gruźlicę. Obecnie zamiast nazwy „nebulizator”
często używa się określenia „inhalator”.
Celem nebulizacji jest dostarczenie do dróg oddechowych terapeutycznej dawki preparatu w formie aerozolu w krótkim czasie. Nieprzerwane podawanie aerozolu pozwala na osiągnięcie w ciągu kilku minut wysokiego stężenia substancji leczniczej w górnych i dolnych drogach oddechowych, a także w płucach, przy
czym ryzyko pojawienia się zjawisk ubocznych jest niewielkie. Dzięki temu rozszerzają się oskrzela (efektywna
bronchodylatacja), znika potrzeba hospitalizacji lub skraca się pobyt w szpitalu.
Firma Little Doctor Internatiоnаl (S) Pte. Ltd. oferuje inhalator LD-221C wyróżniający się małą końcową objętością roztworu do nebulizacji, niezawodnością i prostotą użytkowania, a także tym, że umożliwia on stosowanie wielu preparatów leczniczych. Dziękujemy za zakup naszego produktu.
INFORMACJE OGÓLNE
Inhalator kompresorowy LD przeznaczony jest do leczenia chorób dróg oddechowych oraz płuc przy
pomocy aerozolów wytwarzanych z roztworów preparatów leczniczych. Można z niego korzystać zarówno
w placówkach medycznych, jak i w warunkach domowych.
Niniejsza instrukcja pomoże użytkownikom bezpiecznie i efektywnie korzystać z inhalatora kompresorowego LD.
Aparat należy użytkować zgodnie z zasadami określonymi w niniejszej instrukcji. Nie powinien on być używany do celów innych niż te, które są w niej opisane. Należy przeczytać i zrozumieć całą instrukcję.
Urządzenie składa się z kompresora oraz nebulizatora (komory, w której wytwarzany jest aerozol).
Kompresor, włącznik/wyłącznik i fi ltr powietrza połączone są w jednym bloku. Z kompresora sprężone
powietrze trafi a przez przewód powietrzny do nebulizatora, gdzie wytwarzany jest aerozol. W celu ochładzania kompresora do obudowy urządzenia musi być doprowadzane powietrze.
INFORMACJE DOTYCZĄCE BEZPIECZEŃSTWA
W celu zapewnienia prawidłowego korzystania z produktu należy zawsze przestrzegać
podstawowych zasad bezpieczeństwa, łącznie z ostrzeżeniami i uwag wymienionymi w niniejszej
instrukcji obsługi.
OSTRZEŻENIE
● Przed użyciem należy zapytać lekarza lub licencjonowanego ratownika medycznego/ lekarza
medycyny jak należy używać lekarstwa.
● Nie wolno zakrywać urządzenia kocem, ręcznikiem lub innym rodzajem pokrywy podczas
używania. Może to spowodować przegrzanie lub nieprawidłowe działanie urządzenia.
● Nie wolno używać urządzenia w miejscu w którym może być narażone na działanie łatwopalnego
gazu lub oparów.
Page 17

POL
17
● Nie wolno używać wody mineralnej w nebulizatorze do celów nebulizacji.
● Po każdym użyciu należy zawsze usuwać pozostałe leki ze zbiornika leków. Używać świeżych
leków za każdym razem, kiedy używa się urządzenie.
● Nie zostawiać urządzenia ani jego części w miejscach narażonych na działanie ekstremalnych
temperatur lub zmian wilgotności, takich jak samochód w gorących miesiącach lub w miejscach
narażonych na bezpośrednie działanie promieni słonecznych.
UWAGA
● Ograniczyć używanie urządzenia do 20 minut na raz i odczekać 40 minut przed ponownym
użyciem urządzenia.
● Zapewniać ścisły nadzór podczas używania urządzenia przez lub pobliżu niemowląt, dzieci lub
osoby niepełnosprawne.
● Nie wkładać żadnych przedmiotów do urządzenia.
● Upewnić się, że fi
ltr powietrza jest czysty
. Jeśli fi ltr powietrza zmienił kolor lub nie został użyty
przez 60 dni, należy wymienić fi ltr.
● Upewnić się, że komora inhalacyjna jest prawidłowo zmontowana, fi ltr powietrza prawidłowo
zainstalowany i rurka powietrzna prawidłowo podłączona do urządzenia i komory inhalacyjnej.
Powietrze może uciekać z rury powietrznej podczas używania, jeśli nie jest dobrze podłączona.
● Przed każdym użyciem urządzenia należy sprawdzić kompresor (jednostkę główną) oraz części
nebulizatora. Upewnić się, że żadne części nie są uszkodzone, dysza i rurka powietrzna nie są
zablokowane a kompresor działa prawidłowo.
● Nie wolno używać urządzenia, jeśli rura powietrzna jest zagięta.
● Nie wolno blokować pokrywy fi ltra
powietrza.
● Nie wolno modyfi kowa
ć defl ektora, dyszy w zbiorniku leków lub jakiejkolwiek części zestawu
nebulizatora.
● Nie wolno wlewać więcej niż 10ml leków do zbiorniczka leków.
● Nie wolno używać urządzenia w temperaturze wyższej niż 40 ° C.
● Nie przechylać zestawu nebulizatora tak żeby kąt komory inhalacyjnej był większy niż 45 °. Lek
może się przelać do jamy ustnej.
● Podczas używania urządzenia nie wstrząsać komorą inhalacyjną.
● Nie wolno narażać sprężarki ani podzespołów na silne wstrząsy, takie jak upuszczanie na podłogę.
● To urządzenie jest dopuszczone tylko do użytku przez ludzi.
● Używać urządzenie tylko w celu jego zamierzonego wykorzystania, jak opisano w instrukcji
obsługi. Nie używać dodatków nie zalecanych przez producenta.
● Zutylizować urządzenie, komponenty i akcesoria opcjonalnych zgodnie z obowiązującymi
przepisami lokalnymi. Bezprawna utylizacja może powodować zanieczyszczenie środowiska.
● Upewnić się, że przewód powietrzny jest prawidłowo zamocowany i się nie odpina od urządzenia
(jednostki głównej) i komory inhalacyjnej. Nieznacznie przekręcić rurę powietrzną, wkładając ją do
złączy, aby uniknąć odpięcia się przewodu podczas użytkowania urządzenia.
RYZYKO PORAŻENIA PRĄDEM:
● Nigdy nie podłączać ani nie odłączać wtyczki kabla zasilania mokrymi rękami.
● Nie wolno zanurzać urządzenia (jednostki głównej) w wodzie ani w innych płynach.
Page 18

POL
18
● Nie rozlewać wody ani innych płynów na urządzenie. Te części nie są wodoszczelne. W
przypadku rozlania cieczy na urządzenie należy odłączyć przewód zasilający i dokładnie wytrzeć
ciecz gazą lub innym miękkim materiałem chłonnym.
● Nie wolno używać ani nie przechowywać kompresora w miejscach wilgotnych ani na zewnątrz
pomieszczeń. Używać kompresor w roboczej temperaturze i wilgotności.
● Nie przeciążać gniazd zasilających. Podłączyć urządzenie do odpowiedniego gniazda napięcia.
● Nie używać przedłużaczy. Podłączyć przewód zasilający bezpośrednio do gniazda
elektrycznego.
● Po użyciu urządzenia odłączyć przewód zasilający od gniazda elektrycznego. Nigdy nie zostawiać
tego produktu bez nadzoru po włożeniu.
● Przed czyszczeniem urządzenia odłączyć przewód zasilający od gniazda elektrycznego.
● Przed użyciem należy dokładnie przeczytać całą instrukcję dołączoną do opcjonalnych
akcesoriów
.
● Nie umieszczać kompresora w miejscu tak aby był trudny dostęp odłączenia urządzenie.
● Przełącznik zasilania służy do odłączenia urządzenia od sieci zasilającej.
● Kierunek ruchu wyłącznika zasilania jest zgodny z IEC 60447.
KONSERWACJA I PRZECHOWANIE
● Przechowywać urządzenie w miejscu niedostępnym dla niemowląt i dzieci. Urządzenie może
zawierać małe części, które można połknąć.
● Nie wolno zostawiać roztworu czyszczącego w częściach nebulizatora. Po dezynfekcji należy
umyć części nebulizatora gorącą wodą z kranu.
● Po każdym użyciu umyć części nebulizatora części należy natychmiast wysuszyć po umyciu.
● Nie przechowywać rurki ustnika z pozostałościami leku lub wilgotne. Może być to przyczyną
bakterii powodujących infekcję.
● Przechowywać urządzenie i komponenty w czystej, bezpiecznej lokalizacji.
● Nie należy przenosić ani zostawiać nebulizator z lekami w zbiorniku leków.
●
Nie osuszać urządzenia ani żadnej z części nebulizatora w kuchence mikrofalowej.
● Nie wolno owijać przewodu zasilającego wokół urządzenia (głównej jednostki).
Poniższe czynności konserwacja i naprawa, mogą być wykonane przez osobę wskazaną przez
producenta lub dystrybutora.
SERWIS I KONSERWACJA ODPOWIEDZIALNY
Zmień rurkę inhalacyjną
Operator
Wymień stosowaną część Operator
Wymień fi ltr powietrza Operator
Oczyść powierzchnię urządzenia Operator
Codzienne czyszczenie i dezynfekcja Operator
Wszystkie elementy, które trzeba naprawić lub
zmienić, oddzielając urządzenie
Autoryzowany punkt serwisowy dystrybutora
lub producenta
Page 19

POL
19
OSTRZEŻENIE:
● Nie modyfi kować urządzenia bez zezwolenia producenta
● Nie serwisować ani nie obsługiwać urządzenia podczas pracy z pacjentem.
PIELĘGNACJA I CZYSZCZENIE
● Należy regularnie czyścić urządzenie i wszystkie akcesoria. Zaleca się przecierać wszystkie akcesoria
3% roztworem nadtlenku wodoru z dodatkiem 0,5% roztworu detergentu (na przykład proszek do prania). Następnie nebulizator należy myć mocnym strumieniem wody. Ustniki i dysze można dezynfekować
we wrzątku przez 10 minut lub dezynfekować w temperaturze 150 ° C. Po zakończeniu zabiegu wytrzeć
suchą ściereczka wszystkie części urządzenia.
● Naprawę urządzenia można dokonać tylko w autoryzowanych serwisach.
● W przypadku wygaśnięcia okresu ważności gwarancji obowiązują od czasu do czasu przeglądy auto-
ryzowanych serwisach w celu sprawdzenia stanu technicznego urządzenia. Urządzenie i jego części
należy utylizować zgodnie z lokalnymi przepisami lokalnymi. Producent nie określa żadnych specjalnych
wymóg dotyczących utylizacji tego urządzenia.
SPOSÓB UŻYTKOWANIA
Przygotowanie urządzenia do inhalacji.
UWAGA: Przed pierwszym użyciem aparatu należy umyć go zgodnie z opisem w
punkcie 1 rozdziału “Konserwacja, przechowywanie, naprawa i utylizacja”.
1. Postawić inhalator przed sobą na stole. Upewnić się, że urządzenie jest
wyłączone (przełącznik znajduje się w pozycji “О”), a przewód zasilający nie
jest podłączony do sieci elektrycznej.
2. Zdjąć górną część nebulizatora, przekręcając ją w kierunku przeciwnym do
ruchu wskazówek zegara (rys. 1).
3. Wstawić odpowiedni rozpylacz.
W fabrycznie nowym nebulizatorze zainstalowany jest rozpylacz “B” o kolorze
niebieskim, skutecznie działający na cały układ oddechowy.
W celu zapewnienia skuteczniejszego oddziaływania środków leczniczych
na górne drogi oddechowe zamiast rozpylacza w kolorze niebieskim należy
zainstalować rozpylacz “C” w kolorze czerwonym.
W celu bardziej efektywnego oddziaływania na dolne drogi oddechowe
należy zainstalować rozpylacz “A” w kolorze żółtym, składający się z dwóch
części (rys. 2, cyframi oznaczono kolejność montażu). Wykresy podziału
dyferencjalnego rozmiarów cząsteczek w zależności od masy dla różnych
rozpylaczy pokazane zostały na rys. 4
4.
Wlać do dolnej części nebulizatora roztwór do inhalacji. Dawki nie powinny przekraczać
dawek zaleconych przez lekarza prowadzącego. Ilość roztworu w nebulizatorze określa
się przy pomocy podziałki na korpusie. Maksymalna pojemność zbiornika wynosi 10 ml.
5.
Zamocować górną część nebulizatora, przekręcając ją do oporu zgodnie z
ruchem wskazówek zegara.
Rys. 1
Rys. 2
Page 20

POL
20
6.
Za pomocą złączki połączyć ustnik z górną częścią nebulizatora (rys. 3). W
zależności od rodzaju inhalacji skorzystać z ustnika, końcówki do nosa lub
maski. Maska podłączana jest bezpośrednio do górnej części nebulizatora,
natomiast ustnik lub końcówkę do nosa należy podłączyć przy pomocy złączki.
Trzymać nebulizator w położeniu pionowym.
UWAGA: Zaleca się, by każdy pacjent korzystał z własnego ustnika, własnej
maski i/lub końcówki do nosa.
7.
Podłączyć przewód zasilający do sieci elektrycznej.
8. Podłączyć jeden koniec przewodu powietrznego do gniazda kompresora, a
drugi do nasady nebulizatora.
9. Włączyć inhalator, ustawiając przełącznik w pozycji “I”. URZĄDZENIE
JEST GOTOWE DO INHALACJI.
W zależności od rodzaju używanego rozpylacza, aerozol o różnej wielkości cząsteczek
rozprowadzany jest w następujący sposób
:
wielkość cząsteczek, μm
% masy ogólnej
% masy ogólnej
% masy ogólnej
0
5
10
15
110
wielkość cząsteczek, μm
0
5
10
15
110
wielkość cząsteczek, μm
0
5
10
15
110
(głównie górne drogi oddechowe)
Rozpylacz „С” (czer wony)
Rozpylacz „В” (niebieski)
Rozpylacz „А” (żółty)
(uniwersalny)
(głównie dolne drogi oddechowe)
Dla zapewnienia efektywnego podawania leku
do określonej części dróg oddechowych należy
stosować właściwy rozpylacz.
Rys. 3
Rys. 4
Page 21

POL
21
Nebulizator aktywowany wdechem.
Dzięki specjalnej konstrukcji nebulizatora (komory połączone ze sobą
w określony sposób) przy wdechu i wydechu strumienie powietrza
przepływają różnymi drogami.
Pozwala to na uzyskanie przy wdechu strumienia powietrza o dużej koncentracji aerozolu, a także na zmniejszenie strat aerozolu przy wydechu.
Dzięki nebulizatorowi aktywowanemu wdechem efektywność inhalacji
znacznie wzrasta.
Zakończenie inhalacji.
Po zużyciu roztworu do inhalacji lub po upływie czasu inhalacji zaleconego przez lekarza należy wyłączyć
urządzenie, ustawiając przełącznik w pozycji “O”, a następnie wyjąć z gniazdka wtyczkę przewodu
zasilającego.
Po inhalacji należy pooddychać przez jakiś czas świeżym powietrzem, aby uzyskać lepszy efekt leczenia.
Po każdym użyciu aparatu należy usunąć z niego pozostałości preparatu, wyczyścić i umyć urządzenie
zgodnie z opisem w punkcie 1 rozdziału “Konserwacja, przechowywanie, naprawa i utylizacja”.
KONSERWACJA, PRZECHOWYWANIE, NAPRAWA I UTYLIZACJA
1. Należy regularnie czyścić urządzenie oraz wszystkie akcesoria. Zaleca się przecieranie akcesoriów 3%
roztworem wody utlenionej z dodatkiem 0,5% roztworu środka myjącego (na przykład proszku do
prania). Następnie należy wypłukać dokładnie nebulizator pod strumieniem wody. Ustniki i końcówki
do nosa można sterylizować, wygotowując je przez 10 minut lub poprzez autoklawowanie w
temperaturze do 150°С. Następnie należy wytrzeć do sucha wszystkie części urządzenia przy pomocy
miękkiej szmatki.
2. Należy regularnie sprawdzać, czy fi ltr jest zabrudzony, i wymieniać go w razie konieczności.
ZALECA SIĘ
WYMIENIANIE FILTRA NIE RZADZIEJ NIŻ RAZ DO ROKU.
3. Chronić urządzenie przed bezpośrednimi promieniami słonecznymi i uderzeniami.
4. Nie przechowywać i nie używać aparatu w bezpośredniej bliskości urządzeń grzewczych oraz
otwartego ognia.
5. Chronić urządzenie przed zabrudzeniem.
6. Nie dopuszczać do kontaktu urządzenia z agresywnymi roztworami.
7.
W razie konieczności dokonywać napraw jedynie w wyspecjalizowanych punktach serwisowych.
8. Czas użytkowania kompresora wynosi 5 lat, licząc od rozpoczęcia eksploatacji. Czas użytkowania
materiałów eksploatacyjnych wynosi 1 rok. Po upływie określonego czasu użytkowania, co jakiś
czas, należy sprawdzać stan techniczny urządzenia w wyspecjalizowanych punktach serwisowych i
w razie konieczności zutylizować je zgodnie z zasadami utylizacji obowiązującymi w danym regionie.
Producent nie określił szczególnych warunków utylizacji.
Page 22

POL
22
GWARANCJA
Gwarancja na dane urządzenie jest ważna 36 miesięcy od momentu sprzedaży. Gwarancja nie dotyczy
materiałów eksploatacyjnych (maski, ustniki, przewody itd.). Warunki gwarancji określone są w karcie
gwarancyjnej wydawanej podczas zakupu urządzenia. Adresy punktów zapewniających obsługę gwarancyjną
wskazane są na karcie gwarancyjnej.
ZAWARTOŚĆ KOMPLETU
№
NAZWA MODEL LICZBA, szt.
1 Kompresor — 1
2 Nebulizator (z rozpylaczem inhalacyjnym) LD-N105 1
3
Rozpylacz inhalacyjny LD-N001
1
4
Rozpylacz inhalacyjny LD-N003
1
5 Ustnik LD-N022 2
6 Maska inhalacyjna dla dorosłych LD-N041 1
7 Maska inhalacyjna dla dzieci LD-N040 1
8 Przewód powietrza LD-N051 1
9 Filtr zapasowy LD-N055 5
10 Opakowanie — 1
11 Instrukcja obsługi — 1
12 Karta gwarancyjna — 1
PODSTAWOWE DANE TECHNICZNE
Model LD-221C
Rodzaj kompresorowy
Maksymalna moc 60 W
Wydajność nebulizacji, około
rozpylacz inhalacyjny «A» LD-N001
rozpylacz inhalacyjny «B» LD-N002
rozpylacz inhalacyjny «C» LD-N003
0.31 ml/min
0.43 ml/min.
0.50 ml/min.
Średni rozmiar cząsteczek aerozolu (ММАD)
rozpylacz inhalacyjny «A» LD-N001
rozpylacz inhalacyjny «B» LD-N002
rozpylacz inhalacyjny «C» LD-N003
3.5 μm
4.0 μm
5.0 μm
Maksymalny czas pracy ciągłej 20 minut
Czas chłodzenia
40 minut
Pojemność pojemnika na roztwór do inhalacji 10 ml
Końcowa objętość roztworu inhalacyjnego, nie więcej niż
0.5 ml
Page 23

POL
23
Maksymalne ciśnienie kompresora, nie mniej niż 2.0 bar
Maksymalny poziom hałasu 65 dB
Zasilanie elektryczne: AC 230V, 50Hz
Stopień ochrony przed porażeniem prądem wyrób typu BF
Warunki eksploatacji urządzenia:
Temperatura powietrza od 10°С do 40°С
Wilgotność nie więcej niż 95% Rh
Ciśnienie atmosferyczne
860~1060hPa
Warunki przechowywania i transportu urządzenia:
Temperatura powietrza od - 10°C do +40°C
Wilgotność 95% Rh
Ciśnienie atmosferyczne
500~1060hPa
Waga zestawu (bez opakowania) 1255 g
Wymiary urządzenia, mm
140 х 90 х 190
Rok i miesiąc produkcji Podano na obudowie urządzenia w numerze
seryjnym, po symbolach “А”.
Wyjaśnienie symboli
Stopień ochrony przed penetracją wody
Ważne: Przeczytaj Instrukcję
Zgodny z Dyrektywą EC 93/42/EEC
Stopień ochrony przeciwporażeniowej:
TYP BF
Klasa ochrony przeciwporażeniowej:
CLASS II
IDENTYFIKACJA I USUWANIE USTEREK
Urządzenie może nie włączać się z następujących przyczyn:
• Brak zasilania elektrycznego
• Nieodpowiednie napięcie elektryczne
• Niekompatybilność wtyczki i gniazda sieci elektrycznej
• Przepalenie się bezpiecznika - wymienić
Aerozol może nie być wytwarzany z następujących przyczyn:
•
Brak roztworu do inhalacji w nebulizatorze. Dolać do nebulizatora niezbędną ilość roztworu.
• Zabrudzenie dyszy nebulizatora osadami roztworu do inhalacji. Wyczyścić dyszę inhalatora. Nie
wykorzystywać do czyszczenia przedmiotów metalowych, które mogłyby naruszyć geometrię
dyszy.
• Zagięcia przewodu powietrza. Wyprostować przewód powietrzny tak, by powietrze swobodnie
przepływało do nebulizatora.
Page 24

POL
24
* Nabywane osobno.
Zestaw do inhalacji №1
•
Przeznaczony do użytku z inhalatorami kompresorowymi LD
•
W zestawie:
- nebulizator LD-N105 – 1 szt.
- ustnik inhalacyjny LD-N022 – 2 szt.
- przewód powietrza (2 m) LD-N051 – 1 szt.
- fi ltr LD-N055 – 5 szt.
- rozpylacz inhalacyjny «A» LD-N001 – 1 szt.
- rozpylacz inhalacyjny «C» LD-N003 – 1 szt.
CERTYFIKACJA
Urządzenie zgodne z przepisami europejskimi (Dyrektywa Rady UE 93/42/EEC z dnia 14 czerwca
1993). Sprzęt posiada certyfi kat międzynarodowy ISO 13485:2003. Jakość urządzenia jest potwierdzona i zgodna z następującymi standardami: EN 980, EN 1041, EN 1060-1, EN 1060-3, EN 106011-2, ISO 14971.
Reklamacje i prośby należy kierować na adres:
Little Doctor Europe Sp. z o.o.
ul. Zawiła 57G, 30-390, Kraków, Polska
Serwis tel.: +48 12 2684748, 2684749.
Wyprodukowano pod kontrolą:
Little Doctor International (S) Pte. Ltd., 35 Selegie Road #09-02 Parklane Shopping Mall, Singapore
188307, Singapore. Adres pocztowy: Yishun Central P.O. Box 9293 Singapore 917699.
Producent:
Little Doctor Electronic (Nantong) Co., Ltd., No.8, Tongxing Road Economic & Technical Development
Area, 226010 Nantong, Jiangsu, PEOPLE’S REPUBLIC OF CHINA
Dystrybutor w Polsce:
Little Doctor Europe Sp. z o. o., ul. Zawiła 57G, 30-390 Kraków Polska
Biuro handlowe tel.: +48 12 2684746, 12 2684747, fax: +48 12 268 47 53.
E-mail: biuro@littledoctor.pl
www.LittleDoctor.pl
Autoryzowany przedstawiciel w UE:
Little Doctor Europe Sp. z o.o.
ul. Zawiła 57G, 30-390, Kraków, Polska
CZĘŚCI DO INHALATORA LD*
Page 25

POL
25
Maska inhalacyjna dla dorosłych LD-N041
•
Przeznaczona do użytkowania z inhalatorami kompresorowymi LD i
z inhalatorami ultradźwiękowymi LD
•
Wyprodukowana z PVC.
•
Do użytku indywidualnego.
•
Ilość w opakowaniu – 1 szt.
Maska inhalacyjna dziecięca LD-N040
•
Przeznaczona do użytkowania z inhalatorami kompresorowymi
LD i z inhalatorami ultradźwiękowymi LD
•
Wyprodukowana z PVC.
•
Do użytku indywidualnego.
•
Ilość w opakowaniu – 1 szt.
Ustnik inhalacyjny LD-N022
•
Przeznaczony do użytkowania z inhalatorami kompresorowymi LD
•
Wyprodukowany z plastiku
•
Do użytku indywidualnego
•
Ilość w opakowaniu – 10 szt.
Page 26

UKR
26
ОСНОВНІ ЧАСТИНИ ПРИЛАДУ
#
12
"
13
Page 27

UKR
27
ПРИЗНАЧЕННЯ ЧАСТИН ПРИЛАДУ
№
ПОЗИЦІЇ
НА СХЕМІ
НАЗВА ОПИС / ПРИЗНАЧЕННЯ
1
Компресор
Компресорний блок інгалятора для утворення повітряного тиску.
2
Тумблер Тумблер I/O – вмикання/вимикання мережевого живлення.
3
Штуцер компресору Штуцер компресору для під’єднання інгаляційної трубки.
4
Повітряний забірник
Отвори для забору повітря, для охолодження компресору.
5 Тримач небулайзеру Тримач небулайзеру
6
Гніздо для фільтру
Місце установки фільтру для компресора.
Правила експлуатації та порядок заміни дивіться в розділі
«ДОГЛЯД, ЗБЕРЕЖЕННЯ, РЕМОНТ та УТИЛІЗАЦІЯ» стор. 34.
7
Небулайзер LD-N105
Камера для утворення аерозолю з інгаляційного розчину.
7.1
Верхня частина
небулайзеру
Частина аерозольної камери. Місце під’єднання до муфти.
7.2
Розпилювач
LD-N002
Розпилювач інгаляційний
«B» (синій,
універсальний).
Витратний
матеріал.
7.3
Сопло
Конусоподібний отвір для утворення тонкого повітряного струменю.
7.4
Нижня частина
небулайзеру
Частина аерозольної камери,що призначена для інгаляційного
розчину з посадочним місцем для відбійника.
7.5
Штуцер небулайзеру Штуцер для під'єднання інгаляційної трубки.
8
Маска доросла
LD-N041
Маска інгаляційна доросла. Витратний матеріал.
9
Маска дитяча
LD-N040
Маска інгаляційна дитяча. Витратний матеріал.
10
Мундштук LD-N022 Мундштук інгаляційний. Витратний матеріал.
11
Трубка LD-N051 Інгаляційна трубка довжиною 2 м. Витратний матеріал.
12
Розпилювач
LD-N003
Розпилювач інгаляційний «C» (червоний, переважно для верхніх
дихальних шляхів).
Витратний матеріал.
13
Розпилювач
LD-N001
Розпилювач інгаляційний «A» (жовтий, переважно для нижніх
дихальних шляхів) складається з двох частин: конус та циліндр.
Витратний матеріал.
14
Фільтр LD-N055 Фільтр повітряний для інгалятора. Витратний матеріал.
Page 28

UKR
28
НЕБУЛАЙЗЕРНА ТЕРАПІЯ – ЩО ЦЕ?
Небулайзер – це пристрій для утворення та розпилення аерозолю. Слово «небулайзер» походить від латинського “nebula” (туман, хмара) та вперше було вжито в 1874 році для визначення обладнання, що перетворює рідку речовину в аерозоль для медичних цілей. Одним з
перших портативних “аерозольних апаратів” було створено J. Sаlеs–Girons в Парижі в 1859
році. Перші небулайзери використовували в якості джерела енергії струмінь пару та застосовувались для інгаляції парами смол та антисептиків хворих на туберкульоз. В теперішній
час замість назви «небулайзер» часто використовують «інгалятор».
Мета небулайзерної терапії складається в доставці в дихальні шляхи терапевтичної дози
препарату в аерозольній формі за короткий час. Безперервне подавання аерозолю дозволяє на протязі декількох хвилин утворити високу концентрацію лікарського засобу в верхніх, нижніх дихальних шляхах та легенях з низькою вирогідністю розвитку побічних явищ.
Відповідно досягається ефективна бронходилатація (розширення бронхів), зникає потреба в
шпиталізації або зменшується тривалість перебування у стаціонарі.
Компанія Little Doctor Internatiоnаl (S) Pte. Ltd. пропонує Вам скористатися інгалятором
LD-212C, відмінною особливістю якого є можливість використання ширшого спектру інгаляційних розчинів, малий залишковий об’єм лікарського препарату, надійність та простота
використання. Ми вдячні Вам за Ваш вибір.
ЗАГАЛЬНІ ВІДОМОСТІ
Компресорний інгалятор LD призначений для лікування захворювань дихальних шляхів та легенів аерозолями розчинів лікарських препаратів в лікувальних установах та в домашніх умовах.
Це керівництво призначене для надання допомоги користувачу з безпечної та ефективної
експлуатації інгалятора LD.
Прилад повинен використовуватись у відповідності з правилами які подані в цієї інструкції,
та не повинен використовуватись з іншими цілями, ніж ті що подані тут. Важливо прочитати та зрозуміти всю інструкцію.
Функціонально прилад складається з повітряного компресору та небулайзеру (камери
утворення аерозолю), Повітряний компресор,вмикач/вимикач живлення та повітряний
фільтр поєднані в одному корпусі. Від повітряного компресору стиснене повітря крізь
трубку подається до небулайзеру, де виникає утворення аерозолю. Для охолодження
компресора в корпус приладу примусово подається повітря.
Page 29

UKR
29
ІНФОРМАЦІЯ ЩОДО БЕЗПЕКИ
Щоб забезпечити правильне використання продукту, завжди слід дотримуватися основних
заходів безпеки, включаючи попередження та застереження, наведені в цієї інструкції з
експлуатації.
УВАГА
● Для лікування слід дотримуватися призначень вашого лікаря або ліцензованого лікаряфахівця.
● Під час використання не накривайте компресор ковдрою, рушником або будь-яким іншим
типом покривала. Це може призв
ести до
перегріву або несправності компресора.
● Не використовуйте пристрій в умовах, коли на нього може вплинути горючий газ або пар.
● Не використовуйте мінеральні води в небулайзерах для розпилення.
● Після кожного використання залишок будь-якого лікарського засобу завжди слід видаляти.
Використовуйте свіжі ліки кожного разу, коли використовуєте пристрій.
● Не залишайте пристрій або
його компоненти в місцях, що піддаються впливу високих температур і змінам вологості, наприклад, в автомобілі в спекотні місяці, або в місцях, що піддаються впливу прямих сонячних променів.
ЗАСТЕРЕЖЕННЯ
● Обмежте використання пристрою до 20 хвилин за один раз і зачекайте 40 хвилин, перш ніж
знову використовувати пристрій.
● Заб
е
зпечити суворий контроль при використанні пристрою для або поблизу немовлят, дітей
або людей з обмеженими можливостями.
● Не вставляйте сторонні предмети в пристрій.
● Переконайтеся, що повітряний фільтр чистий. Якщо повітряний фільтр змінив колір або не
використовується протягом 60 днів, замініть фільтр..
● Переконайтеся, що комплект небулайзера правильно зібраний, повітряний філь
тр пра
-
вильно встановлений, а повітряна трубка правильно підключена до компресора та комплекту небулайзера. Повітря може витікати з повітряної трубки під час використання, якщо він
з’єднаний не надійно.
● Перш ніж використовувати пристрій, перевірте компресор (основний блок) та частини небулайзера. Переконайтесь, що деталі не пошкоджені, сопло та повітр
яна тр
убка не заблоковані,
а компресор працює нормально.
● Не використовуйте пристрій, якщо повітряна трубка зігнена.
● Не блокуйте кришку повітряного фільтра.
● Не змінюйте дефлектор, сопло в резервуарі для медикаментів або будь-яку частину набору
для небулайзера.
● Не додавайте в резервуар для медикаментів більше 10 мл ліків.
● Не використовуйте пристрій при темпера
турах, що перевищують 40 °C.
● Не нахиляйте вузол розпилювача так, щоб кут інгаляційної камери перевищував 45 °.
Препарат може потрапляти в рот.
● При використанні пристрою не трясіть камеру для інгаляції.
Page 30

UKR
30
● Не піддавайте компресор або компоненти сильним ударам, наприклад, при падінні на підлогу.
● Цей пристрій призначений тільки для використання людьми.
● Не розбирайте та не намагайтеся відремонтувати пристрій або компоненти.
● Використовуйте пристрій лише для призначеного використання, як описано в інструкції з
експлуатації. Не використовуйте приладдя, не рекомендоване виробником.
● Утилізуйте пристрій, компненти та додаткові аксесуари відповідно до місцевих правил. Утилізація
з порушенням таких правил може спричинити забруднення навколишнього середовища
● Переконайтеся, що повітряна трубка надійно прикріплена до компресора (основного блоку) і
вузла розпилювача, і прикріплення не рознімається. Трохи прокручуйте повітряну трубку, прикріплюючи її до роз’ємів, щоб в подальшому уникнути від’єднання трубки під час використання.
НЕБЕЗПЕКА УРАЖЕННЯ ЕЛЕКТРИЧНИМ СТРУМОМ
● Ніколи не використовуйте пристрій (основний блок), коли шнур живлення вологий.
● Ніколи не підключайте і не відключайте шнур живлення в електричну розетку мокрими
руками.
● Не занурюйте пристрій (основний блок) в воду або інші рідини.
● Не допускайте попадання води або інших рідин на пристрій. Ці деталі не є во
донепроникними. У разі потрапляння рідини на пристрій, від’єднайте кабель живлення і витріть його
марлею або іншим м’яким всмоктуючим матеріалом.
● Не використовуйте та не зберігайте компресор у вологих приміщеннях або на відкритому
повітрі. Використовуйте пристрій при робочіх температурі та вологості.
● Не перевантажуйте електричні розетки. Підк
лючайте пристрій
до відповідної розетки.
● Не використовуйте подовжувачі. Підключіть кабель живлення безпосередньо до електричної розетки.
● Після використання пристрою від’єднайте шнур живлення від електричної розетки. Ніколи
не залишайте цей пристрій підключеним без нагляду.
● Перед очищенням пристрою від’єднайте шнур живлення від електричної розетки.
● Перед використанням уважно прочитайте всі інструкції, які надані з додатковими аксесуарами.
● Не встановлюйте пристрій у місці, де може бути важко його відключити.
● Вимикач живлення використовується для відключення пристрою від електромережі.
● Напрямок руху вимикача живлення відповідає стандарту IEC 60447.
ОБСЛУГОВУВАННЯ ТА ЗБЕРІГАННЯ
● Тримайте пристрій в місцях, недоступних для немовлят та дітей. Пристрій може містити
невеликі деталі, які можуть бути випадково проковтнуті.
● Не залишайт
е очищувальний розчин у частинах небулайзера. Промийте деталі небулай-
зера чистою гарячою водопровідною водою після дезінфекції.
● Після кожного використання промийте частини небулайзера. Просушіть деталі одразу
після промивання.
● Не зберігайте повітряну трубку з вологими залишками або лікарським засобом, що залишився в неї. Це може призвести до бактеріального зараження.
● Зб
еріг
айте пристрій та компоненти в чистому, безпечному місці.
Page 31

UKR
31
● Не переносьте і не залишайте інгалятор з ліками в резервуарі для лікарських засобів.
● Не встановлюйте та не намагайтеся сушити пристрій, компоненти та будь-які частини
небулайзера у мікрохвильовій печі.
● Не обертайте шнур живлення навколо компресора (основного блоку).
Нижче наведені операції з технічного обслуговування і ремонту можуть бути виконані ос
о-
б
ами, вказаними виробником або дистриб’ютором.
СЕРВІС ТА ОБСЛУГОВУВАННЯ ВІДПОВІДАЛЬНІСТЬ
Заміна інгаляційної трубки
Користувач
Заміна використаних частин Користувач
Заміна повітряного фільтру Користувач
Очистка поверхні приладу Користувач
Щоденна очистка та дезінфекція Користувач
Всі компоненти, які потрібно відремонтувати
або змінити, повинні бути від’єднані від
пристрою
Авторизований сервісний центр
дистриб’ютора або виробника
УВАГА:
● Не змінюйте це обладнання без дозволу виробника
● Не піддавайте пристрій ремонту або обслуговуванню під час використання його пацієнтом.
ДОГЛЯД ТА ОЧИЩЕННЯ
● Регу
лярно очищайте пристрій та всі аксесуари. Рекомендується, всі аксесуари обтирати 3%
розчином перекису водню з додаванням 0,5% розчину миючого засобу (наприклад, прального порошку). Після цього небулайзер повинен бути промитий сильним струменем води.
Мундштуки та насадки для носа можна обробляти кип’ятінням протягом 10 хвилин або в автоклаві при температурі 150 ° С. Після обробки витріть вс
і
частини пристрою м’якою тканиною.
● Ремонт пристрою допускається лише в авторизованих установах.
● Після закінчення терміну дії гарантованого строку служби час від часу звертайтесь до
авторизованих ремонтних організацій, щоб перевірити технічний стан пристрою. Пристрій і
його компоненти повинні бути утилізовані відповідно до місцевих національними правилами.
Виробник не в
к
азує будь-яких спеціальних вимог щодо утилізації даного пристрою.
Page 32

UKR
32
ПОРЯДОК ВИКОРИСТАННЯ
Підготовка приладу до інгаляції.
ВАЖЛИВО: Перед використанням приладу в перший раз необхідно провести його повну чистку, як зазначено в п.1 розділу “Догляд, збереження,
ремонт та утилізація”.
1. Встановіть інгалятор перед собою на столі. Переконайтеся, що прилад
вимкнений (тумблер живлення знаходиться в положенні “О”), а кабель
живлення не підключено до електромережі.
2. Зніміть верхню частину небулайзера, повернувши її проти годинної
стрілки (мал.1).
3. Встановіть необхідний розпилювач.
В заводській комплектації всередині небулайзера встановлений розпи-
лювач “В” синього кольору, який ефективний для впливу на всі дихальні
шляхи.
Для більш ефективного впливу лікарських засобів на верхні дихальні
шляхи встановіть, замість синього розпилювача, розпилювач “С” червоного кольору.
Для більш ефективного впливу лікарських засобів на нижні дихальні
шляхи – розпилювач “А” жовтого кольору, який складається з двох частин (мал. 2).
4. Залийте в нижню частину небулайзера інгаляційний розчин. Дозування не
повинне перевищувати рекомендоване Вашим лікарем. Кількість розчину в
небулайзері визначається за допомогою рисок на корпусі. Максимальний об’єм
резервуару 10мл.
5.
Закріпіть на небулайзері верхню частину, повернувши її за годинною
стрілкою до упору.
6. В залежності від типу інгаляції використовуйте мундштук, насадку
для носу, або маску (мал.3).
При використанні маски для інгаляцій або насадки для носу муфту
використовувати не потрібно – вони під’єднується безпосередньо до
верхньої частини небулайзера.
Небулайзер тримайте вертикально або приєднайте до корпусу при-
ладу за допомогою кутового тримача.
ВАЖЛИВО: Кожному пацієнту рекомендовано користуватися індивіду-
альним мундштуком, маскою та/або насадкою для носу.
7.
Підключіть кабель живлення до електромережі.
8. Під’єднайте інгаляційну трубку одним кінцем до штуцера компре-
сора, а іншим до штуцера небулайзера.
9. Ввімкніть інгалятор, перемкнувши тумблер живлення в положення
“І”. ПРИЛАД ГОТОВИЙ ДЛЯ ПРОВЕДЕННЯ ІНГАЛЯЦІЇ.
Мал. 1
Мал. 3
Мал. 2
Page 33

UKR
33
В залежності від типу розпилювача, який використовується, частинки різного розміру
розподіляються в аерозолі наступним чином:
МКГИМУ¼НОДЙКЖИЖИ
ÀÊü¿¼ÇØÉÊȼÍÄ
0
5
10
15
МКГИМУ¼НОДЙКЖИЖИ
ÀÊü¿¼ÇØÉÊȼÍÄ
0
5
10
15
МКГИМУ¼НОДЙКЖИЖИ
ÀÊü¿¼ÇØÉÊȼÍÄ
0
5
10
15
ЛБМБ¾¼ВЙК¾БМСЙАДС¼ЗШЙ
ФЗЫСД
¬КГЛДЗЪ¾¼УdsУБМ¾КЙДЕ
¬КГЛДЗЪ¾¼УdsНДЙЕ
¬КГЛДЗЪ¾¼УdsВК¾ОДЕ
ПЙ¾БМН¼ЗШЙДЕ
ЛБМБ¾¼ВЙКЙДВЙАДС¼ЗШЙ
ФЗЫСД
ЗЫ БРБЖОД¾ЙК АКНО¼¾ЖД ЗЖ¼МНШЖДС Г¼НК½¾
АКЛБ¾ЙК¿КПУ¼НОЖПАДС¼ЗШЙДСФЗЫС¾ЙБК½СА
ЙК¾ДЖКМДНОК¾П¾¼ОД¾А¾К¾АЙДЕМКГЛДЗЪ¾¼У
Page 34

UKR
34
Проведення інгаляції.
Тривалість проведення одного сеансу лікування зазвичай не повинна перевищувати 20 хвилин.
Проконсультуйтесь з Вашим лікарем відносно тривалості процедури інгаляції.
Під час проведення інгаляції будьте завжди спокійні та розслаблені. Дихання повинно бути повільним та глибоким, аби препарат добре заповнював легені та досягав глибоких відділів бронхів.
Затримайте на короткий час дихання, потім повільно видихніть. Не старайтеся дихати дуже
часто. Робіть паузи якщо відчуваєте в них необхідність.
Активований вдиханням небулайзер.
Спеціальна конструкція небулайзеру у вигляді під’єднаних
певним чином камер, визначає різні шляхи повітряних потоків
при вдиханні та видиханні.
Це дозволяє при вдиханні отримати повітряний поток з більшою
концентрацією аерозолю, а при видиханні зменшити втрати
аерозолю. Ефективність інгаляції з використанням активованого
вдиханням небулайзера значно збільшується.
Завершення інгаляції.
Після того як інгаляційний розчин витрачено, або вийшов час проведення інгаляції що був
рекомендований лікарем, вимкніть прилад перевівши тумблер живлення в положення “О” та
вийміть кабель живлення з розетки.
Після проведення інгаляції подихайте деякий час свіжим повітрям для забезпечення кращого
лікувального ефекту.
Після кожного використання приладу залишки препарату повинні бути видалені з нього. Вичистіть
та вимийте прилад як зазначено в п.1 розділу “Догляд, збереження, ремонт та утилізація”.
ДОГЛЯД, ЗБЕРЕЖЕННЯ, РЕМОНТ ТА УТИЛІЗАЦІЯ
1. Провадьте регулярне чищення приладу та всього приладдя. Все приладдя рекомен-
довано протирати 3% розчином перекису водню з додаванням 0,5% розчину миючого засобу (наприклад прального порошку). Після цього необхідно рясно промити
під струменем води небулайзер. Мундштуки та насадки для носу допускають обробку
кип’ятінням на протязі 10 хвилин або автоклавуванням за температури до 150°С. Після
обробки протріть насухо всі частини приладу м’якою тканиною.
2. Регулярно перевіряйте чи не забруднений фільтр та за необхідністю замінюйте його.
Для заміни фільтру відкрийте гніздо для фільтру; встановіть новий фільтр; закрийте
гніздо для фільтру.
РЕКОМЕНДОВАНО ПРОВАДИТИ ЗАМІНУ ФІЛЬТРУ НЕ МЕНШЕ ОДНОГО
РАЗУ НА РІК.
3. Прилад потрібно оберігати від прямих сонячних променів та ударів.
4. Не зберігайте та не використовуйте прилад в безпосередній близкості від нагрівальних
приладів та відкритого вогню.
5. Оберігайте прилад від забруднення.
Page 35

UKR
35
6. Не допускайте контакт приладу з агресивними розчинами.
7. За необхідністю провадьте ремонт тільки в спціалізованих установах
.
8. Термін придатності компресора складає 5 років з моменту його виробництва. Термін при-
датності витратних матеріалів складає 1 рік. Рік виробництва вказано в серійному номері
приладу (див. розділ ОСНОВНІ ТЕХНІЧНІ ХАРАКТЕРИСТИКИ). Після закінчення встановленого терміну придатності необхідно періодично звертатися до спеціалістів (до спеціалізованих ремонтних установ) для перевірки технічного стану приладу, та якщо необхідно,
для проведення його утилізації у відповідності з діючими правилами утилізації у Вашому
регіоні. Спеціальні умови утилізації виробником не зазначені.
ГАРАНТІЙНІ ЗОБОВ’ЯЗАННЯ
На цей прилад встановлено гарантійний термін 36 місяців з дня продажу. Гарантія не розповсюджується на витратні матеріали (маски, мундштуки, трубки та ін.) Гарантійні зобов’язання
оформлюються гарантійним талоном при продажу приладу покупцю. Адреси установ що провадять гарантійне обслуговування вказані в гарантійному талоні.
КОМПЛЕКТНІСТЬ
№
НАЗВА МОДЕЛЬ КІЛЬКІСТЬ, шт.
1 Компресор — 1
2
Небулайзер з розпилювачем
інгаляційним
LD-N105 1
3 Розпилювач інгаляційний LD-N002 1
4 Розпилювач інгаляційний LD-N003 1
5 Мундштук інгаляційний LD-N022 2
6
Маска інгаляційна
LD-N041 1
7
Маска інгаляційна
LD-N040 1
8 Трубка інгаляційна LD-N051 1
9 Запасний фільтр для інгалятора LD-N055 5
10
Упаковка
—1
11 Інструкція з експлуатації — 1
12 Гарантійний талон — 1
Page 36

UKR
36
ОСНОВНІ ТЕХНІЧНІ ХАРАКТИРИСТИКИ
Модель LD-221C
Тип компресорний
Вживана потужність, не більше 60 Вт
Продуктивність отримання аерозолю, приблизно
розпилювач інгаляційний «A» LD-N001
розпилювач інгаляційний «B» LD-N002
розпилювач інгаляційний «C» LD-N003
0.31 мл/хв.
0.43 мл/хв.
0.50 мл/хв.
Середній розмір частинок аерозоля (MMAD)
розпилювач інгаляційний «A» LD-N001
розпилювач інгаляційний «B» LD-N002
розпилювач інгаляційний «C» LD-N003
3.5 мкм
4.0 мкм
5.0 мкм
Максимальний час безперервної роботи 20 хвилин
Час охолодження
40 хвилин
Об’єм резервуару для інгаляційного розчину 10 мл
Залишковий об’єм інгаляційного розчину, не білше
0.5 мл
Максимальний тиск компресору 2.0 бар
Рівень шуму, не більше 65 дБ
Електроживлення: ~230В 50Гц
Ступінь захисту від ураження електричним током Виріб типу BF
Умови експлуатації приладу:
Температура оточуючого повітря Від 10°С до 40°С
Вологість Не більше 95% Rh
Атмосферний тиск 860~1060hPa
Умови збереження та транспортування приладу:
Температура оточуючого повітря Від -10°C до 40°C
Вологість 95% Rh
Атмосферний тиск 500~1060hPa
Маса комплекту (без упаковки), не більше
1255 г
Габаритні розміри електронного блоку
140 мм х 90 мм х 190 мм
Рік виробництва
Рік і місяць виробництва позначенi у серійному
номері після символу «А». Серійний номер розташований на нижній частині корпусу приладу.
РОЗШИФРОВКА СИМВОЛІВ:
Відповідність Директиві 93/42/EEC
Важливо: Прочитайте інструкцію
Представник в Євросоюзі
Виробник
Знак відповідності Техрегламенту України (ПКМУ №753
від 02.10.2013 р.)
Клас захисту II
Виріб типу BF
Page 37

Дата редакції цього Посібника з експлуатації вказана на останній сторінці у вигляді EXXX / YYMM / XX,
де YY - рік, а ММ - місяць редакції.
Технічні характеристики можуть змінюватися без попереднього повідомлення з метою поліпшення експлуатаційних властивостей та якості виробу.
ПОШУК ТА УСУНЕННЯ НЕСПРАВНОСТЕЙ
Відсутність виникнення аерозолю може відбуватись за слідуючих нижчевказаних причин:
1. Відсутність електроживлення в мережі.
Невідповідність напруги електроживлення вимогам.
Поганий контакт вилки з розеткою живлення.
2. Відсутність інгаляційного розчину в небулайзері. Додайте необхідну кількість розчину в
небулайзер.
3. Засмічення сопла небулайзера залишками чи осадом інгаляційного розчину. Прочистіть
сопло небулайзеру. При чищенні не використовуйте металеві предмети, що можуть порушити геометрію сопла.
4. Перекручена інгаляційна трубка. Розправте інгаляційну трубку таким чином, аби поста-
чанню повітря до небулайзеру ніщо не перешкоджало.
ВІДОМОСТІ ПРО СЕРТИФІКАЦІЮ ТА ДЕРЖАВНУ РЕЄСТРАЦІЮ
Виробництво приладу сертифіковане відповідно до стандарту ISO 13485. Прилад відповідає вимогам Директиви MDD 93/42 / ЄЕС, відповідає міжнародним стандартам EN 980, EN 1041, EN 1060-1, EN
1060-3, EN 10601-1-2, ISO 14971.
Цей прилад відповідає вимогам EN 980, EN 1041, EN 1060-1, EN 1060-3, EN 10601-1-2, ISO 14971.
Міністерство охорони здоров’я України Сертифікат відповідності технічниму регламенту №
UA.TR.067.2.16-15 від 29.01.2016.
Претензії споживачів та побажання направляти на адресу офіційного імпортера:
Україна: а/с 123 м. Київ 03049, «Ергоком» ТПК ПП.
Тел. безкоштовної гарячої лінії: 0-800-30-120-80
Polska: Little Doctor Europe Sp. z o.o. ul. Zawila 57G, 30-390, Krakow.
Tel. +48 12 268-47-46
Продукт компанії ЛІТТЛ ДОКТОР ІНТЕРНЕШНЛ (С) Пте. Лтд., Ішун Централ, Поштова скринька
9293, Сінгапур, 917699.
Експортер: Little Doctor International (S) Pte. Ltd. (Літтл Доктор Інтернешнл (С) Пте. Лтд.)
Виробник: Little Doctor Electronic (Nantong) Co., Ltd., No.8, Tongxing Road Economic & Technical
Development Area, 226010 Nantong, Jiangsu, P.R.China (Літтл Доктор Електронік (Нантонг)
Ко.Лтд., Ном.8, Тонгксінг Роад Економік енд Текнікал Девелопмент Еріа, 226010 Нантонг,
Джіангсу, КНР).
Уповноважений представник в Україні: Приватне підприємство „Торгівельно-промислова компанія „Ергоком” вул. Довженка, 10, м. Київ, 03057, Україна.
Тел./факс: (+38 044) 492-79-55/ (+38 044) 404-48-67. Email: info@ergocom.ua www.ergocom.ua
Page 38

КОМПЛЕКТУЮЧІ ДО ІНГАЛЯТОРА LD-207U*
Набір для інгаляції №1
Призначений для використання з компресорними інгаляторами
LD
У набір входять:
• Небулайзер LD-N105 в зборі - 1 шт.
• Мундштук інгаляційний LD-N022 - 2 шт.
• Інгаляційна трубка (2м) LD-N051 - 1 шт.
• Фільтр LD-N055 - 5 шт.
• Розпилювач інгаляційний «A» LD-N001 - 1 шт.
• Розпилювач інгаляційний «C» LD-N003 - 1 шт.
Маска інгаляційна доросла LD-N041
• Призначена для використання з інгаляторами LD
• Матеріал: полівінілхлорид
• Для індивідуального використання
• Кількість в упаковці – 1 шт.
Маска інгаляційна дитяча LD-N040
• Призначена для використання з інгаляторами LD
• Матеріал: полівінілхлорид
• Для індивідуального використання
• Кількість в упаковці – 1 шт.
Мундштук інгаляційний LD-N022
• Призначен для використання з інгаляторами LD
• Матеріал: поліпропілен
• Для індивідуального використання
• Кількість в упаковці – 10 шт.
* При необхідності можна придбати окремо.
Page 39

GUIDANCE AND MANUFACTURE’S DECLARATION –
ELECTROMAGNETIC EMISSION FOR ALL EQUIPMENT AND SYSTEMS
Guidance and manufacture’s declaration – electromagnetic emission
The NB-210C is intended for use in the electromagnetic environment specifi ed below. The cus-
tomer of the user of the NB-210C should assure that it is used in such environment.
Emission test
Compliance
Electromagnetic environment
– guidance
RF emissions CISPR 11 Group 1 The NB-210C uses RF energy only for its inter-
nal function. Therefore, its RF emissions are very
low and are not likely to cause any interference in
nearby electronic equipment.
RF emission CISPR 11 Class B The NB-210C is suitable for use in all establish-
ments, including domestic establishments and those
directly connected to the public low-voltage power
supply network that supplies buildings used for
domestic purposes.
Harmonic emissions
IEC 61000-3-2
Class A
Voltage fl uctuations/ fl icker
emissions IEC 61000-3-3
Complies
Page 40

LITTLE DOCTOR INTERNATIONAL (S) PTE. LTD.
Yishun Central P.O. Box 9293 Singapore 917699,
Fax: 65-62342197, E-mail: ld@singaporemail.com
®
Registered trade marks of Little Doctor International (S) Pte. Ltd.
©
Little Doctor International (S) Pte. Ltd., 2017
E584/1707/02
Little Doctor Europe Sp. z o.o.
57G Zawila Street Krakow 30-390 Poland
WWW.LITTLEDOCTOR.EU
 Loading...
Loading...