Lifescan SureStep Pro User Manual
Bedside Unit Operator’s Guide


The SureStep®Pro Bedside Unit is for in vitro
diagnostic use for the quantitative
measurement of glucose in venous, capillary,
arterial, and neonatal whole blood samples.
It should not be used for the
diagnosis of diabetes.
i
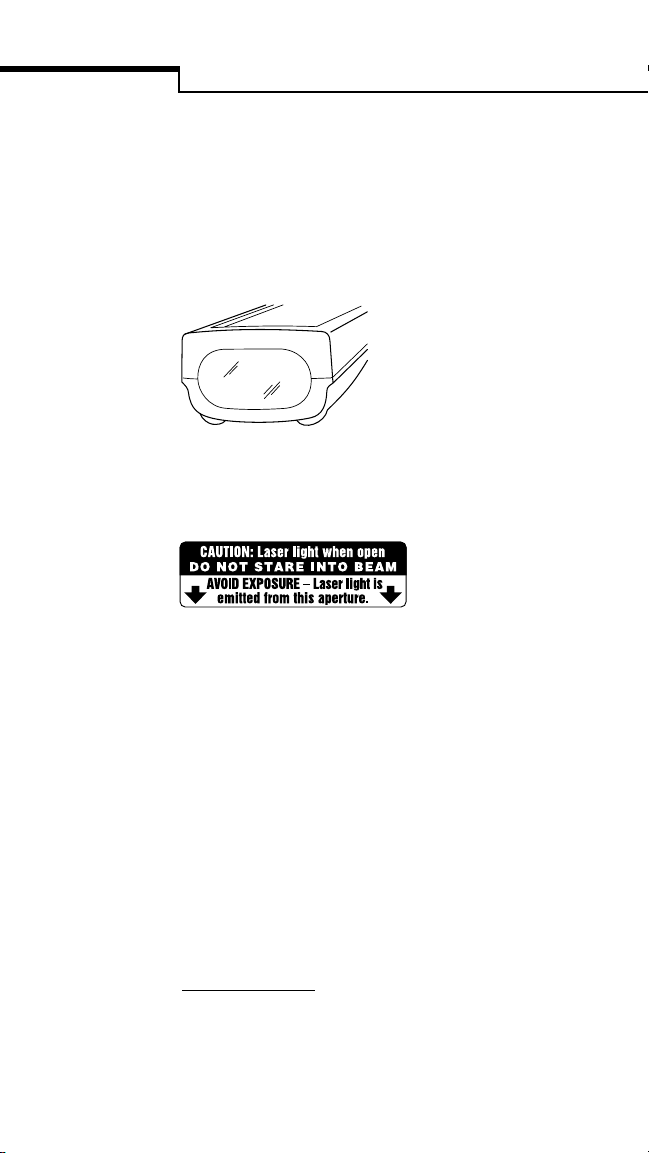
CAUTION
▲
This product contains a barcode scanner. It is
a class II laser which operates at 680 nm and
uses 0.88 mW of energy. This product meets
21 CFR (Code of Federal Regulations)
1040.10* laser light when activated.
Do not stare into the laser beam. Refer to page 8
of this guide for information and instructions on
barcode scanner operation.
©2002, 1996 LifeScan, Inc. All rights reserved.
The system described herein is covered by one or more of the
following US patents: D392,740, D367,109, 5,418,142, 5,515,170,
5,526,120, 5,563,031, 5,605,837, 5,780,304, 5,789,255,
5,922,530, 5,968,836, and 6,335,203. Use of the system described
herein is protected under US patent 4,935,346. Purchase of the
system described herein does not act to grant a license under these
patents. Such a license is granted only when the device is used with
SureStepPro or OneTouch SureStep Test Strips. No test strip supplier
other than LifeScan is authorized to grant such a license, and
LifeScan does not endorse or encourage the use of any strips
manufactured by anyone other than LifeScan.
A Food and Drug Administration (FDA) regulation
*
established for any product that contains a laser.
ii

Table of Contents
Preface . . . . . . . . . . . . . . . . . . . . . . . . . . . . v
About This Guide . . . . . . . . . . . . . . . . . . . vi
What You’ll Find in This Guide. . . . . . . vi
Conventions Used in This Guide . . . . . . . viii
Help . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ix
CHAPTER 1–Introduction . . . . . . . . . . . . . 1
The Bedside Unit . . . . . . . . . . . . . . . . . . . . . 3
General Operating Information . . . . . . . . . . 5
Entering Information . . . . . . . . . . . . . . . . 6
Scanning a Barcode . . . . . . . . . . . . . . . . . 8
System Beep Signals . . . . . . . . . . . . . . . . . 9
SureStepPro Test Strips . . . . . . . . . . . . . . . . 9
Precautions . . . . . . . . . . . . . . . . . . . . . . . . 12
Infection Control . . . . . . . . . . . . . . . . . . 14
CHAPTER 2–Quality Control Test . . . . . . 15
When to Perform a Quality Control Test . . 16
Glucose Control Solutions . . . . . . . . . . . . . 17
Performing a Quality Control Test . . . . . . . 18
Applying Control Solution to Test Strip. 22
CHAPTER 3–Patient Test . . . . . . . . . . . . . 29
Performing a Patient Test . . . . . . . . . . . . . 29
Applying Blood Sample to Test Strip . . . 33
iii

Table of Contents
CHAPTER 4–Data Management . . . . . . . 41
Transferring Data to the Workstation . . . . 41
Using a Direct Infrared Port Connection 42
Using the Connection Module. . . . . . . . 44
Bedside Unit Memory . . . . . . . . . . . . . . . . 49
Accessing Memory to Review Data . . . . 49
CHAPTER 5–Care and Maintenance . . . . 53
General Care . . . . . . . . . . . . . . . . . . . . . . . 53
When to Clean the Unit . . . . . . . . . . . . 53
Cleaning the Outside of the Unit . . . . . . . 54
Cleaning the Test Strip Holder and Lens . . 54
Changing the Batteries . . . . . . . . . . . . . . . 57
Adjusting the Screen Contrast . . . . . . . . . . 60
CHAPTER 6–Special Tests . . . . . . . . . . . . 61
Linearity Test . . . . . . . . . . . . . . . . . . . . . . 61
Unknown Solution Test . . . . . . . . . . . . . . 69
In-Service Test . . . . . . . . . . . . . . . . . . . . . 75
CHAPTER 7–Troubleshooting . . . . . . . . 77
Error Messages . . . . . . . . . . . . . . . . . . . . . 79
Unexpected Messages and Observations . . 85
APPENDIX A–Bedside Unit
Configuration Options . . . . . . . . . . . . . 91
Specifications . . . . . . . . . . . . . . . . . . . . . 97
Bedside Unit Specifications . . . . . . . . . . . . 97
Barcode Specifications . . . . . . . . . . . . . . . . 98
Cleaning Agents . . . . . . . . . . . . . . . . . . . 100
Index . . . . . . . . . . . . . . . . . . . . . . . . . . . 101
iv

Preface
SureStepPro is LifeScan’s integrated blood
glucose management system for testing,
tracking, and managing patient and quality
control data.
The SureStepPro System offers diabetes
caregivers and patients off-meter blood dosing
of the test strip. This design can help reduce
the risk of transmission of blood-borne
pathogens between patients, while providing
accurate and reliable blood glucose results at
the point of patient care.
v

Preface
About This Guide
The SureStepPro Bedside Unit Operator’s Guide
provides detailed instructions on using and
maintaining the SureStepPro Bedside Unit.
Because bedside unit settings will vary
depending on how the unit is configured, you
will benefit by becoming familiar with the
configuration options. For more detailed
information on configuration options, ask your
system administrator or refer to Appendix A,
Bedside Unit Configuration Options, for a list
of bedside unit options configured at the
workstation.
What You’ll Find in This Guide
Chapter 1, Introduction, provides you with an
overview of the system and outlines basic
operating instructions.
Chapter 2, Quality Control Test, guides you
through the steps of performing a quality
control test.
Chapter 3, Patient Test, guides you through
the steps of performing a patient blood test.
Chapter 4, Data Management, covers the
bedside unit/workstation data transfer session.
Details for this procedure can be found in the
DataLink System Administrator’s Guide. Chapter
4 also provides information on reviewing test
results stored in bedside unit memory.
vi

Chapter 5, Care and Maintenance, instructs
you on how to clean the bedside unit and test
strip holder and change the batteries.
Chapter 6, Special Tests, provides instructions
for performing a linearity test and an unknown
solution test. Also included are instructions on
in-service training, when teaching new
operators how to use the bedside unit to
perform a glucose test.
Chapter 7, Troubleshooting, lists error
conditions that may occur or messages that
may appear during operation and offers
solutions to correct the problems.
Appendix A, Bedside Unit Configuration
Options, includes a list of all bedside unit
options that have been configured (selected by
your system administrator) at the workstation.
vii
Preface
Conventions Used in This Guide
NOTE
■
Points out additional information that may be
helpful.
IMPORTANT
◆
Contains information necessary to successfully
perform the test.
CAUTION
▲
Alerts you to situations that could result in
instrument damage, failure in a procedure, or
possible inaccurate results.
Text within shaded boxes appears throughout
this guide. This information describes a
specific workstation option and how it may
affect bedside unit operation.
For example:
1
Enter your operator ID.
Depending on the configuration option setting,
you may or may not be required to enter your
operator ID.
For a list of workstation options that are
reflected in the bedside unit, refer to
Appendix A, Bedside Unit Configuration
Options.
viii

Help
1. Read through the section of the guide
specific to the operation you are performing.
Refer to the table of contents and index to
locate information.
2. See Chapter 7 for error messages and
troubleshooting information.
3. Call LifeScan Healthcare Professional Line at:
1 800 524-7226 (USA)
1 888 353-0800 (Canada)
ix

Preface
x

Introduction
The SureStepPro Bedside Unit is one
component of LifeScan’s professional blood
glucose monitoring system. When used with
SureStepPro Test Strips, the bedside unit
measures a patient’s blood glucose level from a
whole blood sample taken at the bedside. Blood
is applied directly to the test strip before the
strip is inserted into the bedside unit. A confirmation dot on the test strip helps you check
that an adequate volume of sample was applied.
The unit is equipped with a barcode scanner
and a large touch-sensitive display, both of
which allow you to enter operator, patient, and
reagent information.
CHAPTER 1
Before use, each bedside unit must be
configured by your institution’s system
administrator. Your administrator has defined a
set of options from a central workstation
running DataLink
software (or compatible data management
software). This configuration process provides
the flexibility to customize a blood glucose
testing protocol that meets the needs of your
institution.
™
Data Management System
1
Introduction
The bedside unit stores patient and quality
control data in internal memory. A bi-directional
data transfer, performed with the workstation,
sends test results from the bedside unit to the
workstation and the latest configuration information from the workstation to the bedside unit.
LifeScan’s DataLink System for modems or
network provides you with choices for connectivity solutions. Following a data transfer session,
reports can be generated and printed from the
workstation.
The complete SureStepPro Professional Care
Blood Glucose Management System includes a
computer workstation and software, bedside
units, test strips, glucose control solutions,
glucose linearity kit, and carrying case.
*
*Computer products may vary from those shown.
For important information on any of these
LifeScan products, refer to the appropriate
operator’s guide or reagent package insert. It is
important that you read and understand all
instructions before you operate the system.
2
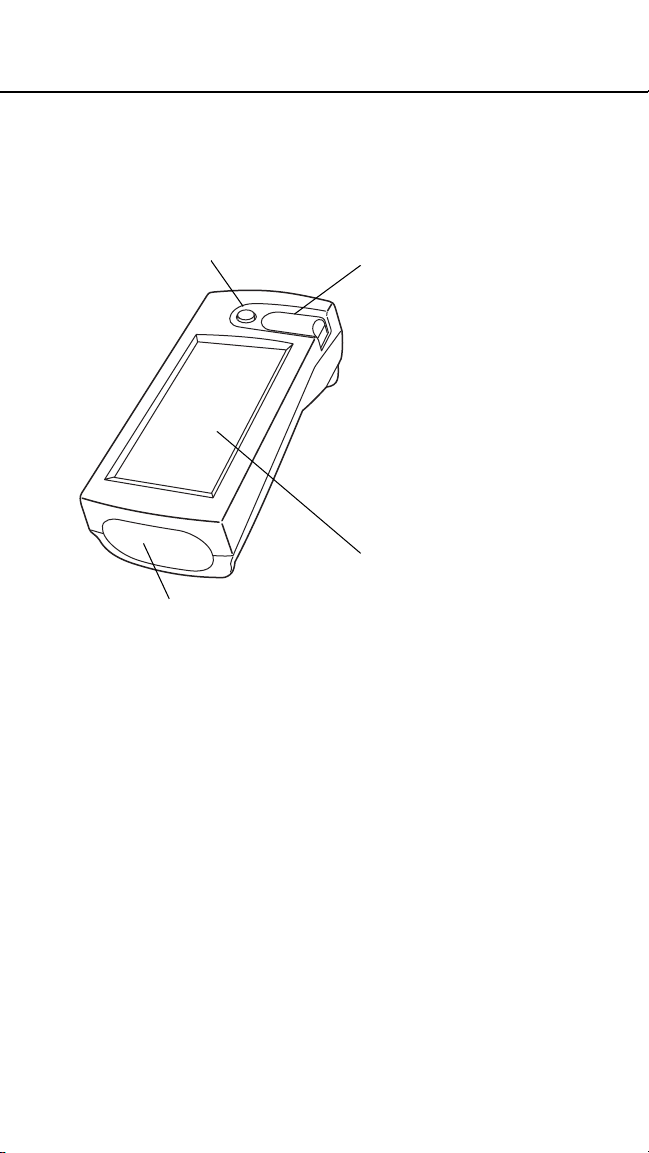
The Bedside Unit
FRONT
Power button turns
the unit on and off.
Barcode scanner enables
direct data entry for operator ID,
patient ID, and control solution
and test strip lot numbers from
their respective barcodes.
Infrared communication port
communicates with the
workstation, via the connection
module.
Test strip holder
covers the le ns area and
holds the test strip. The
holder slides out of the
unit for easy cleaning.
The lens area, located
under the test strip
holder, contains the
optics that reads the
glucose level on the
test strip.
Contact points, under
the test strip holder,
sense the position and
orientation of the test
strip.
LCD screen displays
test results and prompts
that guide you through
QC and patient testing.
The touch-sensitive
screen allows you to
select options and enter
information by simply
touching an area of the
screen.
CAUTION
▲
Do not stare into the barcode scanner or point it
towards anyone’s eyes while the laser light is on.
3
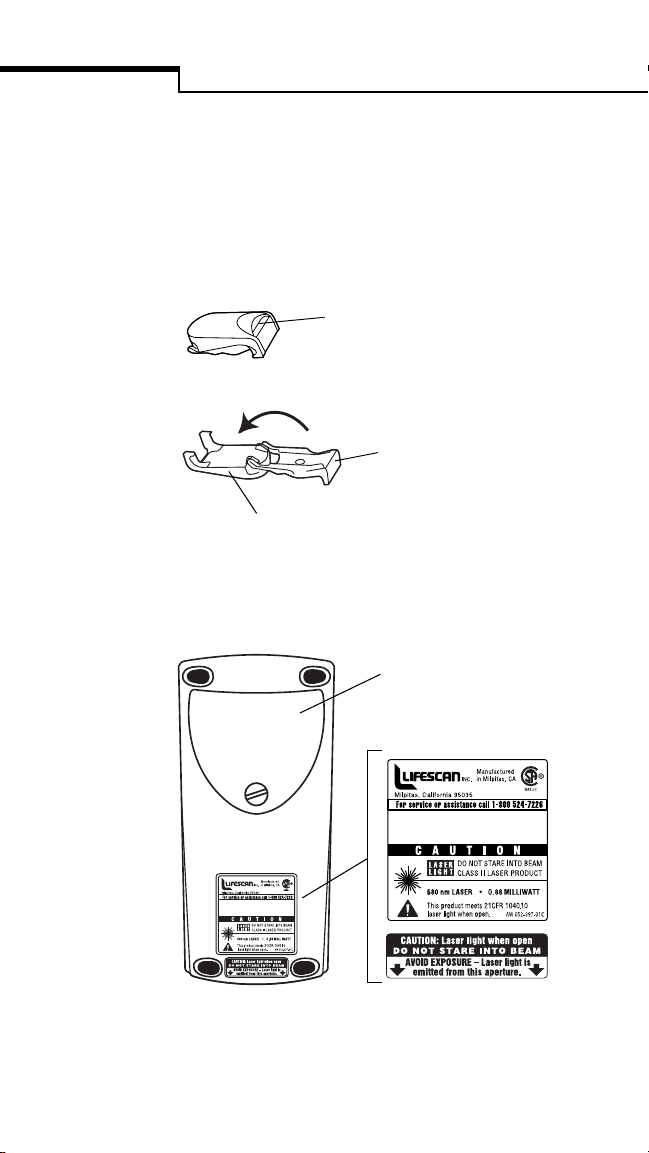
Introduction
TEST STRIP HOLDER
The test strip holder is interchangeable—the
same holder may be used in any SureStep brand
meter.
Closed
The insertion point is the area
where the test strip is inserted.
Opened
The base holds the test
strip in place.
The cover protects the lens.
BACK
Battery Compartment holds
two size C alkaline batteries.
4

General Operating Information
LifeScan recommends that only certified
operators in the hospital or clinic perform
blood glucose testing using the SureStepPro
System.
Before you can operate the bedside unit, it must
be configured. Your system administrator will
perform this function after completing the
initial system setup.
The configuration of a bedside unit and how it
operates is determined by choices the system
administrator makes within DataLink Data
Management software running at the
workstation. It may be helpful for you to
familiarize yourself with the exact
configuration for the bedside unit you are
operating. This will prepare you for what to
expect when performing QC and patient tests.
You may request a Location (or Nursing Unit)
Configuration Report from your system
administrator. This report lists each option
that was selected for all bedside units within a
selected nursing unit.
5
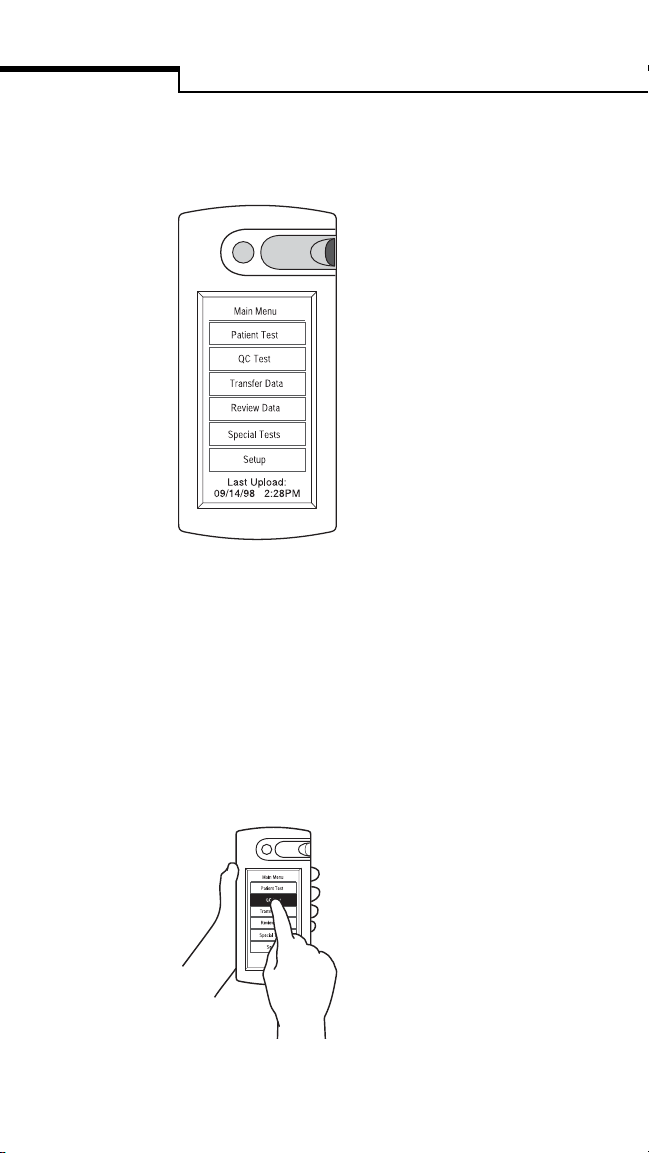
Introduction
A Main Menu lets you select from six different
functions.
Patient Test allows you to run a
patient test.
QC Test allows you to run a quality
control test.
Tran sfe r Da ta transfers data
between bedside unit and workstation.
Review Data displays stored test
results and reagent lot numbers.
Special Tests allows you to run
linearity and unknown test solutions
and perform in-service testing.
Setup allows you to adjust the
bedside unit’s LCD contrast.
Last Upload displays the date and time of the
last successful data transfer session (SureStepPro
2.0 only).
Entering Information
Select an item from a list by touching the
appropriate area of the screen. If a list is too
long to fit on one screen, arrow buttons allow
you to scroll through the list.
6
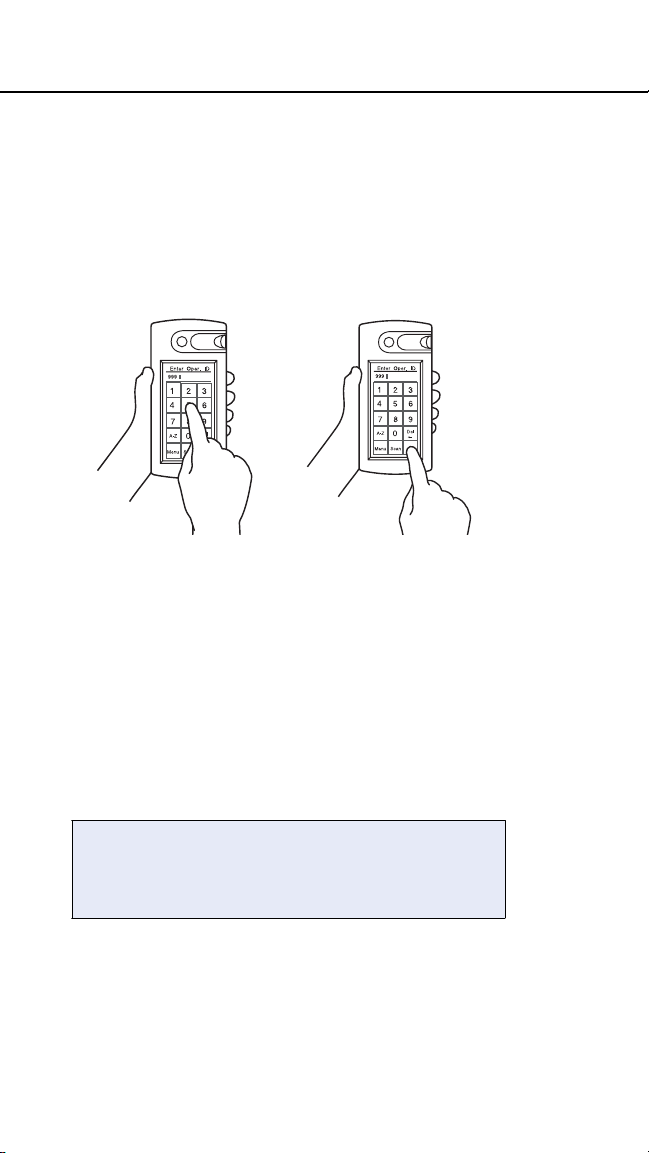
Manually enter IDs or lot numbers by pressing
the appropriate characters on the screen. Both
alpha and numeric characters are available for
data entry. Use the A–Z and 0–9 buttons to
switch between alpha and numeric modes.
Press Ok when you have completed the entry.
To delete the last character or characters
entered, press Del.
NOTE
■
If left inactive, the bedside unit will
automatically shut off. Press the power button
to turn it back on. If you entered your operator
ID before the unit was turned off, it can remain
in memory for up to 1 hour.
A configuration option may be set to maintain
your ID for up to 1 hour after the bedside unit
is turned off.
7
Introduction
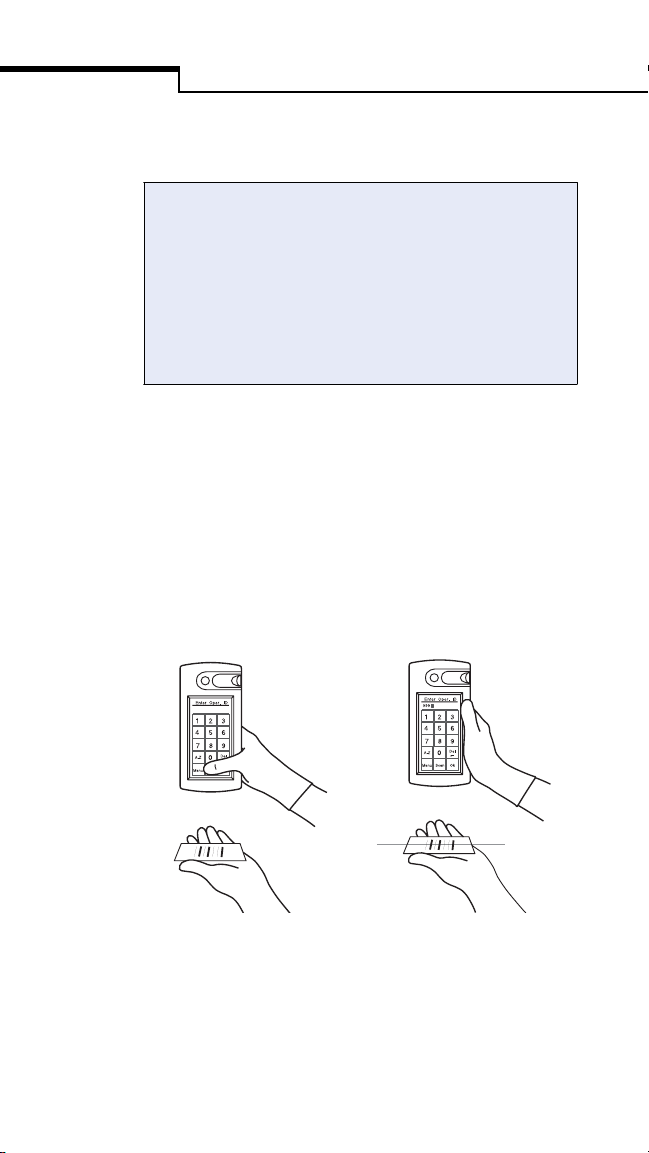
Scanning a Barcode
The bedside unit’s barcode scanner must be
enabled at the workstation. If the scanner is
enabled, the Scanner field at the Status screen
reads ON when you turn on the unit, and the
Scan button appears on data entry screens. If the
scanner is not enabled, the field reads OFF and
the Scan button does not appear on the screens.
To scan a barcode, hold the barcode label
parallel to and approximately 6 to 10 inches
from the unit’s scanner. Press and release the
Scan button on the screen. Scanning begins
when your thumb is lifted from the screen.
After a successful scan, the information appears
in the display field and the bedside unit beeps.
Press Ok to confirm the entry.
Press Release
CAUTION
▲
This product contains a laser. Do not stare into
the laser light or point it towards anyone’s eyes
while scanning a barcode.
8

System Beep Signals
The SureStepPro Bedside Unit uses beeping
signals to indicate various stages in the testing
procedure. These signals do not indicate that
the procedure is being performed correctly.
The unit can detect some, but not all, errors in
the test procedure.
• A single short beep sounds each time a
selection is made or a button is pressed on
the screen.
• Three short beeps sound when a test result is
displayed.
• A single, long beep accompanies error
messages and errors that are detected in the
test procedure, as well as CRITICAL HIGH,
CRITICAL LOW, and HIGH results. This
beep is also used at various times during the
test strip insertion stages of the test.
A configuration option may be set to turn Off
most beeps except error beeps and the touch
screen feedback beep.
SureStepPro Test Strips
SureStepPro Test Strips are the only test strips
specifically designed, developed, and tested for
use with the SureStepPro Bedside Unit.
When blood is applied to the pink test square, a
chemical reaction takes place. A blue color
forms in the confirmation dot on the back of the
test strip. If the confirmation dot is completely
blue, this indicates that an adequate volume of
9
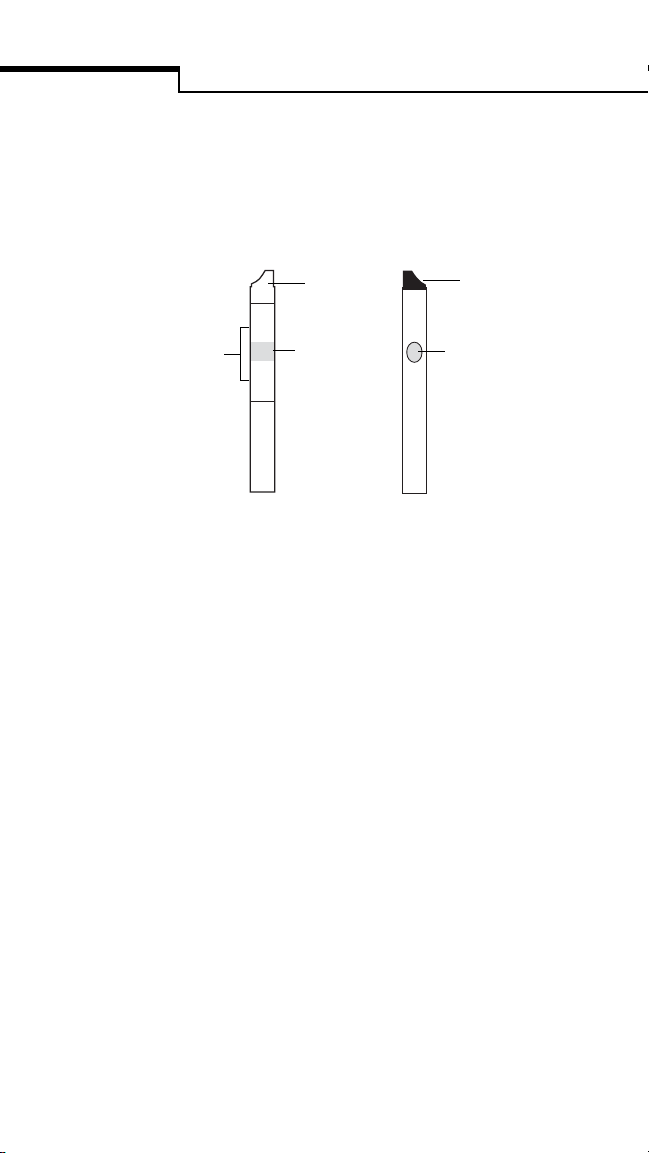
Introduction
sample was applied. The intensity of the blue is
read by the bedside unit—the darker the blue,
the higher the glucose level.
BACK
black tip
confirmation dot
white pad
FRONT
white tip
pink test square
White Tip faces up as the test strip is inserted
into the test strip holder.
Pink Test Square is the area where the blood
must be applied. For detailed information,
refer to “Applying Blood Sample to Test Strip”
on page33.
White Pad absorbs excess blood that may
extend beyond the pink test square. Do not
apply blood directly to the white pad.
Black Tip faces down and helps the unit
determine when the strip has been inserted.
Confirmation Dot is read by the bedside unit
to determine the sample’s glucose concentration.
If it turns completely blue, this indicates that an
adequate volume of blood was applied.
10

IMPORTANT Test Strip Information
◆
For detailed information regarding
SureStepPro Test Strips, read the package
insert.
• Use only SureStepPro Test Strips with the
SureStepPro Blood Glucose Bedside Unit.
• SureStepPro Test Strips are sensitive to heat,
light, and moisture. Keep them tightly
sealed in their original bottle, and store in a
cool, dry place below 30°C (86°F). Do not
refrigerate, freeze, or place in direct heat or
sunlight.
• A lot number and CTL (control) code are
printed on the test strip bottle label. (The
CTL code encodes the test strip expiration
date, control solution ranges, and linearity
reference values.) When entering this
information, always get the lot number and
CTL code from the test strips currently in use.
• Do not use the test strips after the expiration
date printed on the bottle label.
• Discard any unused test strips 4 months after
opening. Write the opened date on the bottle
when you first open it.
• Replace the test strip bottle cap immediately
after removing a test strip and close the cap
tightly.
• Do not transfer test strips to a new bottle or
any other container.
11

Introduction
• Use each test strip immediately after
removing it from the bottle.
• Do not use test strips that are bent, torn, cut,
or damaged in any way.
• Compare the color of the confirmation dot
from an unused test strip to the “Unused”
color dot shown on the Color Chart on the
test strip bottle label. If the color of the test
strip confirmation dot is darker than that
shown on the Color Chart, do not use the
test strip. The test result may be inaccurate.
• You have up to 2 minutes to insert the test
strip after applying blood or control solution.
If you insert the test strip after 2 minutes, you
may get an inaccurate result or an error
message.
Precautions
• Unexpected results should be confirmed by a
laboratory test.
• Various conditions may cause the bedside
unit to produce a very low test result
(20 mg/dL [1.0 mmol/L] or less).
• If the patient has symptoms of low blood
glucose, follow your institution’s policy for
treatment.
• If the patient does not have symptoms of
low blood glucose, check the confirmation
dot on the back of the test strip.
– If the confirmation dot has white patches
or streaks, repeat the test with a new test
strip.
12

– If the confirmation dot is completely
blue, compare it to the sample color dots
on the test strip bottle Color Chart. If the
color comparison indicates very low blood
glucose, repeat the test. If the result is still
lower than expected, perform a quality
control test. A passing control result
indicates the system is working properly.
A control result that is out of range
indicates that the system may not be
working properly. Do not use the system to test
patient blood glucose unless the control solution
result falls within the expected range.
• If patient symptoms are inconsistent with
monitoring results and procedural errors are
ruled out, follow your institution’s policies
for treating the symptoms and confirm the
glucose measurement with a laboratory test.
• If you repeatedly get any error message,
obtain a result from a laboratory test.
Compare the test strip confirmation dot to
the colors on the test strip bottle Color
Chart, and if necessary, take appropriate
precautions while awaiting the laboratory
result. Do not use the Color Chart as a
replacement for a bedside unit result.
• Never make significant changes to a
medication program or ignore signs and
symptoms without consulting a physician.
• Failure to follow the instructions for use may
result in inaccurate results.
13

Introduction
Infection Control
Use universal blood precautions.* All patient
samples and materials with which they come in
contact are considered biohazards and should
be handled as if capable of transmitting
infection. Follow proper precautions in
accordance with your institution’s policies
when disposing of all materials.
*
Centers for Disease Control Report. Guideline
for Infection Control in Health Care Personnel.
Am J Infect Control. 1998;26:289–354.
14

Quality Control Test
Three levels of SureStepPro Glucose Control
Solution are available to check that the
SureStepPro Bedside Unit and SureStepPro
Test Strips are functioning properly.
The SureStepPro bedside units are calibrated at
the factory. Although an internal verification is
performed each time the bedside unit is turned
on, a system calibration (bedside unit and test
strip) is necessary. This is achieved at the start
of each test when you enter the lot number and
code for the current bottle of test strips.
CHAPTER 2
If you are training a new operator on how to
use the bedside unit, use the glucose control
solutions and perform the testing in the
In-Service mode. Refer to “In-Service Test” on
page 75, for more information.
The number of control levels and frequency of
running a QC test have been programmed by your
system administrator. High, Low, and Normal
control levels are available—any one control level
or a combination of levels may have been selected.
15
Quality Control Test
To ensure that a required QC test is performed at
the designated time, a warning or lockout mode
was selected. Both modes display a message
informing you that QC is due. The warning mode
allows you to continue with a patient test; the
lockout mode prevents you from using the bedside
unit for a patient test until QC is successfully
performed.
A second lockout mode prevents the operator who
has not performed QC testing within the
designated number of days from using the bedside
unit to test patient blood.
If a lockout mode was selected and QC is due, a
message appears when you start the bedside unit.
You cannot proceed with patient testing until the
required QC tests have been successfully
performed.
When to Perform a Quality Control Test
• As required by your institution’s quality
control policy or local regulatory requirements.
• When you open a new bottle of test strips.
• If a patient test has been repeated and the
blood glucose results are still lower or higher
than expected.
• After cleaning the bedside unit.
• If there are other indications that the system
is not working properly.
• If you drop the bedside unit.
16

Glucose Control Solutions
Use SureStepPro Glucose Control Solutions
with the SureStepPro Bedside Unit and
SureStepPro Test Strips only.
IMPORTANT Control Solution Information
◆
Read the SureStepPro Control Solutions
package insert for more information.
• Gently shake each vial of control solution
before use.
• Do not use a control solution after the
expiration date printed on the vial label.
• Discard any unused portion 3 months after
opening. Write the opened date on the vial
when you first open it.
• The control ranges printed on the
SureStepPro Test Strip bottle label are for
SureStepPro Control Solutions only.
• Store the control solution at room
temperature below 30°C (86°F). Do not
refrigerate or freeze.
• The control solutions contain dye that
stains clothing.
• The control solutions are not for human
consumption.
• Control solution values can be affected at
altitudes of 3000 feet or greater. Read the
SureStepPro Glucose Control Solutions
package insert for specific information and
correction factors.
17
Quality Control Test
Performing a Quality Control Test
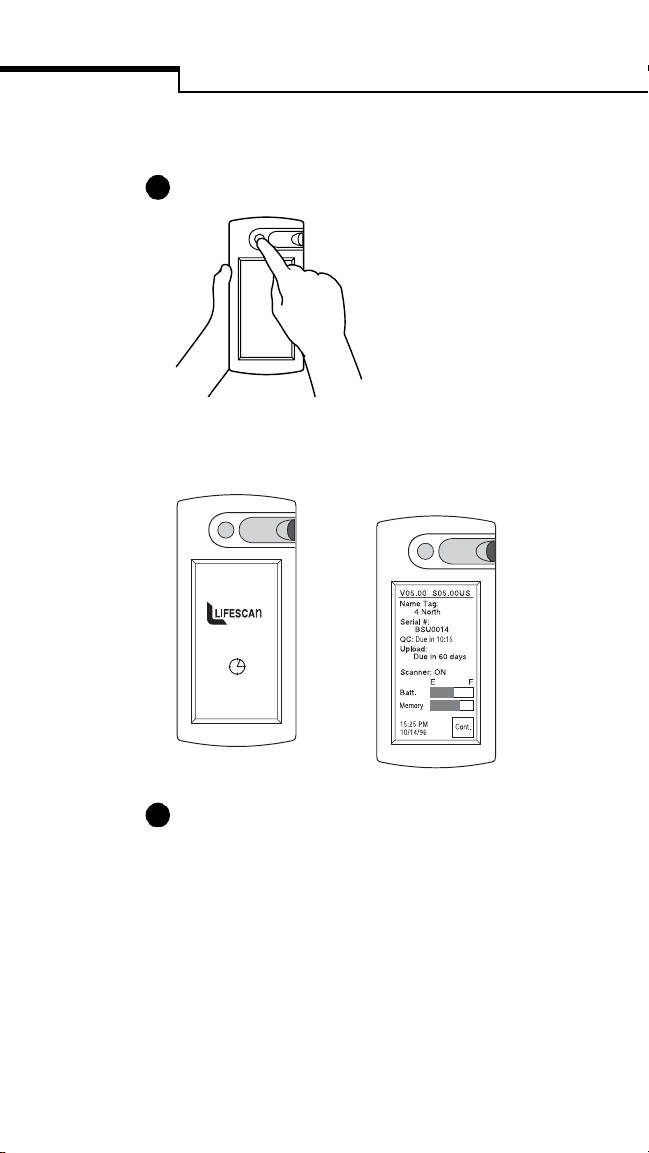
1
Press the power button to turn on the unit.
A start-up screen appears...
...followed by the Status screen.
2
Check the battery status to ensure adequate
power. The battery and memory bars provide
the approximate status of battery power and
bedside unit memory. (If there is insufficient
battery power or memory to perform a test, a
warning message appears.) Press Cont. to
continue.
18
 Loading...
Loading...