Lifescan OneTouch Ultra User Manual

U l t r a E a s y o n Yo u / U l t r a f a c i l e , U l t r a r a p i d e , U l t r a d o u x
O w n e r ’ s B o o k l e t
Guide d’utilisation

OneTouch® Customer Care Line / Ligne InfoSoins OneTouch® :
Canada 1 800 663-5521
U.S.A./É.-U. 1 800 227-8862
Monday–Friday 9am–8pm Eastern Time 6am–5pm Pacific time
Du lundi au vendredi 9 h à 20 h (heure de l’Est) 6 h à 17 h (heure du Pacifique)
www.OneTouch.ca
Distributed by / Distribué par :
LifeScan Canada Ltd./ Produits médicaux LifeScan Canada ltée
Burnaby, B.C./C.-B. V5C 6C6
Manufactured for / Fabriqué pour :
LifeScan Inc.
Milpitas, CA 95035 U.S.A./É.-U.
©LifeScan, Inc. 2003 Milpitas, CA 95035 02/2004
The system described herein is covered by one or more of the following U.S. patents / le système décrit aux présentes est protégé par un ou plusieurs des brevets américains suivants : D428, 150, 5,708,247, 6,045,567, 6,156,051, 6,197,040, 6,241,862 and/et 6,284,125
AW 06052203A

Ultra
DIABETES BLOOD GLUCOSE MONITORING SYSTEM
System Owner’s Booklet

Dear OneTouch® Ultra® System Owner:
You have chosen one of the best blood glucose monitoring systems available. This booklet has important information you must know about the OneTouch® Ultra® System. Please read it carefully.
Blood glucose monitoring plays an important role in diabetes control. A long-term study showed that keeping blood glucose levels close to normal can reduce the risk of diabetes complications by up to 60%.* The results you get with OneTouch® Ultra® System can help you and your healthcare professional monitor and adjust your treatment plan to gain better control of your diabetes.
A warranty registration card is included with your OneTouch® Ultra® System. Please complete it and mail it to us; if you prefer to fill it out online, please visit www.OneTouch.ca
*American Diabetes Association position statement on the Diabetes Control and Complications Trial (1993).
ii
Table of Contents
ABOUT YOUR NEW SYSTEM . . . . . . . . .iv
The Complete OneTouch®
Ultra® Blood Glucose
Monitoring System . . . . . . . . . . . . . .5
OneTouch® Ultra® Blood
Glucose Meter . . . . . . . . . . . . . . . . . .6
OneTouch® Ultra®
Test Strips . . . . . . . . . . . . . . . . . . . .10
BEFORE TESTING . . . . . . . . . . . . . . . . .12
Checking the Display . . . . . . . . . . .12
Coding the Meter . . . . . . . . . . . . . . .12
Checking the System . . . . . . . . . . . .15
TESTING . . . . . . . . . . . . . . . . . . . . . . . . .21
Preparing the OneTouch®
UltraSoft™ Adjustable
Blood Sampler . . . . . . . . . . . . . . . .22
Fingertip Blood Sampling . . . . . . .25
Forearm Blood Sampling . . . . . . . .26
Step-by-Step Test
Procedure . . . . . . . . . . . . . . . . . . . . .30
Used Lancet and
Test Strip Disposal . . . . . . . . . . . . .32
Special Messages . . . . . . . . . . . . . .33
USING THE METER MEMORY . . . . . . . 34
DOWNLOADING TEST RESULTS
TO A PERSONAL COMPUTER . . . . . . . .36
COMPARING METER
AND LABORATORY RESULTS . . . . . . . .38
SETTING THE METER . . . . . . . . . . . . . .40
CARING FOR YOUR OneTouch®
Ultra® SYSTEM . . . . . . . . . . . . . . . . . . .44
Meter . . . . . . . . . . . . . . . . . . . . . . .44
OneTouch® UltraSoft™
Sampler . . . . . . . . . . . . . . . . . . . . .44
Battery . . . . . . . . . . . . . . . . . . . . . .45
DISPLAY MESSAGES AND PROBLEM-SOLVING GUIDE . . . . . . . . .48
SPECIFICATIONS . . . . . . . . . . . . . . . . . .58
GUARANTEE . . . . . . . . . . . . . . . . . . . . . .59
INDEX . . . . . . . . . . . . . . . . . . . . . . . . . . .60
iii

About Your New System
The OneTouch® Ultra® System uses the latest blood glucose monitoring technology. It measures the glucose content of a blood sample by means of an electrical current produced in the test strip and sent to the meter for measurement
Test results are “plasma-calibrated.” This makes it easier for you and your diabetescare team to compare your meter results with laboratory tests.
iv
Your OneTouch® Ultra® Blood Glucose Monitoring System consists of three main products: the OneTouch® Ultra® Blood Glucose Meter, OneTouch® Ultra® Test Strips (sold separately), and OneTouch® Ultra® Control Solution. These products have been designed, tested, and proven to work together to produce accurate blood glucose results. Use no other test strips or control solution with your meter.
1
The OneTouch® Ultra® System is intended for use outside the body (in vitro diagnostic use). It should be used only for testing fresh capillary whole blood samples for glucose (sugar). It should not be used for the diagnosis of diabetes or for testing newborns.
CAUTION: Before using any product to test your blood glucose, read all instructions and practice the test. Do all quality control checks as directed and consult with a diabetes healthcare professional. These recommendations apply to all blood glucose monitoring systems and are supported by the Diabetes Educator Section of the Canadian Diabetes Association.
2
Important Information
•Severe dehydration resulting from excessive water loss may cause false low results. If you believe you are suffering from severe dehydration, consult a healthcare professional immediately.
•Test results below 3.3 mmol/L (60 mg/dL) mean low blood glucose (hypoglycemia). Test results greater than 10.0 mmol/L (180 mg/dL) mean high blood glucose (hyperglycemia). If you get results below 3.3 mmol/L (60 mg/dL) or above 10.0 mmol/L (180 mg/dL), and do not have symptoms, first repeat the test. If you have symptoms or continue to get results that fall below 3.3 mmol/L (60 mg/dL) or above 10.0 mmol/L (180 mg/dL), follow the treatment advice of your healthcare professional.
•If you are experiencing symptoms that are not consistent with your blood glucose test results AND you have followed all instructions described in the OneTouch® Ultra® Owner’s Booklet, call your healthcare professional.
•A red blood cell count (hematocrit) that is either very high (above 55%) or very low (below 30%) can cause false results.
3

4
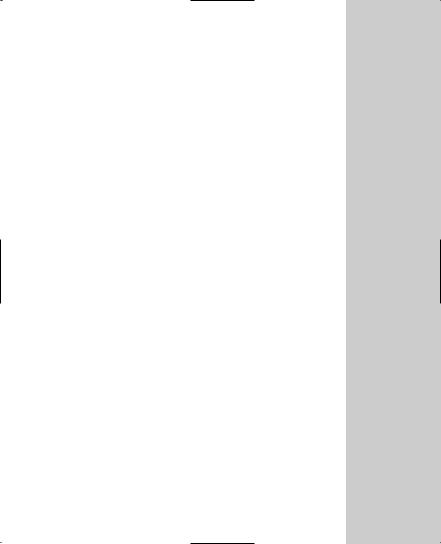
The Complete OneTouch® Ultra® Blood Glucose
Monitoring System
•OneTouch® Ultra® Meter
•Owner’s Booklet
•Quick Reference Guide
•OneTouch® UltraSoft™ Adjustable Blood Sampler
•Optional OneTouch® UltraClear™ Cap
•OneTouch® UltraSoft™ Sterile Lancets
•OneTouch® Ultra® Control Solution
•OneTouch® Ultra® Test Strips (Sold Separately)
•Carrying Case
•Warranty Registration Card
•Logbook
•One 3.0 V Lithium Battery (Installed)
5
OneTouch® Ultra® Blood Glucose Meter
DISPLAY 
Symbols, simple messages, and test results appear here.
M BUTTON
Used to turn meter on to enter:
setting mode
memory mode DATA PORT
Used to download your test results to a
computer.
6

TEST PORT
Insert the OneTouch® Ultra®
Test Strip here.
C BUTTON
Used to:
change date, time and code number
indicate control solution tests
review test results in memory
7

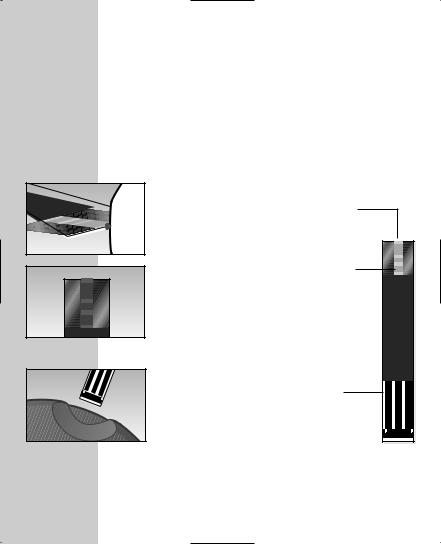
OneTouch® Ultra® Meter Display
CTL
Indicates a control solution test result.
CODE
Appears with the code number of the test strips.
BLOOD DROP SYMBOL
Tells you when to apply the sample.
DECIMAL POINT
Appears in test results only when unit of measure is set to mmol/L.
BATTERY SYMBOL
Warns when the battery is low or must be replaced.
8

KETONES? |
MEM |
Appears when a test result |
Indicates a test result |
is above 13.3 mmol/L |
stored in memory. |
|
|
(240 mg/dL) to suggest |
|
ketone testing. |
|
|
|
|
|
TEST RESULT |
|
|
|
|
AREA |
|
|
|
|
Test results are |
|
|
|
|
displayed here. |
|
|
|
|
MMOL/L |
|
|
|
|
Unit of measure. |
|
|
|
|
Millimoles per |
|
|
|
|
litre (mmol/L) is |
|
|
|
|
the standard unit |
|
|
|
|
in Canada. |
|
|
|
|
MG/DL |
|
|
|
|
Unit of measure |
|
|
|
|
used in other |
MONTH |
DAY |
HOUR |
MINUTES |
parts of the |
|
|
|
|
world. |
9
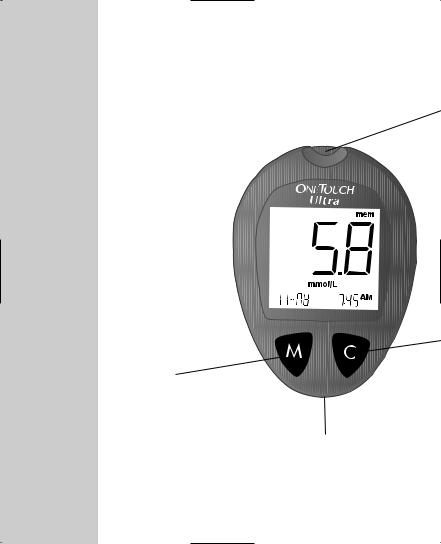
OneTouch® Ultra® Test Strips
(Sold Separately)
The OneTouch® Ultra® System measures the amount of glucose in whole blood. Blood is applied to the TOP EDGE of the OneTouch® Ultra® Test Strip and is automatically drawn into the reaction cell where the reaction takes place.
drop of blood to the channel here in the top the test strip.
Window
here to confirm if enough has been applied.
Bars
this end of the test strip, bars facing up, into the Push it all the way in until
no further.
10

Important Test Strip Information
•Store test strip vials in a cool, dry place below 30°C (86°F). Keep away from direct sunlight and heat. Do not refrigerate.
•Store your test strips in their original vial only. To avoid damage or contamination, do not transfer test strips to any other place.
•Do not use test strips beyond the expiration date printed on the package since they may cause inaccurate results.
•After removing a test strip from the vial, replace the vial cap immediately and close it tightly.
•With clean, dry hands, you may touch the test strip anywhere on its surface.
•Use each test strip immediately after removing it from the vial.
•Count three months from the date you first open a new vial of test strips and write this date on the vial label. Throw test strips and vial away after this discard date.
•Apply only OneTouch® Ultra® Control Solution or a blood sample to the test strip.
•Do not bend, cut, or modify test strips in any way.
•OneTouch® Ultra® Test Strips are for single use only. Never reuse a test strip that has had either blood or control solution applied to it.
•Refer to additional information in the OneTouch® Ultra® Test Strip package. (Sold separately.)
WARNING: Keep the test strip vial away from children; the cap is a choking hazard. Also, the cap may contain drying agents that are harmful if inhaled or swallowed and may cause skin or eye irritation.
11
BEFORE TESTING
Checking the Display
Each time you turn on the OneTouch® Ultra® Meter either by inserting a test strip or pressing the M button, all segments of the display will appear briefly. This tells you that the system is performing several self-checks to confirm that the meter is working properly. To check that all display segments are working, hold the C button down.
Coding the Meter
Code numbers are used to calibrate the OneTouch® Ultra® Meter with OneTouch® Ultra® Test Strips for accurate results. You must code the meter before using it for the first time and then every time you change to another vial of test strips. Each time you test, check to be sure that the code number on the meter display matches the code number on the test strip vial.
CAUTION: Failure to code the meter correctly will cause inaccurate test results.
12
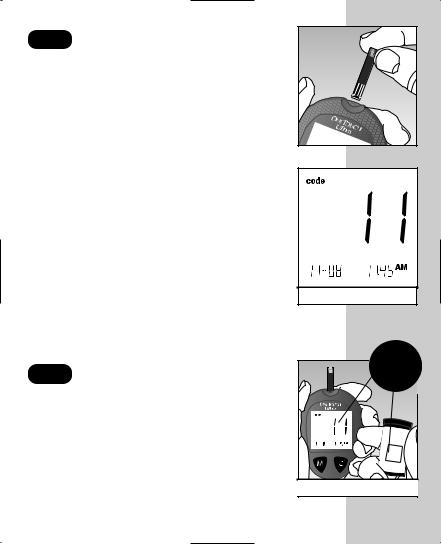
STEP 1 Enter the Code Mode.
Insert a test strip to turn on the meter. Push it all the way in until it will go no further. Avoid bending the test strip. The display check will appear. Then the code number is displayed for three seconds. (The first time you use the meter, three dashes Q will appear, meaning that there is no code stored in the memory.) If three dashes appear any other time, see page 48 of “Display Messages and Problem-Solving Guide.”
(Example)
STEP 2 Match the Code Numbers.
Compare the code number on the meter display with the code number on the test strip vial. If the two code numbers match, you may begin testing. If they do not match, follow Step 3.
13
(Example) |
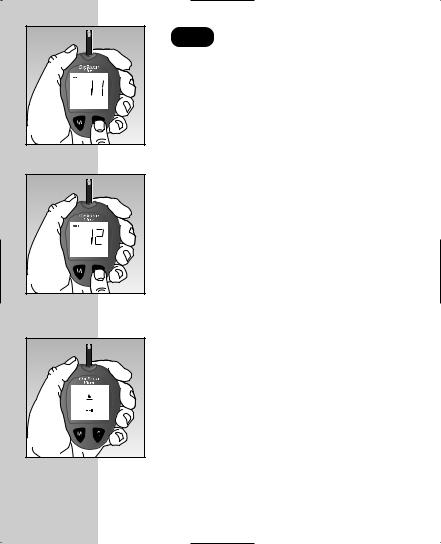
STEP 3 Code the Meter.
Press the C button to select the correct code. Each time you press and release the C button, the number will increase by one. To move more quickly, press and hold the C button.
After selecting the correct code number, it will flash for three seconds and then appear solid for three seconds.
Then the R symbol will appear with the unit of measure, indicating that the OneTouch® Ultra® System is ready
for testing.
14

Checking the System
NOTE: Refer to additional information in the OneTouch® Ultra® Control Solution package.
OneTouch® Ultra® Control Solution is used to check that the meter and the test strips are working together as a system and that you are performing the test correctly.
OneTouch® Ultra® Control Solution contains a measured amount of glucose that reacts with OneTouch® Ultra® Test Strips. Compare your control solution test results with the range printed on the test strip vial label. It is very important that you do this simple check routinely to make sure you get an accurate result.
Before you use the OneTouch® Ultra® Meter to test your blood for the first time, practice the procedure using control solution. When you can do three tests in a row that are within the range, you are ready to test your blood.
15
Important Control Solution Test Information
•Use only OneTouch® Ultra® Control Solution.
•Check the expiration date on the control solution vial. Record the discard date (date opened plus three months) on the vial label. Do not use after expiration or discard date, whichever comes first.
•Control solution, meter, and test strips should be at room temperature (20–25°C/ 68–77°F) before testing.
•Shake the vial, discard the first drop of control solution, and wipe off the tip to ensure a good sample and an accurate result.
•Store control solution tightly closed at temperatures below 30°C (86°F). Do not refrigerate.
16
CAUTION: The control solution range printed on the test strip vial is for OneTouch® Ultra® Control Solution only. It is not a recommended range for your blood glucose level.
When to do a control solution test:
•Once a week.
•When you open a new vial of test strips.
•Whenever you suspect that the meter or test strips are not working properly.
•If your blood glucose test results are not consistent with how you feel.
•After dropping the meter.
17
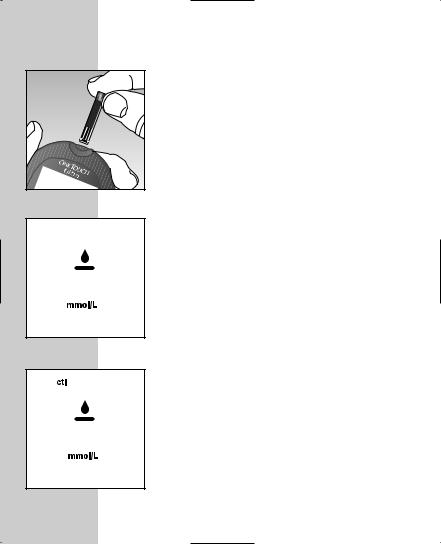
How to do a control solution test:
 Insert Test Strip.
Insert Test Strip.
test strip, contact bars end first up, into the test port. Push it
way in until it will go no further. will turn on and the display
will appear briefly.
code number will appear, followed by the R symbol and unit of measure. Check that the unit of measure is set correctly. Be sure the meter and test strip codes match. If they do not, code the meter correctly.
Press the C button to mark the test as a control solution test in the meter memory. Ë will appear on the display. If you decide not to do a control solution test, press the C button again to remove Ë from the display.
18
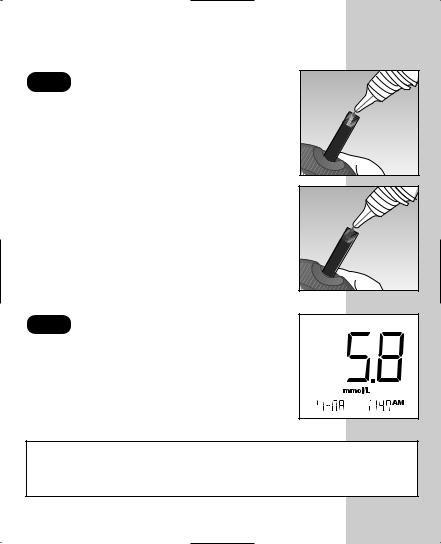
STEP 2 Apply Control Solution.
To ensure an accurate result:
• Shake the vial well
• Discard the first drop
• Wipe the dispenser tip
Hold the drop to the narrow channel in the top edge of the test strip. When the confirmation window is full, the meter will begin to count down from N to A second.
STEP 3 Result Appears in 5 Seconds.
Compare the control solution test result with the range printed on the test strip vial. The result should fall within this range.
NOTE: Mark all control solution tests with Ë to distinguish them from blood glucose tests in the meter memory. Marked control solution tests will not be included in your averages.
19
Comparing control solution results
If test results fall outside the range printed on the test strip vial, repeat the test. Out-of-range results may be caused by one or more of
the following:
•Error in performing the test.
•Failure to shake the control solution vial well.
•Expired or contaminated control solution.
•The meter, test strips, or control solution are too warm or too cold.
•Failure to discard the first drop of control solution and wipe the dispenser tip clean.
•Improper meter coding.
•Test strip deterioration.
•Meter malfunction.
CAUTION: If you continue to get control solution test results that fall outside of the range printed on the vial, the system may not be working properly. Do not use the meter. Call the OneTouch® Customer Care Line at 1 800 663-5521.
20

TESTING YOUR BLOOD
Read this section and the test strip package insert carefully before testing. Make sure you have all items needed to test.
•Meter
•Test Strips
•Sampler
•Optional OneTouch® UltraClear™ Cap (for forearm sampling)
•Sterile Lancet
21
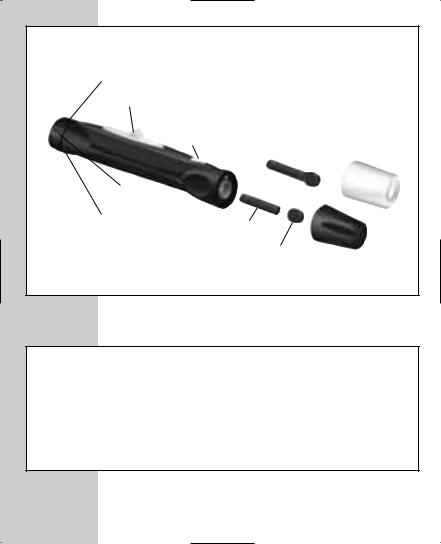
Preparing the OneTouch® UltraSoft™ Sampler
Depth Adjustment Knob
|
|
Lancet with |
|
|
|
|
Ejection/Cocking Control |
Protective Disk |
|||||
|
|
|
|
OneTouch® |
||
|
|
|
|
|||
|
Release Button |
|
UltraClearTM Cap for |
|||
|
|
Forearm Sampling |
||||
|
|
|
|
|||
Depth |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Indicator |
|
|
|
|
|
|
Puncture Settings |
Lancet Point |
|
|
|
||
|
|
|
|
|||
|
|
Protective |
|
|
|
|
|
|
|
|
|
||
|
|
Disk |
Cap |
|||
CAUTION: To reduce the chance of infection:
•Never share a lancet or the OneTouch® UltraSoft™ Sampler with anyone.
•Always use a new, sterile lancet. Lancets are for single use only.
•Keep the OneTouch® UltraSoft™ Sampler clean.
22

STEP 1 Insert a Lancet.
Turn the cap counterclockwise to
Insert the lancet into the lancet holder and push down firmly until it is fully seated. Do not twist the lancet. Twist the protective disk until it separates from the lancet. Replace the OneTouch® UltraSoft™ Cap. Turn it clockwise until it is snug.
Adjust the puncture depth necessary. Twist the depth toward the smaller bumps puncture or toward the deeper puncture.
23
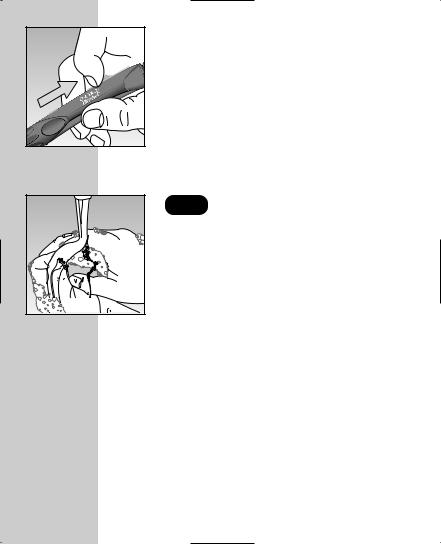
 Cock the Sampler.
Cock the Sampler.
ejection/cocking control back until it does not click, the sampler may cocked when the lancet was
The sampler is now ready for use.
STEP 3 Wash Your Hands and
the Puncture Site.
Use warm, soapy water. Rinse and dry thoroughly.
24
 Loading...
Loading...