Page 1

POWER TO SAVE A LIFE
HEARTSTART FR2+ DEFIBRILLATOR
INSTRUCTIONS FOR USE
M3860A, M3861A
Edition 10
Page 2
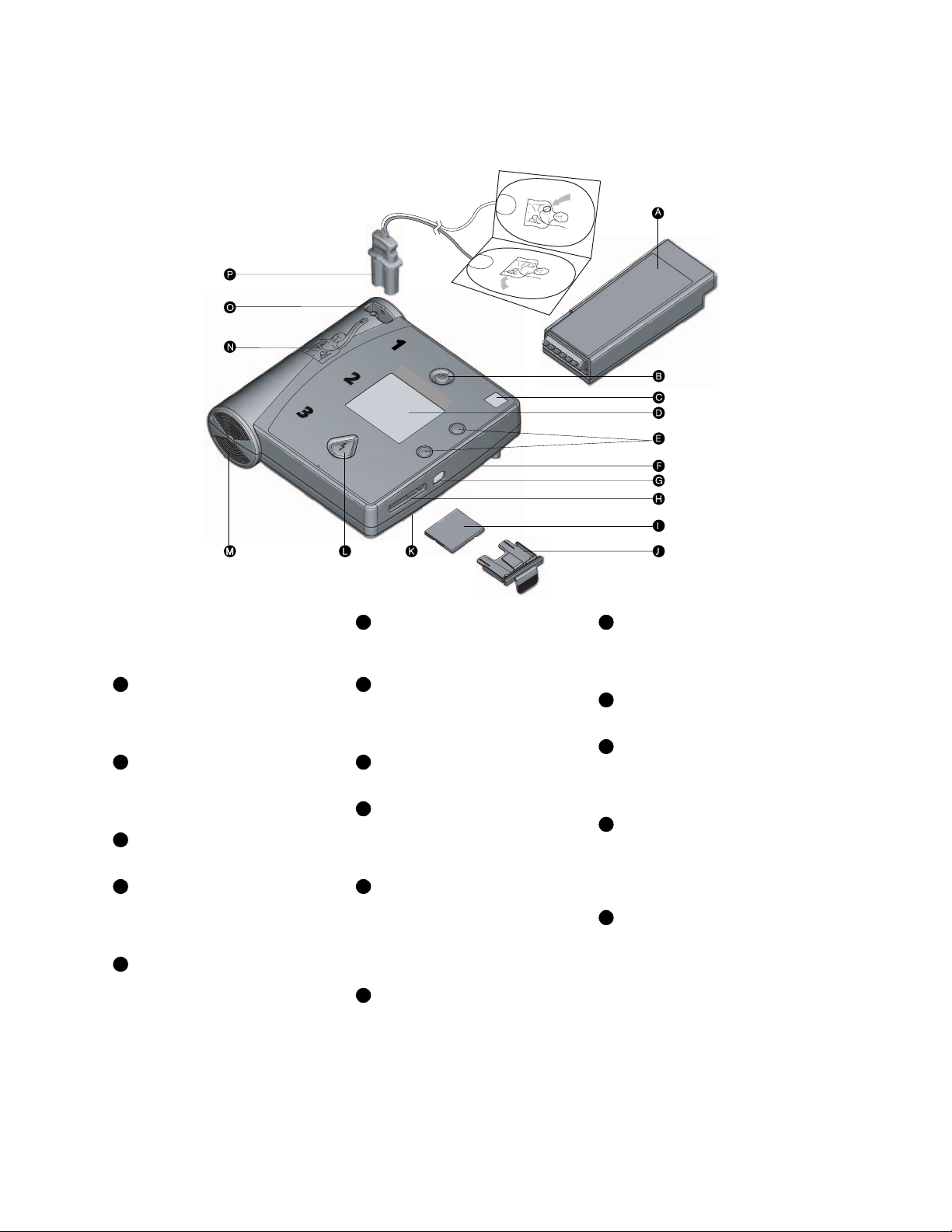
The HeartStart FR2+ Defibrillator
Clockwise from top right.
A
Battery. Standard long-life or
rechargeable battery used to power the
FR2+. (Check local regulations for
disposal and recycling requirements.)
B
On/Off button. Turns on the
FR2+ and starts voice and screen
prompts. Second press turns off the
FR2+.
C
Status Indicator. Shows you the
readiness status of the FR2+.
D
Display screen. Displays text
prompts and incident data. The FR2+
M3860A screen also displays the
patient’s ECG.
E
Option buttons. Adjust the
contrast of the screen display and
control special functions.
F
Beeper port. Broadcasts alert
beeps when required. It is located under
the right edge of the FR2+.
G
Infrared (IR) communications
port. A special lens, or “eye,” used to
transfer data directly to or from another
device.
H
Data card port. Receptacle for
data card tray.
I
Data card (optional). Used to
store and review information about an
incident, including ECG and optional
voice recording.
J
Data card tray. Special sleeve that
holds the data card and fits into the data
card port to help seal the FR2+ against
fluids. The tray should be kept installed
in the FR2+ even if no data card is
used.
K
Microphone. Used optionally to
record surrounding audio during an
incident. It is located under the right
edge of the FR2+.
L
Shock button. Controls shock
delivery. The button flashes when the
HeartStart FR2+ is ready to deliver a
shock.
M
Speaker. Amplifies voice prompts
during use of the FR2+.
N
Pads placement diagram.
Illustrates correct placement of pads.
Diagrams are also shown on the
defibrillator pads.
O
Defibrillator pads connector
socket. Receptacle for connector of the
defibrillator pads cable. An adjacent LED
light flashes to show socket location and
is covered when connector is inserted.
P
Adult defibrillator pads. Selfadhesive pads with attached cable and
connector.
Philips Medical Systems
Page 3
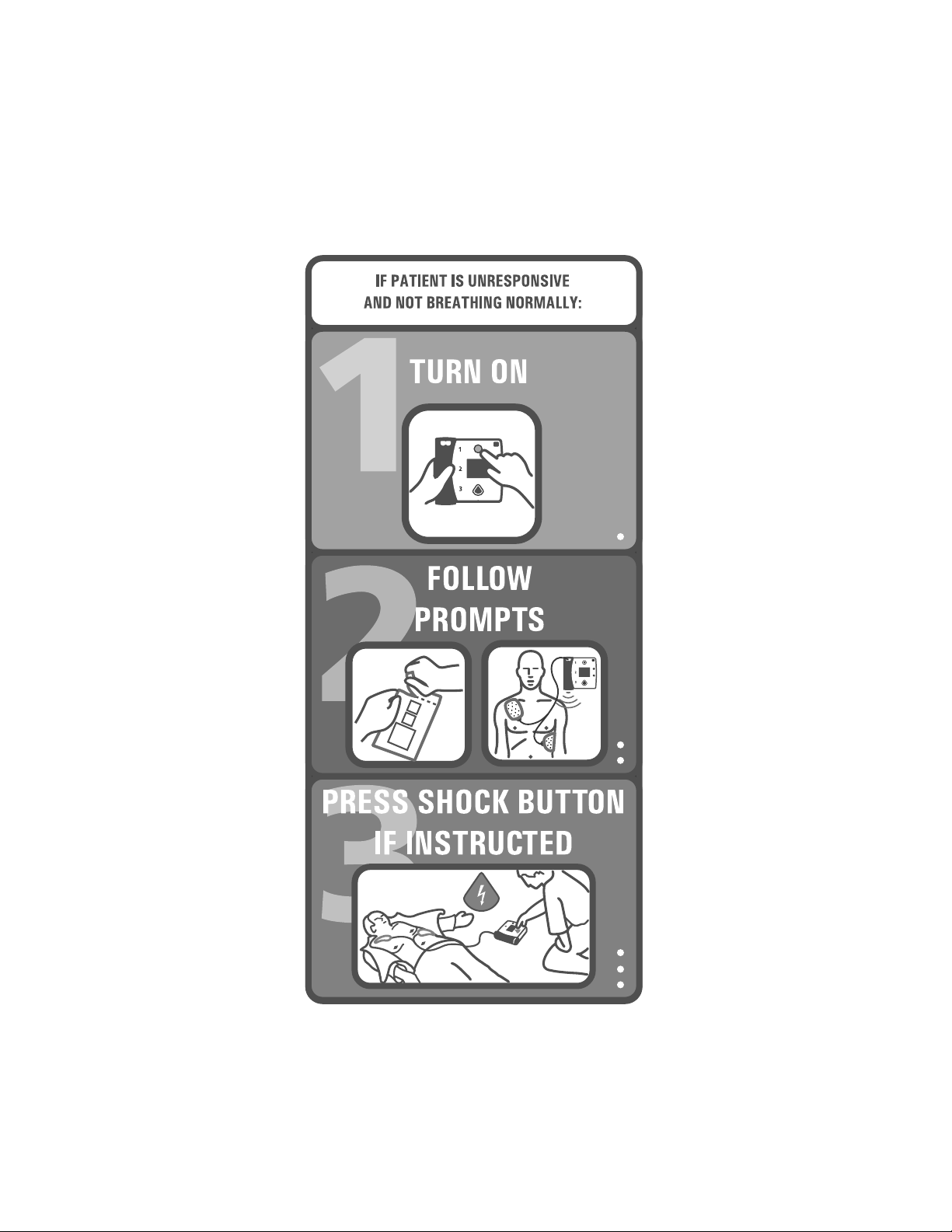
HEARTSTART FR2+ DEFIBRILLATOR
QUICK REFERENCE CARD
Philips Medical Systems
Page 4

Intentionally left blank.
Philips Medical Systems
Page 5

HEARTSTART FR2+
M3860A, M3861A Defibrillator
INSTRUCTIONS FOR USE
Edition 10
Philips Medical Systems
Page 6

Instructions for Use
Authorized EU Representative
Equipment specifications are subject to alteration
without notice. All changes will be in compliance
with regulations governing manufacture of
medical equipment.
Printed in the U.S.A.
Publication date: August 2003
Publication number: M3860-91900
Part number: 011120-0010
© 2003 Philips Electronics North America Corp.
No part of this publication may be reproduced,
transmitted, transcribed, stored in a retrieval
system or translated into any human or computer
language in any form by any means without the
consent of the copyright holder.
Unauthorized copying of this publication may not
only infringe copyright but also reduce the ability
of Philips Medical Systems to provide accurate
and up-to-date information to users and operators
alike.
Philips Medizinsysteme Boeblingen GmbH
Hewlett-Packard Strasse 2
71034 Boeblingen, Germany
(+49) 7031-14-5151
CAUTION
FEDERAL LAW (USA) RESTRICTS THIS DEVICE TO SALE
BY OR ON THE ORDER OF A PHYSICIAN.
The HeartStart FR2+ is designed to be used only with Philipsapproved accessories. The HeartStart FR2+ may perform
improperly if non-approved accessories are used.
Device Tracking
In the U.S.A., this device is subject to tracking requirements by
the manufacturer and distributors. If the defibrillator has been
sold, donated, lost, stolen, exported, or destroyed, notify Philips
Medical Systems or your distributor.
Device Manufacturer
The HeartStart FR2+ Defibrillator is manufactured by Philips
Medical Systems, Seattle, Washington, USA.
Philips Medical Systems reserves the right to
make changes in specifications or to discontinue
any product at any time without notice or
obligation and will not be liable for any
consequences resulting from the use of this
publication.
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 7

Contents
1 Introduction to the HeartStart FR2+
What is the HeartStart FR2+? ................................................................. 1-1
When Is the HeartStart FR2+ Used? .................................................... 1-2
How Does the HeartStart FR2+ Work? ................................................ 1-2
How Is the HeartStart FR2+ Supplied? ................................................ 1-3
2 Preparing Your HeartStart FR2+ for Use
Overview ....................................................................................................... 2-1
Installing the Battery ................................................................................... 2-1
Setting the Clock ........................................................................................ 2-3
Running the Battery Insertion Selftest .................................................... 2-4
Placing and Securing the HeartStart FR2+ .......................................... 2-5
3 Using Your HeartStart FR2+
Overview ....................................................................................................... 3-1
Step 1: Preparation .................................................................................... 3-2
Step 2: ECG Analysis and Monitoring ................................................... 3-3
Step 3: Shock Delivery .............................................................................. 3-4
ECG Display for Ongoing Observation ................................................. 3-5
4 Maintaining, Testing, and Troubleshooting
Your HeartStart FR2+
Overview ....................................................................................................... 4-1
Maintenance ................................................................................................. 4-1
After Using the HeartStart FR2+ ............................................................. 4-2
Cleaning the HeartStart FR2+ ......................................................... 4-3
Operator’s checklist ............................................................................ 4-3
Testing ........................................................................................................... 4-5
Battery insertion selftest .................................................................... 4-5
Periodic selftests ................................................................................. 4-9
Device history ....................................................................................... 4-9
Battery History ..................................................................................... 4-10
Troubleshooting Guide .............................................................................. 4-11
Status indicator summary .................................................................. 4-11
Philips Medical Systems
Recommended action during an emergency ................................ 4-11
Troubleshooting during patient use ................................................ 4-12
General troubleshooting .................................................................... 4-14
1
Page 8

2
5 Clinical and Safety Considerations
Clinical Considerations .............................................................................. 5-1
Indications ............................................................................................. 5-1
Contraindications ................................................................................ 5-1
Safety Considerations ................................................................................ 5-1
General dangers, warnings, and cautions ..................................... 5-2
Defibrillation warnings and cautions ............................................... 5-4
Monitoring cautions ............................................................................ 5-5
Maintenance cautions ........................................................................ 5-5
6 Setup and Advanced Mode Features
Setup Overview ........................................................................................... 6-1
Non-protocol parameters .................................................................. 6-1
Automatic protocol parameters ....................................................... 6-2
Manual override parameters ............................................................. 6-4
Using Setup Features ........................................................................ 6-5
Reviewing current setup .................................................................... 6-6
Revising setup ...................................................................................... 6-6
Receiving setup ................................................................................... 6-7
Reading setup ...................................................................................... 6-8
Sending and Receiving Clock Settings ................................................. 6-8
Using Advanced Mode Features ............................................................. 6-9
Using the manual analyze feature .................................................... 6-10
Using the manual charge feature (M3860A only) ........................ 6-11
7 Data Management and Review
Overview ....................................................................................................... 7-1
Recording Incident Data ............................................................................ 7-1
Recording data in internal memory ................................................. 7-1
Recording data on a data card ........................................................ 7-1
Reviewing Incident Data ............................................................................ 7-3
Reviewing data from internal memory ............................................ 7-3
Reviewing data from a data card ..................................................... 7-4
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 9
Appendices
A Accessories for the HeartStart FR2+
HeartStart Accessories ............................................................................. A-1
Suggested Additional Items ..................................................................... A-2
B Technical Specifications
HeartStart FR2+ Defibrillator Specifications ........................................ B-1
Accessories Specifications ...................................................................... B-6
C Glossary of Symbols and Controls
Instructions for Use ..................................................................................... C-1
HeartStart FR2+ M3860A and M3861A Defibrillator
Symbols and Controls ................................................................................ C-1
Accessories Symbols ................................................................................. C-3
D Glossary of Terms
3
Index
Philips Medical Systems
Contents
Page 10

Notes
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 11
1 Introduction to the HeartStart FR2+
What is the HeartStart FR2+?
The HeartStart FR2+ Defibrillator (“FR2+”) is an automated external
defibrillator. It is compact, lightweight, portable, and battery powered. It is
designed for simple and reliable operation by a trained responder.
NOTE: The HeartStart FR2+ is an enhanced version of the defibrillator
previously sold as the Heartstream FR2. The FR2+ has all the features of the
FR2. All accessories compatible with the FR2 are also compatible with the
FR2+. However, the FR2+ has some new features not present in the FR2
and can be used with certain accessories (labeled FR2+) that are not
compatible with the FR2.
The FR2+ has a Status Indicator that is always active, so you can tell at a
glance if it is ready for use. The front panel of the FR2+ has an On/Off button
at the top and a Shock button at the bottom. A display screen in the center of
the panel provides text prompts and incident information. Voice prompts are
provided through a speaker located at the base of the FR2+. (See the
diagram on the inside front cover for details.)
1
The FR2+ is available in two models, the M3860A and the M3861A. They
share a set of basic features, detailed in Chapter 6. The principle differences
between the two models are identified below:
model M3860A model M3861A
Configurable ECG display on screen Text prompt display on screen, no ECG
display
Configurable manual charge in
advanced mode
NOTE: The FR2+ comes with a factory default setup that can be modified.
No manual charge in advanced mode
(See Chapter 6, “Setup and Advanced Mode Features,” for a description of
Philips Medical Systems
setup defaults and options.)
1-1
Page 12

1-2
When Is the HeartStart FR2+ Used?
The HeartStart FR2+ Defibrillator is used with disposable defibrillator pads
applied to a person who is experiencing the symptoms of sudden cardiac
arrest (SCA): lack of responsiveness and lack of breathing. Defibrillation
should not be performed on anyone who is responsive or is breathing.
Infant/child reduced-energy defibrillator pads are available for use with the
FR2+ on children under the age of 8 or weighing less than 55 pounds (25
kg).
The FR2+ is intended for use by emergency care personnel who have been
specifically trained in the operation of the FR2+ or who are qualified by
training in Basic Life Support (BLS), in Advanced Life Support (ALS), or in
other physician-authorized emergency medical response.
At the discretion of emergency care personnel, the M3860A FR2+ with
ECG display enabled can also be used with the FR2+ ECG assessment
module to display the rhythm of a responsive or breathing patient, regardless
of age. The FR2+ Defibrillator used with the FR2+ ECG assessment module
provides a non-diagnostic display for attended patient monitoring. While
connected to the FR2+ ECG assessment module, the FR2+ displays and
evaluates the patient's ECG and disables its shock capability.
How Does the HeartStart FR2+ Work?
The HeartStart FR2+ Defibrillator is designed to provide external
defibrillation therapy to someone in cardiac arrest. Defibrillation therapy is
the best available way to treat a variety of potentially fatal heart arrhythmias.
The FR2+ is extremely easy to use. When connected to defibrillator pads
that are properly applied to the patient’s bare chest, the FR2+:
1. prompts you to take specific actions,
2. automatically analyzes the patient's heart rhythm and advises you
whether or not the rhythm is shockable, and
3. arms the Shock button, if appropriate, and instructs you to press it to
deliver a biphasic electric pulse designed to defibrillate the heart.
Detailed instructions for use are provided in Chapter 3.
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 13
1-3
How Is the HeartStart FR2+ Supplied?
The HeartStart FR2+ Defibrillator is supplied with a standard long-life
battery, two sets of adult defibrillator pads with integrated cable and
connector, and a data card tray. Other accessories, including an FR2+
rechargeable battery, FR2 infant/child reduced-energy defibrillator pads, and
(for M3860A only, with ECG display enabled) a three-wire FR2+ ECG
assessment module, are available. See Appendix A for a list of accessories
and other recommended supplies.
1
Philips Medical Systems
Introduction to the HeartStart FR2+
Page 14

Notes
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 15
2 Preparing Your HeartStart FR2+ for
Use
Overview
There are a few basic steps to preparing your HeartStart FR2+ Defibrillator
for use:
Install data card (optional).
Install a battery.
Set the clock in the FR2+ (optional).
Run the battery insertion selftest.
Place the FR2+ with recommended accessories in a convenient
location.
The instructions presented here briefly describe the normal sequence of
preparation. It assumes that you are using a fresh battery, that the selftest
passes, that you are not using a data card, and that the factory default
settings will not be changed. Exceptions to this sequence are provided
elsewhere in this manual.
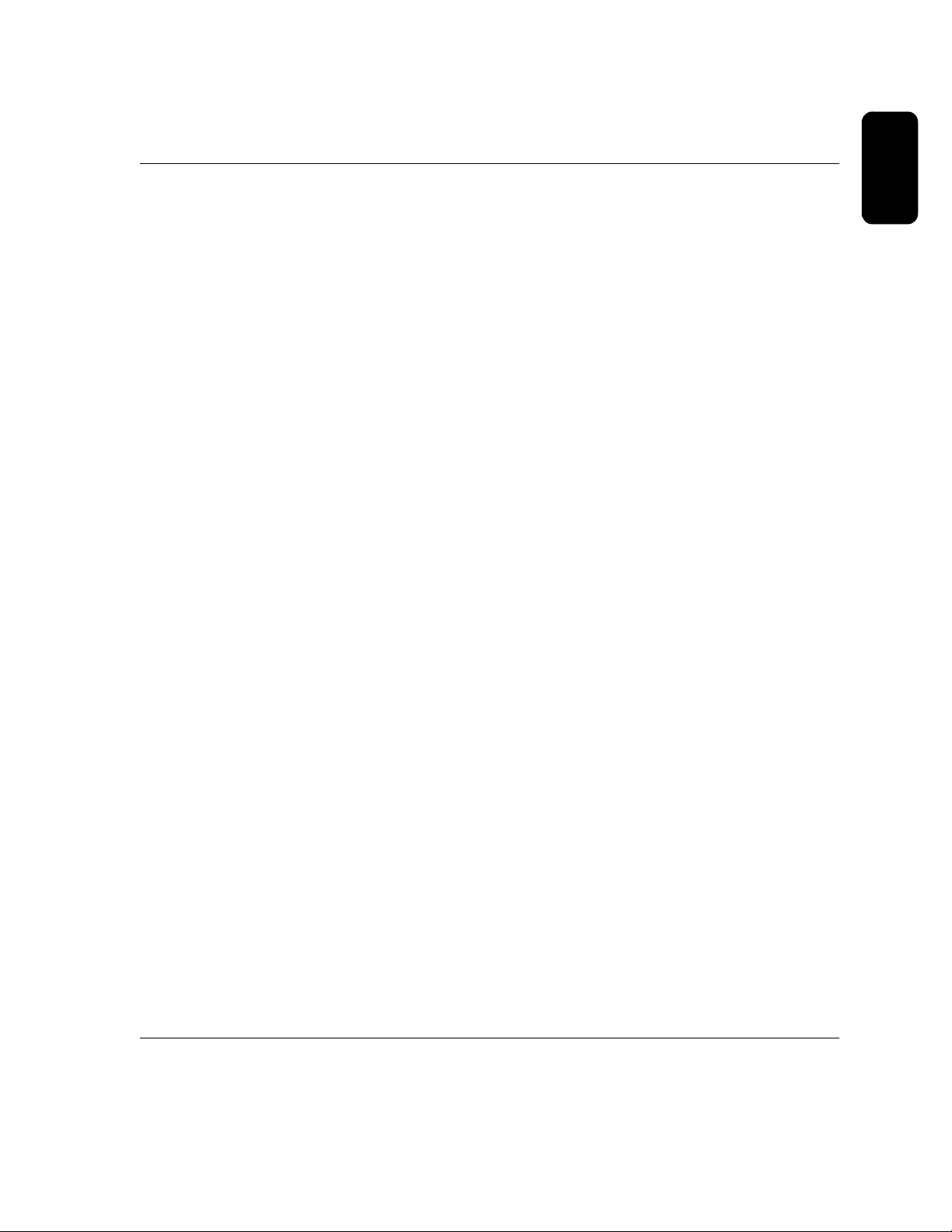
Installing the Battery
2
The HeartStart FR2+ Defibrillator is shipped with a M3863A standard,
long-life battery. The battery is enclosed in a gray plastic case. There is a
yellow latch at one end that holds the battery in place when it is correctly
installed in the FR2+. (The optional M3848A FR2+ rechargeable battery is
enclosed in a blue plastic case and also has a yellow latch. Except where
otherwise noted, the following information applies to both battery types.)
Before installing the battery, make sure the defibrillator pads are not
connected to the FR2+. To install the battery:
1. Hold the battery by the latch end and slide it into the battery
compartment at the top of the FR2+.
2. Slide the battery all the way into the opening, until the latch clicks into
place. The latch will click into place only when the battery is inserted
correctly.
Philips Medical Systems
CAUTION: Follow all instructions supplied with the HeartStart M3863A
standard battery. Install the battery before the install-by date shown on the
battery.
2-1
Page 16

2-2
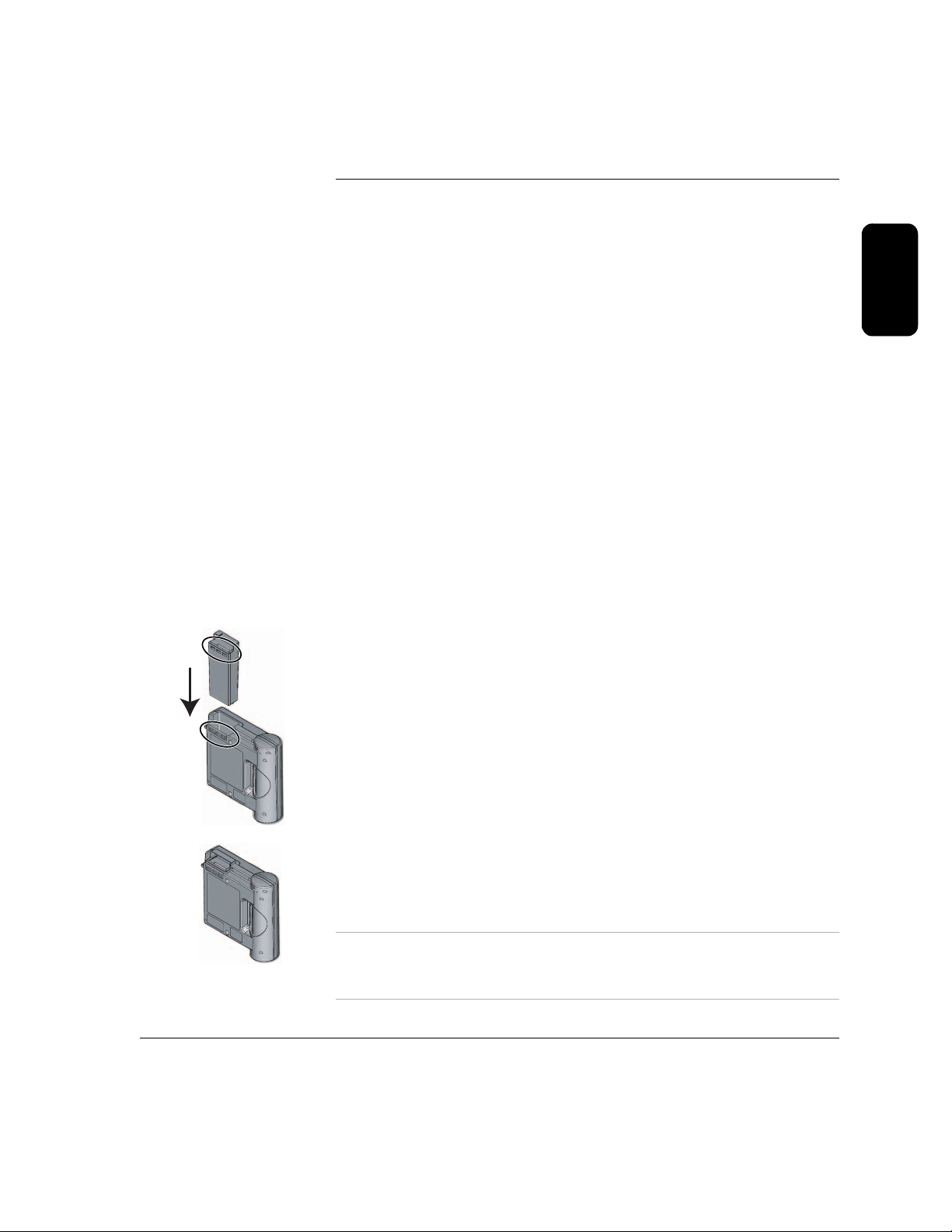
When the battery is installed, the FR2+ automatically turns on. The Status
Indicator displays a flashing black hourglass. The Shock button light and the
indicator light for the defibrillator pads connector socket turn on briefly.
The display screen brings up the main menu. From this menu, you can start
the FR2+ battery insertion selftest, review information from the last time the
FR2+ was used, or go to the next screen for other options. Information about
the optional data card and the battery status is also provided. (See Chapter
7, “Data Management and Review,” for details about reviewing an incident
and using a data card.) For the M3863A standard battery, a
GOOD BATTERY
message should be displayed. For the M3848A FR2+ rechargeable battery,
a “fuel gauge” graphic illustrates remaining power. Throughout the remainder
of this manual, the screen displays illustrated will be for the standard battery
unless otherwise noted.
NOTE: This screen will not be displayed if the FR2+ is connected to
defibrillator pads (that are applied to the patient) when the battery is inserted,
and you will not be able to access the menu items. In addition, the battery
insertion selftest and periodic automatic selftests cannot run while the
defibrillator pads are connected to the patient. Be sure to unplug the pads
connector from the FR2+ after each use. Do not store the FR2+ with the
pads connected.
NOTE: To move around the menus displayed, use the Option buttons as
follows:
• Press the
item to another on the menu.
• Press the
scroll through the settings for that item.
If you select
FR2+, review the history of the battery being used, access setup data, set
the clock, or return to the first menu. (See Chapter 4, “Maintaining, Testing,
and Troubleshooting Your HeartStart FR2+,” for details about the review
options and Chapter 6, “Setup and Advanced Mode Features,” for
information on the setup option.)
M3860A/M3861A HEARTSTART FR2+
LOWER Option button to move the highlight bar from one
UPPER Option button to select the highlighted item or to
NEXT, the menu displayed lets you review the history of the
Philips Medical Systems
Page 17
2-3
NOTE: If you make no selection for 10 seconds, the selftest will
automatically run. If you want to select something different from either of
these menus, you must do so before the selftest begins, or remove and
reinstall the battery to bring up the main menu. You can press the On/Off
button at any time to turn off the FR2+ and return it to standby (ready for
use) mode. To use the FR2+, press the On/Off button again.
Setting the Clock
It is recommended that the first time you prepare your HeartStart FR2+
Defibrillator for use, you check the FR2+’s internal clock to be sure it is set to
the correct date and local time. You can reset it if necessary.
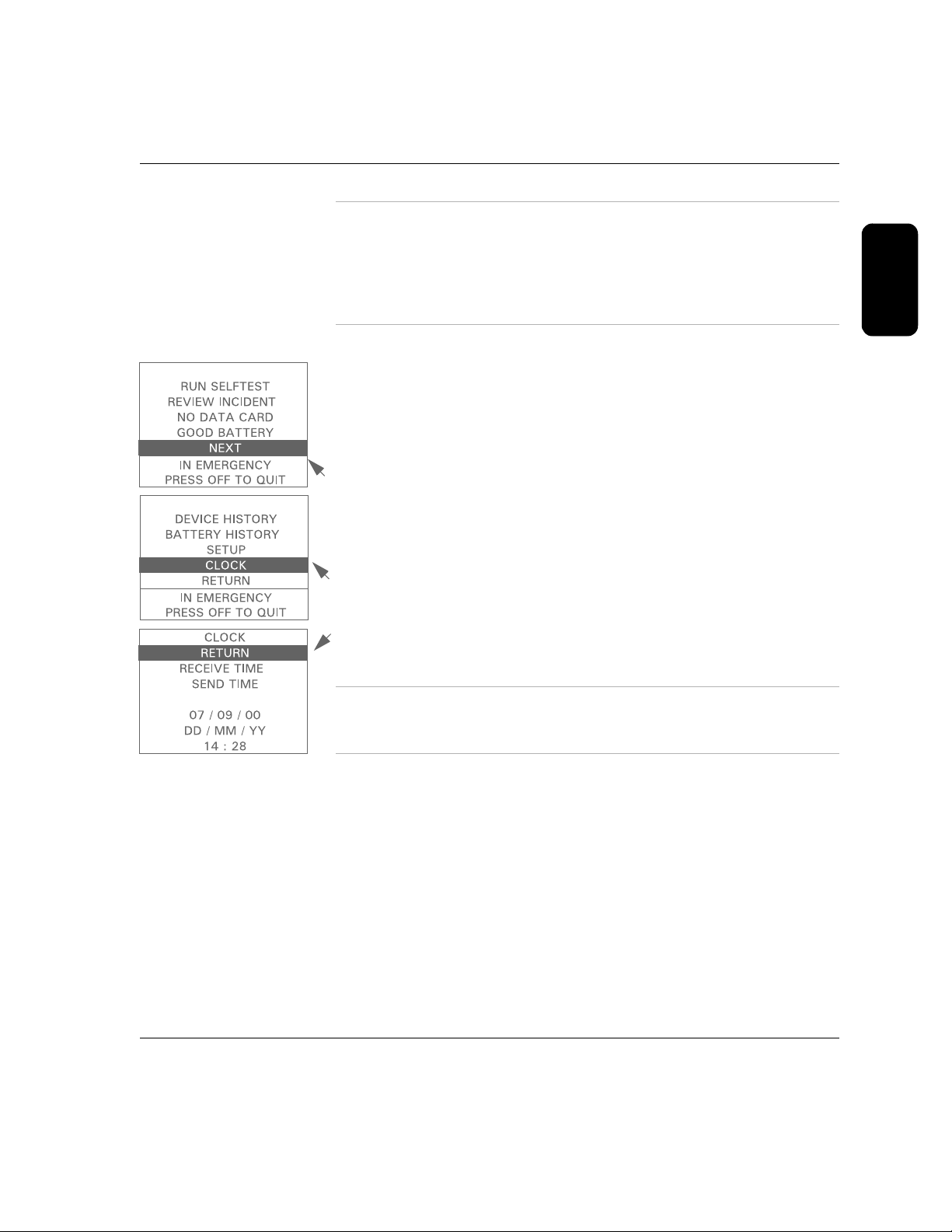
To see the clock settings, select
of installing the battery, and then select
NEXT from the first menu, within 10 seconds
CLOCK. To do this:
2
1. Press the lower Option button to move the highlight bar to
2. Press the upper Option button to bring up the
NEXT screen.
3. Press the lower Option button to move the highlight bar to
4. Press the upper Option button to bring up the
CLOCK screen displays the date and time currently set in the internal
The
CLOCK screen.
NEXT.
CLOCK.
clock of the FR2+.
NOTE: The date is displayed as day (DD), month (MM), and year (YY), as
shown on the screen. The time is displayed using the 24-hour international
clock.
If no changes to the clock settings are needed, select
RETURN and go back
to the first menu. If the date and time are not correct, there are two ways to
set them:
Receive the clock settings from another FR2+ or from a computer using
HeartStart Event Review® software,
*
using the RECEIVE TIME option.
This may be used to synchronize the clocks of several FR2+s. You can
also send the clock settings from one FR2+ to another one, using the
Philips Medical Systems
SEND TIME option. See Chapter 6, “Setup and Advanced Mode
Features,” for instructions.
Manually set the date and the time.
* HeartStart Event Review software was previously sold as CodeRunner software.
Preparing Your HeartStart FR2+ for Use
Page 18

2-4
To manually set the clock:
1. Use the lower Option button to move the highlight bar to the part of the
clock setting you want to change.
2. Press the upper Option button repeatedly to scroll through the settings
until you reach the one you want. If you go past it, keep scrolling until it
comes up again.
3. Use the lower Option button to select the next part you want to change,
and repeat the process, until all parts of the date and time have been set.
4. When you have made all the changes, move the highlight bar to
RETURN
and press the upper Option button to go back to the main menu screen.
NOTE: New clock settings are used by the FR2+ as soon as you set them.
The clock time display is updated each minute this screen is displayed. The
clock seconds, although not displayed, are set to 00 when you move the
highlight bar out of the time settings.
NOTE: If the battery is removed from the FR2+ for more than two hours, the
clock settings will be lost and must be reset.
Running the Battery Insertion Selftest
Except in an emergency, it is recommended that you run this selftest every
time you change the battery. Make sure the defibrillator pads are not
connected to the HeartStart FR2+ Defibrillator before running the battery
insertion selftest.
The selftest has two parts. The first part automatically tests the FR2+
circuitry. The second part is interactive and requires you to respond to
prompts in order to make sure the display, buttons, lights, speaker, and
beeper of the FR2+ are working properly. (See Chapter 4, “Maintaining,
Testing, and Troubleshooting Your HeartStart FR2+,” for details about this
selftest.)
To run the selftest:
1. Make sure the defibrillator pads are not connected to the device.
2. Insert the battery into the battery port. The first screen displayed has
RUN SELFTEST highlighted.
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 19
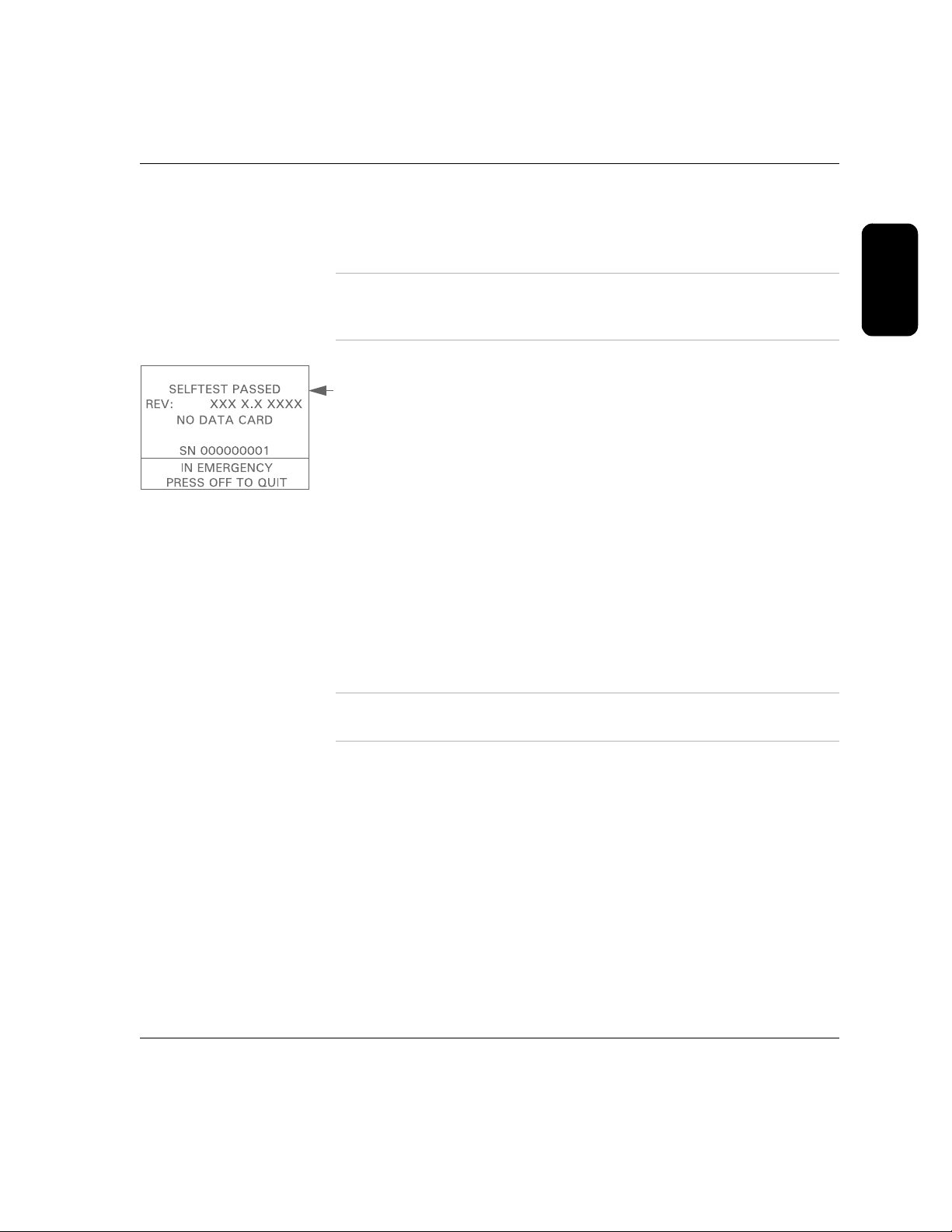
3. Press the upper Option button to activate the test.
OR make no selection for 10 seconds, and the selftest will start
4.
automatically if the FR2+ has been turned off for at least 5 minutes.
2-5
GOOD BATTERY
NOTE: If you connect defibrillator pads (that are applied to the patient) to
the FR2+ during a battery insertion selftest, the selftest will stop and the
FR2+ will go to its standby mode to be ready for use.
When the automatic part of the selftest is successfully completed, the screen
displays a message that the test has passed, and then automatically starts
the interactive part of the selftest. It is important to press the buttons and
verify the indicators to ensure that the FR2+ will be ready for use.
When the entire selftest is complete, the FR2+ automatically turns off and
returns to standby mode. In the standby mode, the Status Indicator displays
a flashing black hourglass. This means that the FR2+ has passed its most
recent self-test and is therefore ready for use, simply by pressing the On/Off
button to turn it on.
Placing and Securing the HeartStart FR2+
Place the HeartStart FR2+ Defibrillator in an accessible area with the Status
Indicator easily visible. Useful accessories for placing and securing the FR2+
include a carrying case, which is suitable for use with a wall mount bracket or
defibrillator cabinet. (See Appendix A for a list of accessories.)
NOTE: Do not store the FR2+ with the defibrillator pads attached. Do not
open the pads package until ready for use.
2
With the battery installed and the FR2+ stored in appropriate environmental
conditions, the FR2+ performs detailed periodic selftests to make sure that it
remains ready for use. (See Appendix B for the environmental storage
specifications.)
While the FR2+ is in the standby mode, the Status Indicator shows the
flashing black hourglass unless the periodic selftests detect a problem. If a
problem is detected, the Status Indicator will show a flashing red X or a solid
Philips Medical Systems
red X and the FR2+ will beep (“chirp”) to alert you to the need for
troubleshooting. (See Chapter 4, “Maintaining, Testing, and Troubleshooting
Your HeartStart FR2+,” for instructions.)
Preparing Your HeartStart FR2+ for Use
Page 20

Notes
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 21
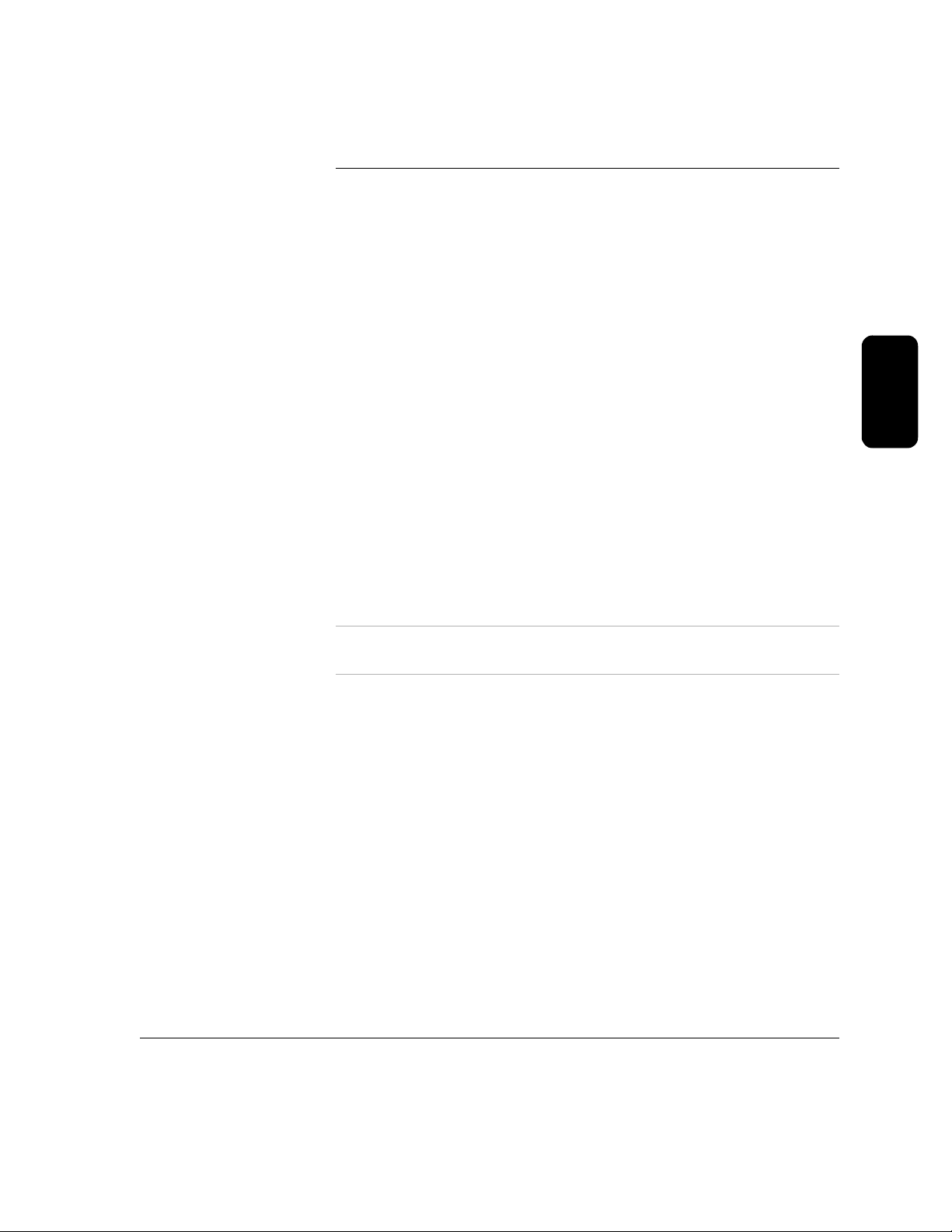
3 Using Your HeartStart FR2+
Overview
This chapter describes how to use the HeartStart FR2+ Defibrillator in an
emergency incident. Some general things to remember are:
Try to relax and stay calm. The FR2+ automatically provides appropriate
voice and display prompts to guide you.
The defibrillator pads must have good contact with the patient’s skin.
The pads have a layer of sticky, conductive gel beneath the protective
backing. To work effectively, the gel must not be dried out.
It may be necessary to dry the patient’s skin or to clip or shave excessive
chest hair to provide good contact between the defibrillator pads and
the patient’s skin.
The following pages provide step-by-step instructions for normal use of the
FR2+ in an emergency. (See Chapter 4, “Maintaining, Testing, and
Troubleshooting Your HeartStart FR2+,” for troubleshooting tips.)
IMPORTANT: Be sure to read the Warnings and Cautions on the last page
of this chapter.
NOTE: These directions apply to both the model M3860A and the model
M3861A FR2+, except where otherwise noted.
3
Philips Medical Systems
3-1
Page 22
3-2
Step 1: Preparation
O
N
O
•
F
F
Press the On/Off button to turn on the HeartStart FR2+ Defibrillator.
Follow the instructions provided by the FR2+ voice and screen prompts
in the order indicated.
Remove clothing from the patient's upper body. Wipe moisture from the
patient's skin and clip or shave excessive chest hair, if necessary.
If the patient appears to be under eight years of age or 55 lbs (25 Kg), use
M3870A FR2 infant/child reduced-energy defibrillator pads, if available. If the
child appears older/larger, use adult defibrillator pads.
TREATMENT TO DETERMINE THE CHILD’S EXACT AGE/WEIGHT.
DO NOT DELAY
Open the defibrillator pads package. Check to see that the pads and
attached cable and connector are undamaged. Pull off the protective
backing from the defibrillator pads and check that the gel has not dried out. If
the pads are damaged or the gel has dried out, use a new set of pads.
Place each pad on the patient. The pads must be placed with the sticky side
on the patient’s skin.
IMPORTANT: Refer to the drawing on each pad for
correct positioning. For adult patients, one pad goes just below the
patient's right collarbone, and the other one goes over the patient’s ribs in
line with the armpit and below the left breast. For children under eight years
old, one pad is centered on the chest between the nipples, and the other on
the back between the scapulae (shoulder bones).
Connect the pads to the FR2+. Insert the defibrillator pads connector firmly
into the connector socket. A flashing light shows you where the socket is
located, at the top left of the FR2+.
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 23

3-3
Step 2: ECG Analysis and Monitoring
Follow the instructions provided by the HeartStart FR2+ Defibrillator’s
voice and screen prompts in the order indicated.
As soon as the FR2+ detects that the defibrillator pads are connected
properly, it automatically begins analyzing the patient’s heart rhythm. Do not
touch the patient during rhythm analysis. The M3860A FR2+ can display the
patient’s ECG on the screen. When the ECG display is enabled, the
patient’s heart rate is also displayed during background monitoring and when
the advanced mode is entered.
If no shock is advised, the FR2+ provides voice and screen prompts to tell
you so. The FR2+ instructs you to perform CPR if needed, and performs
background monitoring of the patient’s ECG while you give appropriate care
to the patient. These instructions are repeated at the programmed Monitor
Prompt interval (the default interval is one minute) while the FR2+ is
monitoring the patient.
NOTE: CPR may interfere with background monitoring. During CPR,
periodically pause for 15 seconds to check the patient and allow the FR2+
to analyze the patient’s heart rhythm without CPR artifact.
3
Monitoring continues until and unless the FR2+ detects a change in the
patient’s heart rhythm that may be a shockable rhythm, detects interference
with rhythm analysis, or is turned off.
If the FR2+ detects a potentially shockable heart rhythm while monitoring, it
automatically goes back to analyzing the rhythm to see if a shock is advised.
If a shock is advised, the FR2+ charges to prepare for shock delivery. It
gives the voice warnings and screen prompts to tell you that a shock is
advised. Make sure that no one is touching the patient or the pads. While the
FR2+ is charging, it continues to analyze the patient’s heart rhythm. If the
rhythm changes and a shock is no longer appropriate, the FR2+ disarms.
Voice and display prompts advise you what action to take.
NOTE: When the FR2+ is fully charged, you can disarm it at any time by
Philips Medical Systems
pressing the On/Off button to turn off the FR2+ and return it to standby
mode. (See the Defibrillator discussion in Appendix B, “Technical
Specifications,” for details on disarming the FR2+.)
Using Your HeartStart FR2+
Page 24
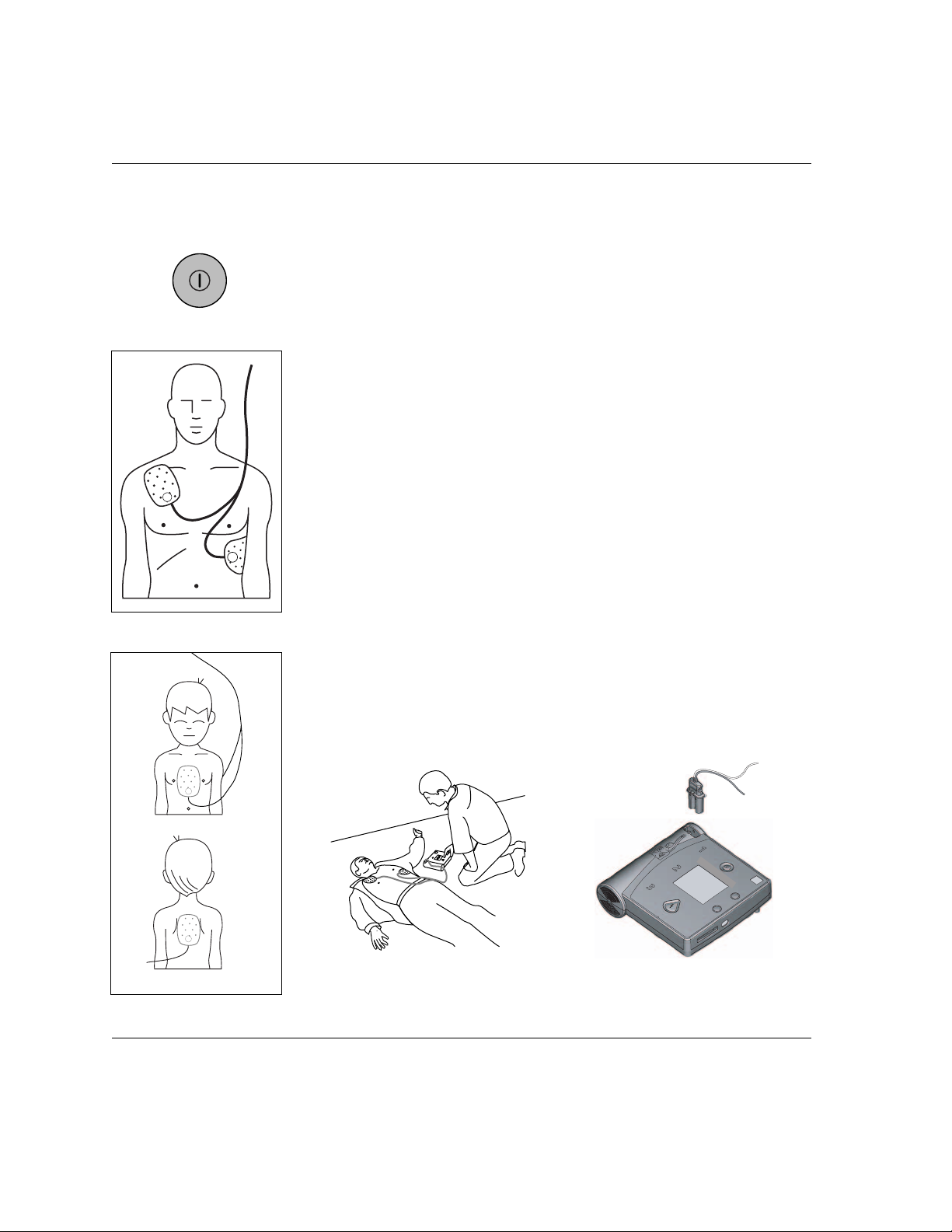
3-4
Step 3: Shock Delivery
Press the Shock button to deliver the shock.
IMPORTANT: You must press the button for a shock to be delivered. The
HeartStart FR2+ Defibrillator will not automatically deliver a shock.
There are four ways you can tell that the FR2+ is ready to deliver a shock:
you hear a voice prompt telling you to deliver a shock,
you see the Shock button flashing,
you hear a steady tone, and/or
you see a screen prompt telling you to press the orange (Shock)
button.
After you press the Shock button, a voice prompt tells you the shock was
delivered. Then FR2+ goes back to analyzing the patient’s heart rhythm to
see if the shock was successful. The FR2+ continues to provide voice and
text prompts to guide you through additional shocks, if appropriate.
NOTE: If you do not press the Shock button within 30 seconds of being
prompted, the FR2+ will disarm itself and provide a pause. The device will
resume analyzing after 30 seconds or when the Resume Analyzing key is
pressed.
Pause for CPR. After the programmed number of shocks in a shock series
are delivered, the FR2+ automatically pauses for a programmed amount of
time to allow you to perform CPR. After the voice and screen prompts tell
you that the FR2+ has paused, there are no further voice prompts during the
rest of the pause, so that you can provide uninterrupted patient care.
During the pause, the FR2+ screen shows a bar that fills in as the pause time
is used up. The screen also shows how much time has gone by since the
FR2+ was turned on,* and how many shocks have been delivered. The
M3860A FR2+ also displays the ECG, if enabled, during this period.
* The FR2+ displays elapsed time to a maximum of 99:59 minutes. If the elapsed time
of use extends beyond this figure, the minutes are represented by “??” but the
seconds are displayed. However, total elapsed time will be recorded on an installed
data card for later review with HeartStart Event Review data management software.
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 25

3-5
ECG Display for Ongoing Observation
At the discretion of emergency care personnel, the M3860A FR2+ with
ECG display enabled can also be used with the M3873A/M3874A FR2+
ECG assessment module. The FR2+ used with the FR2+ ECG assessment
module provides a non-diagnostic ECG display of the patient’s heart rhythm
for attended patient monitoring. The system is intended for use on a
conscious or breathing patient, regardless of age. While connected to the
FR2+ ECG assessment module, the FR2+'s shock capability is disabled,
but the FR2+ continues to evaluate the patient's ECG. The module is
designed for connection to ECG electrodes per AAMI (M3873A) or IEC
(M3874A) color convention.
There are no known contraindications to use of the FR2+ ECG assessment
module.
The module’s colored leadwires are connected to ECG electrodes, which
are then placed on the patient’s bare chest, and the module’s device
connector is inserted in the FR2+’s connector socket.
NOTE: It is not necessary to turn the FR2+ Defibrillator off prior to
connecting the ECG assessment module.
3
Once connected, the FR2+ displays and evaluates the patient's ECG (Lead
II). Follow all prompts from the FR2+. If a data card is used when the module
is connected to the FR2+, all recorded events can be viewed using
HeartStart Event Review data management software
*
on a personal
computer.
Check the patient if:
indicated by the observed ECG display,
the patient becomes unresponsive or stops breathing, or
the FR2+ prompts IF NEEDED, ATTACH DEFIBRILLATION PADS.
If appropriate, unplug the ECG assessment module from the FR2+, attach
the defibrillator pads to the patient, and connect the defibrillator pads to the
FR2+. Verify that the defibrillator pads are at least one (1) inch (2.5 cm)
away from the ECG electrodes.
Philips Medical Systems
The M3873A/M3874A FR2+ ECG assessment module contains no latex
rubber. It is reusable (see the expiration date on the module) and can be
cleaned with a soft cloth dampened with any of the agents recommended for
* HeartStart Event Review software was previously sold as CodeRunner software.
Using Your HeartStart FR2+
Page 26

3-6
cleaning the FR2+ Defibrillator. (See Chapter 4, “Maintaining, Testing, and
Troubleshooting Your HeartStart FR2+.”)
WARNING: During defibrillation, air pockets between the skin and
defibrillator pads can cause patient skin burns. To help prevent air pockets,
make sure defibrillator pads completely adhere to the skin. Do not use
dried-out defibrillator pads.
WARNING: Do not let the defibrillator pads touch each other or other ECG
electrodes, lead wires, dressings, transdermal patches, etc. Such contact
can cause electrical arcing and patient skin burns during defibrillation and
may divert defibrillating current away from the heart.
WARNING: Handling or transporting the patient during heart rhythm
analysis can cause an incorrect or delayed diagnosis. If the FR2+ gives a
SHOCK ADVISED prompt, keep the patient as still as possible for at least 15
seconds so the FR2+ can reconfirm the rhythm analysis before a shock is
delivered.
WARNING: CPR rates significantly above 100 compressions per minute
can cause incorrect or delayed analysis by the FR2+.
WARNING: Defibrillation current can cause operator or bystander injury.
Do not touch the patient during defibrillation. Do not allow the defibrillator
pads to touch any metal surfaces. Disconnect the pads connector from the
FR2+ before using any other defibrillator.
CAUTION: Aggressive handling of the pads in storage or prior to use can
damage the pads. Discard the defibrillator pads if they become damaged.
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 27
4 Maintaining, Testing, and
Troubleshooting Your HeartStart FR2+
Overview
This chapter provides information on HeartStart FR2+ Defibrillator
maintenance, detailed descriptions of the selftests, and a guide to
troubleshooting.
Maintenance
Maintenance of the FR2+ is very simple, but it is a very important factor in its
dependability. The FR2+ performs many maintenance activities itself. These
include daily and weekly selftests to verify readiness for use and more
detailed monthly selftests that also verify the shock waveform delivery
system. In addition, a detailed selftest is run whenever a battery is installed in
the FR2+.
The FR2+ requires no calibration or verification of energy delivery. The FR2+
has no user-serviceable parts.
CAUTION: Improper maintenance may damage the FR2+ or cause it to
function improperly. Maintain the FR2+ only as described in this User's
Guide or as designated by your program's Medical Director.
4
CAUTION: Electrical shock hazard. Dangerous high voltages and currents
are present. Do not open the FR2+, remove its covers, or attempt repair.
There are no user-serviceable components in the FR2+. The FR2+ should be
returned to an authorized service center for repair.
The following table presents a schedule of suggested maintenance for the
FR2+. Different frequency intervals may be appropriate, depending upon the
environment in which the FR2+ is used. The required maintenance frequency
is at the discretion of your program’s Medical Director.
Philips Medical Systems
4-1
Page 28

4-2
daily monthly maintenance task/response
Check the Status Indicator.
If you see the flashing black hourglass: The FR2+ is
ready to use. No action required.
If you see anything other than a flashing black
hourglass, remove and reinstall the battery to run the
selftest.
• If the selftest passes and the Status Indicator
shows the flashing black hourglass, the FR2+ is
ready to use.
• If the selftest fails, install a new battery and run the
selftest. If the selftest passes, the FR2+ is ready to
use. If the selftest fails, contact Philips Medical
Systems.
Check supplies, accessories, and spares for
damage and expiration dating.
Do not use damaged or expired accessories. Replace
them immediately.
LOW BATTERY or REPLACE BATTERY message is
If a
displayed: Replace the battery and run the selftests.
DO NOT ATTEMPT TO CHARGE THE M3863A FR2
STANDARD BATTERY. It is not rechargeable. The
M3848A FR2+ battery is rechargeable. Recharge it,
using the M3849A Charger, for the FR2+
rechargeable battery only.
Check the outside of the FR2+ and the connector
socket for cracks or other signs of damage.
If you see signs of damage: Contact Philips Medical
Systems for technical support.
After Using the HeartStart FR2+
After each use of the FR2+, perform the maintenance tasks described in the
table above, as well as the following post-use checks before returning the
FR2+ to service:
Check the operation of the FR2+ by removing and reinstalling the
battery and running the battery insertion selftest.
when replacing expired defibrillator pads.
Check the outside of the FR2+ and the connector socket for signs of
dirt or contamination. If the FR2+ is dirty or contaminated, clean it
according to the guidelines provided in this manual.
Check the data card if one has been used. If the data card has been
used to record incident data, remove and replace it with a blank data
card. Deliver the recorded data card to appropriate personnel according
to your local guidelines and medical protocol.
M3860A/M3861A HEARTSTART FR2+
NOTE: Perform also
Philips Medical Systems
Page 29
4-3
Check the connector socket to make sure that defibrillator pads are
disconnected from the FR2+ when it is not in use.
Check to make sure the data card tray is installed, even if a data card
is not being used.
Cleaning the HeartStart FR2+
The outside of the FR2+, including the defibrillator pads connector socket,
can be cleaned with a soft cloth dampened in one of several appropriate
cleaning agents (see list below). The following guidelines include some
important reminders:
Do not immerse the FR2+ in fluids.
Make sure a battery (or the M3864A training & administration pack) and
a data card tray are installed when cleaning the FR2+, to keep fluids out
of the device.
Do not use abrasive materials, cleaners, strong solvents such as acetone
or acetone-based cleaners, or enzymatic cleaners.
Clean the FR2+ and the connector socket with a soft cloth dampened
with one of the cleaning agents listed below.
— Isopropyl alcohol (70% solution)
—Soapy water
— Chlorine bleach (30 ml/l water)
— Ammonia-based cleaners
— Glutaraldehyde-based cleaners
— Hydrogen peroxide
4
CAUTION: Do not immerse any portion of the FR2+ in water or other fluids.
Do not allow fluids to enter the FR2+. Avoid spilling any fluids on the FR2+
or accessories. Spilling fluids into the FR2+ may damage it or present a fire
or shock hazard. Do not sterilize the FR2+ or accessories.
Operator’s checklist
The checklist on the following page is for your reference. You may want to
photocopy it or use it as the basis for creating your own checklist.
Inspect the FR2+ as suggested in the maintenance schedule above, or as
specified by your Medical Director. When you use the Checklist, fill in the
Philips Medical Systems
scheduled frequency intervals you will be using for your maintenance
inspections.
Check off each requirement as you complete it, make a note of any problems
you found or corrective action you took, and sign the form.
Maintaining, Testing, and Troubleshooting Your HeartStart FR2+
Page 30
4-4
OPERATOR'S CHECKLIST
HeartStart FR2+ Model No.: ______________________Serial No.: __________________________________
HeartStart FR2+ Location or Vehicle ID: _______________________________________________________
date
scheduled frequency
HeartStart FR2+
Clean, no dirt or contamination;
no signs of damage
Supplies Available
• Two sets defibrillator pads,
sealed, undamaged, within
expiration date
• Ancillary supplies (hand
towel, scissors, razor, pocket
mask, gloves)
• Spare M3863A battery,
within “Install Before” date
• Data cards, undamaged, and
spare data card tray
Status Indicator
Shows alternating
hourglass/square; selftest
passed.
Inspected by
Signature or initials of operator
completing the maintenance
inspection
Remarks, Problems,
Corrective Actions
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 31

4-5
Te s t i n g
The HeartStart FR2+ Defibrillator has several ways of testing itself and
alerting you if it finds a problem. In addition to the selftest performed each
time a battery is installed, the FR2+ also automatically performs periodic
selftests daily.
NOTE: The FR2+ selftests are designed to check that the FR2+ is ready for
use. However, in the event that the FR2+ has been dropped or mishandled, it
is recommended that the battery be removed and reinstalled to initiate a
selftest. If the FR2+ has visible signs of damage, contact Philips Medical
Systems for technical support.
Battery insertion selftest
As described in Chapter 2, “Preparing Your HeartStart FR2+ for Use,” when
4
you insert the battery in the FR2+, be sure that neither the defibrillator pads
nor the FR2+ ECG assessment module are connected to the device. When
you insert the battery, a menu is displayed and a two-part selftest will run
unless you make another selection from the menu within 10 seconds. The
selftest includes an automatic part and an interactive part.
NOTE: Under certain circumstances, the behavior of your FR2+ will be
different.
For example, the menu screen will not appear when a battery is inserted if:
• the defibrillator pads are attached to a patient, indicating that the
FR2+ is in continued use,
• the FR2+ ECG assessment module is connected to the FR2+, or
• the battery is completely depleted.
The menu screen will be displayed, but after 10 seconds the FR2+ will go to
standby mode if you make no selection and:
• less than five minutes have passed since the FR2+ was last used,
indicating that the FR2+ is still in use.
It is recommended that the full selftest (including the interactive portion) be
run under the following circumstances:
Philips Medical Systems
When the FR2+ is first put into service and following each use.
Whenever the battery is replaced.
Whenever expired defibrillator pads are replaced during periodic
maintenance.
Whenever the defibrillator may have sustained physical damage.
Maintaining, Testing, and Troubleshooting Your HeartStart FR2+
Page 32

4-6
When you install the battery, the screen tells you whether or not a data card
is installed. If so, a screen message displays how much recording time is left
until the data card is full. (See Chapter 7, “Data Management and Review,” for
how to review the incident information from the internal memory of the FR2+
or from a data card, if one is used.)
NOTE: The data card is typically capable of storing a number of incidents.
However, it is recommended that it be replaced after every use. In the unlikely
event that the card fills up during an incident, no further data can be
recorded, so it is important for you to monitor the
CARD FULL IN... information
on this screen.
Screen contrast can be adjusted during the battery insertion selftest by using
the Option buttons.
If battery power is low, replace the battery. If a previous selftest has failed, the
screen displays a message that the FR2+ must pass a selftest before being
used.
It is recommended that you always have a spare battery available. However, if
a screen display prompts you to replace the battery or the Status Indicator
shows a flashing red X, but you do not have a spare battery, you can
continue to use the FR2+ until the battery is completely depleted. This may
be necessary in an emergency.
NOTE: It is recommended that the M3848A FR2+ rechargeable battery not
be used as a spare or backup battery.
NOTE: If you connect defibrillator pads (that are applied to the patient) or
the FR2+ ECG assessment module to the FR2+ during a battery insertion
selftest, the selftest will stop and the FR2+ will go to its standby mode to be
ready for use.
During the automatic part of the selftest, the screen displays a bar that fills in
as the test continues. When that part of the test is finished, the FR2+ beeps.
The results of the selftest are automatically recorded on the data card while
the tests are running, if a data card was inserted in the FR2+ prior to
installing the battery.
If the automatic part of the selftest fails:
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 33

4-7
The screen displays a message that the selftest has failed. After a short
time, an error code is displayed. Write down the error code and contact
Philips Medical Systems for technical support.
The Status Indicator shows a flashing or solid red X.
Replace the battery with a new battery and repeat the test. If the second
selftest fails, contact Philips Medical Systems for technical support.
If the automatic part of the selftest passes:
The screen displays a message that the selftest passed, then begins the
interactive part of the test.
The interactive part of the selftest requires you to respond to prompts in
order to make sure the display, buttons, lights, and speaker on the FR2+ are
working properly.
Screen prompts guide you through a series of steps in the interactive part of
the selftest. Some ask you to observe that a feature of the FR2+ works
properly. Others ask you to take certain actions — for example, to press a
button. The screen then displays a message showing that the button’s
operation has been verified. If you do not press the button, or if you do but
the button is not working, the screen displays a message that the button’s
function is not verified.
4
It is important to press the buttons and verify the indicators to ensure that the
FR2+ will be ready for use. If something does not work correctly — for
example, if lights do not come on or you do not hear beeps when expected —
make a note of the problem and contact Philips Medical Systems for
technical support.
Philips Medical Systems
NOTE: Do not use the FR2+ until all parts of the interactive selftest verify
correct performance. Be sure to note and report any problems you find.
Maintaining, Testing, and Troubleshooting Your HeartStart FR2+
Page 34

4-8
The following table describes the parts of the FR2+ tested in the interactive
part of the selftest and any action you are asked to take.
feature test description
speaker
lights
option buttons
display screen
Screen prompt: CHECK SPEAKER SOUND (2 beeps)
> Listen for the two beeps from the speaker.
Screen prompt: CHECK SHOCK BUTTON LIGHT AND PADS CONNECTOR LIGHT
> Check that the lights come on.
Screen prompt: PRESS THE OPTION BUTTONS
> Press the blue upper and lower Option buttons and listen for a beep to
confirm each press. Look at the screen to be sure the button presses
have been verified.
Screen prompt: CHECK DISPLAY. ADJUST CONTRAST IF NEEDED
> Check the test pattern displayed on the screen. Adjust the contrast if
desired using the Option buttons.
NOTE: Screen contrast can be adjusted at any time during the interactive
selftest by repeatedly pressing the appropriate Option button until
desired contrast is achieved.
shock button
Screen prompt: PRESS THE SHOCK BUTTON
> Press the Shock button and listen for a beep to confirm the press.
No shock will be delivered when you press the Shock button during the
test.
> Look at the screen to be sure the button press has been verified.
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 35

feature test description
4-9
on/off button
Screen prompt: PRESS THE ON/OFF BUTTON
> Press the On/Off button and listen for a beep to confirm press.
> Look at the screen to be sure the button press has been verified.
The screen then displays a message that the test is complete.
When the interactive part of the battery insertion selftest is complete, the
FR2+ turns off and goes to standby mode to be ready for use.
If proper operation of all features has not been verified in the interactive
selftest, you may want to rerun the battery insertion selftest. If a feature of
operation cannot be verified, contact Philips Medical Systems for technical
support.
Periodic selftests
In addition to the battery insertion selftest, the FR2+ automatically performs
periodic selftests (PSTs). These daily, weekly, and extensive monthly selftests
check many important functions of the FR2+, including battery capacity and
internal circuitry.
If it detects a problem during one of these periodic selftests, the FR2+
beeps and displays a flashing red X or a solid red X on the Status Indicator.
Device history
4
The FR2+ stores key information about its history in internal memory. To
review the history of your FR2+, select NEXT from the menu screen
displayed when you insert the battery, then select
DEVICE HISTORY from the
next menu displayed.
The device history information is read from the internal memory of the FR2+.
It includes:
USES — how many times the FR2+ has been used (shown in the left
column of numbers) and the total time in minutes it has been used
(shown in the right column of numbers);
Philips Medical Systems
SHOCKS — the total number of shocks it has delivered;
TRAINING — how many times it has been used with the training &
administration pack for training (left column) and the total time in minutes
it has been used for training (right column); and
Maintaining, Testing, and Troubleshooting Your HeartStart FR2+
Page 36

4-10
TESTS — how many tests have been run. Four figures are shown: daily
(upper left), weekly (upper right), and monthly (lower left) periodic
selftests, and battery insertion selftests (lower right).
REV — device language, model, and software revision.
Battery History
Information about use of the battery currently installed in your FR2+ is also
available. To review the history of the battery, select
screen displayed when you insert the battery, then select
from the next menu displayed.
The battery history information is read from the internal memory of the battery.
It includes:
USE MINUTES — the total operating time (in minutes), including selftest
time, for this battery;
CHARGES — the total number of full defibrillation charges that have been
provided by this battery, including selftest charges;
BATTERY — a GOOD BATTERY (M3863A) or a fuel gauge display
(M3848A) showing 25%, 50%, 75% or 100%, or a
REPLACE BATTERY message, as appropriate.
STATUS — the current status of this battery, displayed in a binary code.
Make a note of this code if technical service is needed.
NEXT from the menu
BATTERY HISTORY
LOW BATTERY or
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 37

4-11
Troubleshooting Guide
Status indicator summary
status indicator meaning
Flashing black hourglass The FR2+ passed the battery insertion self-test or the last periodic self-test and is
therefore ready for use.
Flashing red X
accompanied by a chirping
sound.
Solid red X The battery is completely depleted or a self-test failure occurred.
A self-test error has occurred or the battery is low or depleted.
NOTE: Perform CPR (if needed) any time there is a delay before the FR2+
can be used.
Recommended action during an emergency
If the status indicator displays the flashing black hourglass, follow all voice
and screen prompts.
The HeartStart FR2+ Defibrillator is designed to continue working even if the
status indicator displays a flashing red X, although the device may not
perform to all of its specifications. Voice and text prompts should be followed
whenever they are given. If for any reason you cannot hear voice prompts
during use of the defibrillator, periodically check the device screen for text
prompts.
NOTE: After completing emergency use of the FR2+, if you are unable to
clear the problem as described in this Troubleshooting section, and the
Status Indicator does not show the flashing black hourglass, contact Philips
Medical Systems for technical support.
4
In the unlikely event that the device becomes unresponsive during use:
Philips Medical Systems
1. cycle power (press the On/Off button once, wait one second, then press
it again), or
2. remove and reinstall the battery (use a new M3863A FR2 standard
battery, if available, or a charged M3848A FR2+ rechargeable battery).
Maintaining, Testing, and Troubleshooting Your HeartStart FR2+
Page 38

4-12
If neither of these actions clears the problem, do not use the FR2+. Attend to
the patient, providing CPR if needed, until emergency medical personnel
arrive.
Troubleshooting during patient use
symptom possible cause recommended action
STATUS INDICATOR: FLASHING RED X
Screen and voice prompts:
LOW BATTERY
Screen and voice prompts:
REPLACE BATTERY NOW
• The energy remaining in the battery is
low.
• The energy in the battery is nearly
depleted. The FR2+ will turn off if a
new battery is not installed.
STATUS INDICATOR: FLASHING BLACK HOURGLASS
Screen and voice prompts:
APPLY PADS AND
PADS FIRMLY
PRESS
or
PLUG IN CONNECTOR
Or voice prompts:
INSERT CONNECTOR FIRMLY
or
PRESS PADS FIRMLY TO
PATIENT'S BARE CHEST
The defibrillator pads:
• are not properly applied to the patient,
or
• are not making good contact with the
patient's bare chest because of
moisture or excessive hair, or
• are touching each other.
The defibrillator pads connector:
• is not firmly inserted in the connector
socket.
or
POOR PADS CONTACT
Voice and screen prompts:
REPLACE PADS
• The defibrillator pads, cable, or
connector may be damaged.
• The FR2+ has detected a possible
problem with the defibrillator pads or
pads cable.
• Replace the battery with a new
M3863A FR2 standard or a
charged M3848A FR2+
rechargeable battery as soon as
possible.
• Make sure that the defibrillator
pads are sticking completely to
the patient’s skin.
• If the pads are not sticking, dry the
patient's chest and shave or clip
any excessive chest hair.
• Reposition the pads.
• Make sure the pads connector is
completely inserted in the
connector socket.
If the prompt continues after you do
these things, replace the pads.
Replace the defibrillator pads with
new defibrillator pads.
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 39

symptom possible cause recommended action
4-13
Voice prompts:
ANALYZING INTERRUPTED
or
CANNOT ANALYZE
or
STOP ALL MOTION
Voice and screen prompts:
NO SHOCK DELIVERED
Voice prompt:
SHOCK BUTTON NOT
PRESSED
• The patient is being moved or jostled.
• Radio or electrical sources are
interfering with ECG analysis.
• The environment is dry and movement
around the patient is causing static
electricity to interfere with ECG
analysis.
The patient impedance is not appropriate
for the FR2+ to deliver a shock.
Shock has been advised but not delivered
within 30 seconds. (FR2+ has been
disarmed.)
• Stop CPR; do not touch the
patient. Minimize patient motion. If
the patient is being transported,
stop the vehicle if needed.
• Check for possible causes of
radio and electrical interference
and remove them from the area.
• Responders and bystanders
should minimize motion,
particularly in dry environments
that can generate static electricity.
• Make sure the defibrillator pads
are correctly positioned on the
patient according to the diagram
on the back of the pads.
• Make sure the defibrillator pads
connector is completely inserted
in the connector socket.
• Press the defibrillator pads firmly
to the patient's chest.
• Replace the defibrillator pads if
necessary.
• When next prompted, press the
Shock button to deliver shock.
4
Philips Medical Systems
Maintaining, Testing, and Troubleshooting Your HeartStart FR2+
Page 40

4-14
General troubleshooting
symptom possible cause recommended action
Status Indicator:
FLASHING RED X
Audio signal:
CHIRPING
Status Indicator:
FLASHING OR SOLID RED X
Audio signal:
CHIRPING
Screen prompt (displayed
for 10 seconds at the end
of a BIT, before FR2+ turns
off):
NOT READY FOR USE
or
SELFTEST FAILED
• The energy remaining in the battery is
low.
• The FR2+ has been stored outside the
recommended temperature range.
• An error has been detected as part of
the self-test.
• The FR2+ has been unable to perform
its daily self-tests.
A test revealed a failure or error. The
FR2+ performs self-tests every time it is
turned on, when a battery is inserted, and
periodically while it is in standby mode.
• Replace battery with a new
M3863A FR2 standard or a
charged M3848A FR2+
rechargeable battery as soon as
possible.
• Remove and reinstall the battery
and run a battery insertion
self-test. A screen prompt will tell
you if the FR2+
has been stored outside the
recommended temperature range.
See Appendix B for recommended
range.
• Remove and reinstall the battery
and perform the battery insertion
self-test. If it fails, install a new
battery and repeat the test. If it
fails again, do not use the FR2+.
• Make sure defibrillator pads are
not attached to the FR2+.
• Unplug the pads connector from
the FR2+, if connected.
• Remove and reinstall the battery
and check the results of the
battery insertion self-test. If it fails,
install a new M3863A FR2
standard battery or a charged
M3848A FR2+ rechargeable
battery and repeat the test. If it
fails again, do not use the FR2+.
NOTE: You can stop the tests and
use the FR2+ as soon as you see
the Status Indicator change to the
flashing black hourglass. Simply
press the On/Off button to stop the
test and put the FR2+ into standby
mode. The FR2+ is then ready for
use.
Philips Medical Systems
M3860A/M3861A HEARTSTART FR2+
Page 41

symptom possible cause recommended action
4-15
Status Indicator:
SOLID RED X
Audio signal:
NONE
Status Indicator:
SOLID RED X
Audio signal:
CHIRPING
• The battery is missing or completely
depleted.
• The training & administration pack is
being used in the administration
function (the solid red X is normal in
this case) or has been left in the FR2+
by mistake.
• A self-test detected a failure.
• The training & administration
pack is being used in the
ADMINISTRATION function
and more than 10 minutes have
passed without user interaction
(button press or pads change).
• The training & administration pack is
being used in the
TRAINING function
and more than 30 minutes have
passed without user interaction
(button press or pads change).
• Install a new M3863A FR2
standard battery or a charged
M3848A FR2+ rechargeable
battery in the FR2+ and perform
the battery insertion test (BIT).
• Remove the training &
administration pack and install a
battery.
• Remove and reinstall the battery
and perform the battery insertion
self-test. If it fails, install a new
M3863A FR2 standard battery or
a charged M3848A FR2+
rechargeable battery and repeat
the test. If it fails again, do not use
the FR2+.
• To continue using the training &
administration pack, press any
button (except On/Off).
• To return the FR2+ to standby
mode, remove the Pack and install
a battery.
4
Status Indicator:
NONE
Philips Medical Systems
The FR2+ has been physically damaged. • Check for visible damage. Do not
use the FR2+ if it appears to be
damaged.
• Remove and reinstall the battery to
perform the battery insertion
self-test. If it fails, install a new
M3863A FR2 standard battery or
a charged M3848A FR2+
rechargeable battery and repeat
the test. If it fails again, do not use
the FR2+.
Maintaining, Testing, and Troubleshooting Your HeartStart FR2+
Page 42

Notes
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 43

5 Clinical and Safety Considerations
Clinical Considerations
Indications
The HeartStart FR2+ is indicated for use on victims of sudden cardiac arrest
exhibiting the following signs:
Unresponsiveness
Absence of normal breathing
The HeartStart FR2+ is intended for use by personnel who have been
trained in its operation. The user should be qualified by training in basic life
support, advanced life support, or other physician-authorized emergency
medical response.
NOTE: At the discretion of emergency care personnel, the M3860A FR2+
with ECG display enabled can also be used with the FR2+ ECG
assessment module to display the rhythm of a responsive or breathing
patient, regardless of age. There are no known contraindications to use of
the FR2+ ECG assessment module.
5
Contraindications
The HeartStart FR2+ is contraindicated for use (should not be used) on
patients who show either of the following signs:
Responsiveness
Presence of normal breathing
Safety Considerations
You should be aware of the safety concerns listed here when you use the
HeartStart FR2+. Read them carefully. You will also see some of these
messages in other parts of this manual. The messages are labeled Danger,
Philips Medical Systems
Warning, or Caution.
DANGER— immediate hazards that will result in personal injury or
death.
WARNING— conditions, hazards, or unsafe practices that can
result in serious personal injury or death.
5-1
Page 44

5-2
CAUTION — conditions, hazards, or unsafe practices that can
result in minor personal injury, damage to the HeartStart FR2+, or
loss of data stored in the device.
THESE SAFETY CONSIDERATIONS ARE DIVIDED INTO FOUR
GROUPS: SAFETY CONCERNS ABOUT THE HEARTSTART FR2+ IN
GENERAL USE, DEFIBRILLATION, MONITORING, AND MAINTENANCE
ACTIVITIES.
The dangers, warnings, and cautions listed in the following tables apply to
both the model M3860A and the model M3861A HeartStart FR2+, unless
otherwise noted.
General dangers, warnings, and cautions
safety level possible shock or fire hazard, or explosion
DANGER THERE IS A POSSIBILITY OF EXPLOSION IF THE HEARTSTART
FR2+ IS USED IN THE PRESENCE OF FLAMMABLE ANESTHETICS
OR CONCENTRATED OXYGEN.
DANGER THE HEARTSTART FR2+ HAS NOT BEEN EVALUATED OR
APPROVED FOR USE IN HAZARDOUS LOCATIONS AS DEFINED IN
THE NATIONAL ELECTRICAL CODE (ARTICLES 500-503). IN
ACCORDANCE WITH THE IEC CLASSIFICATIONS (SECTION 5.5.),
THE HEARTSTART FR2+ IS NOT TO BE USED IN THE PRESENCE OF
FLAMMABLE SUBSTANCE/AIR MIXTURES.
DANGER DO NOT RECHARGE THE M3863A FR2 STANDARD BATTERY.
WARNING Use the HeartStart FR2+ only as described in this manual. Improper
use of the HeartStart FR2+ can cause death or injury. Do not press
the Shock button if the defibrillator pads are touching each other or
are open and exposed.
CAUTION Hazardous electrical output. The HeartStart FR2+ is for use only by
qualified personnel.
CAUTION Do not immerse any portion of the HeartStart FR2+ in water or other
fluids. Do not allow fluids to enter the HeartStart FR2+. Avoid spilling any
fluids on the HeartStart FR2+ or accessories. Spilling fluids into the
HeartStart FR2+ may damage it or present a fire or shock hazard. Do not
sterilize the HeartStart FR2+ or accessories.
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 45

5-3
safety level possible improper device performance
WARNING Prolonged or aggressive CPR to a patient with defibrillator pads
attached can damage the pads. Replace the defibrillator pads if they
are damaged during use or handling.
WARNING Using damaged or expired equipment or accessories may cause the
HeartStart FR2+ to perform improperly, and/or injure the patient or
the user.
WARNING CPR rates significantly above 100 compressions per minute can
cause incorrect or delayed analysis by the HeartStart FR2+.
WARNING Poor electrode pad-to-patient contact may result in a related
defibrillator prompt or other indication. Check all electrical and
patient connections.
CAUTION The HeartStart FR2+ is designed to be used only with Philips-approved
accessories. The HeartStart FR2+ may perform improperly if
non-approved accessories are used.
CAUTION Follow all instructions supplied with the HeartStart defibrillator pads. Use
the defibrillator pads before the expiration date shown on the package. Do
not reuse the defibrillator pads. Discard them after use.
5
CAUTION Aggressive handling of the defibrillator pads in storage or prior to use can
damage the pads. Discard the defibrillator pads if they become damaged.
CAUTION Follow all instructions supplied with the M3863A FR2 standard battery.
Install the battery before the expiration date shown on the battery.
CAUTION Follow all instructions supplied with the M3848A FR2+ rechargeable
battery. Recharge using the M3849A charger only.
CAUTION Do not use the M3849A charger on aircraft.
CAUTION The HeartStart FR2+ was designed to be sturdy and reliable for many
different field use conditions. However, excessively rough handling can
result in damage to the HeartStart FR2+ or its accessories. Inspect the
unit and accessories periodically according to instructions.
Philips Medical Systems
CAUTION Alteration of the factory default setup of the FR2+ can affect its
performance and should be performed under the authorization of your
Medical Director. Modifications to device operation resulting from
changes to the default settings should be specifically covered in user
training.
Clinical and Safety Considerations
Page 46

5-4
safety level possible improper device performance
CAUTION Use only Philips-approved data cards. The HeartStart FR2+ may perform
improperly if non-approved accessories are used.
safety level possible electrical interference with ECG monitoring
WARNING Radio-frequency (RF) interference from devices such as cellular
phones and two-way radios can cause improper HeartStart FR2+
operation. The HeartStart FR2+ should be used at least 6 feet (2
meters) away from RF devices, as stated in accordance with EN
61000-4-3:1996.
Defibrillation warnings and cautions
safety level possible shock hazard
WARNING Defibrillation current can cause operator or bystander injury. Do not
touch the patient during defibrillation. Disconnect the pads connector
from the HeartStart FR2+ before using any other defibrillator.
safety levels possible ECG misinterpretation
WARNING For safety reasons, some very low-amplitude or low-frequency heart
rhythms may not be interpreted by the HeartStart FR2+ as shockable
VF rhythms. Also, some VT rhythms may not be interpreted as
shockable rhythms.
WARNING Handling or transporting the patient during heart rhythm analysis can
cause an incorrect or delayed diagnosis. If the HeartStart FR2+ gives
SHOCK ADVISED prompt, keep the patient as still as possible for at
a
least 15 seconds so the HeartStart FR2+ can reconfirm the rhythm
analysis before a shock is delivered.
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 47

safety levels possible burns and ineffective energy
WARNING Do not allow the defibrillator pads to touch each other or other ECG
electrodes, lead wires, dressings, transdermal patches, etc. Such
contact can cause electrical arcing and patient skin burns during
defibrillation and may also divert the defibrillation current away from
the heart.
WARNING During defibrillation, air pockets between the skin and defibrillator
pads can cause patient skin burns. To help prevent air pockets, make
sure defibrillator pads completely adhere to the skin. Do not use
dried out defibrillator pads.
safety level possible patient injury
CAUTION The HeartStart FR2+ advanced mode’s MANUAL CHARGE feature is
intended for use only by authorized operators who have been specifically
trained in cardiac rhythm recognition and in defibrillation therapy using
manual charge and shock delivery.
5-5
Monitoring cautions
5
safety level possible misinterpretation of ECG recordings
CAUTION The LCD screen on the HeartStart FR2+ model M3860A is intended only
for basic ECG rhythm identification. The frequency response of the
monitor screen is not intended to provide the resolution needed for
diagnostic and ST segment interpretation.
Maintenance cautions
safety level possible fire or shock hazard
CAUTION Electrical shock hazard. Dangerous high voltages and currents are
present. Do not open the HeartStart FR2+, remove its covers, or attempt
repair. There are no user-serviceable components in the HeartStart FR2+.
Philips Medical Systems
The HeartStart FR2+ should be returned to an authorized service center
for repair.
Clinical and Safety Considerations
Page 48

5-6
safety level possible fire or shock hazard
CAUTION Improper maintenance may damage the HeartStart FR2+ or cause it to
function improperly. Maintain the HeartStart FR2+ only as described in
this User's Guide or as designated by your program's Medical Director.
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 49

6 Setup and Advanced Mode Features
Setup Overview
The “setup” of the HeartStart FR2+ Defibrillator is made up of several
programmable aspects, or parameters, of FR2+ operation. Some setup
parameters govern specific features that are not related to the patient care
protocol, some are used to define the automatic patient care protocol used
by the FR2+, and some provide options for manual override of the protocol
during use.
The FR2+ comes with a factory default setup designed to meet the needs of
most users. If desired, your Medical Director can revise the setup. Even if no
changes are made, however, it is a good idea to understand the setup of your
FR2+ and how the different parameter settings affect the way the device
works.
Non-protocol parameters
The parameters listed in the following table enable features of FR2+
operation that are not related to the patient treatment protocol. The table
describes each of these non-protocol parameters, lists the settings available
for it, and identifies the default setting.
parameter settings default description
speaker volume 1, 2, 3, 4,
5, 6, 7, 8
record voice YES, NO NO Enables or disables the audio recording during incident.
ECG display
(M3860A only)
autosend PST ON, OFF OFF Enables (ON) or disables (OFF) transmission of the
Philips Medical Systems
ON, OFF ON Enables (ON) or disables (OFF) ECG display on the
8 Sets volume of the FR2+’s speaker. 1 is lowest; 8 highest.
The speaker is used for voice prompts and the
charge-done tone.
Voice recording requires use of a data card.
screen.
FR2+ rhythm analysis does not require ECG display to be
on. (ECG display cannot be changed from default OFF
for M3861A.)
results of the FR2+’s periodic selftests (PST) from its
infrared communications port.
6
6-1
Page 50

6-2
Automatic protocol parameters
The HeartStart FR2+ is designed to follow an automatic protocol that guides
you through patient treatment with the defibrillator. The default settings for
programmable parameters used in the automatic protocol can be altered by
your Medical Director if desired.
The setup parameters in the following table are used to define the automatic
patient care protocol used by the FR2+. Many of these parameters interact
with each other, so it is very important to understand how each parameter
affects the protocol. The description of each parameter identifies any
interacting parameters in boldface type.
parameter settings default description
shock series 1, 2, 3, 4 3 Sets the number of shocks that must be delivered to
activate an automatic CPR pause.
The length of the CPR pause after completion of a Shock
Series is defined by the CPR Timer setting.
A new Shock Series begins when a shock is delivered:
• after the FR2+ is turned on
• after the automatic CPR pause, or
• after the Pause Key (if enabled) has been pressed, or
• if the time since the previous shock exceeds the
Protocol Timeout setting.
protocol
timeout
(minutes)
CPR timer
(minutes)
M3860A/M3861A HEARTSTART FR2+
0.5, 1.0, 1.5, 2.0,
2.5, 3.0, 3.5,
∞ (infinite)
0.5, 1.0, 1.5,
2.0, 2.5, 3.0
1.0 Sets the time interval used to determine if a delivered
shock should be counted as part of the current Shock
Series.
1.0 Sets the length of the CPR pause period* that
automatically starts when:
• a Shock Series is completed; or
• the Pause Key (if enabled) is pressed; or
• a No Shock Advised (NSA) decision is made, the NSA
CPR pause is enabled, and the conditions for using the
CPR Timer setting for the NSA CPR pause period are
met (see NSA Action).
After the CPR pause, the FR2+ returns to automatic
rhythm analysis.
* The CPR pause period is lengthened by 10 seconds to
allow time for initial voice prompting.
Philips Medical Systems
Page 51

parameter settings default description
6-3
NSA action
(minutes)
CPR prompt
monitor prompt
interval
(minutes)
MONITOR,
MONITOR
0.5, 1.0, 1.5,
2.0, 2.5, 3.0
LONG, SHORT LONG
1.0, 1.5, 2.0,
1.0 Sets the interval for patient care prompts provided during
2.5, 3.0,
∞ (infinite)
Sets how the FR2+ behaves following a NO SHOCK
ADVISED (NSA) decision:
MONITOR — directs the FR2+ to monitor the patient’s
ECG following an NSA decision and to prompt the user
periodically to provide CPR. The interval for CPR
prompting is set by the Monitor Prompt Interval.
TIME SETTING — directs the FR2+ to provide a CPR
pause period following an NSA decision (NSA CPR
Pause).
• If no shocks have been delivered in the current Shock
Series (e.g., the patient’s initial monitored rhythm is
non-shockable), the length of the CPR pause is defined
by the NSA Action time setting.
• If shocks have been delivered in the current Shock
Series (e.g., the NSA decision follows a shock), the
length of the CPR pause is instead defined by the CPR
Timer setting.
Sets the level of detail provided in the CPR reminder voice
prompts provided at the completion of a Shock Series.
LONG — provides detailed coaching to check airway,
breathing, and pulse before beginning CPR.
SHORT — simply directs user to begin CPR if needed.
FR2+ monitoring of the patient’s ECG following an NSA
decision. Selection of ∞ (infinite) means that no repeat
prompting will be provided during ECG monitoring.
6
Philips Medical Systems
Setup and Advanced Mode Features
Page 52

6-4
Manual override parameters
The HeartStart FR2+ provides several ways of overriding the automatic
protocol. The parameters in the following table are used to enable different
kinds of manual override.
parameter settings default description
advanced
OFF,
ANALYZE,
CHARGE
pause key OFF,
MONITOR,
ALWAYS
OFF
OFF
Enables or disables advanced mode entry for ALS or
tiered-response systems.
OFF — disables advanced mode features.
ANALYZE — enables user-initiated rhythm analysis and
disarm, and (M3860A only) automatically turns on ECG
display when advance mode is entered.
CHARGE (M3860A only) — in addition to enabling the
analyze feature, enables user-initiated charging and
disarming.
Enables or disables user-initiated CPR pause in the
automatic protocol. The length of the pause is defined by
the CPR Timer setting. When an Advanced mode feature
ANALYZE or CHARGE) is enabled and accessed, the
(
Pause key is disabled.
OFF — disables availability of user-initiated pause.
MONITOR — enables user-initiated pause only during FR2+
monitoring of patient rhythm.
ALWAYS — enables user-initiated pause any time except
when the device is already paused.
If enabled, the Pause Key
is accessed by pressing
the lower Option button
indicated by an arrow on
the FR2+ display, as
shown in the sample
screen:
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 53

parameter settings default description
6-5
resume key
advanced use
prompt
interval
(minutes)
ON, OFF OFF
0.5, 1.0, 1.5,
2.0, 2.5, 3.0
Using Setup Features
NOTE: To move around the menus displayed, use the Option buttons as
follows:
• Press the
item to another on the menu.
• Press the
scroll through the settings for that item.
Enables (ON) or disables (OFF) user-initiated interruption
of the CPR pause and return to analyzing. If either the
CPR Timer or the NSA Action setting is programmed to
1.5 minutes or longer, the Resume Key setting is
automatically enabled (
If enabled, the Resume
Key is accessed by
pressing the lower Option
button indicated by an
arrow on the FR2+
display, as shown in the
sample screen:
0.5 Sets the interval for “Press to Analyze” prompts provided
during advanced mode operation.
LOWER Option button to move the highlight bar from one
UPPER Option button to select the highlighted item or to
ON).
6
The FR2+ comes with a factory default setup designed to meet the needs of
most users. The setup feature of the FR2+ lets you review the current setup
of your HeartStart FR2+ or install a revised setup if appropriate. To go to the
SETUP menu:
1. Remove and reinstall the battery to bring up the first menu on the screen.
NOTE: This screen will not be displayed if the FR2+ is connected to
defibrillator pads (that are applied to the patient) when the battery is
inserted, and you will not be able to access the menu items. In addition,
Philips Medical Systems
the battery insertion selftest and periodic automatic selftests cannot run
while the defibrillator pads are connected. Be sure to unplug the pads
connector from the FR2+ after each use. Do not store the FR2+ with
the pads connected.
Setup and Advanced Mode Features
Page 54

6-6
2. Within 10 seconds of installing the battery, press the lower Option
button to move the highlight bar to
NEXT.
3. Press the upper Option button to select
4. Press the lower Option button to move the highlight bar to
5. Press the upper Option button to bring up the
NEXT.
SETUP.
SETUP menu.
The SETUP menu allows you to receive setup directly from another
HeartStart FR2+ or a computer running HeartStart Event Review software,
read setup from a data card, or review current setup.
Reviewing current setup
A good way to understand the setup of your FR2+ is to review the setup it
currently uses.
1. Select
REVIEW SETUP screens is displayed.
2. After reviewing the screen contents, press the upper Option button to
select
3. The last screen allows you to select
menu.
.
REVIEW SETUP from the SETUP menu. The first of a series of
NEXT and move to the next screen.
RETURN and go back to the SETUP
Revising setup
There are several ways to change the setup of your HeartStart FR2+. All of
them require use of products or accessories available separately from Philips
Medical Systems.
Use the M3864A training & administration pack to enable software
within the FR2+ to modify its setup. (Instructions are provided with the
Pack.)
Read a revised setup from a data card containing the setup. (Instructions
are provided later in this chapter.)
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 55

6-7
Use the infrared communications feature of the FR2+ to receive the
revised setup from another FR2+. (Instructions are provided later in this
chapter.)
Use the infrared communications feature of the FR2+ to receive the
revised setup from a computer running HeartStart Event Review
software. (Instructions are provided with the HeartStart Event Review
software.)
CAUTION: Alteration of the factory default setup of the FR2+ can affect its
performance and should be performed under the authorization of your
Medical Director. Modifications to device operation resulting from changes to
the default settings should be specifically covered in user training.
See the tables describing the various setup parameters at the beginning of
this chapter and Appendix D, “Glossary of Terms,” for definitions of setup
items.
Receiving setup
This method uses the infrared communications feature of the HeartStart
FR2+ to receive setup directly from one HeartStart FR2+ to another (which
must have the training & administration pack installed in it) or from a
computer running HeartStart Event Review software. (See instructions
provided with HeartStart Event Review.) To receive setup from another
FR2+, follow these steps:
1. Locate the infrared communications port on each HeartStart FR2+ and
line them up with one another, so that the infrared “eye” in each one has
an uninterrupted view of the “eye” in the other. (See the diagram on the
inside front cover.) The two devices should be no more than 1 meter
apart.
6
2. Make sure the “sending” FR2+ has the training & administration pack
installed and is ready to send. (See the M3864A training &
administration pack Reference Guide for instructions.)
3. Select
RECEIVE SETUP from the setup menu:
4. A new screen comes up. Until the two HeartStart FR2+ devices are
properly positioned, the screen displays
READY TO RECEIVE and
prompts you to check the sending FR2+.
Philips Medical Systems
5. Setup data are automatically transferred as soon as the infrared ports
are correctly aligned.
6. If you select
EXIT before the transfer is complete, the revised setup will
not be received. When the transfer is complete, the screen on the
Setup and Advanced Mode Features
Page 56

6-8
“receiving” FR2+ displays a SETUP COMPLETE message. Your
HeartStart FR2+ immediately uses the new setup.
Receiving setup from a computer running HeartStart Event Review software
is discussed in the directions for use provided with HeartStart Event Review
software.
Reading setup
This method copies setup data from a data card to your HeartStart FR2+. To
read the setup, follow these steps:
1. Insert the data card in the data card tray and install the loaded tray into
the data card slot in the FR2+.
2. Select
READ SETUP from the setup menu.
3. A new screen comes up. If the FR2+ cannot read the data card or
cannot find a valid setup on the data card, the screen displays a
SETUP FILE error message. Otherwise, the FR2+ begins reading the
NO
setup information from the data card immediately.
4. If you select
EXIT before the transfer is complete, the revised setup will
not be copied. When the transfer is finished, the screen displays a
SETUP COMPLETE message. Your FR2+ immediately uses the revised
setup.
Sending and Receiving Clock Settings
To synchronize the clock settings of your HeartStart FR2+ with the clock of
another FR2+ or a computer running HeartStart Event Review software, you
can use the infrared communications feature.
Instructions for synchronizing clock settings using a computer running
HeartStart Event Review are provided with the HeartStart Event Review
software.
To transfer clock settings from one FR2+ to another:
1. Remove and reinstall the battery of both FR2+ devices to bring up the
first menu screen.
2. Select
3. Select
comes up.
4. Locate the infrared communications port on each FR2+ and line them up
with one another, so that the infrared “eye” in each one has an
M3860A/M3861A HEARTSTART FR2+
NEXT to go to the second menu screen.
CLOCK from the second menu screen. The CLOCK screen then
Philips Medical Systems
Page 57

uninterrupted view of the “eye” in the other. (See the diagram on the
back of the first page of this manual.) The two devices should be no
more than 1 meter apart.
6-9
5. Select
SEND TIME from the CLOCK screen on the “sending” HeartStart
FR2+.
6. Select
RECEIVE TIME from the CLOCK screen of the “receiving” FR2+.
7. A new screen comes up. Until the two FR2+ devices are properly
positioned, the screen on the receiving FR2+ displays
RECEIVE and prompts you to check the sending FR2+. The screen on
the sending FR2+ displays
READY TO SEND and prompts you to check
READY TO
the receiving FR2+.
8. Clock settings are automatically transferred as soon as the infrared ports
are correctly aligned.
Using Advanced Mode Features
The HeartStart FR2+ provides an advanced mode that allows responders
who are appropriately trained to override the programmed FR2+ protocol
and take responsibility for certain aspects of the operating sequence used by
the FR2+ to treat the patient.
As described earlier in this chapter, the factory default setup of the FR2+
must be modified to provide access to advanced mode features. This
requires use of the administration function of the M3864A training &
administration pack.
If you are an expert user authorized by your Medical Director to modify setup,
hold down both the Option buttons while installing the training &
administration pack in the FR2+, then select
SETUP from the SETUP menu. Select ADVANCED from the third menu of the
MODIFY SETUP menu.
SETUP. Then select MODIFY
6
Philips Medical Systems
Using the upper Option button, scroll through the available settings for
ADVANCED. The advanced mode options available are based on the FR2+
Setup and Advanced Mode Features
Page 58

6-10
model used. For the M3860A, the user can select ANALYZE, CHARGE, or
OFF. For the M3861A the user can select only ANALYZE or OFF. (Detailed
directions for use are supplied with the training & administration pack.)
CAUTION: Alteration of the factory default setup of the FR2+ can affect its
performance and should be performed under the authorization of your
Medical Director. Modifications to device operation resulting from changes to
the default settings should be specifically covered in user training.
CAUTION: The HeartStart FR2+ advanced mode’s MANUAL CHARGE
feature is intended for use only by authorized operators who have been
specifically trained in cardiac rhythm recognition and in defibrillation therapy
using manual charge and shock delivery.
The
ANALYZE feature is particularly useful for organizations that include
responders who have Basic Life Support (BLS) training as well as more
highly trained responders who may be certified in Advanced Life Support
(ALS). In such situations, the Medical Director may set up a
“tiered-response” system. The HeartStart FR2+ is specifically designed to
provide different product features appropriate to each tier of responder.
In a scenario where a BLS responder is the first on the scene of an incident,
he or she is trained to treat the patient immediately — for example, to check
for breathing and responsiveness; to apply the defibrillator pads and connect
them to the HeartStart FR2+; and to follow the voice and text prompts
provided by the HeartStart FR2+ in its automated (AED) mode. When an
ALS-trained responder arrives, the BLS responder “hands off” the patient’s
care to the more highly trained responder.
Because these second-tier responders have advanced training and
developed clinical skills, they may be authorized to access the advanced
mode features of the HeartStart FR2+. These include user-initiated analysis
and manual charge and disarm control.
Using the manual analyze feature
The Manual Analyze feature is available in both the M3860A and the
M3861A models, when enabled in setup.
To enter the advanced mode during use of an FR2+ that has this feature
enabled, press both Option buttons simultaneously. This brings up a screen
that includes a highlighted line at the bottom, labeled
arrowhead pointing to the lower Option button.
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
ANALYZE, with an
Page 59

6-11
In the M3861A HeartStart FR2+, the patient’s ECG is not displayed; in the
M3860A, the display includes the patient’s ECG and heart rate.
Press the lower Option button (
ANALYZE) to initiate rhythm analysis by the
FR2+. If a shock is advised, the FR2+ automatically charges, and prompts
you to press the Shock button.
After shock delivery, the HeartStart FR2+ returns to the advanced mode
display and monitors the patient’s heart rhythm. If a potentially shockable
rhythm is detected, the text and voice prompts advise you to
ANALYZE.
NOTE: If you do not press the lower Option button (labeled ANALYZE) to
PRESS
initiate rhythm analysis when prompted, the HeartStart FR2+ does not
analyze and prompt if a shock is advised. It is important that you understand
that entering the advanced mode entails taking responsibility for these
functions.
If the rhythm analysis results in a Shock Advised decision, the FR2+ begins
charging, prompts you to press the Shock button, and displays a
DISARM option at the top of the screen. If for any reason you want to cancel
MANUAL
the shock, press the upper Option button to disarm the FR2+.
To return to non-manual, AED mode operation, turn the FR2+ off by pressing
the On/Off button. Then turn the FR2+ on by pressing the On/Off button
again.
Using the manual charge feature (M3860A only)
The manual charge feature is available only in the M3860A, when enabled in
setup.
To enter the advanced mode during use of an FR2+ that has this feature
enabled, press both Option buttons simultaneously. This brings up a screen
that includes a highlighted line at the top, labeled
arrowhead pointing to the upper Option button, and another at the bottom,
labeled
ANALYZE, with an arrowhead pointing to the lower Option button.
MANUAL, with an
6
When the advanced mode is entered, display of the patient’s ECG and heart
rate is automatically initiated.
Philips Medical Systems
Pressing the lower Option button (
analysis as described above. Pressing the upper Option button (
ANALYZE) provides user-initiated rhythm
MANUAL)
brings up a new screen.
Setup and Advanced Mode Features
Page 60

6-12
The highlighted top line is labeled MANUAL CHARGE, with an arrowhead
pointing to the upper Option button.
If the ECG display shows that, in your expert clinical judgment, the patient
has a shockable rhythm, press the upper Option button (
MANUAL CHARGE).
The HeartStart FR2+ will immediately charge for shock delivery.
As soon as charging begins, the screen message changes to
STAND CLEAR, and the label for the arrowhead pointing to the upper Option
button changes to
MANUAL DISARM.
CHARGING,
The FR2+ beeps while it is charging. When the beeping changes to a
continuous tone and the Shock button light flashes, press the Shock button
to deliver a shock. However, if the ECG display shows that the patient’s
rhythm has changed to a non-shockable rhythm, press the upper Option
button to disarm the HeartStart FR2+.
After shock delivery, the HeartStart FR2+ returns to the initial advanced
mode screen. To return to non-manual, AED mode operation, turn the FR2+
off by pressing the On/Off button. Then turn the FR2+ on by pressing the
On/Off button again.
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 61

7 Data Management and Review
Overview
The HeartStart FR2+ is designed to make it easy to manage incident data.
Some information is automatically stored in the internal memory of the
HeartStart FR2+. More detailed data can be stored on a data card if desired.
The incident information stored in the HeartStart FR2+’s internal memory, or
a summary of the information recorded on the data card, can then be
displayed on the HeartStart FR2+ screen for review. In addition, HeartStart
Event Review* software can be used on a personal computer to store and
review the detailed recorded information from a data card.
Recording Incident Data
The HeartStart FR2+ has two ways of recording information about an
emergency incident so that it can be reviewed after the incident: in internal
memory and on an optional data card.
Recording data in internal memory
Summary data for an incident is automatically recorded in internal memory by
the FR2+ while you are using it.
Recording data on a data card
The M3854A data card can be used to store several hours of detailed
incident data, including events and ECG.
IMPORTANT NOTE: To record incident data on a data card, the data card
must be installed before you turn on the FR2+.
CAUTION: The FR2+ is designed to be used only with Philips-approved
accessories. The FR2+ may perform improperly if non-approved accessories
are used.
Philips Medical Systems
* HeartStart Event Review software was previously sold as CodeRunner software.
7-1
7
Page 62

7-2
To install a data card:
1. Make sure the data card is clean and dry.
2. Load the data card into its plastic tray, with the tray’s “tongue” fitting
over the matching yellow area on the data card. The label on the card
should face up. The label has an arrow indicating which side to insert
into the data card port.
3. Make sure the FR2+ is off (in standby mode), or that the battery has
been removed.
4. Hold the loaded tray by its handle and gently insert the tray into the data
card port on the right side of the FR2+. Push the tray all the way into the
port until only the tab remains outside the FR2+ case. Do not force the
tray into the port. If the tray is hard to insert, remove it and make sure that
the arrow label is face up and pointing toward the data card port.
The data card will automatically record incident data the next time the
HeartStart FR2+ is turned on.
To avoid running out of data card space during an incident, it is
recommended that each data card be used to record the information for only
one incident and that it be replaced after each use of the FR2+.
If you record information from more than one incident on a data card, it is
important to review how much time is left on the used data card before
recording a new incident. To do this, load the data card into the data card
tray, insert the tray in the FR2+, then remove and reinstall the battery. The
first screen displayed shows how much recording time remains on the card.
NOTE: During an incident, if for any reason you turn off the FR2+ for less
than five minutes, the FR2+ considers this to be a “continued use” situation,
and:
• the information stored about the incident is saved,
• additional events recorded after the device is turned back on will be
treated as part of the same incident, and
• the selftest will not automatically run if the battery is replaced.
IMPORTANT NOTE: Do not remove the battery while incident data are
being recorded to a data card. To ensure that no incident data are lost, turn
the FR2+ off (return it to standby mode) before replacing the battery.
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 63

7-3
To replace a data card:
IMPORTANT: You must turn the FR2+ off (return it to standby mode) before
you remove the data card, to ensure that no incident data are lost.
1. Press the On/Off button to turn off the FR2+. Never replace the data
card unless the FR2+ is turned off.
2. Remove the loaded data card tray by grasping its handle and pulling it
out of the port.
3. Remove the data card from the tray.
4. Give the data card to the appropriate person in your organization.
5. Because it helps seal the FR2+ against moisture, the data card tray
should always be reinserted into the port of the FR2+. Either load a new
data card into the tray and insert it, or insert the empty data card tray into
the port.
Reviewing Incident Data
Reviewing data from internal memory
Summary information from the last incident that is stored in the internal
memory of the HeartStart FR2+ can be displayed on its screen for review. To
review this information:
1. Remove the data card if one is installed and unplug the pads connector.
2. Remove and reinstall the battery. (Make sure you are using the gray
M3863A FR2 standard battery or the blue M3848A rechargeable
battery, not the yellow training & administration pack.)
3. Select
REVIEW INCIDENT from the menu. A new screen comes up.
4. Observe and record, if desired, the summary information displayed on
the screen:
how long the incident recorded by the FR2+ lasted, and
how many shocks were delivered during the incident.
7
This information stays in the FR2+’s memory and can be displayed for review
until the next time the FR2+ is used. At that time, the data from the new
Philips Medical Systems
incident will be displayed.
Data Management and Review
Page 64

7-4
Reviewing data from a data card
If a data card is installed when the HeartStart FR2+ is turned on for use
during an incident, the HeartStart FR2+ automatically records detailed
information on the data card. To review this information on the HeartStart
FR2+ screen:
1. Make sure the training & administration pack is not installed.
2. Make sure the data card is installed. Unplug the pads connector.
3. Remove and reinstall the battery.
4. Select
REVIEW INCIDENT from the menu. A new screen comes up. This
screen displays:
ELAPSED TIME — how long the incident recorded by the FR2+
lasted,*
SHOCKS DELIVERED — how many shocks were delivered during the
incident, and
FIRST SHOCKS AT — the times at which the first three shocks were
delivered.
NOTE: If the data card does not contain event data, only the summary
information from FR2+ internal memory will be displayed when REVIEW
INCIDENT is selected.
5. To review the events that occurred during the incident, select
EVENTS. A new screen comes up. This and following screens, accessed
by selecting
NEXT EVENTS, display elapsed time information for critical
REVIEW
activities in using the FR2+. These include:
POWER ON — when the FR2+ was turned on,
PADS ON — when the defibrillator pads were connected,
SHOCK ADVISED — when a shock was advised,
ARMED — when the FR2+ charged for shock delivery,
SHOCKED — when a shock was delivered,
SHOCK ABORTED — when a shock was aborted,
PAUSE FOR CPR — when a pause occurred
POWER OFF — when the FR2+ was turned off
* The FR2+ displays elapsed time to a maximum of 99:59 minutes. If the elapsed time of use
extends beyond this figure, the minutes are represented by “??” but the seconds are
displayed. However, total elapsed time will be recorded on an installed data card for later
review with HeartStart Event Review data management software.
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 65

7-5
Additional information may be displayed if your FR2+ is using a revised setup
allowing advanced mode operation.
6. To review the first six seconds of the recorded presenting
incident, select
REVIEW ECG. A new screen comes up. This screen
displays a three-second segment of the presenting
ECG for the
ECG from the
incident.
7. S e l e c t
NEXT ECG SEGMENT to review the second three-second segment
of the presenting
ECG.
Data cards can be reused if desired. Using a personal computer running
HeartStart Event Review software, you can copy the information from a data
card, then erase the card and reuse it in the FR2+.
7
Philips Medical Systems
Data Management and Review
Page 66

Notes
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 67

A Accessories for the HeartStart FR2+
HeartStart Accessories
Accessories for the HeartStart FR2+ available separately from Philips
Medical Systems include the following:
Spare M3863A FR2 standard battery (recommended)
DP2/DP6 adult defibrillator pads
M3870A FR2 infant/child reduced-energy defibrillator pads
Spare M3853A data card tray
M3854A data card and tray
M3868A fabric carrying case
M3869A vinyl carrying case
M3857A wall mount bracket
M3848A FR2+ rechargeable battery
M3849A charger, for the M3848A FR2+ rechargeable battery only;
includes power cord
68-PCHAT fast response kit (pouch containing a pocket mask, a
disposable razor, 2 pairs of gloves, a pair of paramedics scissors, and an
absorbent wipe)
M3873A/M3874A FR2+ ECG assessment module, for use only with an
M3860A FR2+ configured for ECG display, for connection to ECG
electrodes per AAMI (M3873A) or IEC (M3874A) convention
M3864A training & administration pack
M3855A charger, for the training & administration pack only; includes
power cord
PFE7023D/PFE7024D defibrillator cabinets
07-10900 training pads
YC hardshell waterproof carrying case
‡
* †
†
* The M3848A FR2+ rechargeable battery is designed for environments in which the FR2+
Philips Medical Systems
Defibrillator is expected to see frequent use. This battery is not designed for use in aircraft.
It is recommended that this battery not be used as a spare or backup battery and, due to its
shorter standby life, that it not be used as the primary or spare battery in applications
where the FR2+ Defibrillator is infrequently used, such as the home, commercial business,
A
or commercial airlines environments.
† These products can be used only with FR2+ Defibrillators running software version 1.5 or
higher.
‡ IMPORTANT: Never store training pads with the defibrillator.
A-1
Page 68

A-2
Suggested Additional Items
It may be useful to keep some additional items with your HeartStart FR2+ for
use if needed when an incident occurs. Some suggested supplies include:
a pair of paramedic’s shears or scissors
a disposable razor designed for removing chest hair
a pocket mask or face shield
disposable gloves
a towel or antiseptic wipes
a source of oxygen
*
*
*
Your medical director may have other requirements for supplies.
*
*
* Contained in the fast response kit available from Philips Medical Systems
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 69

B Technical Specifications
The specifications for the HeartStart FR2+ provided in this chapter apply to
both the M3860A and M3861A, unless otherwise noted. Additional
information can be found in the
Defibrillator, located online at www.medical.philips.com.
HeartStart FR2+ Defibrillator Specifications
Physical
category nominal specifications
Size 2.6" high x 8.6" wide x 8.6" deep (6.6 cm x 21.8 cm x 21.8 cm).
Weight Approximately 4.7 lbs (2.1 kg) with M3863A FR2 standard battery installed.
Approximately 4.5 lbs (2 kg) with optional M3848A FR2+ rechargeable battery
installed.
Environmental
Technical Reference Manual for the FR2
B
category nominal specifications
Operating Temperature
and Humidity
Standby Temperature
and Humidity
Altitude Meets MIL-810E 500.3, Procedure II (-500 feet to 15,000 feet).
Shock/Drop Abuse
To le r an c e
Vibration Meets MIL-STD-810E 514.4-17.
Sealing With data card tray and battery installed, meets IEC 529 class IP54.
Philips Medical Systems
ESD Meets EN 61000-4-2:1998 Severity Level 4.
EMI (Radiated) Meets EN 60601-1-2 limits (1993), method EN 55011:1998 Group 1 Level B.
32° to 122° F (0° to 50° C).
0% to 95% relative humidity (non-condensing).
32° to 109° F (0° to 43° C).
0% to 75% relative humidity (non-condensing).
Applies to HeartStart FR2+ with battery installed and stored with defibrillator
pads.
Meets MIL-STD-810E 516.4, Procedure IV (after a 1 meter drop to any edge,
corner, or surface, in standby mode).
B-1
Page 70

B-2
category nominal specifications
EMI (Immunity) Meets EN 60601-1-2 limits (1993), method EN 61000-4-3:1998 Level 2 (3 V/m
and 10 V/m at 26 MHz to 1 GHz).
Aircraft: Method Meets RTCA/DO-160D:1997 Section 21 (Category M - Charging).
Defibrillator
category nominal specifications
Waveform Parameters
Biphasic truncated exponential. Waveform parameters are automatically adjusted as a
function of patient defibrillation impedance. In the diagram at left, A is the duration of phase
1 and B is the duration of phase 2 of the waveform, C is the interphase delay, V
voltage, and V
the final voltage.
f
is the peak
p
The HeartStart FR2+ delivers shocks to load impedances from 25 to 180 ohms.
The duration of each phase of the waveform is dynamically adjusted based on
delivered charge, in order to compensate for patient impedance variations, as
shown below:
adult defibrillation
load phase 1 phase 2 delivered
resistance (ohms) duration (ms) duration (ms) energy (J)
25 2.8 2.8 140
50 4.09 4.09 150
100 9.0 6.0 157
125 12.0 8.0 161
150 12.0 8.0 157
pediatric defibrillation
(using M3870A FR2 infant/child reduced-energy defibrillator pads)
load phase 1 phase 2 delivered
resistance (ohms) duration (ms) duration (ms) energy (J)
25 4.1 4.1 35
50 5.8 3.8 48
100 7.2 4.8 55
125 7.2 4.8 54
150 9.0 6.0 55
NOTE: The values given are nominal. Because of the effect of the M3870A FR2
infant/child pads’ attenuation circuitry on the defibrillator’s impedance
compensation feature, the actual phase durations for a given load resistance on
the table above could be those of an adjacent row.
Philips Medical Systems
M3860A/M3861A HEARTSTART FR2+
Page 71

category nominal specifications
Energy Using adult defibrillator pads: 150 J nominal into a 50 ohm load.
Using infant/child reduced-energy defibrillator pads: 50 J nominal into a 50 ohm
load. Sample pediatric energy doses:
age energy dose
newborn 14 J/kg
1 year 5 J/kg
2 − 3 years 4 J/kg
4 − 5 years 3 J/kg
6 − 8 years 2 J/kg
Doses indicated are based on CDC growth charts for the 50th percentile weights
for boys.*
* National Center for Health Statistics in collaboration with the National Center for
Chronic Disease Prevention and Health Promotion. CDC growth charts:
weight-for-age percentiles, revised and corrected November 28, 2000. Atlanta,
GA: Centers for Disease Control and Prevention © 2000.
Charge Control Controlled by Patient Analysis System for automated operation. Can be
programmed for manual initiation using advanced mode of the M3860A.
B-3
B
Charge Time
from “Shock Advised”
< 10 seconds typical, including confirming analysis. Charge time increases near
end of battery service life.
Shock-to-Shock Cycle Time < 20 seconds typical, including analysis, in AED mode.
“Charge Complete”
Shock button flashes, audio tone sounds.
Indicator
Disarm (AED mode) Once charged, the HeartStart FR2+ will disarm if:
• patient’s heart rhythm changes to non-shockable rhythm, OR
• a shock is not delivered within 30 seconds after the FR2+ is armed, OR
• the PAUSE button (if enabled) is pressed, OR
• the On/Off button is pressed to turn off the FR2+, OR
• the defibrillator pads are removed from the patient or the pads connector is
disconnected from the FR2+.
Philips Medical Systems
Technical Specifications
Page 72

B-4
category nominal specifications
Disarm (advanced mode) Once charged, the HeartStart FR2+ will disarm if:
in advanced mode ANALYZE
• the manual disarm button is pressed, OR
• a patient’s heart rhythm changes to non-shockable rhythm, OR
• a shock is not delivered within 30 seconds after the FR2+ is armed, OR
• the On/Off button is pressed to turn off the FR2+, OR
• the defibrillator pads are removed from the patient, OR
• the pads connector is disconnected from the FR2+.
in advanced mode CHARGE (M3860A only)
• the manual disarm button is pressed, OR
• a shock is not delivered within 30 s after charging, OR
• the On/Off button is pressed to turn off the FR2+, OR
• the defibrillator pads are removed from the patient, OR
• the pads connector is disconnected from the FR2+.
Shock Delivery Vector Via adult defibrillator pads placed in the anterior-anterior (Lead II) position or via
FR2 infant/child reduced-energy defibrillator pads placed in the anterior-posterior
position.
ECG Analysis System
category nominal specifications
Function Evaluates impedance of defibrillator pads for proper contact with patient skin, and
evaluates the ECG rhythm and signal quality to determine if a shock is appropriate.
Protocols Follows pre-programmed settings to match local EMS guidelines or medical
protocols. The settings can be modified using the setup options.
Shockable Rhythms Ventricular fibrillation (VF) and certain ventricular tachycardias, including
ventricular flutter and polymorphic ventricular tachycardia (VT). The HeartStart
FR2+ uses multiple parameters to determine if a rhythm is shockable.
NOTE: For safety reasons, some very low-amplitude or low-frequency rhythms
may not be interpreted as shockable VF rhythms. Also, some VT rhythms may not
be interpreted as shockable rhythms. CPR rates significantly above 100
compressions per minute can cause incorrect or delayed analysis by the
HeartStart FR2+.
Asystole On detection of asystole, provides CPR prompt at programmed interval.
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 73

B-5
category nominal specifications
Pacemaker Detection On detection of a pacemaker (in advanced mode or with M3873A/M3874A FR2+
ECG assessment module), provides screen display of
alert. M3860A includes pacemaker artifact in ECG display. In both models,
pacemaker artifact is removed from the signal for rhythm analysis.
PACEMAKER DETECTED
Display
category nominal specifications
Monitored ECG Lead ECG information is received from adult defibrillator pads in anterior-anterior (Lead
II) position or from FR2 infant/child reduced-energy defibrillator pads in
anterior-posterior position. (Displayed on M3860A only.)
ECG information can also be displayed in the M3860A using the FR2+ ECG
assessment module.
NOT E : The ECG display provided by the FR2+ Defibrillator is not is not
intended to provide diagnostic or ST segment interpretation.
B
Display Range
(M3860A only)
Screen Type High-resolution liquid crystal display (LCD) with backlight.
Screen Dimensions 2.8" wide x 2.3" high (70 mm x 58 mm).
Sweep Speed
(M3860A only)
ECG Display 3 second-segments displayed (M3860A only).
Frequency Response
(Bandwidth)
Sensitivity 1.16 cm/mV, nominal.
Heart Rate Displayed
(Normal Sinus Rhythm)
Differential: ±2 mV full scale, nominal.
23 mm/s nominal.
Nondiagnostic rhythm monitor 1 Hz to 20 Hz (-3 dB), nominal.
30 to 300 bpm, updated each analysis period. Displayed (M3860A only) during
monitoring and advanced modes.
Controls and indicators
Philips Medical Systems
LCD Screen High-resolution, backlighted LCD screen, displays text messages and (model
category nominal specifications
M3860A only) ECG.
Technical Specifications
Page 74

B-6
category nominal specifications
Controls On/Off button
Shock button
Option buttons
LED Indicators Connector socket LED, flashes to indicate socket location. LED is covered when
defibrillator pad connector is properly inserted. Shock button LED flashes when
defibrillator is armed.
Audio Speaker Provides voice prompts (volume is adjustable via Setup screen).
Beeper Chirps when a selftest has failed.
Provides various warning beeps during normal use.
Status Indicator Status indicator LCD displays device readiness for use.
Low Battery Detection Automatic during daily periodic selftesting.
Low Battery Indicator Solid or flashing red X Status Indicator on front panel; screen display LOW
BATTERY or REPLACE BATTERY warning, as appropriate.
Accessories Specifications
M3863A FR2 standard battery
category nominal specifications
Battery Type 12 VDC, 4.2 Ah, lithium manganese dioxide. Disposable, long-life primary cell.
Capacity When new, a minimum of 300 shocks or 12 hours of operating time at 77° F
(25° C).
Shelf Life
(prior to installation)
Standby Life
(after installation)
Status Indicators Good battery: flashing black hourglass on the front panel of the FR2+.
Storage Temperature 32° to 109° F (0° to 43° C).
M3860A/M3861A HEARTSTART FR2+
Typically, 5 years from date of manufacture when stored under standby
environmental conditions in original packaging.
Typically, 5 years. >4 years when stored under standby environmental conditions
(battery installed, FR2+ unused).
Low battery: flashing red X on the front panel of the FR2+.
Dead battery: solid red X on the front panel of the FR2+.
Philips Medical Systems
Page 75

(Optional) M3848A FR2+ rechargeable battery
category nominal specifications
Battery Type 11.3 VDC, 6.5 Ah, lithium ion. Rechargeable cell using the M3849A charger.
Capacity When freshly charged and used at 77° F (25° C) , provides a minimum of 80
(typically 100) shocks, or 3.5 hours (typically 5 hours) of ECG display time only,
before recharging is indicated.
Status Indicators Good battery: bar graph on display screen indicating remaining power level.
Low battery: flashing red X on the front panel of the FR2+ (When low battery
indicator appears, there is still enough energy to deliver 9 shocks plus 15 minutes
of ECG display time).
Dead battery: solid red X on the front panel of the FR2+.
Storage Temperature 32° to 109° F (0° to 43° C).
B-7
B
Standby Life
(after installation)
6 months when installed fully charged in a defibrillator labeled FR2+.
(Optional) M3849A charger
category nominal specifications
Application For use with M3848A FR2+ rechargeable battery only.
Power Requirements 100 to 240 VAC, 47 to 63 Hz, 30 Watts
Environmental
Requirements
Conformance Testing International: EN60335-1:1994 Class 1
32° to 122° F (0° to 50° C).
0% to 95% relative humidity (non-condensing).
North America: UL 1310 Class 2
M3870A and DP2/DP6 defibrillator pads
category nominal specifications
Pads, Cable, and Connector Disposable and self-adhesive. DP2/DP6 adult defibrillator pads have a nominal
Philips Medical Systems
active surface area of 100 cm
integrated 122 cm (48 inch), typical, cable and connector. M3870A FR2
infant/child reduced-energy defibrillator pads have a nominal active surface area of
2
each and are provided in a sealed package with an integrated 122 cm (48
44 cm
inch), typical, cable and connector incorporating attenuating electronics.
2
each and are provided in a sealed package with an
Technical Specifications
Page 76

B-8
category nominal specifications
Defibrillator Pad
Requirements
Use only DP2/DP6, M3870A, M3713A, and M3716A defibrillator pads with the
HeartStart FR2+. Place the pads on the patient as illustrated on each pad.
(Optional) M3854A data card
category nominal specifications
Capacity 8 hours of event and ECG data, or 60 minutes with voice recording.
(Optional) M3864A training & administration pack
category nominal specifications
Battery Type 12 V, 1.1 Ah, nickel metal hydride. Disposable, rechargeable cell using the
M3855A charger.
Capacity Provides 4 hours of operating time at 77 °F (25 °C).
Status Indicators Low battery: flashing red X on the front panel of the FR2+.
Dead battery: solid red X on the front panel of the FR2+.
Storage Temperature 50° to 104° F (10° to 40° C).
(Optional) M3855A charger
category nominal specifications
Application For use with M3864A training & administration pack only.
Power Requirements With appropriate power cord, any AC mains power input or inverter-type power
sources.
Environmental
Requirements
Conformance Testing International: EN60335-1:1994 Class I
M3860A/M3861A HEARTSTART FR2+
32° to 113° F (0° to 45° C).
35% to 85% relative humidity (non-condensing).
North America: UL 1310 Class 2
Philips Medical Systems
Page 77

(Optional) M3873A/M3874A FR2+ ECG assessment
module
category nominal specifications
Application For use with the FR2+ M3860A with ECG display enabled and running version
1.5 software or higher (denoted by FR2+ on the front panel or rear label).
Length and Weight 100 inches (182 cm); ≤ 1 lb. (2.2 kg).
B-9
B
Operating Temperature and
Humidity
Storage Temperature and
Humidity
Patient Lead Wire
Designation
Typical (Lead II) Connection Lead II vectors:
Battery Type 3 V, 1 Ah, poly-carbonmonofluoride lithium (LiCFx). Non-replaceable disposable
Service Life Typi c al ly , 5 yea r s .
Performance with FR2+
Defibrillator
32° to 122° F (0° to 50° C);
0% to 95% relative humidity (non-condensing).
32° to 109° F (0° to 43° C).
0% to 75% relative humidity (non-condensing).
M3873A (AAMI): M3874A (IEC):
positive lead − red positive lead − green
negative lead − white negative lead − red
reference lead − black. reference lead − yellow
positive − left leg
negative − right arm
reference − left arm.
Other limb vectors can be obtained by different electrode positions.
primary cell.
Meets environmental specifications cited for FR2+ Defibrillator on page B1
through B2.
Philips Medical Systems
Technical Specifications
Page 78

Notes
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 79

C Glossary of Symbols and Controls
Instructions for Use
symbol description
Meets the requirements of the European medical device
directives.
Printed on recycled paper.
HeartStart FR2+ M3860A and M3861A
Defibrillator Symbols and Controls
Control panel and back label
symbol description
O
•
F
N
F
O
On/Off button. Turns the HeartStart FR2+ on or off;
disarms HeartStart FR2+, stops automatic self-test. When
the optional training & administration pack is being used in
the Training function, this button is used to select and exit
training scripts.
C
Shock button. Delivers shock to patient when the
HeartStart FR2+ is charged.
Upper and lower Option buttons. Allow you to move
around in and select an item from a display menu, provide
adjustment of display screen contrast.
Defibrillation protection. Defibrillation protected, type BF
Philips Medical Systems
patient connection.
C-1
Page 80

C-2
symbol description
High voltage.
Refer to operating instructions.
Meets the requirements of the European medical device
directives.
This product has passed relevant safety tests by CSA, a
nationally recognized test lab.
IP54 With data card tray and battery installed, meets IEC 529
class IP54.
HeartStart FR2+ display screen
symbol description
HR XXX Heart rate.
XX
XX:XX Time. How much time (minutes:seconds) has passed since
TEMPERATURE
SETUP
REV: XXX X.X
XXXX
M3860A/M3861A HEARTSTART FR2+
Number of shocks delivered.
the HeartStart FR2+ was turned on.
Temperature. Recommended storage temperature range
has been exceeded since the last battery insertion
self-test.
Setup. Setup has been lost from memory; factory default
setup is being used. Contact Medical Director for revised
setup.
Software. The version of software used in your HeartStart
FR2+.
Philips Medical Systems
Page 81

Status indicator
symbol description
C-3
Flashing black hourglass. Ready for use.
Solid red X. Not ready for use. (See Chapter 4,
“Maintaining, Testing, and Troubleshooting Your HeartStart
FR2+.”)
Flashing red X. Troubleshooting required. (See Chapter 4,
“Maintaining, Testing, and Troubleshooting Your HeartStart
FR2+.”)
Accessories Symbols
M3870A and DP2/DP6 defibrillator pads
symbol description
These pads are disposable and are for single patient use
only.
Pouch contents: one pair of defibrillator pads.
C
Store the pads at temperatures between 0° and 43° C
(32° and 110° F).
Refer to operating instructions.
Philips Medical Systems
Non-sterile.
This product does not contain natural rubber latex.
Glossary of Symbols and Controls
Page 82
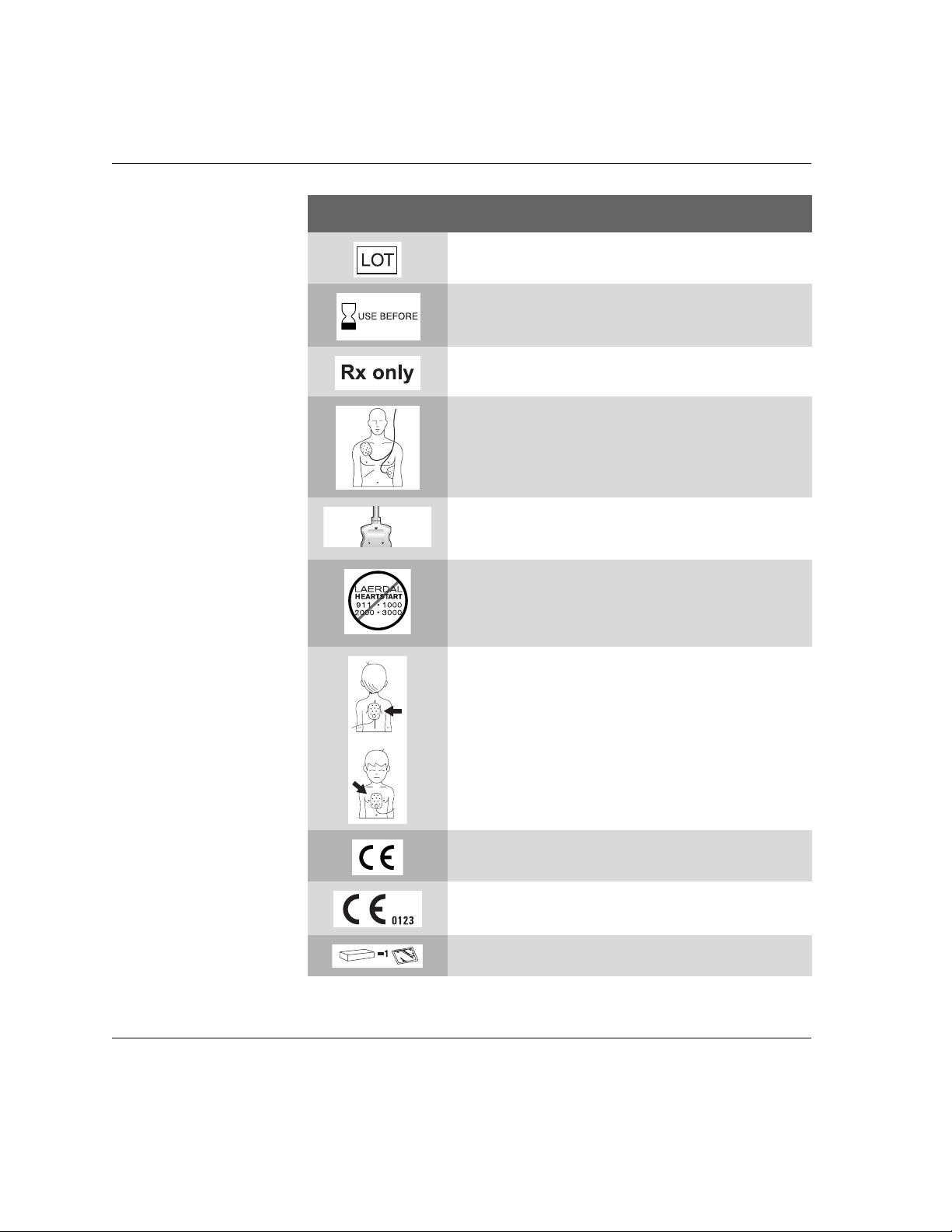
C-4
symbol description
Lot number.
Use the pads before the date shown. Date format is
YYYY-MM.
CAUTION: Federal law (USA) restricts this device to sale
by or on the order of a physician.
Pad placement for adults. (DP2/DP6)
For use with Philips Heartstream/HeartStart and Laerdal
HeartStart ForeRunner, FR, and FR2 AEDs. (DP2/DP6)
Not for use with Laerdal HeartStart models 911, 1000,
2000, 3000. (DP2/DP6)
M3860A/M3861A HEARTSTART FR2+
Pad placement for infants and children younger than 8
years or lighter than 55 pounds (25 Kg). (M3870A)
Meets the requirements of the EMC directives. (DP2/DP6)
Meets the requirements of the European medical device
directives. (M3870A)
Box contents = 1 pouch. (M3870A)
Philips Medical Systems
Page 83

symbol description
C-5
2
6
Box contents = 2 pouches. (DP2)
Box contents = 6 pouches. (DP6)
This side up.
Fragile.
Protect from moisture.
Printed on recycled paper.
M3863A standard battery and
M3848A FR2+ rechargeable battery
symbol description
C
Do not crush.
Do not expose to high heat or open flames. Do not
incinerate.
Do not mutilate or open.
Philips Medical Systems
Install before the date shown on this label. Date format is
MM-YYYY.
Glossary of Symbols and Controls
Page 84

C-6
symbol description
Refer to operating instructions.
Lithium manganese dioxide battery chemistry (M3863A)
Lithium ion battery chemistry (M3848A)
12 volts direct current output.
Insert into FR2+ in this direction.
Meets the requirements of the European medical device
directives.
Fragile.
M3849A charger
for the M3848A FR2+ rechargeable battery
symbol description
M3860A/M3861A HEARTSTART FR2+
Protect from moisture.
Contains one battery.
Printed on recycled paper.
Philips Medical Systems
Refer to operating instructions.
Page 85

symbol description
High voltage.
Protect from moisture.
On/Off indicator.
Charger status indicator.
Meets the requirements of the EMC directives.
This product has passed relevant safety tests by CSA, a
nationally recognized test lab.
C-7
C
This product has been certified by the Australian
Communication Authority.
N11695
V00341
Printed on recycled paper.
Electrical input.
Electrical output
Philips Medical Systems
Glossary of Symbols and Controls
Page 86

C-8
M3873A/M3874A FR2+ ECG assessment module
symbol description
Use the cable and electrodes before the respective dates
shown on this label.
Ship and store the product within the temperature ranges
shown.
Place the electrodes as shown.
Meets the requirements of the EMC directives.
M3864A training & administration pack
symbol description
M3860A/M3861A HEARTSTART FR2+
Lot number.
Refer to operating instructions.
Printed on recycled paper.
Refer to operating instructions.
Philips Medical Systems
Page 87

symbol description
Do not crush.
Do not expose to high heat or open flames. Do not
incinerate.
Do not mutilate or open.
Nickel metal hydride battery chemistry.
12 volts direct current output.
Insert into FR2+ in this direction.
C-9
C
Meets the requirements of the EMC directives.
Kit contains Training & Administration Pack, Instructions
for Use, and set of training pads.
Ship and store the product within the temperature ranges
shown.
This side up.
Fragile.
Philips Medical Systems
Protect from moisture.
Glossary of Symbols and Controls
Page 88

C-10
symbol description
Printed on recycled paper.
M3855A charger
for the M3864A training & administration pack)
symbol description
Refer to operating instructions.
High voltage.
Protect from moisture.
On/Off indicator.
M3860A/M3861A HEARTSTART FR2+
Charger status indicator.
Meets the requirements of the EMC directives.
Electrical input.
Electrical output
Printed on recycled paper.
Philips Medical Systems
Page 89

D Glossary of Terms
The terms listed in this Glossary are defined in the context of the HeartStart
FR2+ and its use.
advanced mode ..................... A programmable treatment mode that permits an authorized user to control
when the FR2+ starts rhythm analysis and (model M3860A only) when to
begin defibrillator charging for shock delivery.
AED .......................................... Automated external defibrillator.
AED mode ............................... The standard FR2+ treatment mode, with voice and screen prompts guiding
the responder through connecting the defibrillator pads, waiting for rhythm
analysis, and delivering a shock if needed. In this mode, heart rhythm analysis
and monitoring, and shock decision and charging for shock delivery are
automatically performed by the FR2+.
ALS ........................................... Advanced Life Support.
analysis ................................... See “SMART analysis.”
arrhythmia ............................... An unhealthy, often irregular, beating of the heart.
battery ..................................... See “standard battery” and “rechargeable battery.”
BLS ........................................... Basic Life Support.
continued use ........................ A condition in which use of the HeartStart FR2+ is interrupted for less than
five minutes (e.g., for battery replacement). When the battery is reinserted or
the unit is turned on again, the information stored about the interrupted
incident is saved, any additional events recorded after the battery is
reinstalled are treated as part of the same incident, and the selftest does not
automatically run when the battery is reinstalled.
D
CPR timer ................................ A programmable period provided by the HeartStart FR2+ during which the
responder can administer CPR.
defibrillation ............................ Termination of cardiac fibrillation by applying electrical energy
defibrillation charge .............. Electrical energy stored in the capacitor of the HeartStart FR2+ as it arms for
shock delivery.
defibrillation shock ................ See “SMART biphasic waveform.”
Philips Medical Systems
defibrillator pads .................... The self-adhesive electrode pads applied to the adult patient’s bare chest or
pediatric (under 8 years of age or less than 55 lb./25 kg) patient’s bare chest
and back, and used to detect the patient’s heart rhythm and transfer the
D-1
Page 90

D-2
defibrillation shock. Use only DP2/DP6, M3870A, M3713A, and M3716A
defibrillator pads with the HeartStart FR2+.
ECG .......................................... Electrocardiogram, a display or printout of the electrical rhythm of the heart
as detected through defibrillator pads.
event ........................................ An action recognized or performed by the HeartStart FR2+ as a step in the
sequence of using the device in an incident. Examples include: applying the
pads and connecting them to the HeartStart FR2+, analyzing heart rhythm,
delivering a shock, etc.
fibrillation ................................ A disturbance of the normal heart rhythm that results in chaotic, disorganized
activity that cannot effectively pump blood. Ventricular fibrillation (fibrillation
in the lower chambers of the heart) is associated with sudden cardiac arrest.
heart rhythm
(ECG) analysis .................. A system used by the FR2+ to determine if the patient’s heart rhythm is
shockable — ventricular fibrillation (VF) or certain ventricular tachycardias
(VTs). See “
HeartStart Event Review ...... A dedicated data management software system (formerly sold as
SMART analysis.”
CodeRunner) for use with the HeartStart FR2+. It is available from Philips
Medical Systems on CD or on the world wide web at
http://www.medical.philips.com/goto/eventreview.
impedance .............................. Electrically, this is the total opposition offered by the body to the flow of the
electrical shock waveform delivered by the HeartStart FR2+. The FR2+
automatically monitors the electrical impedance between the defibrillator
pads placed on the patient’s bare skin, and adjusts the shock waveform
appropriately.
incident .................................... The series of events involved in treating a patient with the HeartStart FR2+.
infrared
communications
............... A method of sending information using a special part of the light spectrum. It
is used to transmit information to and from the HeartStart FR2+ and another
FR2+ or a computer running HeartStart Event Review software.
manual charge ....................... A feature of the advanced mode used by an authorized ALS-certified
responder that allows the user to arm the HeartStart FR2+ for shock delivery.
manual disarm ....................... A feature of the advanced mode used by an authorized ALS-certified
responder that allows the user to dump the HeartStart FR2+ charge
internally.
monitoring .............................. A mode of background analysis to determine if patient rhythm has changed
to a shockable rhythm.
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 91

non-shockable
................................ A heart rhythm that the HeartStart FR2+ determines is not appropriate for
rhythm
shock delivery.
NSA .......................................... No Shock Advised decision, made by the HeartStart FR2+ based on
analysis of the patient’s heart rhythm.
pacemaker .............................. External or implanted cardiac pulse generator that stimulates the heart
electronically.
pads ......................................... See “defibrillator pads.”
D-3
pause ....................................... A defined period during which the HeartStart FR2+ does not perform rhythm
analysis.
pediatric defibrillation ............ Defibrillation of a child under eight years of age or 55 lbs. (25 Kg). It is
recommended that FR2 infant/child reduced-energy defibrillator pads be
used for pediatric patients.
periodic selftests ................... Daily, weekly, and monthly tests automatically conducted by the FR2+ when
it is in the standby mode. The tests monitor many key functions and
parameters of the FR2+, including battery capacity and the state of its
internal circuitry.
presenting ECG ...................... The heart rhythm seen by the HeartStart FR2+ when it is first connected to
the patient (via the defibrillator pads) and begins rhythm analysis.
prompts ................................... The voice commands and screen text used to guide the responder through
use of the HeartStart FR2+ to treat the patient.
protocol ................................... A sequence of operations performed by the HeartStart FR2+ to direct
patient care in the AED mode.
protocol timeout .................... A programmable interval between shocks, used by the HeartStart FR2+ to
decide if the shocks are part of the same shock series.
read (data) .............................. A feature of the HeartStart FR2+ that allows it to read setup data from a
M3854A data card.
receive (data) ......................... A feature of the HeartStart FR2+ that allows use of its infrared (IR)
communications port to receive revised setup and clock settings directly
from another device.
D
rechargeable battery .............. The M3848A FR2+ rechargeable battery, used with the M3849A charger
Philips Medical Systems
record voice ............................ An optional feature of the HeartStart FR2+ that allows sound recording to a
only.
data card during use of the device in an incident. Activation of this feature
requires revision of the HeartStart FR2+’s default settings.
Glossary of Terms
Page 92

D-4
rhythm analysis ...................... See “SMART analysis.”
send (data) ............................. A feature of the HeartStart FR2+ that allows use of its infrared (IR)
communications port to send data directly to another FR2+ or a computer
running HeartStart Event Review Web software.
sensitivity ................................ A measure of the ability of the HeartStart FR2+ to reliably detect and identify
shockable heart rhythms.
setup ........................................ The settings of all programmable operating parameters of the HeartStart
FR2+. The factory default setup can be modified using the M3864A training
& administration pack.
shock series ........................... One or more shocks, each separated by no more than a preset time
(programmed Protocol Timeout). After completion of a shock series, the
HeartStart FR2+ automatically pauses for CPR.
shockable rhythm .................. Ventricular fibrillation and certain ventricular tachycardias associated with
sudden cardiac arrest.
shock waveform ..................... See “SMART biphasic waveform.”
SMART analysis ...................... The proprietary algorithm used by the FR2+ to analyze the patient’s heart
rhythm and determine whether a shock is advised.
SMART biphasic
waveform
............................ The patented, low-energy defibrillation shock waveform used by the FR2+. It
is an impedance-compensated biphasic waveform with 150 Joules, nominal,
delivered to a 50 ohm load. When delivered via FR2 infant/child
reduced-energy defibrillator pads, the energy is attenuated to 50 Joules,
nominal.
specificity ................................ A measure of the ability of the HeartStart FR2+ to reliably detect and identify
non-shockable heart rhythms.
standard battery...................... The M3863A battery, 12 VDC, 4.2 Ah, lithium manganese dioxide,
disposable, long-life primary cell.
standby mode ........................ The operating mode of the HeartStart FR2+ when a battery has been
installed, and the unit is turned off and ready for use when needed. Shown by
flashing black hourglass on the Status Indicator.
status indicator ...................... This is a special window in the upper right-hand corner of the front panel of
the HeartStart FR2+ that lets you know the status of the device.
sudden cardiac
arrest ................................... The sudden cessation of the heart’s pumping rhythm.
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 93

D-5
training &
administration pack
.......... An optional accessory for the FR2+ that enables training and administrative
functions. The integral battery should be charged only using the M3855A
charger.
waveform ................................ See “SMART biphasic waveform.”
write (data) .............................. A feature of the HeartStart FR2+ that allows it to record setup information on
a data card.
D
Philips Medical Systems
Glossary of Terms
Page 94

Notes
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
Page 95

Index
A
accessories
additional, recommended
adult defibrillator pads
available to order
charger for M3848A
rechargeable battery
charger for training &
administration pack
data card
data card tray
fabric carrying case
fast response kit
FR2+ ECG assessment module
A-1
infant/child defibrillator pads
rechargeable battery
standard battery
training & administration pack
vinyl carrying case
wall mount bracket
adult defibrillator pads
advanced mode
definition
features
manual analyze
manual charge
manual disarm
programmable settings
tiered-response features
user qualifications
AED mode, definition
AED, definition
Philips Medical Systems
ALS, definition
applying to child patient
arrhythmia, definition
A-1
6-4, D-1
6-9
A-1
A-1
A-1
A-1
6-10
6-11, 6-12, D-2
6-12, D-2
6-9
D-1
D-1
D-1
A-2
A-1
A-1
A-1
A-1
A-1
A-1
A-1
A-1
A-1
A-1
6-4
6-9
D-1
3-2
asystole detection
autosend PST
definition
programmable settings
B
battery
description
insertion selftest
replacing during use
battery history
description of data
reviewing
battery insertion self-test
4-14
failure
battery insertion selftest
description
interactive
recording test results
BLS, definition
C
calibration 4-1
cautions, warnings, and dangers
5-2
charge
disarming
time from Shock Advised
chirping
cleaning
4-11
FR2+ ECG assessment module
3-5
agents to use
guidelines
B-4
6-1
6-1
2-1, D-1
2-4
4-5, 7-2
4-10
4-10
INDEX
2-4
4-7
4-6
D-1
B-3, B-4
B-3
4-3
4-3
I-1
Page 96

I-2
clock
receiving settings
sending settings
setting independently
synchronizing
continued use
contrast, adjusting
controls and indicators
specifications
controls and symbols
CPR prompt
definition
programmable settings
CPR timer
definition
programmable settings
6-3
6-2, D-1
2-3, 6-8
6-8
2-4
2-3
4-5, 7-2, D-1
4-8
B-5
C-1
6-3
6-2
D
dangers, warnings, and cautions
5-2
data card
reading setup
recommended use
recording time available
removing
replacing
defibrillation charge
defibrillation therapy
defibrillation, definition
6-8
4-6
4-6
7-3
7-3
D-1
1-2
D-1
damage during CPR
description
positioning correctly
specifications
device history
description of data
reviewing
disarming the FR2+
in advanced mode
in AED mode
manual
display screen
adjusting contrast
specifications
DP2/DP6 adult defibrillator pads
A-1
D-1
4-9
B-3
6-12, B-4
3-6
3-2
B-7
4-9
B-4
4-8
B-5
E
ECG analysis
see SMART analysis
ECG analysis system
description
ECG assessment module
ECG display
definition
programmable settings
specifications
ECG, definition
event, definition
D-2
3-5
6-1
6-1
B-5
D-2
D-2
defibrillator
specifications
defibrillator pads
applying to adult patient
applying to patient
checking before use
connecting to the FR2+
M3860A/M3861A HEARTSTART FR2+
B-2
3-2
3-2
3-2
3-2
F
fibrillation, definition D-2
flashing black hourglass
see Status Indicator
flashing red X
see Status Indicator
Philips Medical Systems
Page 97

I-3
G
glossary
of terms
of symbols and controls
D-1
C-1
H
heart rhythms
shockable
HeartStart M3870A FR2
infant/child reduced-energy
defibrillator pads
hourglass Status Indicator
how to install the battery
how to remove the data card
how to review battery history
how to review device history
how to review the presenting ECG
7-5
how to run the battery insertion
selftest
how to use the advanced mode
How to use the M3873A/M3874A
ECG assessment module
B-4
A-1
C-2
2-1
7-3
4-10
4-9
2-4
6-9
3-5
indications and contraindications
1-2, 5-1
infrared communications
description
receiving setup
installing the battery
D-2
6-6
2-1
L
LCD di splay
see display screen
M
M3848A rechargeable battery A-1,
B-7
M3849A charger for M3848A
rechargeable battery
M3854A data card
M3855A charger for M3864A
training & administration pack
A-1, B-8
M3857A wall mount bracket
M3860A and M3861A FR2+,
description
M3863A battery
A-1, B-8
1-1
A-1, B-6
A-1, B-7
INDEX
A-1
I
impedance
automatic adjustment of shock
waveform
troubleshooting
impedance, definition
incident data
definition of data on data card
Philips Medical Systems
definition of internal memory data
7-3
reviewing from data card
reviewing from internal memory
7-3
incident, definition
B-2
4-13
D-2
7-4
7-4
D-2
M3864A training & administration
A-1, B-8
pack
M3868A carrying case
M3870A FR2 infant/child
reduced-energy defibrillator pads
A-1, B-7
M3873A/M3874A ECG
assessment module
B-8
main menu
maintenance
after using the FR2+
cleaning
daily selftests
2-2
4-3
4-1
A-1
3-5, A-1,
4-2
Index
Page 98

I-4
monthly selftests 4-1
operator’s checklist
recommended schedule
manual mode
see advanced mode
monitoring, description
4-3
4-1
D-2
N
non-shockable rhythms D-3
NSA action
definition
programmable settings
NSA, definition
6-3
6-3
D-3
O
On/Off button, description of uses
C-1
operating temperature
Option buttons
description of uses
to adjust display screen contrast
4-8
B-1
C-1
P
pacemaker
definition
detection
pads placement, adult and pediatric
3-2
patient impedance
pause for CPR, description
pause key
definition
programmable settings
pause, definition
pause, time indication
D-3
B-4
B-2
3-4
6-4
6-4
D-3
3-4
pediatric defibrillation
choosing defibrillator pads
periodic selftests
definition
description
frequency
Status Indicator alerts
presenting ECG
definition
description
prompt interval
monitor settings
prompt intervals
advanced use settings
definition
prompts, definition
protocol timeout
definition
programmable settings
protocol, definition
D-3
4-9
4-1
D-3
7-5
6-5
6-2, D-3
R
record voice
definition
programmable settings
recording incident data
in internal memory
replacing battery during use
replacing data card
responder
qualifications and training
resume key
definition
programmable settings
reviewing incident data
from data card
from internal memory
6-1, D-3
6-5
6-5
D-3
D-3
7-3
7-4
7-1
4-9
6-5
6-2
6-1
6-5
7-3
3-2
7-2
1-2
Philips Medical Systems
M3860A/M3861A HEARTSTART FR2+
Page 99

I-5
rhythm analysis
see SMART analysis
S
safety considerations 5-1
selftests
battery insertion
4-1
daily
monthly
periodic
sensitivity, definition
setup
definition
reading setup
receiving setup
shock
see SMART biphasic waveform
Shock button, description of use
C-1
shock series
definition
programmable settings
shock waveform
see SMART biphasic waveform
shockable rhythms
SMART analysis
definition
during CPR
specifications
SMART biphasic waveform
definition
energy delivered
shock delivery vector
shock waveform
Philips Medical Systems
specification
4-1
2-5, 4-1
2-4
D-4
D-4
6-8
6-6
6-2, D-4
6-2
D-4
D-4
3-6
B-4
D-4
B-3
B-4
B-2
B-2
speaker volume
definition
programmable settings
specifications
battery
controls and indicators
defibrillator
defibrillator pads
display screen
ECG analysis performance
ECG analysis system
environmental
physical
specificity, definition
standby mode, definition
standby temperature
Status Indicator
description
flashing black hourglass
flashing red X
in standby mode
solid red X
sterilization
storage conditions
sudden cardiac arrest, definition
D-4
symbols
on accessory packaging
on battery
on FR2+ control panel and back
label C-1
on FR2+ display screen
on FR2+ status indicator C-2
on Instructions for Use C-1
symbols and controls
6-1
6-1
B-6
B-5
B-2
B-7
B-5
B-5
B-4
B-1
B-1
D-4
D-4
B-1
D-4
C-2
4-12, C-2
2-5
C-2
4-3
B-1
C-3
C-4
C-2
C-1
INDEX
solid red X
see Status Indicator
Index
Page 100

I-6
T
temperature
operating
standby
tiered-response features
training & administration pack
charger
description
troubleshooting
B-1
B-1
6-9
A-1
D-5
4-11
U
user, qualifications and training 1-2
W
warnings, cautions, and dangers
5-2
waveform
see SMART biphasic waveform
X
X Status Indicator
flashing or solid
4-11
solid
4-11
M3860A/M3861A HEARTSTART FR2+
Philips Medical Systems
 Loading...
Loading...