Page 1

A
Guide
To
Freeze
Drying
for the
Laboratory
An Industry Service Publication
Page 2

2
Page 3

Foreword
Introduction
This booklet has been developed to serve as a basic
guide to the freeze drying process. The information
presented is generic in nature and is the result of
research and experience by Labconco personnel and
users of freeze drying equipment. It is our intention to
provide a non-biased review of preparation techniques
and freeze drying methods. The purpose of this booklet is
to help you make an informed choice of equipment for
your laboratory applications.
Our Method
We begin our discussion of freeze drying for the
laboratory by examining the three steps in the process:
prefreezing, primary drying and secondary drying. Next,
we examine a typical freeze drying cycle and the methods
available to facilitate the freeze drying process using
equipment designed for use by laboratories. Finally,
suggestions to optimize successful results are discussed,
including determination of end point, contamination,
backfilling of dried samples and product stability.
A glossary of terms used throughout this booklet to
explain the freeze drying process follows the text, along
with a bibliography.
applications for many years, most commonly in the food
and pharmaceutical industries. There are, however, many
other uses for the process including heat-sensitive
sample preparation, plant material research, the
stabilization of living materials such as microbial
cultures, long term storage of HPLC samples,
preservation of whole animal specimens for museum
display, restoration of books and other items damaged by
water, and the concentration and recovery of reaction
products.
conditions conducive to the freeze drying process. This
equipment is currently available and can accommodate
freeze drying of materials from laboratory scale projects
to industrial production.
solvent from a frozen product by a process called
sublimation. Sublimation occurs when a frozen liquid
goes directly to the gaseous state without passing
through the liquid phase. In contrast, drying at ambient
temperatures from the liquid phase usually results in
changes in the product, and may be suitable only for
some materials. However, in freeze drying, the material
does not go through the liquid phase, and it allows the
preparation of a stable product that is easy to use and
aesthetic in appearance.
freeze dried products do not need refrigeration, and
can be stored at ambient temperatures. Because the cost
of the specialized equipment required for freeze drying
can be substantial, the process may appear to be an
expensive undertaking. However, savings realized by
stabilizing an otherwise unstable product at ambient
temperatures, thus eliminating the need for
refrigeration, more than compensate for the investment
in freeze drying equipment.
Freeze drying has been used in a number of
Specialized equipment is required to create the
Freeze drying involves the removal of water or other
The advantages of freeze drying are obvious. Properly
3
Page 4
Principles of Freeze Drying
Solid
Phase
Liquid
Phase
Va
por
Phase
F
U
S
I
O
N
L
I
N
E
V
A
P
O
R
I
Z
A
T
I
O
N
L
I
N
E
S
U
B
L
I
M
A
T
I
O
N
L
I
N
E
CRITICAL
POINT
TRIPLE
POINT
A
B
C
D
PRESSURE
TEMPERATURE
prefreezing, primary drying, and secondary drying.
from the solid phase to the gaseous phase, material to be
freeze dried must first be adequately prefrozen. The
method of prefreezing and the final temperature of the
frozen product can affect the ability to successfully freeze
dry the material.
preserving structures to be examined microscopically,
but resulting in a product that is more difficult to
freeze dry. Slower cooling results in larger ice crystals
and less restrictive channels in the matrix during the
drying process.
makeup of the product. The majority of products that are
subjected to freeze drying consist primarily of water, the
solvent, and the materials dissolved or suspended in the
water, the solute. Most samples that are to be freeze dried
are eutectics which are a mixture of substances that
freeze at lower temperatures than the surrounding water.
When the aqueous suspension is cooled, changes occur
in the solute concentrations of the product matrix. And
as cooling proceeds, the water is separated from the
solutes as it changes to ice, creating more concentrated
areas of solute. These pockets of concentrated materials
have a lower freezing temperature than the water.
Although a product may appear to be frozen because of
all the ice present, in actuality it is not completely frozen
until all of the solute in the suspension is frozen. The
mixture of various concentration of solutes with the
solvent constitutes the eutectic of the suspension. Only
when all of the eutectic mixture is frozen is the
suspension properly frozen. This is called the eutectic
temperature.
product to below the eutectic temperature before
beginning the freeze drying process. Small pockets of
unfrozen material remaining in the product expand
and compromise the structural stability of the freeze
dried product.
undergoes glass formation during the freezing process.
Instead of forming eutectics, the entire suspension
becomes increasingly viscous as the temperature is
lowered. Finally the product freezes at the glass
transition point forming a vitreous solid. This type of
product is extremely difficult to freeze dry.
to freeze dry a frozen suspension. While these factors can
be discussed independently, it must be remembered that
they interact in a dynamic system, and it is this delicate
balance between these factors that results in a properly
freeze dried product.
established in which ice can be removed from the frozen
product via sublimation, resulting in a dry, structurally
intact product. This requires very careful control of the
two parameters, temperature and pressure, involved in
the freeze drying system. The rate of sublimation of ice
from a frozen product depends upon the difference in
The freeze drying process consists of three stages:
Prefreezing: Since freeze drying is a change in state
Rapid cooling results in small ice crystals, useful in
Products freeze in two ways, depending on the
It is very important in freeze drying to prefreeze the
The second type of frozen product is a suspension that
Primary drying: Several factors can affect the ability
After prefreezing the product, conditions must be
4
vapor pressure of the product compared to the vapor
pressure of the ice collector. Molecules migrate from the
higher pressure sample to a lower pressure area. Since
vapor pressure is related to temperature, it is necessary
that the product temperature is warmer than the cold
trap (ice collector) temperature. It is extremely
important that the temperature at which a product is
freeze dried is balanced between the temperature that
maintains the frozen integrity of the product and the
temperature that maximizes the vapor pressure of the
product. This balance is key to optimum drying. The
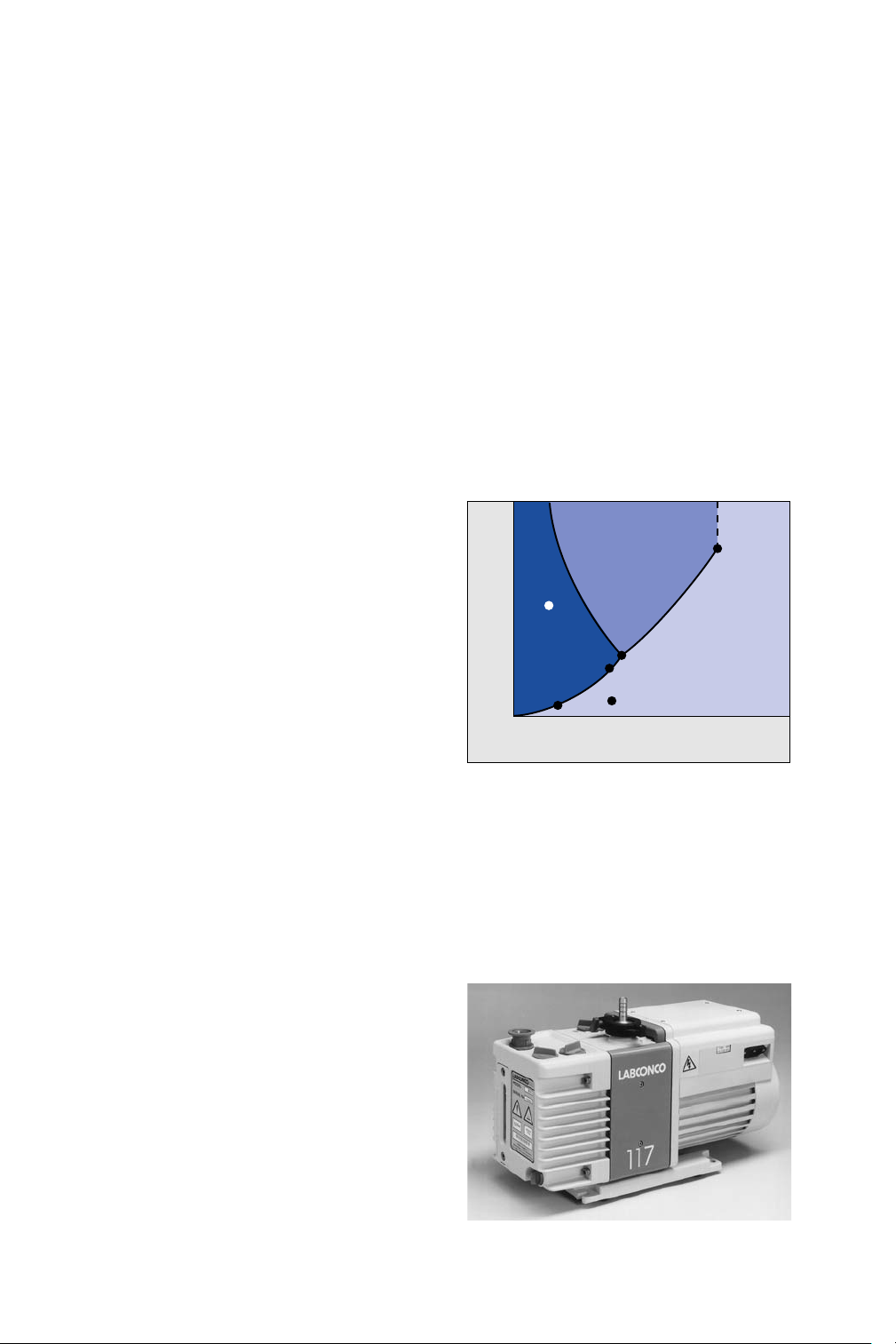
typical phase diagram shown in Figure 1 illustrates this
point. Most products are frozen well below their eutectic
or glass transition point (Point A), and then the
temperature is raised to just below this critical
temperature (Point B) and they are subjected to a
reduced pressure. At this point the freeze drying process
is started.
Figure 1
A typical phase diagram.
Some products such as aqueous sucrose solutions can
undergo structural changes during the drying process
resulting in a phenomenon known as collapse. Although
the product is frozen below its eutectic temperature,
warming during the freeze drying process can affect the
structure of the frozen matrix at the boundary of the
drying front. This results in a collapse of the structural
matrix. To prevent collapse of products containing
A vacuum pump is essential to evacuate the
environment around the product to be freeze dried.
Page 5

sucrose, the product temperature must remain below a
critical collapse temperature during primary drying. The
collapse temperature for sucrose is -32° C.
No matter what type of freeze drying system is used,
conditions must be created to encourage the free flow of
water molecules from the product. Therefore, a vacuum
pump is an essential component of a freeze drying
system, and is used to lower the pressure of the
environment around the product (to Point C). The other
essential component is a collecting system, which is a
cold trap used to collect the moisture that leaves the
frozen product. The collector condenses out all
condensable gases, i.e; the water molecules, and the
vacuum pump removes all non-condensable gases.
The collecting system acts as a cold trap to collect
moisture leaving the frozen product.
It is important to understand that the vapor pressure
of the product forces the sublimation of the water vapor
molecules from the frozen product matrix to the
collector. The molecules have a natural affinity to move
toward the collector because its vapor pressure is lower
than that of the product. Therefore, the collector
temperature (Point D) must be significantly lower than
the product temperature. As can be noted in Table 1,
raising the product temperature has more effect on
the vapor pressure differential than lowering the
collector temperature.
Table 1
Vapor Pressure (mBar) Temperature (°C)
6.104 0
2.599 -10
1.034 -20
0.381 -30
0.129 -40
0.036 -50
0.011 -60
0.0025 -70
0.0005 -80
Vapor Pressure/Temperature Relationships
A third component essential in a freeze drying system
is energy. Energy is supplied in the form of heat. Almost
ten times as much energy is required to sublime a gram
of water from the frozen to the gaseous state as is
required to freeze a gram of water. Therefore, with all
other conditions being adequate, heat must be applied to
the product to encourage the removal of water in the
form of vapor from the frozen product. The heat must be
very carefully controlled, as applying more heat than the
evaporative cooling in the system can remove warms the
product above its eutectic or collapse temperature.
Heat can be applied by several means. One method is
to apply heat directly through a thermal conductor shelf
such as is used in tray drying. Another method is to use
ambient heat as in manifold drying.
Secondary drying: After primary freeze drying is
complete, and all ice has sublimed, bound moisture is
still present in the product. The product appears dry, but
the residual moisture content may be as high as 7-8%.
Continued drying is necessary at the warmer
temperature to reduce the residual moisture content to
optimum values. This process is called isothermal
desorption as the bound water is desorbed from
the product.
Secondary drying is normally continued at a product
temperature higher than ambient but compatible with
the sensitivity of the product. All other conditions, such
as pressure and collector temperature, remain the same.
Because the process is desorptive, the vacuum should be
as low as possible (no elevated pressure) and the collector
temperature as cold as can be attained. Secondary drying
is usually carried out for approximately 1/3 to 1/2 the
time required for primary drying.
How Freeze Drying Works
Refer to the phase diagram (Figure 1) and a typical
sublimation cycle (Figure 2). The product is first cooled
to below its eutectic temperature (Point A). The collector
is cooled to a temperature approximately 20° C cooler
than the product temperature, generally around -50 to
-105° C. The product should be freeze dried at a
temperature slightly lower than its eutectic or collapse
temperature (Point B) since the colder the product, the
longer the time required to complete primary drying, and
the colder the collector temperature required to
adequately freeze dry the product.
After the product is adequately frozen and the
collector temperature achieved, the system is evacuated
using a vacuum pump (Point C). At this point, primary
drying of the product begins and continues until the
entire frozen matrix appears dry. Heat input to the
product may be achieved by several means such as
increasing the shelf temperature in the case of tray
drying, or using a liquid bath for manifold drying. While
the collector and vacuum pump create the conditions for
allowing sublimation to occur, heat input is really the
driving force behind the whole process.
Heat input to the sample can be enhanced by
controlling the pressure in the system at some level
above the ultimate capability of the vacuum pump. Some
5
Page 6

freeze dryers incorporate vacuum control systems that
A
B
C
D
As drying proceeds product
temperature remains below
shelf temperature
When primary drying is complete
product temperature equals
shelf temperature
ERUTAREPMETRESNEDNOC
ERUTAREPMETTCUDORP
ERUTAREPMETFLEHS
TIME
PRESSURE
TEMPERATURE
Water
Vapor
Dry Cake
Frozen Sample
Interface
Build-up
of Frozen
Material
automatically regulate the pressure to the preset level.
This allows additional gas molecules to reside in the
system thereby improving the conduction of heat to the
sample. This improves the sublimation rate, reducing
process time and associated energy costs. Care must be
product, drying becomes more and more difficult. The
water molecules must now travel through the dried
portions of the product which impedes their progress. As
the drying front moves farther and farther down the
matrix, the application of heat to the product becomes
more important (Figure 3).
taken to prevent the pressure within the system from
exceeding the ice vapor pressure of the product or
melting of the sample may occur.
can increase the surface area to volume ratio by
spreading out the frozen product inside the vessel
(Figure 4). Shell freezing is accomplished by rotating the
vessel in a low temperature bath causing the product to
Figure 2
freeze in a thin layer on the inside surface of the vessel.
The thickness of the frozen suspension depends on the
volume of the product in comparison to the size of the
vessel. Shell freezing is primarily used in conjunction
with manifold drying.
drying because the pressure must be maintained at a low
level to ensure adequate water vapor flow from the
product to the collector. A pressure gauge (commonly
called a vacuum gauge) is used to monitor the pressure
in the system during the drying process. Pressure can be
expressed in several different units which are compared
in Table 2. Some gauges measure condensable gases,
while others do not. Those gauges that do not measure
the condensable gases give an indication of the total
pressure in the system. Gauges that do sense the
condensable gases indicate a change in pressure during
Typical Sublimation Cycle found in system utilizing Tray
Dryer with shelves.
drying. These sensors can be used as an indication of
the rate of drying, as well as the endpoint of the
drying process.
Heat input to the product must be very carefully
controlled especially during the early stages of drying.
Figure 4
The configuration of the product container and the
volume of the contained product can affect the amount of
heat that can be applied. For small volumes of material,
evaporative cooling compensates for high levels of heat
and drying is accelerated.
The volume and configuration of the suspension to be
freeze dried often determines how the material is freeze
dried. For example, the greater the ratio of the surface
area to the volume of the suspension, the faster drying
occurs. This is because a greater area for the water
molecules to leave the product exists compared to the
distance they have to travel to reach the surface of the
frozen matrix. Drying occurs from the top of the product
and initially the removal of water molecules is efficient.
However, as the drying front moves down through the
Shell freezing as a method of prefreezing the product
The vacuum system is very important during freeze
Figure 3
Ambient room temperature provides heat to encourage
removal of water vapor from frozen samples when
manifold drying.
6
Shell freezing can increase the surface area to volume
ratio by spreading out the frozen product inside the vessel.
Table 2
Microns mm Hg Torr mBar
1000 111.33
100 0.1 0.1 0.133
10 0.01 0.01 0.03
Pressure Relationships
Page 7

Freeze Drying Methods
Three methods of freeze drying are commonly used:
(1) manifold drying, (2) batch drying, and (3) bulk
drying. Each method has a specific purpose, and the
method used depends on the product and the final
configuration desired.
Manifold Method. In the manifold method, flasks,
ampules or vials are individually attached to the ports of
a manifold or drying chamber. The product is either
frozen in a freezer, by direct submersion in a low
temperature bath, or by shell freezing, depending on the
nature of the product and the volume to be freeze dried.
The prefrozen product is quickly attached to the drying
chamber or manifold to prevent warming. The vacuum
must be created in the product container quickly, and the
operator relies on evaporative cooling to maintain the
low temperature of the product. This procedure can only
be used for relatively small volumes and products with
high eutectic and collapse temperatures.
Manifold drying has several advantages over batch tray
drying. Since the vessels are attached to the manifold
individually, each vial or flask has a direct path to the
collector. This removes some of the competition for
molecular space created in a batch system, and is most
ideally realized in a cylindrical drying chamber where the
distance from the collector to each product vessel is the
same. In a “tee” manifold, the water molecules leaving
the product in vessels farthest from the collector
experience some traffic congestion as they travel past the
ports of other vessels.
vessels to ambient temperature or via a circulating bath.
For some products, where precise temperature control is
required, manifold drying may not be suitable.
system allowing drying of different products at the same
time, in different sized vessels, with a variety of closure
systems. Since the products and their volumes may differ,
each vessel can be removed from the manifold separately
as its drying is completed. The close proximity to the
collector also creates an environment that maximizes
drying efficiency.
similar sized vessels containing like products are placed
together in a tray dryer. The product is usually prefrozen
on the shelf of the tray dryer. Precise control of the
product temperature and the amount of heat applied to
the product during drying can be maintained. Generally
all vials in the batch are treated alike during the drying
process, although some variation in the system can
occur. Slight differences in heat input from the shelf can
be experienced in different areas. Vials located in the
front portion of the shelf may be radiantly heated
through the clear door. These slight variations can result
in small differences in residual moisture.
same time, under the same atmospheric conditions. The
vials can be stoppered in a vacuum, or after backfilling
with inert gas. Stoppering of all vials at the same time
ensures a uniform environment in each vial and uniform
product stability during storage. Batch drying is used to
prepare large numbers of ampules or vials of one
product.
Heat input can be affected by simply exposing the
Several vessels can be accommodated on a manifold
Batch Method. In batch drying, large numbers of
Batch drying allows closure of all vials in a lot at the
In the manifold drying method, flasks are individually
attached to the ports of a drying chamber.
7
Batch drying in a tray dryer permits precise control of
product temperature and heat input.
Bulk Method. Bulk drying is generally carried out in
a tray dryer like batch drying. However, the product is
poured into a bulk pan and dried as a single unit.
Although the product is spread throughout the entire
surface area of the shelf and may be the same thickness
as product dried in vials, the lack of empty spaces within
the product mass changes the rate of heat input. The
heat input is limited primarily to that provided by
contact with the shelf as shown in Figure 5.
Bulk drying does not lend itself to sealing of product
under controlled conditions as does manifold or batch
drying. Usually the product is removed from the freeze
dry system prior to closure, and then packaged in air
tight containers. Bulk drying is generally reserved for
stable products that are not highly sensitive to oxygen
or moisture.
Page 8

Figure 5
Some heat input at
edges where drying
occurs faster than
in middle
In bulk drying, heat is provided primarily through
conduction from shelf.
Determining Drying Endpoints
Several means can be used to determine the endpoint
of primary drying. The drying boundary in batch drying
containers has moved to the bottom of the product
container and inspection reveals that no ice is visible in
the product. No visible ice indicates only that drying at
the edges of the container is complete and gives no
indication of the conditions in the center of the product.
An electronic vacuum gauge can be used to measure
condensable gases in the system. When the pressure
indicated by the electronic gauge reaches the minimum
pressure attainable by the system, as no more water
vapor is leaving the product.
As the heat input to the product is increased,
evaporative cooling keeps the product temperature well
below the temperature of its surrounding atmosphere.
When primary drying is complete, the product
temperature rises to equal the temperature of its
environment. In manifold systems and tray dryers with
external collectors, the path to the collector can be shut
off with a valve and the pressure above the product
measured with a vacuum gauge. If drying is still
occurring, the pressure in the system increases.
Contamination in a
Freeze Dry System
Two types of contamination can occur in a freeze dry
system. One results from freeze drying microorganisms
and the other results from freeze drying corrosive
materials.
The potential for contamination of a freeze drying
system by microorganisms is real in any system where
microorganisms are freeze dried without a protective
barrier such as a bacteriological filter. Contamination is
most evident in batch tray dryer systems where large
numbers of vials are dried in a single chamber. Evidence
for contamination may be found by sampling the surfaces
of the vials, shelves and collector. The greatest degree of
contamination is usually found on the vials and on the
collector. Although some vial contamination may be due
to sloppiness in dispensing the material originally,
contamination on the collector is due to microorganisms
traveling from the product to the collector through the
vapor stream.
The potential for contamination must be considered
every time microorganisms are freeze dried, and
precautions must be taken in handling material after the
freeze dry process is completed. Recognizing that the
vials are potentially contaminated, the operator should
remove the vials to a safe area such as a laminar flow
hood for decontamination. Decontamination of the freeze
dry system depends upon the type of freeze dry system
used. Some tray dryer systems are designed for
decontamination under pressure using ethylene oxide
sterilization. Ethylene oxide is considered hazardous,
corrosive and detrimental to rubber components. Its use
should be carefully monitored.
Coupled with the risk of contamination in a freeze dry
system is the risk of cross contamination when freeze
drying more than one product at time. It is not good
practice to mix microbiological products in a freeze dry
system unless some type of bacteriological filter is
used to prevent the microbial product from leaving the
vial itself.
While freeze drying of corrosive materials does not
necessarily present a risk to the operator, it does present
a risk of damaging the freeze dry system itself. Although
freeze dry systems are designed using materials that
resist corrosion and prevent the build up of corrosive
materials, care should be taken to clean the system
thoroughly following each use to protect it from damage.
Backfilling
For many freeze dried products, the most ideal system
of closure is while under vacuum. This provides an
environment in which moisture and oxygen, both
detrimental to the freeze dried material, are prevented
from coming in contact with the product. In some cases,
vacuum in a container may be less than ideal, especially
when a syringe is used to recover the product, or when
opening the vessel results in a rush of potentially
contaminating air. In these cases, backfilling the product
container with an inert gas such as argon or nitrogen is
often beneficial. The inert gas must be ultrapure,
containing no oxygen or moisture.
Backfilling of the product container is generally useful
in a batch tray dryer type system. The backfilling should
also be carried out through a bacteriological filter. It is
important that the gas flow during backfilling be slow
enough to allow cooling of the gas to prevent raising the
collector temperature. Backfilling can be carried out to
any desired pressure in those tray dryers that have
internal stoppering capability, and the vials then
stoppered at the desired pressure.
8
Page 9

Stability of Freeze Dried Products
Several factors can affect the stability of freeze
dried material. Two of the most important are moisture
and oxygen.
All freeze dried products have a small amount of
moisture remaining in them termed residual moisture.
The amount of moisture remaining in the material
depends on the nature of the product and the length of
secondary drying. Residual moisture can be measured by
several means: chemically, chromatographically,
manometrically or gravimetrically. It is expressed as a
weight percentage of the total weight of the dried
product. Residual moisture values range from <1% to 3%
for most products.
By their nature, freeze dried materials are hygroscopic
and exposure to moisture during storage can destabilize
the product. Packaging used for freeze dried materials
must be impermeable to atmospheric moisture. Storing
products in low humidity environments can reduce the
risk of degradation by exposure to moisture. Oxygen is
also detrimental to the stability of most freeze dried
material so the packaging used must also be
impermeable to air.
The detrimental effects of oxygen and moisture are
temperature dependent. The higher the storage
temperature, the faster a product degrades. Most freeze
dried products can be maintained at refrigerator
temperatures, i.e. 4-8° C. Placing freeze dried products at
lower temperatures extends their shelf life. The shelf life
of a freeze dried product can be predicted by measuring
the rate of degradation of the product at an elevated
temperature. This is called accelerated storage. By
choosing the proper time and temperature relationships
at elevated temperatures, the rate of product degradation
can be predicted at lower storage temperatures.
Contact Labconco for a complimentary catalog of Freeze
Dry Systems designed for laboratory use.
about freeze drying equipment. It is available upon
request from your laboratory supply dealer or Labconco
Corporation at (800) 821-5525, (816) 333-8811 or
www.labconco.com.
The Labconco catalog provides additional information
9
Page 10

Glossary
Accelerated Storage: Exposure of freeze dried products to
elevated temperatures to accelerate the degradation process that
occurs during storage.
Batch Freeze Drying: Freeze drying multiple samples of the
same product in similar sized vessels at the same time in a shelf
tray dryer.
Bulk Freeze Drying: Freeze drying a large sample of a single
product in one vessel such as the bulk drying pans designed for
shelf tray dryers.
Collapse: A phenomenon causing collapse of the structural
integrity of a freeze dried product due to too high a temperature
at the drying front.
Collapse Temperature: The temperature above which
collapse occurs.
Collector: A cold trap designed to condense the water vapor
flowing from a product undergoing freeze drying.
Internal Collector: A collector located in the same area as the
product. All water vapor has a free path to the collector.
External Collector: A collector located outside the product
area connected by a small port through which all water vapor
must pass. Allows isolation of the product from the collector
for drying end point determinations and easier defrosting.
Ethylene Oxide: A colorless, odorless gas used for gas
sterilization of tray dryer systems.
Eutectics: Areas of solute concentration that freeze at a lower
temperature than the surrounding water. Eutectics can occur at
several different temperatures depending on the complexity of
the product.
Eutectic Temperature: The temperature at which all areas of
concentrated solute are frozen.
Evaporative Cooling: Cooling of a liquid at reduced pressures
caused by loss of the latent heat of evaporation.
Freeze Drying: The process of drying a frozen product by
creating conditions for sublimation of ice directly to
water vapor.
Glass Transition Temperature: The temperature at which
certain products go from a liquid to a vitreous solid without ice
crystal formation.
Isothermal Desorption: The process of desorbing water from a
freeze dried product by applying heat under vacuum.
Lyophilization: The freeze drying process.
Manifold Freeze Drying: A freeze drying process where each
vessel is individually attached to a manifold port resulting in a
direct path to the collector for each vessel.
Prefreezing: The process of cooling a product to below its
eutectic temperature prior to freeze drying.
Pressure Gauge (Vacuum Gauge): An instrument used to
measure very low pressures in a freeze drying system.
Thermocouple Gauge: A pressure gauge that measures only
the condensable gases in the system. This gauge can be used
as an indicator of drying end points.
McLeod Gauge: A mercury gauge used to measure total
pressure in the system (i.e. condensable and noncondensable gases.)
Primary Drying: The process of removing all unbound
water that has formed ice crystals in a product undergoing
freeze drying.
Residual Moisture: The small amount of bound water that
remains in a freeze dried product after primary drying. Residual
moisture is expressed as the weight percentage of water
remaining compared to the total weight of the dried product.
The amount of residual moisture in a freeze dried product can
be reduced during secondary drying.
Secondary Drying: The process of reducing the amount of
bound water in a freeze dried product after primary drying is
complete. During secondary drying, heat is applied to the
product under very low pressures.
Shell Freezing: Freezing a product in a thin layer that coats the
inside of the product container. Shell freezing is accomplished
by swirling or rotating the product container in a low
temperature bath.
Sublimation: The conversion of water from the solid state (ice)
directly to the gaseous state (water vapor) without going
through the liquid state.
Vapor Pressure: The pressure of the vapor in equilibrium with
the sample.
10
Page 11

Bibliography
1. Barbaree, J.M. and A. Sanchez. 1982. Cross-contamination
during lyophilization. Cryobiology 19:443-447.
2. Barbaree, J.M., A. Sanchez and G.N. Sanden. 1985.
Problems in freeze-drying: I. Stability in glass-sealed
rubber stoppered vials. Developments in Industrial
Microbiology 26:397-405.
3. Barbaree, J.M., A. Sanchez and G.N. Sanden. 1985.
Problems in freeze-drying: II. Cross-contamination
during lyophilization. Developments in Industrial
Microbiology 26:407-409.
4. Flink, J.M. and Knudsen, H. 1983. An Introduction to
Freeze Drying. Strandberg Bogtryk/Offset, Denmark.
5. Flosdorf, E.W. 1949. Freeze-Drying. Reinhold Publishing
Corporation, New York.
6. Greiff, D. 1971. Protein structure and freeze-drying: the
effects of residual moisture and gases. Cryobiology 8:145-
152.
7. Greiff, D. and W.A. Rightsel. 1965. An accelerated storage
test for predicting the stability of suspensions of measles
virus dried by sublimation in vacuum. Journal of
Immunology 94:395-400.
8. Greaves, R.I.N., J. Nagington, and T.D. Kellaway. 1963.
Preservation of living cells by freezing and by drying.
Federation Proceedings 22:90-93.
9. Harris, R.J.C., Ed. 1954. Biological Applications of Freezing
and Drying. Academic Press, New York.
10. Heckly, R.J. 1961. Preservation of bacteria by lyophilization.
Advances in Applied Microbiology 3:1-76.
11. Heckly, R.J. 1985. Principles of preserving bacteria
by freeze-drying. Developments in Industrial Microbiology
26:379-395.
12. Jennings, Thomas. 2002. Lyophilization: Introduction and
Basic Principles. CRC Press, Florida.
13. King, C.J. 1971. Freeze-Drying of Foods. CRC Press,
Cleveland.
14. May, M.C. E. Grim, R.M. Wheeler and J. West. 1982.
Determination of residual moisture in freeze-dried
viral vaccines: Karl Fischer, gravimetric, and
thermogravimetric methodologies. Journal of Biological
Standardization 10:249-259.
15. Mellor, J.D. 1978. Fundamentals of Freeze-Drying.
Academic Press, London.
16. Nail, S.L. 1980. The effect of chamber pressure on heat
transfer in the freeze-drying of parental solutions.
Journal of the Parental Drug Association 34:358-368.
17. Nicholson, A.E. 1977. Predicting stability of
lyophilized products. Developments in Biological
Standardization 36:69-75.
18. Parkes, A.S., and A.U. Smith, Eds. 1960. Recent Research
in Freezing and Freeze-Drying. Charles C. Thomas,
Springfield.
19. Rey, L.R., Ed. 1960. Traite de Lyophilization.
Hermann, Paris.
20. Rey, L.R., Ed. 1964. Aspects Theorique et Industriels de la
Lyophilisation. Hermann, Paris.
21. Rowe, T.W.G. 1970. Freeze-drying of biological materials:
some physical and engineering aspects. Current Trends in
Cryobiology: 61-138.
22. Seligman, E.B. and J.F. Farber. 1971. Freeze-drying and
residual moisture. Cryobiology 8:138-144.
11
Page 12

Labconco Corporation
8811 Prospect Avenue
Kansas City, Missouri 64132-2696
816-333-8811, 800-821-5525
FAX: 816-363-0130
www.labconco.com
© 2010 by Labconco Corporation Printed in the U.S.A. 3-53-8/10-James-3M-R8
 Loading...
Loading...