Invivo Expression MRI Service manual
MRI Patient Monitoring System
Model 865214
EXPRESS SERVICE MANUAL
*989803162701*
989803162701 Rev. D
Proprietary Information
This document contains proprietary information, which is protected by copyright.
Manufacturer
Invivo Corporation 12501 Research Parkway Orlando, FL 32826
(407) 275 3220
(800) 331 3220 www.invivocorp.com
Warranty
The information contained in this document is subject to change without notice. Invivo Corporation makes no warranty of any kind with regard to this material, including, but not limited to, the implied warranties or merchantability and fitness for a particular purpose. Invivo Corporation shall not be liable for errors contained herein or for incidental or consequential damages in connection with the furnishing, performance, or use of this material.
Limits of Liability
Invivo has taken care to ensure the accuracy of this document. However, Invivo assumes no liability for errors or omissions and reserves the right to make changes without further notice to any products herein to improve reliability, function, or design. Invivo may make improvements or changes in the product(s) or program(s) described in this document at any time.
Printing History
New revisions of this document will incorporate all material updated since the previous revision. Update packages may be issued between revisions and contain replacement and additional pages to be merged by a revision date at the bottom of the page. The documentation part number (P/N) and revision indicate the current edition.
P/N 989803162701 Rev. D
Copyright © 2010, Invivo Corporation
All rights reserved. Printed in U.S.A

Contents
1 |
Introduction ..................................................................................................................... |
|
1 |
||
|
1.1 |
About this Manual ........................................................................................................... |
1 |
||
|
1.2 |
Text Conventions ............................................................................................................. |
1 |
||
2 |
Unpacking the System ...................................................................................................... |
3 |
|||
3 |
System Overview.............................................................................................................. |
|
5 |
||
|
3.1 |
Cart Configuration............................................................................................................ |
5 |
||
|
3.2 |
Patient Management Configuration................................................................................ |
5 |
||
|
|
3.2.1 Battery Operation: Cart, PMC, and DCU.................................................................... |
6 |
||
|
|
|
3.2.1.1 |
Installation and Removal ...................................................................... |
7 |
|
|
3.2.2 Battery Operation: WECG and WSpO2 Modules...................................................... |
8 |
||
|
|
|
3.2.2.1 |
Installation and Removal ...................................................................... |
9 |
|
|
3.2.3 |
Safe Battery Use............................................................................................................ |
9 |
|
|
|
3.2.4 |
Battery Charging Instructions.................................................................................... |
10 |
|
|
|
3.2.5 |
Battery Disposal Instructions .................................................................................... |
10 |
|
|
|
|
3.2.5.1 |
Guidelines in Europe........................................................................... |
11 |
|
|
|
3.2.5.2 Guidelines in the United States .......................................................... |
11 |
|
4 |
System Installation ......................................................................................................... |
|
13 |
||
|
4.1 |
Power Supply ................................................................................................................. |
|
13 |
|
|
|
4.1.1 |
Part Number 989803169201..................................................................................... |
13 |
|
|
|
4.1.2 |
Part Number 989803168201..................................................................................... |
15 |
|
|
4.2 |
Cart Configuration.......................................................................................................... |
16 |
||
|
4.3 |
Patient Management Configuration (PMC) ................................................................... |
17 |
||
|
4.4 |
Display Control Unit (DCU) ............................................................................................ |
18 |
||
|
|
4.4.1 Installed on the Cart ................................................................................................... |
18 |
||
|
|
4.4.2 |
Stand Alone Operation............................................................................................... |
19 |
|
|
4.5 |
Additional Installation Options ...................................................................................... |
21 |
||
i

5 |
System Power Up ........................................................................................................... |
|
|
23 |
||
|
5.1 |
Removing AC Mains Power |
............................................................................................ |
23 |
||
6 |
Test and Inspection ........................................................................................................ |
|
|
25 |
||
|
6.1 |
When to Test.................................................................................................................. |
|
|
25 |
|
|
6.2 |
Test and Inspection Matrix Checklist............................................................................. |
25 |
|||
|
6.3 |
Performance Assurance Test Checklist.......................................................................... |
26 |
|||
|
6.4 |
Recommended Frequency of ............................................................................Testing |
27 |
|||
|
6.5 |
Test Process Description................................................................................................ |
|
28 |
||
|
|
6.5.1 |
Test Equipment Requirements ................................................................................. |
28 |
||
|
|
6.5.2 |
Initial AC Power Up..................................................................................................... |
|
28 |
|
|
|
|
6.5.2.1 |
ETCO2 Option .............................................................................Only |
28 |
|
|
|
6.5.3 |
Operational Tests........................................................................................................ |
|
28 |
|
|
|
|
6.5.3.1 |
System Communication ..................................................Verification |
29 |
|
|
|
|
6.5.3.2 |
WECG Communication........................................................................ |
29 |
|
|
|
|
6.5.3.3 |
WSpO2 Communication...................................................................... |
30 |
|
|
|
|
6.5.3.4 |
NIBP Verification ................................................................................. |
31 |
|
|
|
|
|
6.5.3.4.1 .................................. |
Pneumatic Leak Test High (1 st row) |
31 |
|
|
|
|
6.5.3.4.2 .................................. |
Pneumatic Leak Test Low (2 nd row) |
31 |
|
|
|
|
6.5.3.4.3 .................................................... |
NIBP Offset Verification |
31 |
|
|
|
|
6.5.3.4.4 ............................................... |
Calibration Accuracy Check |
32 |
|
|
|
|
6.5.3.4.5 ........................................................... |
Overpressure Adult |
32 |
|
|
|
|
6.5.3.4.6 ..................................................... |
Overpressure Neonatal |
33 |
|
|
|
|
6.5.3.4.7 .................................................................. |
Adult Cuff Test |
33 |
|
|
|
|
6.5.3.4.8 ........................................................ |
Adult Dead Man Cuff |
34 |
|
|
|
|
6.5.3.4.9 ..................................................................... |
Neo Natal BP |
34 |
|
|
|
|
6.5.3.4.10 ................................................. |
Neo Natal Dead Man Cuff |
35 |
|
|
|
|
6.5.3.4.11 ........................................................ |
Leak Time Out, Adult |
35 |
|
|
|
|
6.5.3.4.12 ................................................. |
Leak Time Out, Neo Natal |
35 |
|
|
|
|
6.5.3.4.13 ................................................................... |
Calibrate Error |
36 |
ii

|
|
|
6.5.3.5 |
Invasive Pressure Verification............................................................. |
36 |
|
|
|
|
6.5.3.6 |
Agents Verification.............................................................................. |
37 |
|
|
|
|
|
6.5.3.6.1 |
Agents Flow Test ............................................................... |
37 |
|
|
|
|
6.5.3.6.2 |
O2 Sensor Verification ...................................................... |
39 |
|
|
|
6.5.3.7 ETCO2 Flow Test Verification.............................................................. |
39 |
||
|
|
|
6.5.3.8 |
Respiration Verification ...................................................................... |
40 |
|
|
|
|
6.5.3.9 |
Temperature Verification ................................................................... |
40 |
|
|
|
6.5.4 |
Electrical Safety Testing ............................................................................................. |
40 |
||
7 |
Removal and Replacement ............................................................................................. |
|
41 |
|||
|
7.1 |
The Cart WPU................................................................................................................. |
|
|
41 |
|
|
|
7.1.1 WPU Removal from the Cart ..................................................................................... |
41 |
|||
|
|
7.1.2 WPU Replacement into the Cart............................................................................... |
45 |
|||
|
|
7.1.3 |
Exchange Network Setup........................................................................................... |
48 |
||
|
|
|
7.1.3.1 |
Network Channel Options................................................................... |
48 |
|
|
|
|
7.1.3.2 |
Exchange Parameter Disabling ........................................................... |
49 |
|
|
7.2 |
The PMC WPU................................................................................................................ |
|
|
49 |
|
|
|
7.2.1 WPU Removal from the PMC .................................................................................... |
49 |
|||
|
|
7.2.2 WPU Replacement into the PMC.............................................................................. |
54 |
|||
|
|
7.2.3 |
Exchange Network Setup........................................................................................... |
60 |
||
|
|
|
7.2.3.1 |
Network Channel Options................................................................... |
60 |
|
|
|
|
7.2.3.2 |
Exchange Parameter Disabling ........................................................... |
61 |
|
8 |
Service Parts................................................................................................................... |
|
|
63 |
||
|
8.1 |
Non Exchange Service Parts .......................................................................................... |
63 |
|||
|
8.2 |
Exchange Service Parts .................................................................................................. |
|
65 |
||
|
8.3 |
System Preventative Maintenance Parts....................................................................... |
66 |
|||
|
8.4 |
Service Numbers and Replacement/Exchange Parts..................................................... |
66 |
|||
9 |
List of Symbols................................................................................................................ |
|
|
69 |
||
iii

iv

1 INTRODUCTION
This Express Service manual is intended for use by qualified service personnel for the repair and maintenance of the Expression MRI Patient Monitoring System. This manual contains information regarding the installation, intended use, accessories, and troubleshooting of a fully equipped Expression MRI Patient Monitoring System. The terms “Cart” and “Patient Management Configuration (PMC)” are used throughout this document to refer to the configurations of the Expression MRI Patient Monitoring System. Specific differences between available configurations, where applicable, are noted in the text. Some information in this manual may depict monitoring features that your system does not contain. For information on features and enhancements that are not included in your system, contact Invivo Corporation at (800) 331 3220 or your Invivo sales representative. For additional information about your accessories, please consult the documentation that accompanied the accessory.
1.1About this Manual
This manual contains the following sections:
Section 1: Introduction, page 1.
Section 2: Unpacking the System, pages 3–4.
Section 3: System Overview, pages 5–11.
Section 4: System Installation, pages 13–21.
Section 5: System Power Up, page 23.
Section 6: Test and Inspection, pages 25–40.
Section 7: Removal and Replacement, pages 41–61.
Section 8: Service Parts, pages 63–67.
Section 9: List of Symbols, pages 69–72.
1.2Text Conventions
The manual uses the following conventions for Warnings, Cautions, and Notes:
WARNING: A Warning calls attention to a condition or possible situation that could cause injury to the user and/or patient.
CAUTION: A Caution calls attention to a condition or possible situation that could damage or destroy the product or the user’s work.
Note: A Note calls attention to notable details or to conventions used within this text.
WARNING: THIS PAGE CONTAINS COPYRIGHTED MATERIALS THAT ARE CONFIDENTIAL AND/OR PROPRIETARY. ANY RELEASE OR DISTRIBUTION
OF THIS MATERIAL, WITHOUT PERMISSION, IS A VIOLATION OF LAW. 1 of 82

Section 1: Introduction
WARNING: THIS PAGE CONTAINS COPYRIGHTED MATERIALS THAT ARE CONFIDENTIAL AND/OR PROPRIETARY. ANY RELEASE OR DISTRIBUTION
OF THIS MATERIAL, WITHOUT PERMISSION, IS A VIOLATION OF LAW. 2 of 82

2 UNPACKING THE SYSTEM
This section provides instructions regarding the unpacking and inspection of the Expression MRI Patient Monitoring System. Remove the system components from the shipping container(s). Verify the presence of all ordered items against the included packing list and purchase request. Carefully examine all components for any damage that may have occurred during shipment. Save the packing materials and related shipping documents, as these will be required to process a claim with the carrier if damage during shipment occurred. To resolve any issues or concerns with your order or product, or to report shipping damage, contact Invivo Customer Service; see Warranty.
CAUTION: The Expression MRI Patient Monitoring System must be used and stored according to the environmental specifications in Appendix A of the Instructions for Use (IFU) manual. Failure to follow these specifications can affect the accuracy of the Expression MRI Patient Monitoring System.
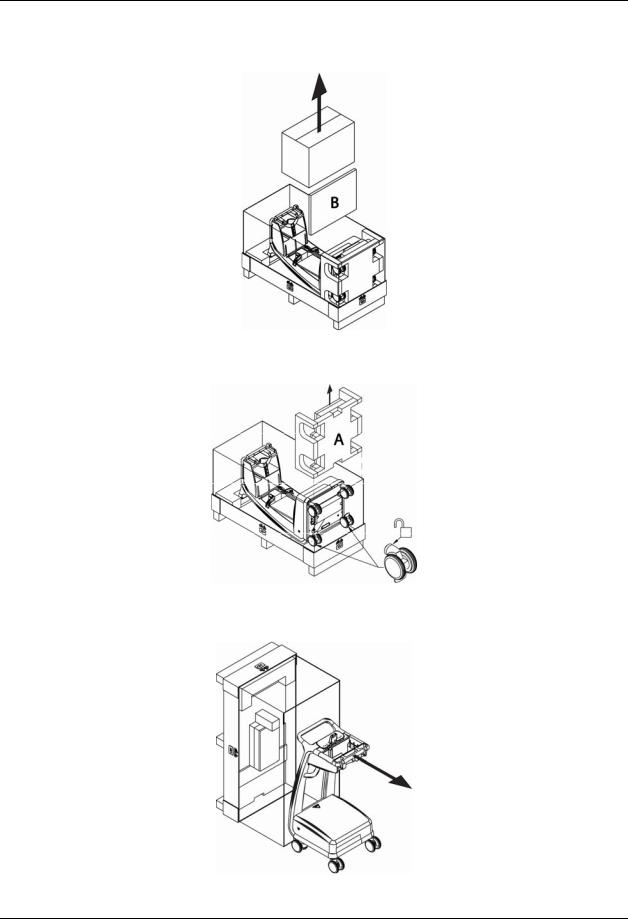
1. Open the 4 Snaps that secure the lid to the crate. Remove the lid and Foam Insert C from the crate.
WARNING: THIS PAGE CONTAINS COPYRIGHTED MATERIALS THAT ARE CONFIDENTIAL AND/OR PROPRIETARY. ANY RELEASE OR DISTRIBUTION
OF THIS MATERIAL, WITHOUT PERMISSION, IS A VIOLATION OF LAW. 3 of 82
Section 2: Unpacking the System
2. Remove the Accessories Box and Foam Insert B.
3. Remove Foam Insert A and unlock the wheels of the Cart.
4. Carefully raise the crate and then roll out the Cart.
WARNING: THIS PAGE CONTAINS COPYRIGHTED MATERIALS THAT ARE CONFIDENTIAL AND/OR PROPRIETARY. ANY RELEASE OR DISTRIBUTION
OF THIS MATERIAL, WITHOUT PERMISSION, IS A VIOLATION OF LAW. 4 of 82

3 SYSTEM OVERVIEW
Depending on the system, the Expression MRI Patient Monitor has several possible configurations.
3.1Cart Configuration
The Cart configuration consists of the following primary components:
One Cart, including the Wireless Processing Unit (WPU)
One Display Control Unit (DCU)
One Wireless ECG (WECG) Module
One Wireless WSpO2 (WSpO2) Module
Two Wireless Module Batteries
Four Cart / DCU Batteries
One DCU Power Converter Kit
One Power Supply
The DCU may be docked on the Cart or located remotely; see the Expression IFU for details. When docked on the Cart and with the Cart connected to AC mains power, the DCU and Cart batteries are charged through the battery charger contained within the Cart. For added flexibility, the system can be configured with two additional DCUs. The additional DCUs can be located in the MR system room, MR control room, or MR holding area. Refer to the Expression IFU for warnings, cautions, and instructions regarding the DCU.
3.2Patient Management Configuration
The Patient Management Configuration (PMC) consists of the following primary components:
One Patient Management Configuration (including the WPU)
One DCU
One WECG module
One WSpO2 module
Two Wireless Module Batteries
Four PMC / DCU Batteries
One DCU Power Converter Kit
Power Supply(s): one with dual DC Outputs, or two with single DC Outputs
Several options are available for the placement of the PMC, including:
Horizontal mounting to a patient examination table;
Vertical mounting to a patient examination table;
Mounting to an anesthesia cart; and,
Mounting to a stationary surface.
With the PMC connected to AC mains power, the batteries within the PMC are charged through the internal battery charger. The accompanying DCU can be located in the MR system room, MR control room, or MR holding area. For added flexibility, the system can also be configured with two
WARNING: THIS PAGE CONTAINS COPYRIGHTED MATERIALS THAT ARE CONFIDENTIAL AND/OR PROPRIETARY. ANY RELEASE OR DISTRIBUTION
OF THIS MATERIAL, WITHOUT PERMISSION, IS A VIOLATION OF LAW. 5 of 82
Section 3: System Overview
additional DCUs. Refer to the Expression IFU manual for warnings, cautions, and instructions regarding the DCU.
3.2.1Battery Operation: Cart, PMC, and DCU
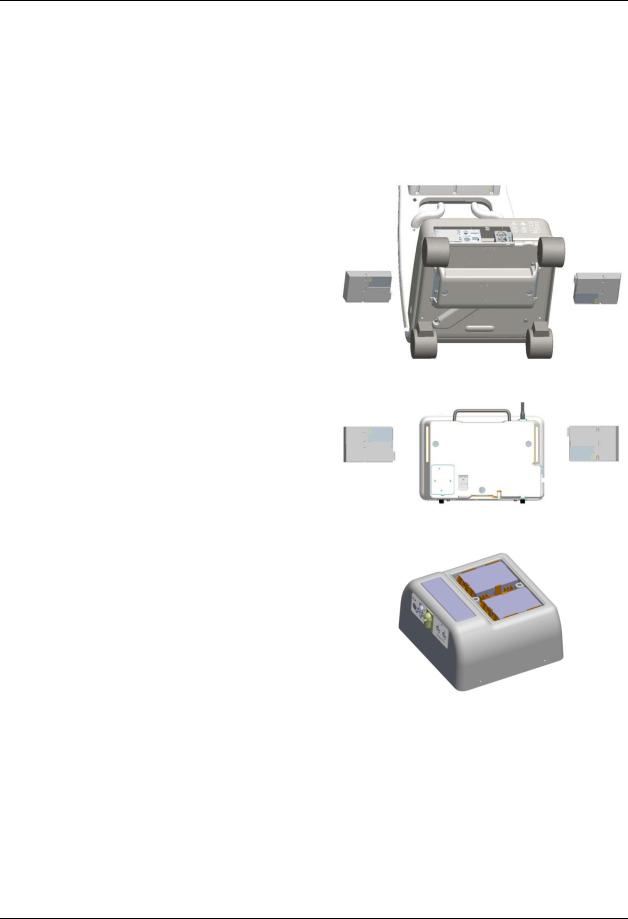
The Cart, PMC, and DCU batteries are interchangeable. Depending upon your equipment type, the number and location of the batteries (P/N 989803169491) will differ:
The Cart has two batteries located on the underside of the unit.
The DCU has two batteries located on the left and right sides of the unit.
The PMC has two batteries located beneath the latched top cover on the unit.
The lower portion of the DCU contains the System Status area, dedicated to displaying the battery and wireless communication status; see Section 6.5.3. (Refer to Expression IFU manual for a full explanation of the Battery Status Display.) Maximum operation time of the Cart, PMC, and DCU batteries is approximately 8 hours when anesthetic agents and CO2 are turned off, and NIBP, ECG, and SpO2 parameters are running at 5 minute intervals. Battery operation time will be reduced by up to 2 hours when performing certain operations such as anesthetic agents monitoring, printing charts and trends, or running short automatic NIBP cycle times.
WARNING: THIS PAGE CONTAINS COPYRIGHTED MATERIALS THAT ARE CONFIDENTIAL AND/OR PROPRIETARY. ANY RELEASE OR DISTRIBUTION
OF THIS MATERIAL, WITHOUT PERMISSION, IS A VIOLATION OF LAW. 6 of 82

Section 3: System Overview
When the AC mains power is interrupted and batteries in the Cart, PMC, and DCU are inserted, the Expression automatically switches to internal batteries.
Note: To prevent unintentional power interruption, Invivo recommends keeping the batteries inserted in the equipment, even when operating under AC mains power.
3.2.1.1Installation and Removal
WARNING: The batteries used within the Cart, PMC, and DCU contain some ferrous materials that are attracted to the MR magnetic field. DO NOT install or remove the batteries from the devices when these units are closer than the 1,000 Gauss (0.1 T) field line as measured from the center line of the MR bore. The batteries will be attracted to the magnetic field, possibly causing patient or user injury.
CAUTION: Never force a battery into the battery compartment; damage to the battery or the Expression MRI Patient Monitoring System will occur.
To install batteries into the Cart, PMC, and DCU, the batteries must be oriented properly to latch into place in the battery compartments, as the battery contours fit the device geometry. Slide the batteries into their respective compartments and they will automatically latch into place. If the battery does not latch automatically when fully inserted into the battery compartment, then the battery is not positioned properly. In this case, reorient the battery then retry installation.
To install batteries in the PMC, follow the steps below.
WARNING: THIS PAGE CONTAINS COPYRIGHTED MATERIALS THAT ARE CONFIDENTIAL AND/OR PROPRIETARY. ANY RELEASE OR DISTRIBUTION
OF THIS MATERIAL, WITHOUT PERMISSION, IS A VIOLATION OF LAW. 7 of 82
Section 3: System Overview
Step |
Battery Installation – PMC |
|
|
1. |
Move the PMC away from the MR system, outside of the 1,000 |
|
Gauss (0.1T) field line (as measured from the center line of the MR |
|
bore), prior to installing batteries. |
|
|
2. |
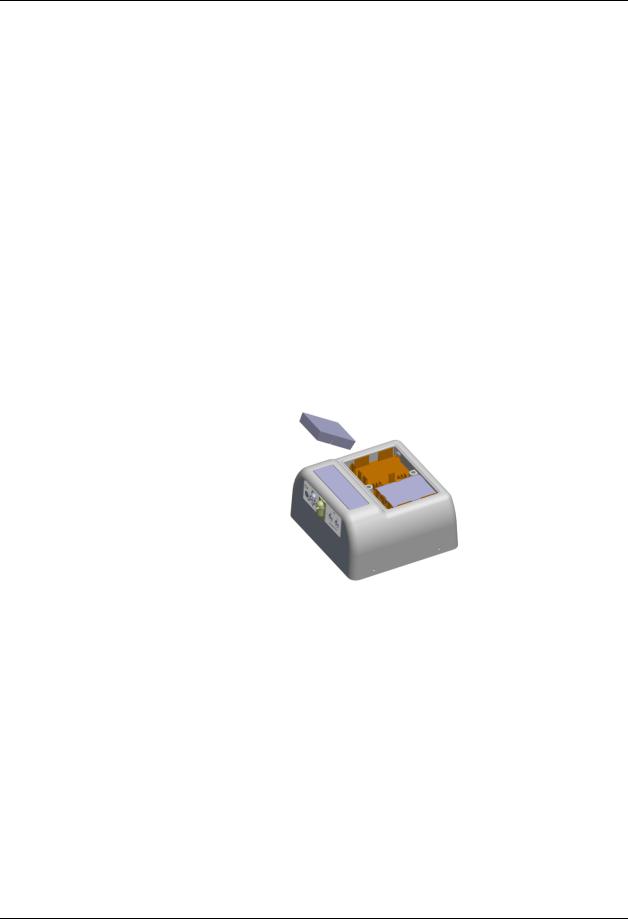
Remove the Battery Compartment Cover by lifting the latches. |
|
|
3. |
Slide the batteries into their respective holder until seated (refer to |
|
the following figures). |
|
|
4. |
Install the Battery Compartment Cover. |
|
|
5. |
Assure that the Battery Compartment Cover is latched into place. |
|
|
To remove batteries from the Cart and DCU, press the battery eject button on the device and the battery will partially eject for easy removal.
To remove batteries from the PMC, follow the steps below.
Step |
Action |
|
|
1. |
Move the PMC away from the MR system, outside of the 1,000 |
|
Gauss (0.1T) field line (as measured from the center line of the MR |
|
bore), prior to removing batteries. |
|
|
2. |
Remove the Battery Compartment Cover by lifting the latches. |
|
|
3. |
Pull the latch holding the battery with one hand. |
|
|
4. |
Using your other hand, pull the battery out (from the side opposite |
|
the connector) of the PMC. |
|
|
3.2.2Battery Operation: WECG and WSpO2 Modules
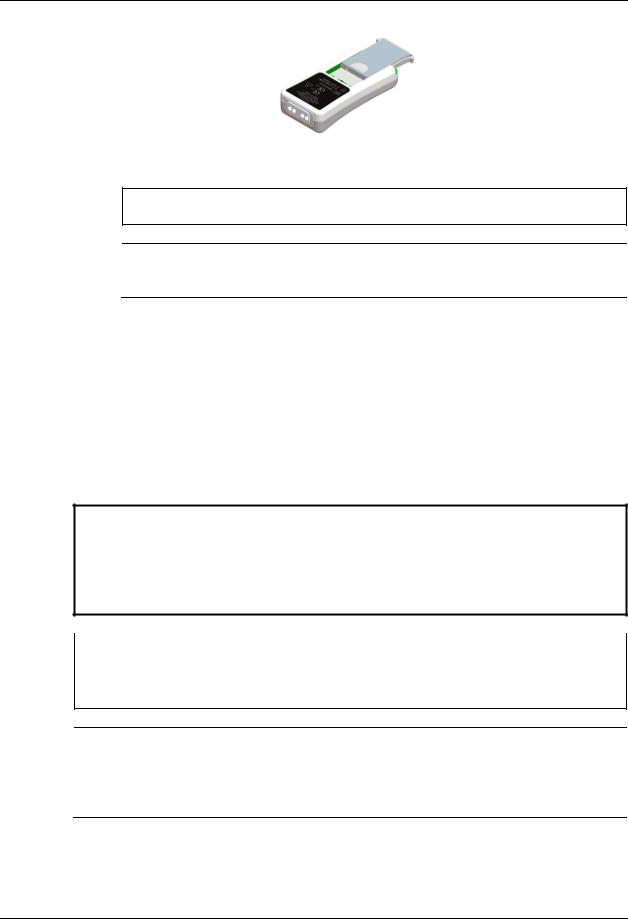
The WSpO2 and WECG modules have interchangeable batteries (P/N 9065). These batteries slide and latch into the battery slots in the WECG and WSpO2 modules.
WARNING: THIS PAGE CONTAINS COPYRIGHTED MATERIALS THAT ARE CONFIDENTIAL AND/OR PROPRIETARY. ANY RELEASE OR DISTRIBUTION
OF THIS MATERIAL, WITHOUT PERMISSION, IS A VIOLATION OF LAW. 8 of 82
Section 3: System Overview
3.2.2.1Installation and Removal
CAUTION: The 9065 Battery and wireless module should not be placed in the
Field of View to minimize the chance of image artifact.
Note: The WECG and WSpO2 module batteries are non magnetic and can be removed and replaced from the modules while in the MR magnetic field.
To install batteries into the WSpO2 and WECG modules, slide a battery into the battery slot in the module until the battery latches (on each side) into place.
To remove batteries from the WSpO2 or WECG modules, simultaneously press the latches on both sides of the battery then slide the battery out of the module.
3.2.3Safe Battery Use
The following warnings, cautions, and notes shall be observed to ensure the safety of operators and patients.
WARNING: Stop using any battery that exhibits abnormal heat, odor, color, deformation or is in an abnormal condition. If a battery is punctured or liquid leaks onto your skin or clothing, wash skin and clothing with fresh water immediately. If liquid leaks from a battery and gets into your eyes, do not rub your eyes. Wash eyes well with clean water and consult a doctor immediately.
CAUTION: |
If the battery terminals become dirty, wipe them with a clean dry cloth |
|
before use. |
Keep the battery terminals away from metal objects.
Note: Batteries have life cycles. If the time that the battery is powering the
equipment becomes much shorter than usual, the battery life is at an end. Remove a battery with an expired life cycle from the equipment immediately. Replace the battery with a new Invivo specified battery. Refer to Section 8 for part numbers.
WARNING: THIS PAGE CONTAINS COPYRIGHTED MATERIALS THAT ARE CONFIDENTIAL AND/OR PROPRIETARY. ANY RELEASE OR DISTRIBUTION
OF THIS MATERIAL, WITHOUT PERMISSION, IS A VIOLATION OF LAW. 9 of 82

Section 3: System Overview
3.2.4Battery Charging Instructions
Charge the batteries before use. When installing the system for the first time, all batteries must be charged at least 12 hours with AC power to the Expression turned off so that the batteries are fully charged and conditioned for operation.
The Cart, PMC, and DCU batteries are charged by integrated intelligent battery chargers. These intelligent charging devices automatically provide the appropriate profile needed to efficiently charge and condition the batteries. When the Cart, PMC, and DCU are plugged into AC power and turned off, the battery charger is functional and will automatically charge the batteries. When the Cart, PMC, and DCU are turned ON, they operate from AC power and charge the batteries simultaneously.
When the Cart, PMC, and DCU batteries are removed from the system, the user can determine battery capacity by pressing the battery’s Power Level button then observing the LEDs. The LEDs indicate the battery capacity, in 20 percent increments, from 20 – 100 percent. Indications of battery capacity are also available via the DCU; see the Expression IFU manual. The minimum battery voltage value for normal operation is 14.8V.
The Wireless ECG and SpO2 module batteries must be charged by the stand alone Invivo battery charger (P/N 9023). Refer to the instructions and cautions provided with the battery charger for more information. Indications of battery capacity are also available via the DCU; see the Expression IFU manual. The minimum battery voltage value for normal operation is 3.4V.
3.2.5Battery Disposal Instructions
The Expression MRI Patient Monitoring System uses lithium polymer and lithium ion batteries that are subject to strict disposal regulations for user and environmental safety.
CAUTION: Never heat or throw the battery into a fire. Heating the battery will damage the safety circuitry, which can cause rupture or ignition of the battery.
Batteries must be stored in a dry place, at a temperature between 0°C to 40°C.
The Expression MRI Patient Monitoring System uses rechargeable batteries that contain hazardous material. These batteries must be recycled or disposed of properly. Refer to disposal guidelines listed below. Do not disassemble or incinerate the battery.
WARNING: THIS PAGE CONTAINS COPYRIGHTED MATERIALS THAT ARE CONFIDENTIAL AND/OR PROPRIETARY. ANY RELEASE OR DISTRIBUTION
OF THIS MATERIAL, WITHOUT PERMISSION, IS A VIOLATION OF LAW. 10 of 82

Section 3: System Overview
3.2.5.1Guidelines in Europe
The European Community (EC) has issued two directives regarding battery disposal: 91/157/EEC and 93/86/EEC. Each member country implements these independently. Thus, in each country the manufacturers, importers, and users are responsible for the proper disposal or recycling of batteries. Confirm proper disposal requirements with your healthcare facility or distributor.
3.2.5.2Guidelines in the United States
The U.S. Federal Environmental Protection Agency (EPA) hazardous waste regulations, as conveyed by the Resources Conservation and Recovery Act (RCRA), neither specifically list nor exempt lithium batteries. The only metal of possible concern in the battery is the lithium metal that is not listed or characterized as a toxic hazardous waste. Significant amount of spent cells and batteries that are untreated and not fully discharged are considered as reactive hazardous waste. Thus, hazardous waste of spent cells and batteries can be disposed after they are first neutralized through an approved secondary treatment prior to disposal (as required by U.S. Land Ban Restriction of the Hazardous and Solid Waste Amendments of 1984). Disposal of spent batteries must be performed by authorized, professional disposal company, which has the knowledge in the requirements of the Federal, the State and the Local authorities regarding hazardous materials, transportation, and waste disposal. Confirm proper disposal requirements with your healthcare facility, distributor, and/or local EPA office.
WARNING: THIS PAGE CONTAINS COPYRIGHTED MATERIALS THAT ARE CONFIDENTIAL AND/OR PROPRIETARY. ANY RELEASE OR DISTRIBUTION
OF THIS MATERIAL, WITHOUT PERMISSION, IS A VIOLATION OF LAW. 11 of 82

Section 3: System Overview
WARNING: THIS PAGE CONTAINS COPYRIGHTED MATERIALS THAT ARE CONFIDENTIAL AND/OR PROPRIETARY. ANY RELEASE OR DISTRIBUTION
OF THIS MATERIAL, WITHOUT PERMISSION, IS A VIOLATION OF LAW. 12 of 82
4 SYSTEM INSTALLATION
This section provides instructions regarding the installation of the Expression MRI Patient Monitoring System. Observe the following warnings, cautions and notes when installing, setting up, and using the Expression MRI Patient Monitoring System.
WARNING: |
Do not use the Expression MRI Patient Monitoring System in the presence of |
|
|
|
flammable anesthetics. |
|
Always verify proper communication of the Expression with the corresponding |
|
|
|
DCU prior to patient use. |
|
|
|
CAUTION: |
|
Avoid the use of cellular phones or other radio frequency transmitters in the |
|
|
proximity of an operating Expression MRI Patient Monitoring System. |
|
Where the integrity of the external protective conductor in the installation or its |
|
|
|
arrangement is in doubt, the system shall be operated from batteries. |
|
|
|
|
|
|
Note: |
|
Do not use two Expression MRI Patient Monitoring Systems in the same MR room, as |
|
|
this will lead to communication errors. |
A minor but noticeable degradation in the Wireless ECG and Wireless SpO2 radio communications will occur in the presence of high powered radios.
4.1Power Supply
Depending upon the equipped Power Supply, observe the following warnings, cautions, and notes when installing, setting up, and using the Expression MRI Patient Monitoring System.
4.1.1Part Number 989803169201
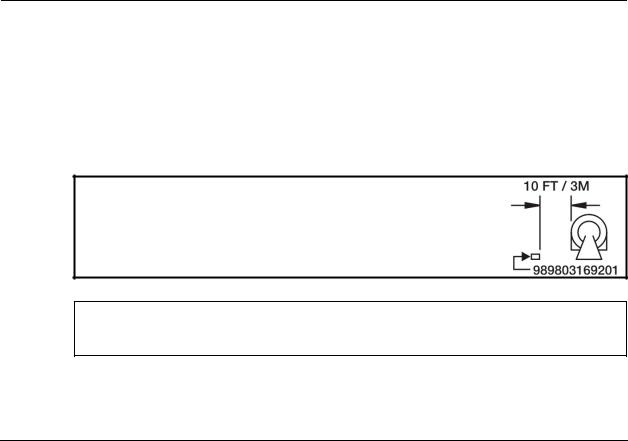
WARNING: Ensure that the Power Supply (P/N 989803169201) remains 10 feet (3 meters) or more from the MR system. Mount the Power Supply to a horizontal surface using the pre applied Velcro strips on the bottom of the supply.
CAUTION: Avoid use of electrical extension cords or Multiple Portable Socket Outlets which may create a safety hazard by compromising the grounding integrity of the Expression MRI Patient Monitoring System.
WARNING: THIS PAGE CONTAINS COPYRIGHTED MATERIALS THAT ARE CONFIDENTIAL AND/OR PROPRIETARY. ANY RELEASE OR DISTRIBUTION
OF THIS MATERIAL, WITHOUT PERMISSION, IS A VIOLATION OF LAW. 13 of 82
Section 4: System Installation
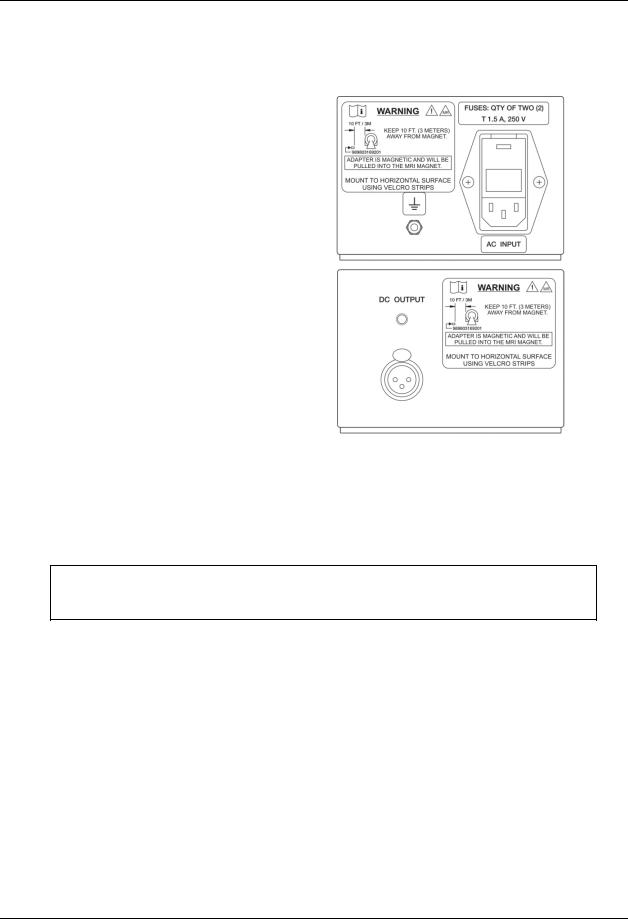
Input / Output Configuration of Power Supply, P/N 989803169201:
AC Input:
DC Output:
The ground terminal (as designated by the symbol) on Power Supply P/N 989803169201 allows Invivo authorized service personnel to connect a ground strap to prevent ESD discharge during servicing, and for testing leakage currents.
symbol) on Power Supply P/N 989803169201 allows Invivo authorized service personnel to connect a ground strap to prevent ESD discharge during servicing, and for testing leakage currents.
CAUTION: Performance of the Expression MRI Patient Monitoring System and any other devices within the room may be degraded if the ground terminal is used against Invivo’s intended use, as listed above.
WARNING: THIS PAGE CONTAINS COPYRIGHTED MATERIALS THAT ARE CONFIDENTIAL AND/OR PROPRIETARY. ANY RELEASE OR DISTRIBUTION
OF THIS MATERIAL, WITHOUT PERMISSION, IS A VIOLATION OF LAW. 14 of 82
Section 4: System Installation
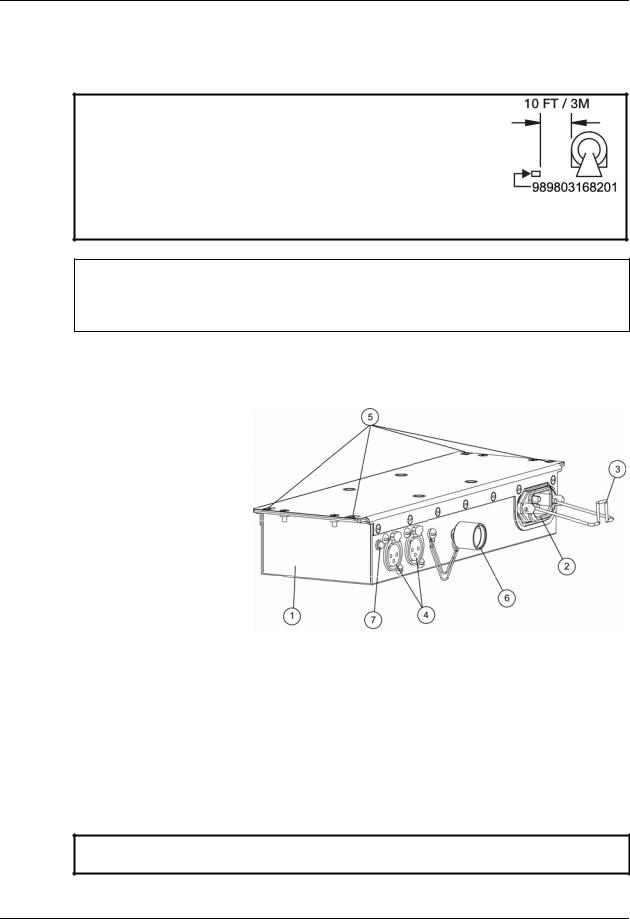
4.1.2Part Number 989803168201
WARNING: Ensure that the Power Supply (P/N 989803168201) remains 10 feet (3 meters) or more from the MR system, mounted to a horizontal surface using the pre applied Velcro strips and/or secured to the floor (or another immoveable surface) with fasteners through the unit’s mounting flange holes, in order to prevent the Power Supply from being pulled into the MRI Magnet.
CAUTION: If longer distances are required, only use approved AC electrical extension cord, P/N 989803168221; avoid use of extension cords or multiple portable socket outlets, which may create a safety hazard by compromising the grounding integrity of the MRI Patient Monitoring System.
Input / Output Configuration of Power Supply, P/N 989803168201:
Item Description
Power Supply 989803168201
AC Input Connector
Strain Relief
DC Output Connector (2)
Mounting Flange Holes (4)
Shield Cap
LED Indicator (Illuminated = Power ON)
WARNING: If using only one DC Output, cover the unused output with the Shield Cap in order to prevent noise artifacts from appearing on the MR image.
WARNING: THIS PAGE CONTAINS COPYRIGHTED MATERIALS THAT ARE CONFIDENTIAL AND/OR PROPRIETARY. ANY RELEASE OR DISTRIBUTION
OF THIS MATERIAL, WITHOUT PERMISSION, IS A VIOLATION OF LAW. 15 of 82

Section 4: System Installation
4.2Cart Configuration
Observe the following warnings, cautions, notes, and instructions when installing, setting up, and using the Cart configuration for the Expression MRI Patient Monitoring System.
The Expression MRI Patient Monitoring System Cart may be
.
used inside the MR system room in a location at or outside the 5,000 (5,000 or less) Gauss (0.5T) field line of the MR system, as measured from the center line of the MR bore.
Failure to properly place the Expression and its accessories WARNING: in the MR system room will result in system or accessory
failure, and possible patient or user injury. Always secure the Cart’s wheel locks when the unit is placed within the MR system room.
If the Expression MRI Patient Monitoring System Cart rolls to the face of the MR system due to magnetically induced pull force, DO NOT ATTEMPT TO DISLODGE THE EXPRESSION BY PULLING FROM THE DOCKED DCU OR GUIDE HANDLE AT THE TOP OF THE SYSTEM. Dislodge the Expression by gently
pulling from the base of the system at its lowest point. This will prevent the CAUTION: base of the unit from experiencing higher MR pull forces in the vertical
direction.
Field strength variations in a particular MR system room (which may be due to active shielding technology, manufacturer variability, future enhancements, etc) can make distinguishing the 5,000 Gauss level (as measured from the center line of the MR bore) difficult. These variations may require moving the Cart away from the MR system if system abnormalities or malfunctions are observed. Prior to clinical use, ensure that the allowable distance of the Expression components from the MR system is maintained for proper operation.
WARNING: THIS PAGE CONTAINS COPYRIGHTED MATERIALS THAT ARE CONFIDENTIAL AND/OR PROPRIETARY. ANY RELEASE OR DISTRIBUTION
OF THIS MATERIAL, WITHOUT PERMISSION, IS A VIOLATION OF LAW. 16 of 82
Section 4: System Installation
Complete the following actions to install the Cart configuration:
Step |
Action |
|
|
1. |
Install the batteries into the Cart; see Section 3.2.1. |
|
|
2. |
Locate the Power Supply and attach the AC Power Cord to the AC Input of the Power |
|
Supply; see Section 4.1. |
|
|
3. |
Connect the male end of the 25 ft. DC power cable (P/N AC517B) to the DC Output of |
|
the Power Supply. |
|
|
4. |
Position the Power Supply in the MR system room near an approved AC outlet at a |
|
distance of at least 10 feet (3 meters) from the MR system. |
|
|
5. |
Plug the female end of the DC power cable into the Power Input of the Cart. |
|
|
6. |
Position the Cart within the parameters specified (in the warnings and cautions, |
|
above) and then lock the wheels. |
|
|
7. |
Plug the AC Power Cord into the AC outlet. |
|
|
4.3Patient Management Configuration (PMC)
Observe the following warnings and instructions when installing, setting up, and using the PMC for the Expression MRI Patient Monitoring System.
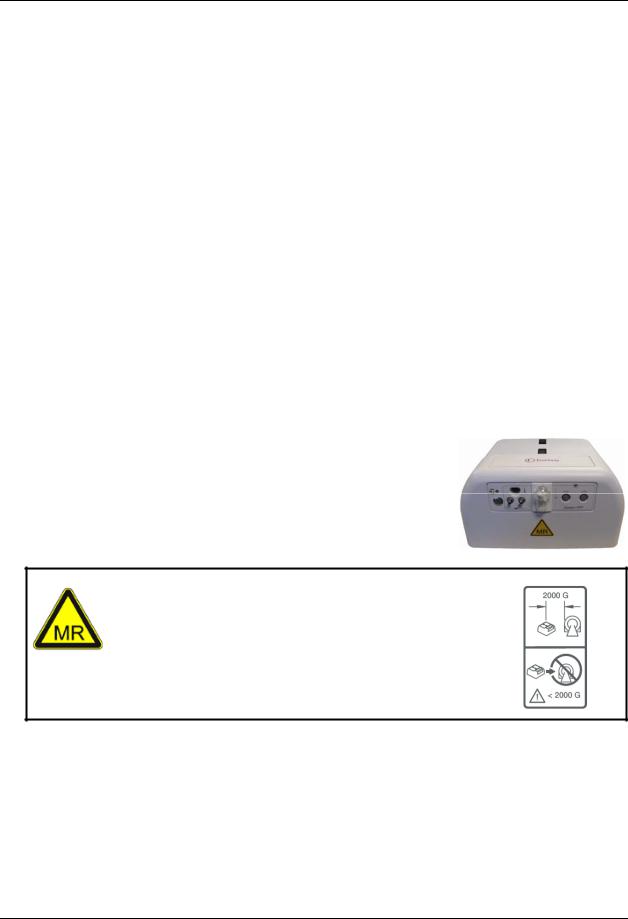
The Patient Management Configuration (PMC) may be used inside the MR system room at or outside the 2,000 Gauss (0.2T) field line as measured from the center line of the MR bore. Always ensure that no portion of the PMC is closer
than 2,000 Gauss (0.2T) field line and that the PMC is WARNING: securely fastened to the mounting surface. System failure or
patient injury may result if the PMC is brought closer than 2,000 Gauss (0.2T) field line.
WARNING: THIS PAGE CONTAINS COPYRIGHTED MATERIALS THAT ARE CONFIDENTIAL AND/OR PROPRIETARY. ANY RELEASE OR DISTRIBUTION
OF THIS MATERIAL, WITHOUT PERMISSION, IS A VIOLATION OF LAW. 17 of 82
 Loading...
Loading...