
Invacare®SoftAIR®Super/
SoftAIR®Excellence
enAlternatingmattresssystems
UserManual................................3
noAlternerendemadrassystem
Bruksanvisning..............................33
daAlternerendemadrassystem
Brugsanvisning..............................65
svAlternerandemadrassystem
Bruksanvisning..............................97
fiVaihtelevatpatjajärjestelmät
Käyttöohje.................................129
ThismanualMUSTbegiventotheuseroftheproduct.
BEFOREusingthisproduct,readthismanualandsaveforfuturereference.

©2016Invacare®Corporation
Allrightsreserved.Republication,duplicationormodificationinwholeorinpartisprohibitedwithout
priorwrittenpermissionfromInvacare.Trademarksareidentifiedby™and®.Alltrademarksare
ownedbyorlicensedtoInvacareCorporationoritssubsidiariesunlessotherwisenoted.
—
Medenerett.Deterforbudtårepublisere,kopiereellerendreheleellerdeleravdenneveiledningen
utenatdetpåforhånderinnhentetskriftligtillatelsefraInvacare.Varemerkererangittmed™og
®.AllevaremerkereiesavellerlisensierestilInvacareCorporationellertilhørendedatterselskaper
medmindreanneterangitt.
—
Medforbeholdafallerettigheder.Gengivelse,kopieringellerændringdelvistelleridenshelheder
forbudtudenforudgåendeskriftligtilladelsefraInvacare.Varemærkerermarkeretmed™og®.Alle
varemærkerejesafellerergivetilicenstilInvacareCorporationellerdennevirksomhedsfilialer,
medmindreandetfremgår.
—
Medensamrätt.Återpublicering,dupliceringellerändringavhelaellerdelarärintetillåtetutan
föregåendeskriftligttillståndfrånInvacare.Varumärkenidentifierasav™och®.Allavarumärkentillhör
ellerärlicensieradetillInvacareCorporationellerdessdotterbolagomingetannatanges.
—
Kaikkioikeudetpidätetään.Kokomateriaalintaisenosanuudelleenjulkaisu,jäljentäminentai
muuttaminenonkiellettyäilmanInvacarenetukäteenantamaakirjallistalupaa.Tavaramerkitilmaistaan
symboleilla™ja®.KaikkitavaramerkitovatInvacareCorporationintaisentytäryhtiöidenomistamiatai
lisensoimia,elleitoisinoleilmoitettu.

Contents
ThismanualMUSTbegiventotheuseroftheproduct.
BEFOREusingthisproduct,readthismanualandsaveforfuture
reference.
1General........................................4
1.1Generalinformation.............................4
1.2Symbolsinthisusermanual.......................4
1.3Intendeduse..................................4
1.4Warranty....................................5
1.5ServiceLife...................................5
2Safety..........................................7
2.1Safetyinformation..............................7
2.2Symbolsontheproduct..........................8
3Components....................................9
3.1Overview....................................9
3.2Description...................................9
4Setup..........................................11
4.1Safetyinformation..............................11
4.2Installingthemattresssystem......................11
4.3Activatingthecontrolunit........................12
4.4Removingthemattresssystem.....................13
5Usage..........................................14
5.1Safetyinformation..............................14
5.2Usingthecontrolunitpanel.......................14
5.3Usingthemattresssystem........................16
5.4CPRProcedure................................16
5.5AlarmFunctions...............................17
5.6Transportingapatientonthemattress...............18
6Transport......................................19
6.1Safetyinformation..............................19
7Maintenance....................................20
7.1Inspection....................................20
7.2CleaningandCare..............................20
7.3Replacingtheairfilter...........................22
7.4Replacingfuse(1ampslowblowfuse)................22
8AfterUse.......................................23
8.1Storage......................................23
8.2Re-use......................................23
8.3Disposal.....................................23
9Troubleshooting.................................24
9.1Identifyingandrepairingfaults......................24
10Technicaldata..................................26
10.1GeneralData.................................26
10.2Guidanceandmanufacturer’sdeclaration.............28
Invacare®SoftAIR®Super/SoftAIR®Excellence
1General
1.1Generalinformation
Essentialnursingcareispivotalinpressureulcerprevention.The
SoftAIR®mattresseswillpositivelycontributetotheoutcomeofa
pressureulcerpreventioncareplan.
Education,clinicaljudgementandactionbasedplanningbasedon
vulnerabilityarefundamentalfactorsinpreventionofpressureulcers.
Arangeofassessmentscalescanbeusedasaformalmethodof
assessingriskfrompressureulcerdevelopment,andshouldbe
usedinconjunctionwithaninformalassessment(informednursing
judgement).Informalassessmentisconsideredtobeofgreater
importanceandclinicalvalue.
Pleaseheedallthenotes,particularlythesafetyinformation,and
actaccordingly.
Formoreinformationabouttheproduct,contactyourlocalInvacare
representative.Foraddressandwebsiteseebackpageofthismanual.
1.2Symbolsinthisusermanual
InthisUserManualwarningsareindicatedbysymbols.Thewarning
symbolsareaccompaniedbyaheadingthatindicatestheseverity
ofthedanger.
WARNING
Indicatesapotentiallyhazardoussituationwhichifnot
avoidedcouldresultindeathorseriousinjury.
CAUTION
Indicatesapotentiallyhazardoussituationwhichifnot
avoidedcouldresultinproductdamage,minorinjury
orboth.
IMPORTANT
Indicatesahazardoussituationwhichifnotavoided
couldresultindamagetotheproduct.
Givesusefultips,recommendationsandinformation
forefficient,trouble-freeuse.
Thisproductcomplieswiththedirective93/42/EEC
formedicalproducts.Thelaunchdateforthisproduct
isspecifiedintheCEdeclarationofconformity.
Manufacturer
1.3Intendeduse
Thispressureredistributionmattressandcontrolunitareintended
tobeusedinconjunctionwithanappropriatelysizedbedframe.
Itcanbeusedsafelyinstaticmodeforstaticpressureredistribution,
orindynamicmodeshouldanalternatingpressuresupportsurface
berequired.
Thisproducthasbeendesignedtodelivereffectivepressure
reductiontousers,whentheproductisinnormalusewhichis
definedbyInvacareLtdaswhenthesupportsurfaceiscoveredwith
acotton,cottoncombinationorlinenbedsheet,andanyoneof
thesewouldbetheonlyitemdeployedbetweenthesupportsurface
andtheuser.
Indications
TheSoftAIR®mattressesaresuitableforthepreventionand
treatmentofpressureulcersinthe'VeryHighRisk'patient.Itis
suitableforuseinallhomecare,residential,nursingandacutecare
settingsandisappropriateforthetreatmentofpressureulcersup
tograde4aswellasseverepressureulcers.
4
1533195-A

General
1.4Warranty
Weprovideamanufacturer’swarrantyfortheproductinaccordance
withourGeneralTermsandConditionsofBusinessintherespective
countries.Guaranteeclaimscanonlybemadethroughtheprovider
fromwhomtheappliancewasobtained.
StandardInvacareTerms
ThisistocertifythatyourSoftAIR®mattressiswarrantedby
InvacareLtdforaperiodstatedintheTable“TechnicalData“of
thisuserguide.Thisissubjecttotheindividualcountry‘sSales
Agreements.
PleasecontactyourlocalInvacareSalesOfficeforfurtherinformation.
TheWarrantyofyourInvacareSoftAIR®productisvalidfromtime
ofshipping.
Ifadefectorfaultisdiscoveredpleasecontactandnotifytheprovider
fromwhomtheappliancewasobtainedimmediately.
Themanufacturerwillnotacceptresponsibilityfordamagecaused
bymisuseornon-observanceoftheinstructionssetoutinthisuser
guide.
Duringtheperiodofthewarrantyanyproductsthathavebecome
defectiveduetofaultyworkmanshipormaterialswillberenewed
withoutcharge.
Thewarrantywillbeforfeitedshouldanyunauthorizedalterationbe
madetotheequipment.
BothwarrantyandfireretardancyCertificationwillbecome
nullandvoidifnon-InvacaresparesareusedonanyInvacare
SoftAIR®Mattressproducts.
Thepurchaser’sstatutoryrightsundertheConsumerProtection
Actarenotaffected.
QualityandFlameRetardancy
Qualityisfundamentaltothecompany’soperation,working
withinthedisciplinesofISO9001(QualitymanagementsystemsRequirements)andISO13485(Medicaldevices-Qualitymanagement
systems-Requirementsforregulatorypurposes).
TheInvacareSoftAIR®mattressesfeaturetheCEmark,in
compliancewiththeMedicalDeviceDirective93/42/EECClass1.
Invacare®iscontinuouslyworkingtowardsensuringthatthe
company’simpactontheenvironment,locallyandglobally,isreduced
toaminimum.
•Wecomplywiththecurrentenvironmentallegislation(e.g.
WEEEandRoHSdirectives).
•WeonlyuseREACHcompliantmaterialsandcomponents.
Thecontrolunitistestedaccordingto3rdversionsafetystandard
IEC/EN60601-1(Medicalelectricalequipment-Part1:General
requirementsforbasicsafetyandessentialperformance)&EMC
standardIEC/EN60601-1-2(Collateralstandard:Electromagnetic
compatibility-Requirementsandtests).
TheSoftAIR®mattressisfiresafetytestedandcertifiedinaccordance
withEN597–1&–2.
ForfurtherinformationpleasecontactInvacareinyourcountry
(addressesseebackpageofthismanual).
1.5ServiceLife
Weestimatealifeexpectancyoffiveyears(5yrs)forthese
products,providedtheyareusedinstrictaccordancewiththe
intendeduseassetoutinthisdocumentandallmaintenanceand
servicerequirementsaremet.Theestimatedlifeexpectancycanbe
exceedediftheproductiscarefullyusedandproperlymaintained,and
providedtechnicalandscientificadvancesdonotresultintechnical
1533195-A5

Invacare®SoftAIR®Super/SoftAIR®Excellence
limitations.Thelifeexpectancycanalsobeconsiderablyreduced
byextremeorincorrectusage.
Thefactthatweestimatealifeexpectancyfortheseproductsdoes
notconstituteanadditionalwarranty.
61533195-A
Safety
2Safety
2.1Safetyinformation
WARNING!
–Donotusethisproductoranyavailableoptional
equipmentwithoutfirstcompletelyreadingand
understandingtheseinstructionsandanyadditional
instructionalmaterialsuchasusermanuals,service
manualsorinstructionsheetssuppliedwiththis
productoroptionalequipment.Invacareproduct
manualsareavailableatyourlocaldealerorInvacare
inyourcountry,addressesareonthebackpageofthis
manual.Ifyouareunabletounderstandthewarnings,
cautionsorinstructions,pleasecontactahealthcare
professional,dealerortechnicalpersonnelbefore
attemptingtousethisequipment–otherwise,injury
ordamagemayoccur.
WARNING!
Therearesiginificantrisksofreciprocalinterference
posedbythepresenceofthesystemduringspecific
investigationsortreatments.
–Intheeventofelectromagneticorotherinterference
betweenthesystemandotherdevices,movethe
equipmentawayfromthesensitivedevicesorcontact
themanufacturer.
WARNING!
–Unplugthecontrolunitfromthemainspowersupply
todisconnectthepower.
WARNING!
Invacareproductsarespecificallydesignedand
manufacturedforuseinconjunctionwithInvacare
accessories.Accessoriesdesignedbyother
manufacturershavenotbeentestedbyInvacareandare
notrecommendedforusewithInvacareproducts.
Theintroductionofcertainthirdpartyproductsbetween
themattresssurfaceandtheusermayreduceorimpede
theclinicaleffectivenessofthisproduct.
’Thirdpartyproducts’mayinclude,butarenotlimited
toitemsincludingunderblankets,plasticsheetsand
sheepskins,etc.
WARNING!
Riskofdevelopingpressureulcers
–Bedsheetsmustbelooselyfitted,withcreases
smoothedout.Caremustalwaysbetakentoensure
thatthesupportsurfaceincontactwiththeuseris
keptfreefromcrumbsandotherfooddebris,andthat
dripcables,stents,andotherforeignobjectsdonot
becomeentrappedbetweentheuserandthepressure
reducingsurfaceofthemattress,asthismayresultin
thedevelopmentofpressureulcers.
1533195-A7
Invacare®SoftAIR®Super/SoftAIR®Excellence
WARNING!
Riskoffireorexplosion!
Acigarettecanburnaholeinthebedsurfaceand
causedamagetothemattress.Also,patientclothing,
bedsheets,etc,maybecombustibleandcauseafire.
Failuretoobservethiswarningcanresultinasevere
fire,propertydamageandcausephysicalinjuryordeath.
Thereisanexplosionriskifusedwithflammable
anesthetics.
Thereisapossiblefirehazardwhenusedwithoxygen
administeringequipmentotherthannasalmaskorhalf
bedtenttype.
–Donotsmokewhileusingthisdevice.
–Anoxygententmaynotextendbelowmattress
supportlevel.
IMPORTANT!
Theinformationcontainedinthisdocumentissubject
tochangewithoutnotice.
–Checkallpartsforshippingdamageandtestbefore
using.
–Incaseofdamage,donotuse.Contact
Invacare/Carrierforfurtherinstructions.
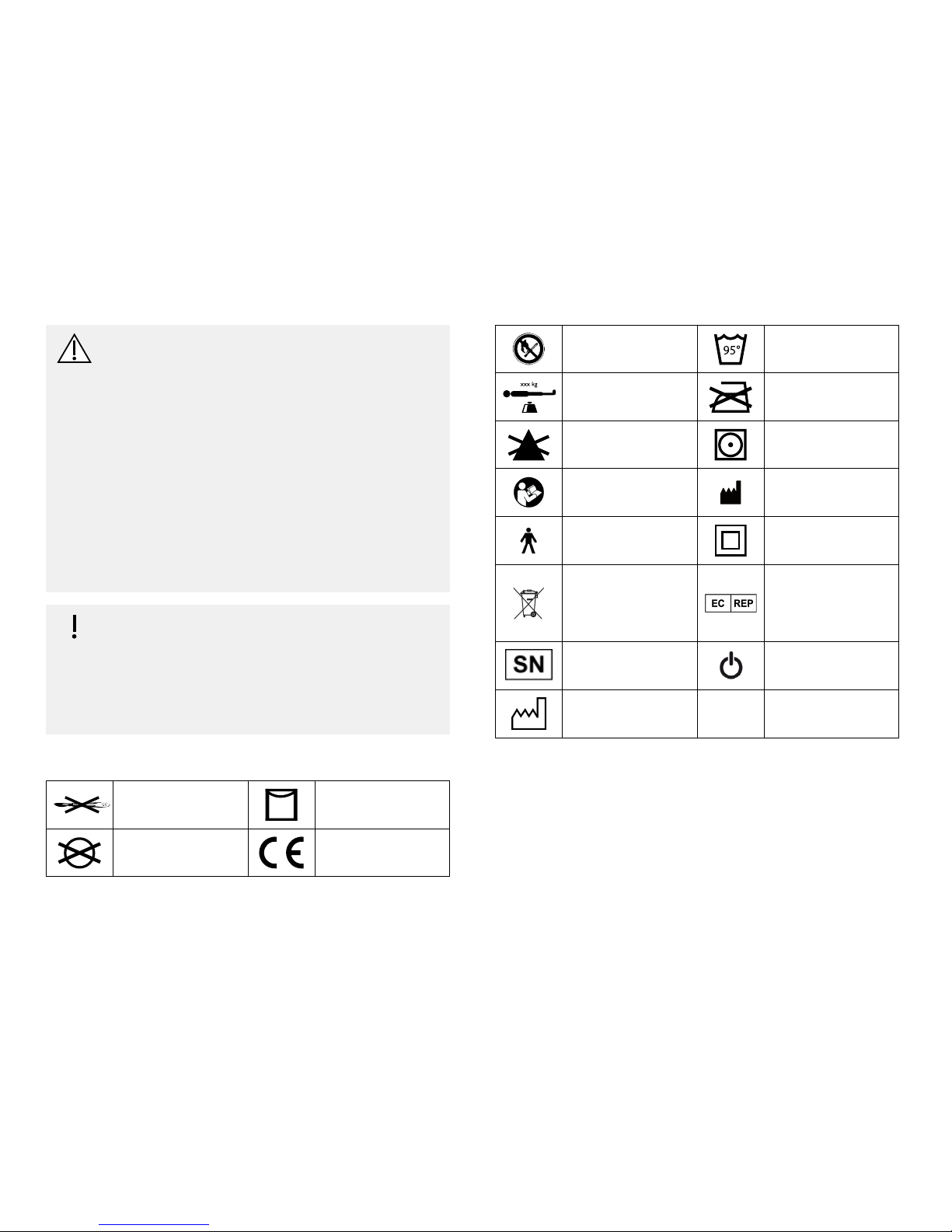
2.2Symbolsontheproduct
Donotpierceor
cut
Linedry
Donotdryclean
Declarationof
conformity
Donotputnear
flame
95°
Maximum
95°C
xxx kg
Userweightlimit*
Donotiron
DonotbleachTumbledrylowheat
RefertousermanualManufacturer
TypeBappliedpart
ClassIImedical
equipment
WEEEconformAuthorised
Representative
intheEuropean
Community
Serialnumber
Power
Dateofmanufacture
*Minimum/Maximumuserweightaspersection10Technicaldata,
page26.
81533195-A
Components
3Components
3.1Overview
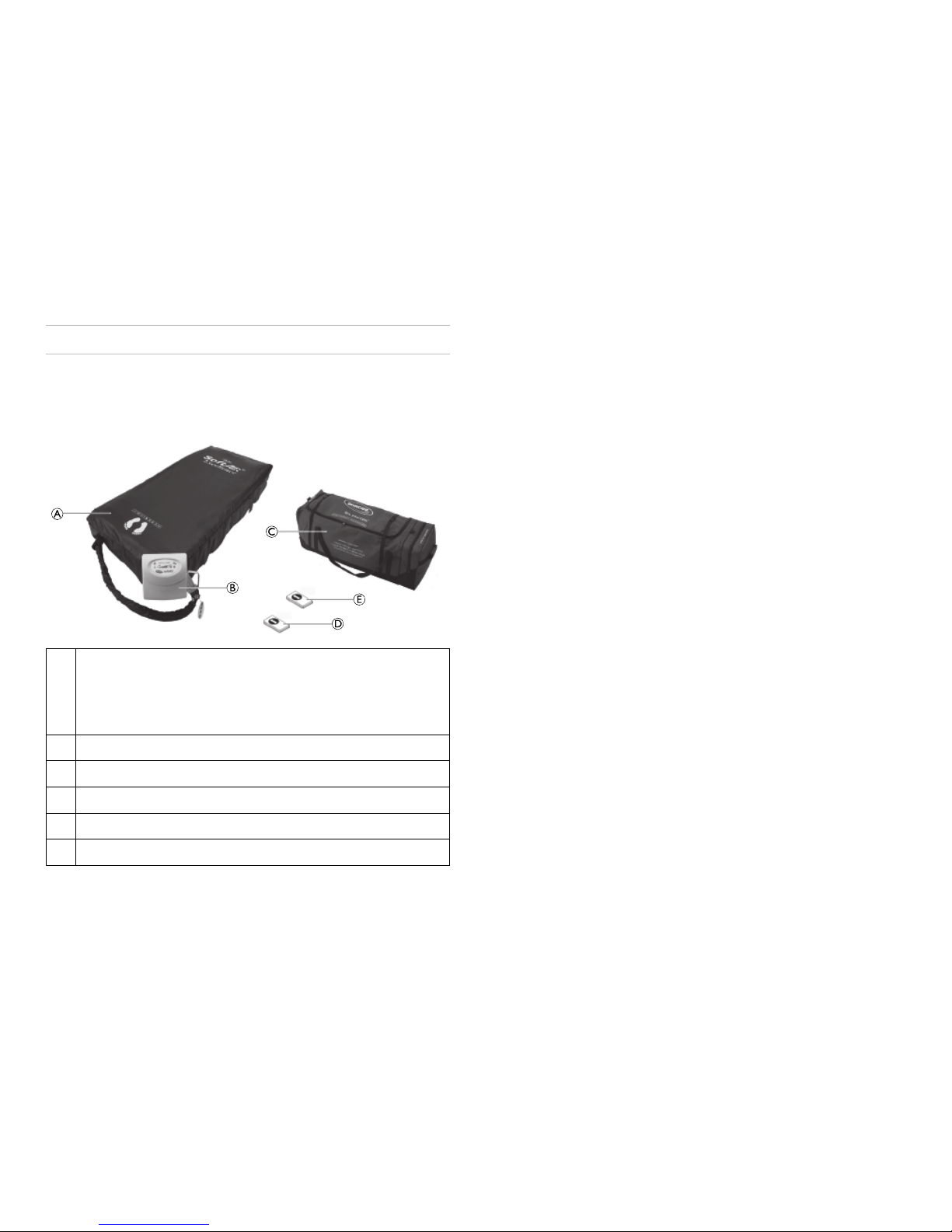
Thefollowingcomponentsareincludedwithinthescopeofdelivery:
SoftAIR®Excellence/SoftAIR®Super
A
B
C
D
E
A
SoftAIR®MattressSystemincludingMattressBase,CPRTag,
AirHoses,HandlewithTransportCapattached,Topcover
withquickreleasezip.
Twoinflatablesideformers(onlySoftAIR®Excellence)
B
DigitalControlUnit
C
CarryBag
D
QuickSetupGuide
E
UserManual
MedicalGradePowerCord(notshown)
3.2Description
SoftAIR®AlternatingMattressSystems
TheSoftAIR®ExcellenceandSoftAIR®Superarealternating
MattressSystemsthatprovidealternatingpressuretopatientswho
arevulnerableto,orsufferfrom,pressureulcers.Theyaredesigned
toreplaceyourexistingmattressandcanbeusedonbothstandard
andprofilingbedframes.
DigitalControlUnit
TheControlUnitprovidestheairsupplytotheMattress.
•Itiscontrolledviaatouchpanel.Thereisavisualandaudible
alarmwhenpressurefailsorpowerisinterrupted.AlarmMute
silencesthealarmforamaximumof20minutes–thealarm
resumesifcauseoffailureisnotresolved.TheAlarmwillsound
foruptotwohoursfollowinganinterruptiontopower.As
soonaspowerisrestored,thebatterymemorybackupwill
restorethepreviouspressure/usersetting.
•TheControlUnitincludesabatteryfordigitalmemoryback
up.Thisbatteryiscontinuouslyre-chargedandwilllastthelife
timeoftheproduct.
•Buttonsonthecontrolpaneladjusttheeightalternating
pressuresettings.
•ThesystemwillautomaticallyreverttoAlternationModeupto
20minutesafterstaticmodehasbeenselected.See5.2Using
thecontrolunitpanel,page14
forfurtherinstruction.
Thevisibleandaudiblealarmfunctionhasanumberofindications
dependingonthecauseofthefailure.Seechapter5.5Alarm
Functions,page17.
Onthesideofthecontrolunitarefourmaleairconnectorsfor
connectingtheHandle.
1533195-A9

Invacare®SoftAIR®Super/SoftAIR®Excellence
TherapidreleaseHandleincludesanattachedtransportcapwhich
canbeinsertedintotheopeningtosealairinthesystemforupto
72hoursasatransportfeature.
ThemainssupplytotheControlUnitcanbeeasilydisconnectedand
isdesignedtodetachiftuggedtoofirmly-protectingtheinternal
wiringoftheunit.
Shouldthisoccur,thealternationsequenceissuspendedandthe
Mattresscellsremaininflatedand/ordeflatedbasedonthecurrent
cycle.ThePowerfailureindicatorwillsound.
SoftAIR®Super
TheSoftAIR®SuperMattresscomprises21highdensitycellswhich
allfeatureapermanentlyinflatedinternalcelltopreventthepatient
“bottomingout”intheeventoflowpressureduetoincorrect
settings,electricalorcellfailure.
Thissystemincludesthreestaticheadcellstoprovidestatic“pillow”
supportforoptimumusercomfort,whileairpressureintheother
18cellsisalternatedovera10~12minutecycle.Thisprovides
regularperiodsofpressurereductiontoaidbloodandlymphatic
flowtovulnerabletissue.
SoftAIR®Excellence
TheSoftAIR®ExcellenceMattresscomprises19highdensitycells
whichallfeatureapermanentlyinflatedinternalcelltopreventthe
patient“bottomingout”intheeventoflowpressureduetoincorrect
settings,electricalorcellfailure.
Thissystemincludesthreestaticheadcellstoprovidestatic“pillow”
supportforoptimumusercomfort,whileairpressureintheother
16cellsisalternatedovera10~12minutecycle.Thisprovides
regularperiodsofpressurereductiontoaidbloodandlymphatic
flowtovulnerabletissue.
TheSoftAIR®Excellencehasahingedbacktoallowthemattressto
conformtotheprofileofthebedwhenarticulated.TheSoftAIR®
Excellencealsoincludesanindependentheelzoneoffivemicrocells
forindividualizedtherapytothissensitivearea.
Inaddition,twopermanentlyinflatedsideformersassistinlateral
supportfortheuserandcarer,whileincreasingtheeffectiveness
oftheoneinthreealternationcycle.Thehingedmattressbase
conformstoprofilingbedmovement.
101533195-A

Setup
4Setup
4.1Safetyinformation
WARNING!
Electricalshockhazard!
–Onlyplugintoagroundedpoweroutletandusethe
powercordsuppliedwiththesystem.
–Donotexposetheelectroniccontrolunittoany
liquidwhileitispluggedin.
–Alwaysusefusesofthesameratingasspecifiedin
theTechnicalsection.Usingfuseswithhigherratings
couldresultindamageand/orinjury.
–Theelectroniccontrolunitisaprecisionelectronic
product.Handleandtransportwithcare.Droppingor
othersuddenimpactsmayresultindamagetotheunit.
–Donotopenthecontrolunit.
–Donotattempttorepairorservicethecontrolunit
withoutbeingauthorizedtodoso.
–Donotplaceanyobjectsoritemssuchasblanketson
oroverthecontrolunit.
–ThepowercordtotheControlUnitmustbe
positionedtoavoidatriphazardand/ordamagetothe
cord.Carefulconsiderationisrequiredwhenrouting
thepowercable.InvacareLtdrecommendsplacing
thecordunderthebedframeandattachingittoan
electricaloutletattheheadofthebed.
–Donotinsertitemsintoanyopeningsofthecontrol
unit.Doingsomaycausefireorelectricshockby
shortingtheinternalcomponents.
–Keepthecontrolunitawayfromallheatsourcesand
radiatorsduringoperation.
WARNING!
–Donotmodifythisequipmentwithoutauthorization
ofthemanufacturer.
–Ifthisequipmentismodified,appropriateinspection
andtestingmustbeconductedtoensurecontinued
safeuseoftheequipment.
4.2Installingthemattresssystem
Itisrecommendedthatallpackingmaterialsandinstructionsbekept
inthecarrybagprovidedintheeventtheproducthastobeshipped
toanapprovedInvacareServiceCenter.Themattressistreated
astheappliedpart.CarefullyremovetheControlUnit,Mattress
andaccessoriesfromtheshippingcartons.Inspectallitemsfor
anydamagethatmayhaveoccurredduringshipping.Anydamaged
ormissingpartsmustbereportedtoanInvacareServiceCenter
immediately.
1.Removeallpackagingbeforeuse.
2.Placethemattressdirectlyontheframeofthebed.
Themattressisdesignedforbedswithadjustablelying
surface.
1.Removeallcovers,sheetsandmattressfromthebed.
CAUTION!
Riskofinjuryordamage
–Priortoattachingthecontrolunittothefootboard
ofthebed,checkthatthebedendisrobustenough
orthattheunitisplacedonanevenenoughsurface
tosupportthecontrolunitsafelywithoutrisktothe
carerorpatientordamagetotheproductitself.
1533195-A
11

Invacare®SoftAIR®Super/SoftAIR®Excellence
IMPORTANT
–PriortoactivationensuretheCPRvalve(4plugs)
locatedneartheheadendofthemattressisfully
engagedandthattherapidreleasehandleisfirmly
connectedtothecontrolunit.
2.Onastandardbed,positiontheMattressontopofbedframe,
topcoverfacingupwardsandairhosesatfootofbedforcontrol
unitpositioning.
3.Attachtothebedbysecuringthetwo(2)adjustablestrapsunder
eachendofthebed.Ensurebucklesaresecurelyfastenedand
strapsarepulledtight.
Oronaprofilingbed:
4.Securetheadjustablestrapsaroundthemoveablesectionsof
thebedframe.
IMPORTANT!
Riskofdamagetothemattress
–Ensuretherearenosharpobjectswhichmaycome
intocontactwiththemattresssystem.
–CheckthattheattachmentoftheMattressdoesnot
interferewiththemovementoroperationofthebed.
–Donotsecurestrapstobedsiderailsasstrapswill
tear.
–Ensurethatthepositioningofthesystemdoesnot
interferewiththeabilitytodisconnecttheelectrical
power.
4.3Activatingthecontrolunit
1.Positionthecontrolunitbyhangingthehooksoverthefoot
boardofthebedorsiderails,ensuringthattheyarerobust
enoughtoholdthecontrolunit.
CAUTION!
–Ensuretheairhosedoesnotkinkbetweenthebed
frameandcontrolunit.
12
1533195-A

Setup
IMPORTANT!
–PriortopumpactivationensuretheCPRvalve(4
plugs)locatedneartheheadendofthemattressis
fullyengaged.
–Alsoensurethattherapidreleasehandleisfirmly
connectedtothecontrolunit.
2.Connectthehandletothecontrolunit.
3.Insertthepowercordintothecontrolunitthenplugintoa
grounded220V50Hzelectricaloutlet.
IMPORTANT
–PriortoactivationensuretheCPRvalve(4plugs)
locatedneartheheadendofthemattressisfully
engagedandthattherapidreleasehandleisfirmly
connectedtothecontrolunit.
4.Pressthepowerbuttonforatleasttwo(2)secondstoactivate
thecontrolunit.
ThepressureLEDswillflashindicatingthesystemhas
activated.
5.Allow40–50minutesforthemattresstofullyinflate.Once
ready,youshouldseethefourthpressureLEDplusalternating
modeLEDilluminatetoindicatethatthesystemisreadyfor
use(systemautomaticallydefaultstoAlternatingModeafter
start-up).
6.OncetheMattressisfullyinflatedthebeddingcanbeplaced.
Fitsheetslooselyenoughtoallowforfreemovementofthe
mattressaircells.
7.Ensureeachsheetcornerisplacedthroughretainingbuckle.
4.4Removingthemattresssystem
1.SwitchoffthecontrolunitanddisconnectfrommainssupplyA.
2.RemovetherapidreleasehandleBfromthecontrolunitand
disconnecttheCPRtagC.
3.Placecontrolunitandpowercordontopofthemattressand
detachmattressfromthebedframe.
4.Onceairhasbeenreleasedfromallcells,rollupthemattress
andreturnallitemstothecarrybagforsafekeeping.
IMPORTANT!
–Priortorestartingthesystem,ensuretheCPRtag
isreplacedandallfoursealingconnectorsarefirmly
attached;andthattherapidreleasehandleisreplaced
andfirmlyconnectedtothecontrolunit.
–Usetargetdesigntolineupeachplugwithits
correspondingsocket.
A
B
C
1533195-A13

Invacare®SoftAIR®Super/SoftAIR®Excellence
5Usage
5.1Safetyinformation
Operatingconditions,see10.1GeneralData,page26.
WARNING!
Itisveryimportantforthepatienttoreposition
themselves,ortoberepositioned,onaregularbasis.
Thismustbebasedontheclinicaljudgementofa
qualifiedhealthcareprofessional.Thisrelievespressure
whichhelpspreventbothtissuecompressionand
potentialulcerformation.
–Alwaysconsultaqualifiedhealthcareprofessional
beforeusingtheSoftAIR®mattress.
–Monitorthepatientfrequently.
CAUTION!
–Makesurethattheprintedsideofthemattresscover
alwaysfacesupwards.
–Makesurethatthedistancebetweenthesurfaceofthe
mattressandthetopofthesiderailisatleast220mm.
IMPORTANT!
Strikethroughcanoccurinmattresscovers.
–Medicalequipmentincludinginfusionpumpsand
monitorsshouldbeattachedtoappropriatebed
accessories.
–Forhomeusecommoncausesofdamageinclude
cigaretteburnsandtheclawsofpetsthatpuncture
covers,allowingfluidingressandstaining.
IMPORTANT!
Riskofdamagetothemattresscover
–Topreventaccidentalcoverdamage,donotplace
hypodermicneedles,venflons,scalpelsorother
similarlysharpobjectsontothemattress.
–Ensurethatallvenflonsaretapeddowncorrectlywith
nosharpedgesexposed.
–Whenusingbridgingboardsorotherpatienttransfer
aids,careshouldbetakennottodamagethemattress
cover.Alltransferaidsshouldbecheckedforany
sharpedgesorburrsbeforeuseasthesecandamage
themattresscover.
–Makesurethatthemattressesarenotjammedor
damagedbysharpedgeswhenusedonbedswithan
adjustableframe.
–WhenusingtheSoftAIR®mattressonaprofilingbed
ensurethatthekneebreakisusedbeforethebackrest.
5.2Usingthecontrolunitpanel
WARNING!
–Ensurethismanualisreadandunderstoodfullybefore
operatingthecontrolunitpanel.
WARNING!
Thisequipmentisnotsuitableforuseinthepresenceof
aflammableanestheticmixturewithairorwithoxygen
ornitrousoxide.
14
1533195-A

Usage
A
B
C
D
E
E
F G
A
PowerButton
Turnssystempoweronandoff.Pressfortwo(2)seconds
B
AlarmLED
Thisredlightflashes,andanaudiblealarmsounds,toalert
whencontrolunitorMattresspressurefails.Thealarmhas
fivedifferentsignalstoindicatethecauseofthefailure.The
audiblealarmalsosoundswhenpowerisswitchedoff–press
AlarmMutetosilence.Referto9.1Identifyingandrepairing
faults,page24
.
C
AlarmMuteButton
Silencestheaudiblealarm(on/off).Audiblealarmwill
resumeafter20minutesifcauseoffailureisnotresolved.
D
ModeButton
PresstoselecteitherAlternationMode(alternativecells
cyclicallyinflatinganddeflating)orStaticMode(allcellsfully
inflatedwithnodynamicalternation).
StaticModewillautomaticallyrevertbacktoAlternation
Modeafterupto20minutes.
E
PressureArrowButtons
Pressarrowstoincreaseordecreasepressuresetting.Eight
availablepressuresettingsfromsofttohard(18mmHgto60
mmHg;6mmHgperstep).ThegreenLEDsilluminateto
indicatewhichoftheeightsettingsisoperational.
F
MaxFirmButton
Presstofacilitaterapidinflationtomaximumpressuresetting
(60mmHg).After≤30minutes,thesystemautomatically
revertsbacktothepreviouspressuresettingforpatient
safety.
G
ControlunitLock/UnlockButton
Pressforatleastfive(5)secondstolockthecontrolunit
settings–abeepsoundsandtheamberLEDilluminatesto
indicatesystemislocked.Whenlocked,onlytheAlarmMute
andLock/Unlockbuttonsremainoperational.Pressagain
foratleasttwo(2)secondstounlock(beepsoundsand
amberLEDturnsoff).
Thecontrolunitwillautomaticallyunlockintheeventof
apowerfailure.
1533195-A15

Invacare®SoftAIR®Super/SoftAIR®Excellence
5.3Usingthemattresssystem
EstablishingPressure(supine/faceupposition)
1.Whenmattressisfullyinflatedplacetheuserontothemattress.
2.PressPressurebuttontoselectthebestsettingforeffective
pressurereliefandsupport,basedonpatientweightandcomfort
requirements.
3.Assesswhetherthepatientiscomfortableandthesystemis
functioningcorrectlybyperforminga‘bottomingout’test.
BottomingOutTest
Whenalteringthepressuresetting,ensurethepatientisnot
‘bottomingout’(insufficientlysupportedbytheaircellsandtherefore
comingincontactwithbedbase).
1.Ensuresystemisinalternationmodebutisnotundergoingan
alternation.
2.Withthepatientlyinginasupineposition,unziptopcoverjust
pastsacral(bottom)region.
3.Slideyourhandalongadeflatedcellunderthepatientssacral
area(bottom).Theinnerstaticcellwillremaininflatedbutyour
handshouldslideeasilybetweenpatientandbase.
4.Ifhandcanpassunderpatientthenpatientisadequately
suspendedandpressurecanbelowered.
5.RepeatBottomingOuttestafterpressurehasbeenlowered.
Intheeventofasystemmalfunction,thealarmwillactivate
andpressureLEDswillflash.
EstablishingPressure(inclinedposition)
Whenmovingthepatienttoamoreuprightposition,pressuremay
needtobeincreased(byapproximately20%)toprovideadded
supportandtoavoid‘bottomingout’.
IMPORTANT!
–Returntotheoriginalpressuresettingwhenthe
patientreturnstothesupineposition,andperforma
BottomingOuttest.
–Waitaminimumof10~12minutesbetweenpressure
adjustmentandpatientassessment,asitmaytakea
fullcycleforthesystemtoadjusttoanynewsetting.
5.4CPRProcedure
A
1.FirmlypulltheyellowrapidreleaseCPRtagAfromthesideof
themattresstodeflatethewholesystem.
2.Switchoffthecontrolunit.
161533195-A

Usage
Mattresswillstarttodeflate.
3.
WhenCPRiscompletereplacetheyellowCPRTagensuring
thefoursealingconnectorsarefirmlyattachedandrestartthe
controlunitfollowingchapter4.3Activatingthecontrolunit,
page12.Usingthetargetdesigntolineupeachplugwithit's
correspondingsocket.
IMPORTANT!
–Waitforthemattresssystemtogainoptimalpressure.
–PerformaBottomingOuttestafterinflatingthe
mattressfollowingrapiddeflation.
5.5AlarmFunctions
TheredAlarmLEDflashes,andanaudiblealertsounds,toindicate
thecontrolunitormattresspressurehasfailed.TheLEDwillremain
illuminateduntilappropriatepressureisrestored.Theaudiblealarm
canbesilencedbypressingtheAlarmMutebutton.
Thesystemhasfivedifferentalarmsignals,identifiedbyfive
differentPressureSettingilluminationsequences.Thesignalsand
correspondingPressureSettingLEDdisplaysareillustratedbelow.
DisplayAlarm
Signal
Description
Initial
Failure
Mattresshasfailed
toreachminimum
operationalpressure
within50minutes
Low
Pressure
Pressurehasfallen
5mmHgormorebelow
thesettingminimum
High
Pressure
Pressurehasexceeded
thesettingmaximumby
10mmHgormore
AlternatingMode
failure
Mattresshasfailedto
commencealternation
ACpower
failure
Nopressureoutputdue
tomainspowerfailure
Ifalarmactivatesandthesystemfailstoinflateorloses
pressure,referto9.1Identifyingandrepairingfaults,page24.
1533195-A17

Invacare®SoftAIR®Super/SoftAIR®Excellence
5.6Transportingapatientonthemattress
1.Switchmodesfromalternatingtostaticandwaitupto10–15
minutesforcellstoinflatetomaximumpressure(ButtonDon
controlunit).
Ifaquickerresponseisrequired(upto5-10minutes)
thentheMaxinflatebuttoncanbeused.
2.Turnoffcontrolunitandthenremovetherapidreleasehandle
fromthecontrolunit.
3.Allowairtoescapeforacoupleofsecondsbeforesealing
withtheattachedtransportcap.ThiswillsoftentheMattress
surfaceforpressurereliefandcomfort.Ifpatientisresponsive,
questiontheircomfortlevelbasedoncurrentpressureand
adjustaccordingly.
Whenintransportationmode(e.g.sealedwiththe
transportationcap)themattresswillstayinflatedfora
minimumof72hours.
CAUTION!
Airpressureisreleasedfromallinternalstatic
cellsaswellasalternatingsections.
–Regularlyperforma‘BottomingOut’testtoensure
thepatientisappropriatelysupported.
181533195-A

Transport
6Transport
6.1Safetyinformation
IMPORTANT!
–Takecarewhenhandlingmattressestoensureno
damagetothecover.Itisrecommendedthattwo
peoplelift/carrymattresses.
–Avoidcontactwithjewellery,nails,abrasivesurfaces
etc.
–Donotdragmattresses.
–Avoidcontactwithwall,doorframes,doorcatches
orlocksetc.
–Donottransportinrollcagesunlesscompletely
protectedfromthesharpedgesofthecage.
1533195-A19

Invacare®SoftAIR®Super/SoftAIR®Excellence
7Maintenance
7.1Inspection
Itisrecommendedtocheckmattresses(aircellsandcover)for
strike-through(thismayincludefluidingress,stains,ripsordamage)
afterthereleaseofeachpatientoraftereachperiodofusebya
suitablyqualifiedandcompetentperson.
Checkmattresses
1.Unzipthecovercompletely.
2.Checkforanystainingonthewhiteundersideofthecover.
3.Checkforanystainingontheinterioraircells.
4.Replaceanystaineditemsanddisposeofasperlocalauthority
procedure.
7.2CleaningandCare
InfectionControlandroutinecleaningmustbecarriedoutin
accordancewithyourlocalInfectionControlPolicy.Itissuggested
thatalldisinfectionbedonewithacommonlyhighgradedisinfectant
orasolutionofSodiumHypochloriteorsimilar(upto10,000ppm
availablechlorine).
IMPORTANT!
Allcleaningagentsanddisinfectantsusedmustbe
effective,compatiblewithoneanotherandmustprotect
thematerialstheyareusedtoclean.
–Forfurtherinformationondecontaminationin
HealthcareEnvironments,contactyouhygiene
specialistorlocalinfectioncontrolpolicy.
Thetopcoverseamsaresealedtopreventmoistureingress
andbacterialgrowthintheseamstitching.
IMPORTANT!
–Donotusehightemperatureautoclaveforcleaning.
IMPORTANT!
Itisrecommendedthesystemiscleanedbetween
patientsandapproximatelyeverytwoweeksifin
constantuse.
Pleasecontactyourhygienespecialistintheeventofcontamination.
Cleaningmattresstopcover
Removeallcoversforlaundering.
Heavysoilage
IMPORTANT!
–Toestablishtheamountofdisinfectanttouse,
determinetheamountofwaterinthewasherandthen
followthemanufacturers’instructionsfordilution.
–Useonlyapproveddisinfectants.
1.Ifpresent,cleanupallspillagesofbodilyfluidsi.e.blood,urine,
faeces,sputum,woundexudaterandallotherbodilysecretions
assoonaspossibleusingacommonlyuseddisinfectantor1%
ChlorineSolution(10,000ppm).Thisisdependentoninfection
controlprotocolsandlocalmarketrequirements.
Largespillagesofbloodshouldbeabsorbedandremoved
withpapertowelsfirst,followedbyasbelow.
2.Launderthecoverswithamaximumtemperatureof
95°Cusingadiluteddetergentsolution(Instructionsonlabel).
3.Soakthetopcoverinthedisinfectantduringthewashcycle.
4.Afterlaunderingrinsethecoverwellincleanwateranddry
thoroughlybeforeuse.
201533195-A

Maintenance
IMPORTANT!
–Polyurethanecoatedfabricscanabsorbliquidsfor
shortperiodscausingatemporarychangetothe
polyurethanecharacteristics.Themattresscover
swellstemporarilyandismorevulnerabletophysical
damageforaperiodafteritiscompletelysurface
dried,bywhichtimeitwillreverttoitspreviousstate.
IMPORTANT!
–Maybetumbleddriedonalowheatsetting,however
thecycleshouldbedisruptedtoensurethatnowater
istrapped.
5.Hangmattresscoversfromalineorbaranddripdryinaclean
indoorenvironment.
IMPORTANT!
1%ChlorineSolutionusedonaregularbasiscandiminish
thelifeofthecoverifnotrinsedanddriedproperly.
Lightsoilage
Iftherearevisiblesignsofbodyfluidsandorsubstancesonthetop
cover,thetopcovershouldbesanitized.
1.Applyanintermediatelevelofcommonlyuseddisinfectant(or
asolutionofSodiumHypochloriteorsimilarupto10,000ppm
availablechlorine)tothetopcoveruppersurfaceeitherby
sprayingorbyhandapplication.Thisisdependentoninfection
controlprotocolsandlocalmarketrequirements.
IMPORTANT!
–Ensurethesurfaceiscompletelycoveredwiththe
disinfectantandremainsincontactwiththesurface
accordingtomanufacturer’sinstructions.
2.Removedisinfectantandrinsethoroughly.
3.Allowtoairdrybeforeuse.
Cleaningmattressbase
IMPORTANT!
–DonotmachinewashordrytheMattressbase.
1.Wipedowntheoutsideshellwithacommonlyuseddisinfectant
solution(orasolutionofSodiumHypochloriteorsimilarupto
10,000ppmavailablechlorine).Thisisdependentoninfection
controlprotocolsandlocalmarketrequirements.
IMPORTANT!
–Ensurethatallsurfacescomeincontactwiththe
disinfectant.
2.Rinseoffwellwithacleandampclothandairdry.
Disinfectingtheaircells
IMPORTANT!
–DonotdisassembletheMattressunlesscleaningis
required.
–Donotdisconnectthepipesfromindividualaircells.
–Donotmachinewashordrytheaircells.
1.Disconnectaircellsfromthebasebyunfasteningthepressstuds
ateachend.
2.Disconnectairpipesfrommainairhoses.
3.Slideeachcelloutfromthecellstraps.
1533195-A
21

Invacare®SoftAIR®Super/SoftAIR®Excellence
4.Swabwithaclothdampedwithwarmwatercontainingdetergent
orasolutionofSodiumHypochloriteorsimilar(upto10,000
ppmavailablechlorine).Thisisdependentoninfectioncontrol
protocolsandlocalmarketrequirements.
5.Drythoroughlywithasoftclothbeforerefastening.
Cleaningthehandle
1.Theexteriorhandlecanbeperiodicallywipedusingacloth
dampenedwithdisinfectant.
Cleaningthecontrolunit
WARNING!
Electricalshockhazard!
Thecontrolunithasnoprotectionagainstingressof
water.
–Ensurethecontrolunitisdisconnectedfromthe
mainselectricitysupplybeforecleaning.
–Donotspraydisinfectantdirectlyontothecontrol
unit,orimmersethecontrolunitinanytypeofliquid.
1.Wipedowncontrolunitandhosefittingsusingaclothdampened
withwarmwatercontainingdetergent(orwithasolutionof
SodiumHypochloriteorsimilar).Thisisdependentoninfection
controlprotocolsandlocalmarketrequirements.
2.Drythoroughlybeforeuse.
Preventiveinspectionsandcalibrationofthecontrolunit
arenotrequired.
Themanufacturerwillprovidecircuitdiagrams,component
partlists,anddescriptionstoassistservicepersonnelin
repairingtheequipment.
7.3Replacingtheairfilter
IMPORTANT!
Goodfiltermaintenanceiscriticaltomaintainyour
SoftAIRsysteminoptimaloperatingcondition.Failure
tokeepthefilterscleanwillresultinsystemdowntime
andincreaserepaircosts.Itisrecommendedthattheair
filterbereplacedannually.Replacementairfiltersare
availablefromanInvacareServiceCentre.
1.Switchoffthepowersupplytothecontrolunit.
2.Disconnectthepowercordandairhoses.
3.Placethecontrolunitonaflatsurfacewithitsbackpanel
uppermost(placesoftclothunderunittopreventscratches).
4.Carefullyremovetheairfiltercover.Removeanddiscardthe
filterandfitwithnewfilter.
5.Refittheairfiltercovertothecontrolunit.
Thecontrolunitisnowreadyforre-connection.
7.4Replacingfuse(1ampslowblowfuse)
CAUTION!
–Ensurethereplacementoffusesiscarriedoutin
accordancewithlocallegislation.
1.Switchoffthepowersupplytothecontrolunit.
2.Removethepowercordfromtheelectricalsocketontheside
ofthecontrolunit.
3.InsertasmallFlatHeadscrewdriverintothegrooveandturn
anti-clockwise(1/4turn).
4.Removethe“blown”fusefromthefuseholderclipanddiscard.
5.Insertanewfuseintotheplug.Pushagainsttheforceofthe
springandturnclockwisewiththescrewdriver(1/4turn).
22
1533195-A

AfterUse
8AfterUse
8.1Storage
IMPORTANT!
–Storemattressesinadryenvironment.
–Storemattresseswithinaprotectivecover.
–Ensuremattressiscarefullyrolledandstoredin
protectivebagprovidedonclean,dry,off-flooringfree
fromsharpedgestoavoidanypossibledamage.
–Neverstoreotheritemsontopofamattress.
–Donotstoremattressesnexttoradiatorsorother
heatingdevices.
–Protectmattressesfromdirectsunlight.
Environmentalconditionsforstorage,see10.1GeneralData,page26.
8.2Re-use
Acleaningrecordshouldbekeptaspartofcleaningthesystem.
Theproductissuitableforrepeateduse.Thenumberoftimesitcan
beuseddependsonhowoftenandinwhichwaytheproductisused.
1.Beforereuse,cleantheproductthoroughly,seechapter7.2
CleaningandCare,page20
.
8.3Disposal
Thedisposalandrecyclingofuseddevicesandpackagingmustcomply
withtheapplicablelegalregulation.
1533195-A23

Invacare®SoftAIR®Super/SoftAIR®Excellence
9Troubleshooting
9.1Identifyingandrepairingfaults
ThissectionprovidesbasictroubleshootingsupportfortheSoftAIR®MattressSystems.
WARNING!
Electricshockhazard!
Openingthecontrolunitcouldcausepersonalinjuryorequipmentdamage.
–Donottrytoopenthecontrolunit.Ensurethereplacementoffusesiscarriedoutinaccordancewithlocallegislation.
Alarm/Fault
CauseSolution
Controlunitdoesnot
operate;nodisplaylights
illuminate
TheControlUnitmaynotbe
attachedtoapowersource
Afusemayneedreplacingin
thecontrolunit
1.ChecktheControlUnitisconnectedtomainspoweroutletwiththe
correctvoltage.
2.ChecktheControlUnitisswitchedon.
3.Checkthemainsplugfuse(3AMP)thencheckbothcontrolunitfuses(1amp
slowblowfuse)–fusescanbereleasedusingascrewdrivertopushandturn.
AlarmLED
+audiblealarm
Initialfailure
1.Resetthealarm–turnoffPowerandpresstheAlarmMutebutton.
2.ChecktheHandleisintact,ensuringallfoursealingconnectorsarefirmly
fittedtoControlUnitandtheairhoses.ChecktheCPRTagisattachedand
allfoursealingconnectorsarefirmlysecure.
3.Checkallairhosesalongtheinsideofthemattress–eachshouldbefirmly
connected.Checkeachaircellissecurelyattachedtoitsconnectingairpipe.
4.Checkallcells,pipesandhosesforanyairleakage.
5.SwitchonPower.
24
1533195-A

Troubleshooting
Alarm/Fault
CauseSolution
AlarmLED
+audiblealarm
Pressuretoolow
1.Resetthealarm–turnoffPowerandpresstheAlarmMutebutton.
2.ChecktheHandleisintact,ensuringallfoursealingconnectorsarefirmly
fittedtoControlUnitandtheairhoses.ChecktheCPRTagisattachedand
allfoursealingconnectorsarefirmlysecure.
3.Checkallairhosesalongtheinsideofthemattress–eachshouldbefirmly
connected.Checkeachaircellissecurelyattachedtoitsconnectingairpipe.
4.Checkallcells,pipesandhosesforanyairleakage.
5.Checkthattheairfiltercoveriscorrectlysecuredandtheairfilterisclean.
6.SwitchonPower.
AlarmLED
+audiblealarm
Pressuretoohigh
1.Resetthealarm–turnoffPowerandpresstheAlarmMutebutton.
2.Disconnecttheairhosestoreducepressure–reconnectwhenpressure
hasdecreased.
3.CheckfortwistsintheairhosesbetweenMattressandControlUnit.
4.SwitchonPower.
AlarmLED
+audiblealarm
AlternatingModeFailure(no
alternation)
1.Resetthealarm–turnoffPowerandpresstheAlarmMutebutton.
2.Disconnecttheairhosestoreducepressure–reconnectwhenpressure
hasdecreased.
AlarmLED
+audiblealarm
ACpowerfailure1.PresstheAlarmMutebuttontosilencetheaudiblealarm.
2.Checkthepowercableisfirmlypluggedintothemainsoutletandthe
ControlUnit;andcheckthemainspowerisswitchedon.
3.ChecktheControlUnitfuse(1ampslowblowfuse)–fusecanbereleased
usingascrewdrivertopushandturn.
Patientissinkingor
“bottomingout”whilst
lyingflatontheMattress
Thepressuremaybesettoo
lowforthepatient’sweight
1.IncreasethepressuresettingbypressingupthePressurearrow.
2.Tocheckeffectivesystemperformance,conducta“BottomingOut”test,
→5.3Usingthemattresssystem,page16.
Iftheproblemisnotresolved,pleasecontactanauthorisedInvacareLtdServiceCentre.
1533195-A25

Invacare®SoftAIR®Super/SoftAIR®Excellence
10Technicaldata
10.1GeneralData
SoftAIR®SuperSoftAIR®Excellence
CycleControlPurposedesigneddistributorvalvesupplyingoperatingairtotheinflatablecells
CycleTime10–12minutes
SupplyVoltage220-240V,50/60Hz,0.2Aforcontrolunit
FuseRating
1A(x1)SlowBlowFuse
BatterySourceVARTA,V80H,1.2VDC,70mAh
PowerRating
12VA
NumberofCells21(cellincell)including3staticheadcells19(cellincell)including3staticheadcells
and5microcellsplus2sidebolsters
CellHeight200mm230mm
Minimum/maximumUserWeight≤30–200kg
MattressDimensions:
Length
Width
Height
2000mm
830mm/880mm
200mm
2000mm
830mm/880mm
250mm(includingsideformers)
Mattressweight
9.2kg/10.5kg9.5kg/10.8kg
ControlUnitDimensions:
Length
Width
Height
290mm
170mm
270mm
261533195-A

Technicaldata
SoftAIR®SuperSoftAIR®Excellence
Controlunitweight3.2kg
CellMaterial0.15mmTPUfilmlaminatedon210deniernylonfabric
BaseMaterial
Nylonfabric420denierwitha0.1mmTPUcoating
CoverMaterial100%Polyurethanesurface,100%Polyesterinside
HoseConnection
Pushonconnectionhandle
Emergency
CPRTag
ModeofOperation
Non-continuous
OperatingEnvironment
Airhumidity
Ambienttemperature
Altitude
30%to70%
10ºCto40ºC
≤2000m
Storage/TransportationEnvironment
Airhumidity
Ambienttemperature
10%to70%
-10°Cto60°C
Warranty
Controlunit
Mattress
2years
2years
Allproductspecificationsaresubjecttochangewithoutnotice.
1533195-A27

Invacare®SoftAIR®Super/SoftAIR®Excellence
10.2Guidanceandmanufacturer’sdeclaration
TheSoftAIR®Super&Excellenceareintendedforuseintheelectromagneticenvironmentspecifiedbelow.Thecustomerortheuserofthe
SoftAIR®SuperorExcellenceshouldensurethatitisusedinsuchanenvironment.
Electromagneticemissions
Emissiontest
Compliance
Electromagneticenvironment–guidance
RFemissions
CISPR11
Group1
TheSoftAIR®Super&ExcellenceuseRFenergyonlyfortheirinternalfunctions.
Therefore,itsRFemissionsareverylowandarenotlikelytocauseanyinterference
innearbyelectronicequipment.
RFemissions
CISPR11
ClassB
Harmonicemissions
IEC61000-3-2
ClassA
TheSoftAIR®Super&Excellencearesuitableforuseinallestablishments,including
domesticestablishmentsandthosedirectlyconnectedtothepubliclow-voltage
powersupplynetworkthatsuppliesbuildingsusedfordomesticpurposes.
Voltagefluctuations/flicker
emissions
IEC61000-3-3
Complies
Electromagneticimmunity
Immunitytest
IEC60601testlevelCompliancelevel
Electromagneticenvironment–
guidance
Electrostaticdischarge(ESD)
IEC61000-4-2
±6kVcontact
±8kVair
±6kVcontact
±8kVair
Floorsshouldbewood,concreteorceramic
tile.Iffloorsarecoveredwithsynthetic
material,therelativehumidityshouldbeat
least30%.
Electricalfasttransient/burst
IEC61000-4-4
±2kVforpowersupplylines±2kVforpowersupplylinesMainspowerqualityshouldbethatofatypical
commercialorhospitalenvironment.
281533195-A

Technicaldata
Immunitytest
IEC60601testlevelCompliancelevel
Electromagneticenvironment–
guidance
Surge
IEC61000-4-5
±1kVline(s)toline(s)±1kVdifferentialmodeMainspowerqualityshouldbethatofatypical
commercialorhospitalenvironment.
Voltagedips,short
interruptionsandvoltage
variationsonpowersupply
inputlines
IEC61000-4-11
<5%UT(>95%dipinUT)
for0.5cycle
40%UT(60%dipinUT)
for5cycles
70%UT(30%dipinUT)
for25cycles
<5%UT(>95%dipinUT)
for5sec
<5%UT(>95%dipinUT)
for0.5cycle
40%UT(60%dipinUT)
for5cycles
70%UT(30%dipinUT)
for25cycles
<5%UT(>95%dipinUT)
for5sec
Mainspowerqualityshouldbethatofatypical
commercialorhospitalenvironment.Ifthe
useroftheSoftAIR®SuperorExcellence
requirescontinuedoperationduringpower
mainsinterruptions,itisrecommendedthat
theSoftAIR®SuperorExcellencebepowered
fromanuninterruptiblepowersupplyora
battery.
Powerfrequency(50Hz)
magneticfield
IEC61000-4-8
3A/m3A/mPowerfrequencymagneticfieldsshouldbeat
levelscharacteristicofatypicallocationina
typicalcommercialorhospitalenvironment.
NOTE:UTisthea.c.mainsvoltagepriortoapplicationofthetestlevel.
1533195-A29

Invacare®SoftAIR®Super/SoftAIR®Excellence
Immunitytest
IEC60601testlevelCompliancelevel
Electromagneticenvironment–guidance
ConductedRF
IEC61000-4-6
3V
rms
150kHzto80MHz
3V
rms
RadiatedRF
IEC61000-4-3
3V/m
80MHzto2.5GHz
3V/m
PortableandmobileRFcommunicationsequipment
shouldbeusednoclosertoanypartoftheCT515,
includingcables,thantherecommendedseparation
distancecalculatedfromtheequationapplicableto
thefrequencyofthetransmitter.
Recommendedseparationdistance:
d=1.167√P
d=1.167√P80MHzto800MHz
d=2.333√P800MHzto2.5GHz
WherePisthemaximumoutputpowerratingofthe
transmitterinwatts(W)accordingtothetransmitter
manufactureranddistherecommendedseparation
distanceinmeters(m).
FieldstrengthsfromfixedRFtransmitters,as
determinedbyanelectromagneticsitesurvey
a)
,
shouldbelessthanthecompliancelevelineach
frequencyrange
b)
.
Interferencemayoccurinthevicinityofequipment
markedwiththefollowingsymbol:
301533195-A

Technicaldata
NOTE1:At80MHzand800MHz,thehigherfrequencyrangeapplies.
NOTE2:Theseguidelinesmaynotapplyinallsituations.Electromagneticpropagationisaffectedbyabsorptionandreflectionfromstructures,
objectsandpeople.
a)
Fieldstrengthsfromfixedtransmitters,suchasbasestationsforradio(cellular/cordless)telephonesandlandmobileradios,amateurradio,
AMandFMradiobroadcastandTVbroadcastcannotbepredictedtheoreticallywithaccuracy.Toassesstheelectromagneticenvironment
duetofixedRFtransmitters,anelectromagneticsitesurveyshouldbeconsidered.Ifthemeasuredfieldstrengthinthelocationinwhichthe
SoftAIR®SuperorExcellenceisusedexceedstheapplicableRFcompliancelevelabove,theSoftAIR®SuperorExcellenceshouldbeobservedto
verifynormaloperation.Ifabnormalperformanceisobserved,additionalmeasuresmaybenecessary,suchasreorientingorrelocatingthe
SoftAIR®SuperorExcellence.
b)
Overthefrequencyrange150kHzto80MHz,fieldstrengthsshouldbelessthan3V/m.
1533195-A31

Invacare®SoftAIR®Super/SoftAIR®Excellence
RecommendedseparationdistancesbetweenportableandmobileRFcommunicationsequipmentandtheSoftAIR®Super&
ExcellenceAlternatingControlUnit
TheSoftAIR®Super&ExcellenceareintendedforuseinanelectromagneticenvironmentinwhichradiatedRFdisturbancesarecontrolled.The
customerortheuseroftheSoftAIR®SuperorExcellencecanhelppreventelectromagneticinterferencebymaintainingaminimumdistance
betweenportableandmobileRFcommunicationsequipment(transmitters)andtheSoftAIR®SuperorExcellenceasrecommendedbelow,
accordingtothemaximumoutputpowerofthecommunicationsequipment.
Ratedmaximumoutputpowerof
transmitter(W)
Separationdistanceaccordingtofrequencyoftransmitter(m)
150kHzto80MHz
d=1.167√P
80MHzto800MHz
d=1.167√P
800MHzto2.5GHz
d=2.333√P
0.010.1170.1170.233
0.10.3690.3690.738
11.1671.1672.333
103.6893.6897.379
10011.66711.66723.333
Fortransmittersratedatamaximumoutputpowernotlistedabove,therecommendedseparationdistancedinmeters(m)canbeestimated
usingtheequationapplicabletothefrequencyofthetransmitter,wherePisthemaximumoutputpowerratingofthetransmitterinwatts(W)
accordingtothetransmittermanufacturer.
NOTE1:At80MHzand800MHz,theseparationdistanceforthehigherfrequencyrangeapplies.
NOTE2:Theseguidelinesmaynotapplyinallsituations.Electromagneticpropagationisaffectedbyabsorptionandreflectionfromstructures,
objectsandpeople.
321533195-A
 Loading...
Loading...