Hospira Plum A+ Service manual

INFUSION SYSTEM
For use with list number 12391-36
Technical Service Manual
430-10996-001 (Rev. 12/05)

©Hospira, Inc.
This document and the subject matter disclosed herein are proprietary information. Hospira retains all the exclusive rights of dissemination, reproduction, manufacture, and sale. Any party using this document accepts it in confidence, and agrees not to duplicate it in whole or in part nor disclose it to others without the written consent of Hospira.
430-10996-001 (Rev. 12/05) |
Plum A+® Infusion System |
|
Change History |
Part Number |
Description of Change |
430-10996-001 |
Original issue |
(Rev. 12/05) |
|
Technical Service Manual |
430-10996-001 (Rev. 12/05) |
CHANGE HISTORY
This page intentionally left blank.
430-10996-001 (Rev. 12/05) |
Plum A+® Infusion System |
Contents
Section 1
INTRODUCTION . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-1 |
||
1.1 |
SCOPE . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-1 |
|
1.2 |
CONVENTIONS . . . . . . . . . . . . . . . . . . . . . . . . |
1-2 |
|
1.3 |
COMPONENT DESIGNATORS . . . . . . . . . . . . . . . . . . |
1-2 |
|
1.4 |
ACRONYMS AND ABBREVIATIONS . . . . . . . . . . . . . . . . |
1-3 |
|
1.5 |
USER QUALIFICATION . . . . . . . . . . . . . . . . . . . . . |
1-5 |
|
1.6 |
ARTIFACTS . . . . . . . . . . . . . . . . . . . . . . . . . |
1-5 |
|
1.7 |
ELECTROMAGNETIC COMPATIBILITY. . . . . . . . . . . . . . . |
1-5 |
|
1.8 |
INSTRUMENT INSTALLATION PROCEDURE . . . . . . . . . . . . |
1-6 |
|
|
1.8.1 |
UNPACKING . . . . . . . . . . . . . . . . . . . . . . |
1-6 |
|
1.8.2 |
INSPECTION . . . . . . . . . . . . . . . . . . . . . . |
1-6 |
|
1.8.3 |
SELF TEST. . . . . . . . . . . . . . . . . . . . . . . . |
1-7 |
1.9 |
BIOMED SETTINGS . . . . . . . . . . . . . . . . . . . . . . |
1-9 |
|
|
1.9.1 |
ALARMS LOG . . . . . . . . . . . . . . . . . . . . . . |
1-11 |
|
1.9.2 |
SETTING THE TIME AND DATE . . . . . . . . . . . . . . . |
1-12 |
Section 2
WARRANTY . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-1
Section 3
SYSTEM OPERATING MANUAL . . . . . . . . . . . . . . . . . . . . 3-1
Section 4
THEORY OF OPERATION . . . . . . . . . . . . . . . . . . . . . . . |
4-1 |
|
4.1 |
GENERAL DESCRIPTION . . . . . . . . . . . . . . . . . . . . |
4-1 |
4.2 |
ELECTRONIC SUBSYSTEM OVERVIEW . . . . . . . . . . . . . . . |
4-2 |
|
4.2.1 CPU SUBSYSTEM . . . . . . . . . . . . . . . . . . . . . |
4-2 |
|
4.2.1.1 CPU . . . . . . . . . . . . . . . . . . . . . . . |
4-3 |
4.2.1.2SYSTEM MEMORY ADDRESS MAP . . . . . . . . . . . 4-3
4.2.1.3PROGRAMMABLE READ-ONLY MEMORY . . . . . . . . 4-3
4.2.1.4STATIC RANDOM ACCESS MEMORY . . . . . . . . . . 4-3
4.2.1.5 |
CONTROL LOGIC . . . . . . . . . . . . . . . . . . |
4-3 |
4.2.1.6 |
LCD CONTROLLER . . . . . . . . . . . . . . . . . |
4-4 |
4.2.1.7 |
LCD BACKLIGHT CONTROL . . . . . . . . . . . . . |
4-4 |
4.2.1.8 |
LCD CONTRAST CONTROL . . . . . . . . . . . . . . |
4-4 |
4.2.1.9 |
REAL-TIME CLOCK . . . . . . . . . . . . . . . . . |
4-5 |
4.2.1.10VOLTAGE MONITOR WATCHDOG TIMER . . . . . . . . 4-5
4.2.1.11ANALOG-TO-DIGITAL CONVERTER . . . . . . . . . . 4-6
4.2.1.12DIGITAL-TO-ANALOG CONVERTER . . . . . . . . . . 4-8
4.2.1.13FRONT PANEL KEYPAD MATRIX . . . . . . . . . . . . 4-8
4.2.1.14FRONT PANEL ON/OFF KEY . . . . . . . . . . . . . . 4-8
4.2.1.15 FRONT PANEL LED INDICATORS . . . . . . . . . . . 4-8
4.2.1.16KEYPAD LOCKOUT INTERFACE . . . . . . . . . . . . 4-9
4.2.1.17NURSE CALL INTERFACE. . . . . . . . . . . . . . . 4-9
4.2.1.18 AUDIBLE INDICATORS . . |
. . . . . . |
. . . . |
. . |
. |
4-9 |
4.2.1.19 DATAPORT INTERFACE . . |
. . . . . . |
. . . . |
. . |
. |
4-9 |
4.2.1.20 POWER SUPPLY INTERFACE |
. . . . . . |
. . . . |
. . |
. |
4-10 |
4.2.1.21 MECHANISM INTERFACE . |
. . . . . . |
. . . . |
. . |
. |
4-11 |
Technical Service Manual |
iii |
430-10996-001 (Rev. 12/05) |
CONTENTS
4.2.2 POWER SUPPLY SUBSYSTEM . . . . . . . . . . . . . . . . 4-12
4.2.2.1MAIN SWITCHING REGULATOR. . . . . . . . . . . . 4-13
4.2.2.2MAIN REGULATOR FAULT DETECTION . . . . . . . . . 4-13
4.2.2.3 |
SYSTEM POWER . . . . . . . . . |
. . . . . |
. |
. . |
. |
4-14 |
4.2.2.4 |
AUXILIARY SUPPLIES . . . . . . . |
. . . . . |
. |
. . |
. |
4-14 |
4.2.2.5 |
POWER CONTROL . . . . . . . . |
. . . . . |
. |
. . |
. |
4-14 |
4.2.2.6BATTERY VOLTAGE MEASUREMENT . . . . . . . . . . 4-15
4.2.2.7BATTERY CHARGE/DISCHARGE CURRENT
MEASUREMENT . . . . . . . . . . . . . . . . . . 4-15
4.2.2.8 BATTERY CHARGER |
. . . |
. . . . . . . . . |
. . |
. |
. |
4-16 |
4.2.3 MECHANISM SUBSYSTEM . |
. . . |
. . . . . . . . . |
. . |
. |
. |
4-16 |
4.2.3.1MOTORS/MOTOR DRIVE. . . . . . . . . . . . . . . 4-16
4.2.3.2MOTOR POSITION SENSORS . . . . . . . . . . . . . 4-18
4.2.3.3 |
V2_5 REFERENCE VOLTAGE |
. . . . |
. . . . . |
. . |
. . 4-19 |
||
4.2.3.4 |
AIR SENSORS . . . . . . |
. . . . |
. . . . . |
. . |
. |
. |
4-19 |
4.2.3.5 |
PRESSURE SENSORS . . . |
. . . . |
. . . . . |
. . |
. |
. |
4-21 |
4.2.3.6PRESSURE SENSOR CALIBRATION . . . . . . . . . . . 4-22
4.2.3.7CASSETTE TYPE/PRESENCE SELECTION . . . . . . . . . 4-22
|
|
4.2.3.8 |
SERIAL EEPROM . . . . . . . . . . . . . . |
. |
. |
. . 4-23 |
4.3 |
PRINTED WIRING ASSEMBLIES . . . . . . . . . . . . . |
. |
. |
. . 4-23 |
||
|
4.3.1 |
POWER SUPPLY PWA . . . . . . . . . . . . . . . |
. |
. |
. . 4-23 |
|
|
4.3.2 |
PERIPHERAL PWA . . . . . . . . . . . . . . . . |
. |
. |
. . 4-24 |
|
|
4.3.3 |
CPU PWA . . . . . . . . . . . . . . . . . . . . |
. |
. |
. . 4-24 |
|
|
4.3.4 |
DRIVER PWA . . . . . . . . . . . . . . . . . . |
. |
. |
. . 4-25 |
|
|
4.3.5 |
SWITCH PWA . . . . . . . . . . . . . . . . . . |
. |
. |
. . 4-26 |
|
|
4.3.6 |
APP PWA . . . . . . . . . . . . . . . . . . . . |
. |
. |
. . 4-26 |
|
4.4 REMOTE MOUNTED PERIPHERALS . . . . . . . . . . . . |
. |
. |
. . 4-26 |
|||
|
4.4.1 |
LCD . . . . . . . . . . . . . . . . . . . . . . |
. |
. |
. . 4-26 |
|
|
4.4.2 |
SEALED LEAD ACID BATTERY . . . . . . . . . . . |
. |
. |
. . 4-27 |
|
4.5 |
MECHANICAL OVERVIEW . . . . . . . . . . . . . . . |
. |
. |
. . 4-27 |
||
|
4.5.1 |
CASSETTE . . . . . . . . . . . . . . . . . . . |
. |
. |
. . 4-27 |
|
|
4.5.2 |
MECHANISM ASSEMBLY . . . . . . . . . . . . . |
. |
. |
. . 4-29 |
|
|
|
4.5.2.1 |
MOTOR AND VALVE ASSEMBLIES . . . . . . . |
. |
. |
. . 4-29 |
|
|
4.5.2.2 |
A/B VALVE SUBSYSTEM . . . . . . . . . . . |
. |
. |
. . 4-30 |
|
|
4.5.2.3 |
INLET/OUTLET VALVE SUBSYSTEM . . . . . . |
. |
. |
. . 4-31 |
|
|
4.5.2.4 |
PLUNGER DRIVE SUBSYSTEM. . . . . . . . . |
. |
. |
. . 4-31 |
Section 5
MAINTENANCE AND SERVICE TESTS . . . . . . . . . . . . . . . . . . |
5-1 |
|
5.1 ROUTINE MAINTENANCE . . . . . . . . . . . . . . . . . . . |
5-1 |
|
5.1.1 |
CLEANING . . . . . . . . . . . . . . . . . . . . . . . |
5-1 |
5.1.2 |
SANITIZING . . . . . . . . . . . . . . . . . . . . . . |
5-2 |
5.2 PERFORMANCE VERIFICATION TEST . . . . . . . . . . . . . . . |
5-2 |
|
5.2.1 |
EQUIPMENT REQUIRED . . . . . . . . . . . . . . . . . . |
5-3 |
5.2.2 |
INSPECTION . . . . . . . . . . . . . . . . . . . . . . |
5-3 |
5.2.3 |
TEST SETUP . . . . . . . . . . . . . . . . . . . . . . . |
5-4 |
5.2.4 |
SELF TEST . . . . . . . . . . . . . . . . . . . . . . . |
5-4 |
5.2.5 |
CASSETTE ALARM TEST . . . . . . . . . . . . . . . . . . |
5-6 |
5.2.6 |
FREE FLOW TEST . . . . . . . . . . . . . . . . . . . . . |
5-6 |
5.2.7 |
DISPLAY TEST . . . . . . . . . . . . . . . . . . . . . . |
5-6 |
5.2.8 |
KEYPAD VERIFICATION/FUNCTIONAL TEST . . . . . . . . . . |
5-7 |
5.2.9 |
ALARM LOUDNESS TEST . . . . . . . . . . . . . . . . . |
5-7 |
5.2.10 |
LOCKOUT SWITCH TEST. . . . . . . . . . . . . . . . . . |
5-9 |
5.2.11 |
PROXIMAL OCCLUSION TEST. . . . . . . . . . . . . . . . |
5-9 |
5.2.12 |
PROXIMAL AIR-IN-LINE TEST . . . . . . . . . . . . . . . . |
5-10 |
430-10996-001 (Rev. 12/05) |
iv |
Plum A+® Infusion System |
CONTENTS
|
5.2.13 |
DISTAL AIR-IN-LINE TEST . . . . . . . . . . . . . . |
. |
. |
. |
5-10 |
|
5.2.14 DISTAL OCCLUSION TEST . . . . . . . . . . . . . . |
. |
. |
. |
5-12 |
|
|
5.2.15 DELIVERY ACCURACY TEST . . . . . . . . . . . . . |
. |
. |
. |
5-14 |
|
|
5.2.16 |
NURSE CALL TEST . . . . . . . . . . . . . . . . . |
. |
. |
. |
5-14 |
|
5.2.17 ELECTRICAL SAFETY TEST . . . . . . . . . . . . . . |
. |
. |
. |
5-15 |
|
|
5.2.18 |
END OF THE PVT . . . . . . . . . . . . . . . . . . |
. |
. |
. |
5-15 |
5.3 |
PERIODIC MAINTENANCE INSPECTION . . . . . . . . . . . |
. |
. |
. |
5-15 |
|
5.4 |
BATTERY OPERATION OVERVIEW . . . . . . . . . . . . . |
. |
. |
. |
5-16 |
|
Section 6
TROUBLESHOOTING . . . . . . . . . . . . . . . . . . . . . . . . |
6-1 |
||
6.1 |
TECHNICAL ASSISTANCE. . . . . . . . . . . . . . . . . . . . |
6-1 |
|
6.2 |
WARNING MESSAGES . . . . . . . . . . . . . . . . . . . . . |
6-1 |
|
6.3 |
ALARM MESSAGES AND ERROR CODES . . . . . . . . . . . . . . |
6-2 |
|
|
6.3.1 |
OPERATIONAL ALARM MESSAGES . . . . . . . . . . . . . |
6-2 |
|
6.3.2 |
ERROR CODES REQUIRING TECHNICAL SERVICE . . . . . . . . |
6-7 |
6.4 |
TROUBLESHOOTING PROCEDURES. . . . . . . . . . . . . . . . |
6-13 |
|
Section 7
REPLACEABLE PARTS AND REPAIRS . . . . . . . . . . . . . . . . . . |
7-1 |
||
7.1 |
REPLACEABLE PARTS . . . . . . . . . . . . . . . . . . . . . |
7-1 |
|
7.2 |
REPLACEMENT PROCEDURES . . . . . . . . . . . . . . . . . . |
7-3 |
|
|
7.2.1 |
SAFETY AND EQUIPMENT PRECAUTIONS . . . . . . . . . . . |
7-3 |
|
7.2.2 |
REQUIRED TOOLS AND MATERIALS . . . . . . . . . . . . . |
7-3 |
|
7.2.3 |
RUBBER FOOT PAD REPLACEMENT . . . . . . . . . . . . . |
7-4 |
|
7.2.4 |
BATTERY, BATTERY DOOR, AND DOOR PAD REPLACEMENT . . . |
7-6 |
|
7.2.5 AC POWER CORD, RETAINER, AND VELCRO STRAP |
|
|
|
|
REPLACEMENT . . . . . . . . . . . . . . . . . . . . . |
7-8 |
|
7.2.6 |
PERIPHERAL ASSEMBLY REPLACEMENT . . . . . . . . . . . |
7-10 |
7.2.7PERIPHERAL ASSEMBLY COMPONENT REPLACEMENT . . . . . . 7-12
7.2.7.1VOLUME CONTROL KNOB REPLACEMENT. . . . . . . . 7-14
7.2.7.2PERIPHERAL COVER REPLACEMENT . . . . . . . . . . 7-14
7.2.8SEPARATING THE FRONT ENCLOSURE, REAR ENCLOSURE,
AND MAIN CHASSIS ASSEMBLY . . . . . . . . . . . . |
. |
. |
. |
7-15 |
7.2.9 FRONT ENCLOSURE, REAR ENCLOSURE, OR MAIN CHASSIS |
|
|
|
|
REPLACEMENT . . . . . . . . . . . . . . . . . . |
. |
. |
. |
7-17 |
7.2.9.1 SHOE GASKET REPLACEMENT . . . . . . . . . |
. |
. |
. |
7-18 |
7.2.9.2FRONT/REAR ENCLOSURE GASKET REPLACEMENT . . . . 7-19
7.2.10REAR ENCLOSURE COMPONENT REPLACEMENT . . . . . . . . 7-19
7.2.10.1POLE CLAMP EXTRUSION, BACKING PLATE,
AND INSULATOR REPLACEMENT . . . . . . . . . . . 7-21
7.2.10.2POLE CLAMP SHAFT/KNOB ASSEMBLY
AND SHAFT TIP REPLACEMENT . . . . . . . . . . . . 7-21
7.2.10.3REAR ENCLOSURE AND HANDLE GASKETS
REPLACEMENT . . . . . . |
. . . . . . . . . |
. . |
. |
7-22 |
7.2.11 MINIPOLE ASSEMBLY REPLACEMENT |
. . . . . . . . . |
. . |
. |
7-23 |
7.2.11.1 COTTER RING REPLACEMENT |
. . . . . . . . . |
. . |
. |
7-23 |
7.2.11.2BAG HANGER REPLACEMENT. . . . . . . . . . . . . 7-24
7.2.11.3CLUTCH HOUSING REPLACEMENT. . . . . . . . . . . 7-24
7.2.11.4 CLUTCH SPRING REPLACEMENT . . . . . . . . . . . 7-25
7.2.12MAIN CHASSIS ASSEMBLY COMPONENT REPLACEMENT . . . . . 7-25
7.2.12.1POWER SUPPLY PWA REPLACEMENT . . . . . . . . . . 7-27
7.2.12.2KEYPAD REPLACEMENT . . . . . . . . . . . . . . . 7-27
7.2.12.3DISPLAY REPLACEMENT . . . . . . . . . . . . . . . 7-28
Technical Service Manual |
v |
430-10996-001 (Rev. 12/05) |
FIGURES
7.2.12.4 |
CPU PWA REPLACEMENT . . |
. . . |
. . . . . |
. . . |
. |
7-29 |
7.2.12.5 |
PIEZO ALARM REPLACEMENT |
. . . |
. . . . . |
. . . |
. |
7-31 |
7.2.12.6MECHANISM ASSEMBLY REPLACEMENT . . . . . . . . 7-32
7.2.12.7CASSETTE DOOR AND FLUID SHIELD REPLACEMENT . . . 7-34
7.2.12.8 OPENER HANDLE ASSEMBLY REPLACEMENT . . . . . . 7-37
Section 8
SPECIFICATIONS . . . . . . . . . . . . . . . . . . . . . . . . . . 8-1
Section9
DRAWINGS . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-1
Appendix. . . . . . . . . . . . . . . . . . . . . . . . . . . . A-1
Index . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . I-1
Figures
Figure |
1-1. |
Display and Keypad . . . . . . . . . . . . . . . . . . |
. . |
1-8 |
Figure |
1-2. |
Biomed Settings . . . . . . . . . . . . . . . . . . . . |
. . 1-10 |
|
Figure |
1-3. |
Alarms Log . . . . . . . . . . . . . . . . . . . . . . |
. . 1-11 |
|
Figure |
1-4. |
Setting the Time and Date . . . . . . . . . . . . . . . . |
. . 1-12 |
|
Figure |
4-1. |
Serial Interface to ADC . . . . . . . . . . . . . . . . . |
. . |
4-7 |
Figure 4-2. |
System Startup and Shutdown Timing, Battery Powered . . . . . |
. . 4-15 |
||
Figure |
4-3. |
Stepper Motor Coils. . . . . . . . . . . . . . . . . . . |
. . 4-17 |
|
Figure |
4-4. |
Air Sensor Block Diagram . . . . . . . . . . . . . . . . |
. . 4-19 |
|
Figure 4-5. |
Pressure Sensor Excitation and Amplifier Block Diagram . . . . . |
. . 4-21 |
||
Figure |
4-6. |
Major Elements of the Dual-Channel Cassette . . . . . . . . . |
. . 4-28 |
|
Figure |
4-7. |
Fluid Path in the Cassette. . . . . . . . . . . . . . . . . |
. . 4-29 |
|
Figure |
4-8. |
Mechanism Valve Pins and Sensor Locations. . . . . . . . . . |
. . 4-30 |
|
Figure |
5-1. |
Display and Keypad . . . . . . . . . . . . . . . . . . |
. . |
5-5 |
Figure |
5-2. |
Rear View . . . . . . . . . . . . . . . . . . . . . . |
. . |
5-8 |
Figure 5-3. |
Special Cassettes with Bubble Sensor Tips Removed . . . . . . . |
. . 5-11 |
||
Figure |
5-4. |
Distal Occlusion Test Setup . . . . . . . . . . . . . . . . |
. . 5-13 |
|
Figure |
7-1. |
Bottom View . . . . . . . . . . . . . . . . . . . . . |
. . |
7-5 |
Figure |
7-2. |
Battery, Battery Door, and Door Pad . . . . . . . . . . . . . |
. . |
7-7 |
Figure |
7-3. |
AC Power Cord, Retainer, and Velcro Strap . . . . . . . . . . |
. . |
7-9 |
Figure |
7-4. |
Rear View . . . . . . . . . . . . . . . . . . . . . . |
. . 7-11 |
|
Figure |
7-5. |
Peripheral Assembly Replacement . . . . . . . . . . . . . |
. . 7-12 |
|
Figure |
7-6. |
Peripheral Assembly Components . . . . . . . . . . . . . |
. . 7-13 |
|
Figure 7-7. |
Separating the Front Enclosure, Main Chassis, and Rear Enclosure . . |
. . 7-16 |
||
Figure |
7-8. |
Front Enclosure Gaskets . . . . . . . . . . . . . . . . . |
. . 7-18 |
|
Figure |
7-9. |
Rear Enclosure Components. . . . . . . . . . . . . . . . |
. . 7-20 |
|
Figure |
7-10. |
Minipole Assembly . . . . . . . . . . . . . . . . . . . |
. . 7-23 |
|
Figure |
7-11. |
Main Chassis Assembly Components . . . . . . . . . . . . |
. . 7-26 |
|
Figure 7-12. |
Keypad, Display, CPU PWA, and Piezo Alarm . . . . . . . . . |
. . 7-30 |
||
Figure |
7-13. |
CPU PWA Replacement . . . . . . . . . . . . . . . . . |
. . 7-31 |
|
Figure |
7-14. |
Mechanism Assembly Replacement . . . . . . . . . . . . . |
. . 7-33 |
|
Figure |
7-15. |
Fluid Shield Replacement . . . . . . . . . . . . . . . . |
. . 7-35 |
|
Figure 7-16. |
Cassette Door and Opener Handle Replacement . . . . . . . . |
. . 7-36 |
||
Figure |
9-1. |
Illustrated Parts Breakdown . . . . . . . . . . . . . . . . |
. . |
9-5 |
Figure |
9-2. |
Front Enclosure, Rear Enclosure, and Main Chassis . . . . . . . |
. . |
9-7 |
Figure |
9-3. |
Front Enclosure Assembly . . . . . . . . . . . . . . . . |
. . |
9-9 |
430-10996-001 (Rev. 12/05) |
vi |
Plum A+® Infusion System |
FIGURES
Figure |
9-4. |
Rear Enclosure Assembly . . . . . . . . . . . . |
. . . . |
. |
. |
. |
9-11 |
Figure |
9-5. |
Peripheral Assembly . . . . . . . . . . . . . |
. . . . |
. |
. |
. |
9-13 |
Figure |
9-6. |
Main Chassis Assembly . . . . . . . . . . . . |
. . . . |
. |
. |
. |
9-15 |
Figure 9-7. |
AC Power Cord, Retainer, and Battery Assembly . . . |
. . . . |
. |
. |
. |
9-21 |
|
Figure |
9-8. |
CPU PWA and Main Chassis. . . . . . . . . . . |
. . . . |
. |
. |
. |
9-23 |
Figure |
9-9. |
Mechanism Assembly . . . . . . . . . . . . . |
. . . . |
. |
. |
. |
9-25 |
Figure |
9-10. |
Power Supply PWA Schematic . . . . . . . . . . |
. . . . |
. |
. |
. |
9-27 |
Figure |
9-11. |
Peripheral PWA Schematic . . . . . . . . . . . |
. . . . |
. |
. |
. |
9-37 |
Figure |
9-12. |
CPU PWA Schematic . . . . . . . . . . . . . |
. . . . |
. |
. |
. |
9-45 |
Figure |
9-13. |
Driver PWA Schematic. . . . . . . . . . . . . |
. . . . |
. |
. |
. |
9-65 |
Figure |
9-14. |
Switch PWA Schematic . . . . . . . . . . . . |
. . . . |
. |
. |
. |
9-71 |
Figure |
9-15. |
APP PWA Schematic . . . . . . . . . . . . . |
. . . . |
. |
. |
. |
9-73 |
|
|
Tables |
|
|
|
|
|
Table 1-1. |
Conventions . . . . . . . . . . . . . . . . |
. . . . |
. |
. |
. |
1-2 |
|
Table 1-2. |
System Configuration Data . . . . . . . . . . . |
. . . . |
. |
. |
. |
1-10 |
|
Table 4-1. |
Analog Inputs . . . . . . . . . . . . . . . . |
. . . . |
. |
. |
. |
4-7 |
|
Table 4-2. |
Keypad Map . . . . . . . . . . . . . . . . |
. . . . |
. |
. |
. |
4-8 |
|
Table 4-3. |
CPU-Power Supply Interface . . . . . . . . . . |
. . . . |
. |
. |
. |
4-10 |
|
Table 4-4. |
CPU-Mechanism Interface Signals . . . . . . . . |
. . . . |
. |
. |
. |
4-11 |
|
Table 4-5. |
Power Supply PWA Interface Connections. . . . . . |
. . . . |
. |
. |
. |
4-23 |
|
Table 4-6. |
Peripheral PWA Interface Connections . . . . . . . |
. . . . |
. |
. |
. |
4-24 |
|
Table 4-7. |
CPU PWA Interface Connections . . . . . . . . . |
. . . . |
. |
. |
. |
4-25 |
|
Table 4-8. |
Driver PWA Interface Connections . . . . . . . . |
. . . . |
. |
. |
. |
4-25 |
|
Table 4-9. |
APP PWA Interface Connections . . . . . . . . . |
. . . . |
. |
. |
. |
4-26 |
|
Table 5-1. |
Cleaning Solutions . . . . . . . . . . . . . . |
. . . . |
. |
. |
. |
5-2 |
|
Table 6-1. |
Warning Messages . . . . . . . . . . . . . . |
. . . . |
. |
. |
. |
6-2 |
|
Table 6-2. |
Operational Alarm Messages and Corrective Actions . . |
. . . . |
. |
. |
. |
6-3 |
|
Table 6-3. |
Error Codes Requiring Technical Service . . . . . . |
. . . . |
. |
. |
. |
6-7 |
|
Table 6-4. |
Troubleshooting with the PVT . . . . . . . . . . |
. . . . |
. |
. |
. |
6-13 |
|
Table 9-1. |
Drawings . . . . . . . . . . . . . . . . . |
. . . . |
. |
. |
. |
9-1 |
|
Table 9-2. |
IPB for the Infuser . . . . . . . . . . . . . . |
. . . . |
. |
. |
. |
9-2 |
|
Technical Service Manual |
vii |
430-10996-001 (Rev. 12/05) |
TABLES
This page intentionally left blank.
430-10996-001 (Rev. 12/05) |
viii |
Plum A+® Infusion System |

Section 1
INTRODUCTION
The Hospira Plum A+® infusion system is an advanced medication management system designed to meet the fluid delivery requirements of today’s evolving healthcare environments. With its primary line, secondary line, and piggyback fluid delivery capabilities, the Plum A+® is suited for a wide range of medical, surgical, and critical care applications. Full compatibility with LifeCare® Plum® Series administration sets and accessories make the Plum A+® a convenient and cost-effective infusion system.
1.1
SCOPE
This manual is organized into the following sections:
Section 1 Introduction
Section 2 Warranty
Section 3 System Operating Manual
Section 4 Theory of Operation
Section 5 Maintenance and Service Tests
Section 6 Troubleshooting
Section 7 Replaceable Parts and Repair
Section 8 Specifications
Section 9 Drawings
Appendices
Index
Technical Service Bulletins
If a problem in device operation cannot be resolved using the information in this manual, contact Hospira (see Section 6.1).
Specific instructions for operating the device are contained in the Plum A+® System Operating Manual. Provision is made for the inclusion of the system operating manual in Section 3 of this manual.
Note: The terms “infusion system”, “infuser”, and “device” are used interchangeably throughout the manual.
Note: Figures are rendered as graphic representations to approximate actual product. Therefore, figures may not exactly reflect the product.
Note: Screen representations are examples only, and do not necessarily reflect the most current software version.
Technical Service Manual |
1 - 1 |
430-10996-001 (Rev. 12/05) |

SECTION 1 INTRODUCTION
1.2
CONVENTIONS
The conventions listed in Table 1-1 are used throughout this manual.
Table 1-1. Conventions
Convention |
Application |
Example |
|
|
|
Italic |
Reference to a section, figure, |
(see Section 6.1) |
|
table, or publication |
|
|
|
|
[ALL CAPS] |
In-text references to keys |
[START] |
|
and touchswitches |
|
|
|
|
ALL CAPS |
Screen displays |
CASSETTE TEST IN PROGRESS |
|
|
|
Bold |
Emphasis |
CAUTION: Use proper ESD grounding |
|
|
techniques when handling components. |
|
|
|
Throughout this manual, warnings, cautions, and notes are used to emphasize important information as follows:
WARNING: A WARNING CONTAINS SPECIAL SAFETY EMPHASIS AND MUST BE OBSERVED AT ALL TIMES. FAILURE TO OBSERVE A WARNING MAY RESULT IN PATIENT INJURY AND BE LIFE-THREATENING.
CAUTION: A CAUTION usually appears in front of a procedure or statement. It contains information that could prevent hardware failure, irreversible damage to equipment, or loss of data.
Note: A note highlights information that helps explain a concept or procedure.
EN-2 Indicates International Electrotechnical Commission (IEC) compliance.
1.3
COMPONENT DESIGNATORS
Components are indicated by alpha-numeric designators, as follows:
Battery |
BT |
Diode |
D |
Resistor |
R |
Capacitor |
C |
Fuse |
F |
Switch |
SW |
Crystal |
Y |
Integrated Circuit |
U |
Transistor |
Q |
The number following the letter is a unique value for each type of component (e.g., R1, R2).
Note: Alpha-numeric designators may be followed with a dash (-) number that indicates a pin number for that component. For example, U15-13 is pin 13 of the encoder chip [U15] on the interface PWA.
430-10996-001 (Rev. 12/05) |
1 - 2 |
Plum A+® Infusion System |

1.4 ACRONYMS AND ABBREVIATIONS
1.4
ACRONYMS AND ABBREVIATIONS
Acronyms and abbreviations used in this manual are as follows:
A |
Ampere |
AC |
Alternating current |
A/D |
Analog-to-digital |
ADC |
Analog-to-digital converter |
APP |
Air, pressure, and pin |
CCFT |
Cold cathode fluorescent tube |
cm |
Centimeter |
CMOS |
Complementary metal-oxide semiconductor |
CPU |
Central processing unit |
DAC |
Digital-to-analog converter |
DC |
Direct current |
DIP |
Dual in-line package |
DMA |
Direct memory access |
DMM |
Digital multimeter |
DPM |
Digital pressure meter |
ECG |
Electrocardiogram |
EEG |
Electroencephalogram |
EEPROM |
Electrically erasable programmable read-only memory |
EMC |
Electromagnetic compatibility |
EMG |
Electromyogram |
EMI |
Electromagnetic interference |
ESD |
Electrostatic discharge |
ETO |
Ethylene oxide |
FPGA |
Field programmable gate array |
FSR |
Force sensing resistor |
hr |
Hour |
Hz |
Hertz |
ID |
Identification |
I/O |
Input/output |
IPB |
Illustrated parts breakdown |
IV |
Intravenous |
KB |
Kilobyte |
Kg |
Kilogram |
kHz |
Kilohertz |
KVO |
Keep vein open |
Technical Service Manual |
1 - 3 |
430-10996-001 (Rev. 12/05) |
SECTION 1 INTRODUCTION
lbs |
Pounds |
LCD |
Liquid crystal display |
LED |
Light emitting diode |
L/S |
Line select |
mA |
Milliampere |
MB |
Megabyte |
mcg |
Microgram |
MHz |
Megahertz |
min |
Minute |
mL |
Milliliter |
mmHg |
Millimeter of mercury |
MMIO |
Memory-mapped input/output |
MOSFET |
Metal-oxide semiconductor field-effect transistor |
ms |
Millisecond |
nF |
Nanofarad |
ng |
Nanogram |
Op-amp |
Operational amplifier |
pF |
Picofarad |
PROM |
Programmable read-only memory |
PVT |
Performance verification test |
PWA |
Printed wiring assembly |
PWM |
Pulse width modulator |
RAM |
Random access memory |
rms |
Root-mean-square |
RTC |
Real-time clock |
SCC |
Serial communication controller |
SCP |
Serial communication port |
SLA |
Sealed lead acid |
SMT |
Surface mount technology |
SPI |
Serial peripheral interface |
SRAM |
Static random access memory |
TQFP |
Thin quad flat pack |
V |
Volt |
VAC |
Volts AC |
VCC |
Collector supply voltage |
VCO |
Voltage controlled oscillator |
VDC |
Volts DC |
VSC |
5 VDC supply circuitry |
VSO |
Voltage sweep oscillator |
VTBI |
Volume to be infused |
430-10996-001 (Rev. 12/05) |
1 - 4 |
Plum A+® Infusion System |

1.5 USER QUALIFICATION
WDI Watchdog input
A Microampere
L Microliter
V Microvolt
sec Microsecond
1.5
USER QUALIFICATION
The infusion system is intended for use at the direction or under the supervision of licensed physicians or certified healthcare professionals who are trained in the use of the infuser and the administration of parenteral and enteral fluids and drugs, and whole blood or red blood cell components. Training should emphasize preventing related IV complications, including appropriate precautions to prevent accidental infusion of air. The epidural route can be used to provide anesthesia or analgesia.
1.6
ARTIFACTS
Nonhazardous, low-level electrical potentials are commonly observed when fluids are administered using infusion devices. These potentials are well within accepted safety standards, but may create artifacts on voltage-sensing equipment such as ECG, EMG, and EEG machines. These artifacts vary at a rate that is associated with the infusion rate. If the monitoring machine is not operating correctly or has loose or defective connections to its sensing electrodes, these artifacts may be accentuated so as to simulate actual physiological signals. To determine if the abnormality in the monitoring equipment is caused by the infuser instead of some other source in the environment, set the device so that it is temporarily not delivering fluid. Disappearance of the abnormality indicates that it was probably caused by electronic noise generated by the infuser. Proper setup and maintenance of the monitoring equipment should eliminate the artifact. Refer to the appropriate monitoring system documentation for setup and maintenance instructions.
1.7
ELECTROMAGNETIC COMPATIBILITY
The Plum A+® infusion system has been tested and found to comply with electromagnetic compatibility (EMC) limits for the Medical Device Directive 93/42/EEC (EN 55011 Class B and EN 60601-1-2:2001). These limits are designed to provide reasonable protection against harmful interference in a typical medical installation.
The equipment generates, uses, and can radiate radio frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference to other devices in the vicinity (see the system operating manual).
CAUTION: Portable and mobile RF communications equipment, such as cellular telephones, two-way radios, Bluetooth® devices, and microwave ovens in close proximity to the infusion system may affect wireless and wired communications and degrade performance of the system. Operation of the infuser under such conditions should be avoided.
Technical Service Manual |
1 - 5 |
430-10996-001 (Rev. 12/05) |

SECTION 1 INTRODUCTION
There is a shared responsibility between manufacturers, customers, and users to assure that medical equipment and systems are designed and operated as intended. Medical electrical equipment requires special precautions regarding electromagnetic compatibility.
The electromagnetic environment should be managed to permit the infusion system to perform as intended without disturbing other equipment. The infusion system should not be used adjacent to or stacked with other equipment. If the device must be used adjacent to or stacked with other equipment, monitor the equipment to assure there is no electromagnetic interference, and verify normal infuser operation.
1.8
INSTRUMENT INSTALLATION PROCEDURE
CAUTION: Infusion system damage may occur unless proper care is exercised during product unpacking and installation. The battery may not be fully charged upon receipt of the infuser. Do not place the infuser in service if it fails the self test.
Accessory equipment connected to the analog and digital interfaces must be certified according to the respective IEC standards (e.g., IEC 60950 for data processing equipment, and IEC 60601-1 for medical equipment). Furthermore, all configurations shall comply with the system standard IEC 60601-1-1. Any person who connects additional equipment to the signal input or output part configures a medical system, and is therefore responsible for ensuring that the system complies with the requirements of IEC 60601-1-1. If in doubt, contact Hospira Technical Support Operations (see Section 6.1).
The instrument installation procedure consists of unpacking, inspection, and self test.
1.8.1
UNPACKING
Inspect the shipping container as detailed in Section 1.8.2. Use care when unpacking the infusion system. Retain the packing slip and save all packing material in the event it is necessary to return the infuser to the factory. Verify the shipping container contains a copy of the system operating manual.
1.8.2
INSPECTION
Inspect the shipping container for damage. Should any damage be found, contact the delivering carrier immediately.
CAUTION: Inspect the infuser for evidence of damage. Do not use the device if it appears to be damaged. Should damage be found, contact Hospira (see Section 6.1).
Inspect the infusion system periodically for signs of defects such as worn accessories, broken connections, or damaged cable assemblies. Also inspect the infuser after repair or during cleaning. Replace any damaged or defective external parts.
430-10996-001 (Rev. 12/05) |
1 - 6 |
Plum A+® Infusion System |

1.8 INSTRUMENT INSTALLATION PROCEDURE
1.8.3
SELF TEST
CAUTION: Do not place the infuser in service if the self test fails.
Note: Do not place the infuser in service if the battery is not fully charged. To make certain the battery is fully charged, connect the infuser to AC power for six hours
(see Section 8).
Note: If an alarm condition occurs during the self test, cycle the power and repeat the self test. If the alarm condition recurs, note the message and take corrective action
(see Section 6). Repeat the self test. If the alarm condition continues to recur, remove the infuser from service and contact Hospira.
To perform the self test, see Figure 1-1, and proceed as follows:
1.Connect the AC power cord to a grounded AC outlet. Verify the charging/line indicator CHARGE illuminates and an alarm beep sounds.
2.Without a cassette installed, press the ON/OFF key to turn on the infuser.
3.The LCD screen briefly displays the SELF TEST screen (see Figure 1-1).
Note: If the SELF TEST screen does not appear, contact Hospira.
4.After the self test is complete, the message “INSERT PLUM SET CLOSE LEVER” appears.
5.Verify the time and date. To set the time and date, see Section 1.9.2.
6.Open the cassette door and insert a primed cassette. Close the cassette door. The cassette test is complete when the “CASSETTE TEST IN PROGRESS” message disappears.
Note: The message “MECHANISM INITIALIZATION IN PROGRESS” may briefly appear prior to the “CASSETTE TEST IN PROGRESS” message.
7.A “NEW PATIENT?” message may appear. Press the [YES] softkey.
8.Press the ON/OFF key to turn off the infuser.
Technical Service Manual |
1 - 7 |
430-10996-001 (Rev. 12/05) |
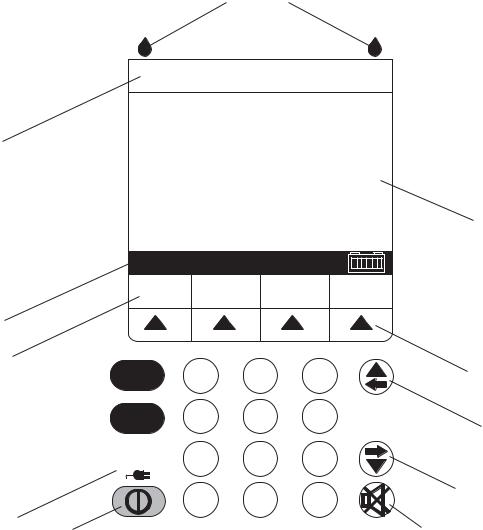
SECTION 1 INTRODUCTION
LINE FLOW
INDICATORS
A B
STATUS
REGION
MESSAGE
REGION
SOFTKEY
LABEL REGION
CHARGE/LINE INDICATOR
ON/OFF
HOSPIRA Plum A+
Release X.XX - MM/DD/YY
Copyright HOSPIRA
2005
System Self Test
In Progress
START |
1 |
2 |
3 |
STOP |
4 |
5 |
6 |
CHARGE |
7 |
8 |
9 |
|
CLEAR |
0 |
, |
|
|
Figure 1-1. Display and Keypad
WORKING
REGION
SOFTKEYS
UP/LEFT
DOWN/RIGHT
SILENCE
05K01050
430-10996-001 (Rev. 12/05) |
1 - 8 |
Plum A+® Infusion System |

1.9 BIOMED SETTINGS
1.9
BIOMED SETTINGS
The biomed settings screens contain the following options that can be changed or reviewed by qualified personnel:
-Alarms log
-Set time and date
All infusers (new or refurbished) are shipped with factory settings (see Table 1-2).
Note: Biomed screens do not time out for the Infuser Idle alarm or No Action alarm.
Note: The battery will not be detected in the biomed service mode.
Note: Upon entry to biomed mode, any drug library waiting for installation will be installed, and the infuser will power off at completion.
To access the biomed settings, proceed as follows:
1.Open the door and turn on the device. The infusion system will perform a self test.
2.After the self test is complete, the message “INSERT PLUM SET CLOSE LEVER” appears.
3.Press the comma [,] key, then [START], and verify the BIOMED SETTINGS screen is displayed (see Figure 1-2).
Note: The [CHANGE BATTERY] softkey appears on the biomed settings screen.
Technical Service Manual |
1 - 9 |
430-10996-001 (Rev. 12/05) |

SECTION 1 INTRODUCTION
Table 1-2. System Configuration Data
Data |
Options Range |
Factory Setting |
|
|
|
Maximum macro IV mode delivery rate |
0.1 - 99.9 mL/hr and |
999 mL/hr |
|
100 - 999 mL/hr |
|
|
|
|
Macro distal occlusion alarm |
1 to 15 psi |
6 psi |
(pressure level) |
|
|
|
|
|
Deliver together enable |
Concurrent or Piggyback |
Piggyback |
|
|
|
Delayed start/standby enable |
Yes or No |
Yes |
|
|
|
Continue rate |
Rate or KVO |
KVO |
|
|
|
Nurse callback default |
Yes or No |
No |
|
|
|
Time |
(24 hr) 00:00 - 23:59 in |
Factory time |
|
one minute increments |
|
|
|
|
Date |
1/1/2002 - 12/31/2098 |
Factory date |
|
|
|
BIOMED SETTINGS
Alarm Log
Alarms Log
Set Time and Date
Select, then Choose
Change
Choose
Battery
05K03002
Figure 1-2. Biomed Settings
430-10996-001 (Rev. 12/05) |
1 - 10 |
Plum A+® Infusion System |

1.9 BIOMED SETTINGS
1.9.1
ALARMS LOG
Note: The alarms log will retain the latest 40 alarm and malfunction codes, listed in order from the most current to the oldest.
To view the alarms log, see Figure 1-3, then proceed as follows:
1.Access the biomed settings screen as described in Section 1.9.
2.Select Alarms Log, and press [CHOOSE].
3.Use the [PAGE UP] and [PAGE DOWN] softkeys to view the alarms log.
4.Press [BACK] to exit the alarms log and return to the main biomed settings screen.
ALARMS LOG
6/23/05 11:43:01 E437 S/W Failure # 202 6/23/05 09:18:10 N190 Neg. Prox. Occl. A 6/22/05 23:44:11 N102 Infuser Idle 2 minutes 6/22/05 21:43:14 N161 Line A VTBI complete 6/22/05 11:44:20 N106 Distal occlusion 6/22/05 09:43:07 N161 Line A VTBI complete 6/22/05 06:23:20 N160 Line B VTBI complete 6/22/05 03:40:13 N101 No action alarm
|
|
|
|
|
|
Page |
Page |
|
Back |
|
Up |
Down |
|
|
|
|
|
||
|
|
|
|
|
05K03008
Figure 1-3. Alarms Log
Technical Service Manual |
1 - 11 |
430-10996-001 (Rev. 12/05) |

SECTION 1 INTRODUCTION
1.9.2
SETTING THE TIME AND DATE
Note: The infuser will automatically display February 29 on leap years.
Note: Daylight savings and time zone changes must be made manually.
To set the time and date, see Figure 1-4, then proceed as follows:
1.Access the biomed settings screen as described in Section 1.9.
2.Select Set Time and Date, and press [CHOOSE].
3.Select the parameter to be changed.
4.Enter the desired value.
5.Repeat step 3 and step 4 for each parameter to be changed.
6.Verify the time and date are correct, then press [ENTER] to return to the biomed settings screen.
7.If there are no other changes to the biomed settings, turn off the infuser.
BIOMED SETTINGS
Set Time and Date |
|
||||
|
|
|
|
||
Time |
14 |
: 22 hr:min |
|||
Year |
2005 |
|
|
||
Month |
02 |
|
|
||
Day |
14 |
|
|
||
|
|
|
|
||
Enter value using keypad |
|
||||
|
|
|
|
|
|
|
|
Enter |
|
Cancel/ |
|
|
|
|
Back |
||
|
|
|
|
|
|
|
|
|
|
|
05K13004 |
Figure 1-4. Setting the Time and Date |
|||||
430-10996-001 (Rev. 12/05) |
1 - 12 |
Plum A+® Infusion System |

Section 2
WARRANTY
Subject to the terms and conditions herein, Hospira, Inc., herein referred to as Hospira, warrants that (a) the product shall conform to Hospira's standard specifications and be free from defects in material and workmanship under normal use and service for a period of one year after purchase, and (b) the replaceable battery shall be free from defects in material and workmanship under normal use and service for a period of 90 days after purchase. Hospira makes no other warranties, express or implied, as to merchantability, fitness for a particular purpose, or any other matter.
Purchaser's exclusive remedy shall be, at Hospira's option, the repair or replacement of the product. In no event shall Hospira's liability arising out of any cause whatsoever
(whether such cause be based in contract, negligence, strict liability, other tort, or otherwise) exceed the price of such product, and in no event shall Hospira be liable for incidental, consequential, or special damages or losses or for lost business, revenues, or profits. Warranty product returned to Hospira must be properly packaged and sent freight prepaid.
The foregoing warranty shall be void in the event the product has been misused, damaged, altered, or used other than in accordance with product manuals so as, in Hospira's judgment, to affect its stability or reliability, or in the event the serial or lot number has been altered, effaced, or removed.
The foregoing warranty shall also be void in the event any person, including the Purchaser, performs or attempts to perform any major repair or other service on the product without having been trained by an authorized representative of Hospira and using Hospira documentation and approved spare parts. For purposes of the preceding sentence, "major repair or other service" means any repair or service other than the replacement of accessory items such as batteries, flow detectors, detachable AC power cords, and patient pendants.
In providing any parts for repair or service of the product, Hospira shall have no responsibility or liability for the actions or inactions of the person performing such repair or service, regardless of whether such person has been trained to perform such repair or service. It is understood and acknowledged that any person other than a Hospira representative performing repair or service is not an authorized agent of Hospira.
Technical Service Manual |
2 - 1 |
430-10996-001 (Rev. 12/05) |
SECTION 2 WARRANTY
This page intentionally left blank.
430-10996-001 (Rev. 12/05) |
2 - 2 |
Plum A+® Infusion System |

Section 3
SYSTEM OPERATING MANUAL
A copy of the system operating manual is included with every Plum A+® infusion system.
Insert a copy here for convenient reference. If a copy of the system operating manual is not available, contact Hospira Technical Support Operations (see Section 6.1).
Technical Service Manual |
3 - 1 |
430-10996-001 (Rev. 12/05) |
SECTION 3 SYSTEM OPERATING MANUAL
This page intentionally left blank.
430-10996-001 (Rev. 12/05) |
3 - 2 |
Plum A+® Infusion System |

Section 4
THEORY OF OPERATION
This section describes the Plum A+® theory of operation. The theory of operation details the general description, electronic subsystem overview, printed wiring assemblies, remote mounted peripherals, and mechanical overview of the infuser. Related drawings are provided in Section 9.
4.1
GENERAL DESCRIPTION
The infusion system includes the following features:
-Dose calculation
-Loading dose
-Multi-step programming
-Therapy selection
-Nurse call
-Delayed start setting
-Standby mode
-Drug label library
-Piggyback and concurrent delivery modes
-Titration
-0.1-99.9 mL/hr flow rate range for both lines
(in 0.1 mL/hr increments)
-100-999 mL/hr flow rate range for both lines
(in 1 mL/hr increments)
-Anti free-flow protection
-Air removal/backpriming
-Battery gauge
-Air detection (proximal and distal)
-Serial communication
-Alarm history
-Volumes infused (A, B, total volumes)
-KVO at dose end (1 mL/hr
or less depending on delivery rate) or continue rate to continue
-Variable distal pressure setting
-Nonpulsatile volumetric accuracy
-Microprocessor control
-Large display
-Panel back illumination on mains power
-Lockout switch
-Standard fullfill, partfill, syringe, and vial use
-Enteral and parenteral fluid delivery
-Blood and blood product delivery
-Wide range of standard and specialty administration sets
Technical Service Manual |
4 - 1 |
430-10996-001 (Rev. 12/05) |

SECTION 4 THEORY OF OPERATION
Alarms include the following: |
|
- Distal occlusion |
- Lockout violation |
- Proximal occlusion |
- VTBI complete |
- Proximal air-in-line |
- Valve/cassette test failure |
- Distal air-in-line |
- Nurse call |
- Low battery |
- No action alarm |
- Door open while pumping |
- Infuser idle for two minutes |
4.2
ELECTRONIC SUBSYSTEM OVERVIEW
This section describes the function and electronic circuitry of three main subsystems in the infuser: CPU subsystem, power supply subsystem, and mechanism subsystem. Schematic diagrams of subsystem PWAs are in Section 9.
Note: An asterisk (*) denotes an active low or negative true logic signal.
4.2.1
CPU SUBSYSTEM
The CPU subsystem contains the main microcontroller, which is responsible for controlling the display/keypad interface, external communications interfaces, and system management.
The CPU subsystem provides the following functions:
-External memory devices access
-LCD interfaces
-Real-time clock generator interface
-System watchdog
-Analog-to-digital and digital-to-analog converter interface
-Keypad interfaces
-Control and monitor status signals, such as LEDs, audible alarms, volume control, nurse call switch, and lockout switch
-Serial communication with host computer (DataPort)
-Power supply subsystem interface
-Mechanism subsystem interface
430-10996-001 (Rev. 12/05) |
4 - 2 |
Plum A+® Infusion System |

4.2 ELECTRONIC SUBSYSTEM OVERVIEW
4.2.1.1
CPU
The central processing unit (CPU) is a Motorola MC68302. The CPU has a closely coupled 16 bit data bus and 24 bit address bus; MC68000 microprocessor core; a system integration block for peripherals; and an RISC communications processor. The MC68302 is packaged in a 144 pin thin quad flat pack (TQFP) package and operates from a 3.3 VDC power supply. The on-chip peripheral devices are isolated from the system through the dual port RAM. The 1152 byte dual port RAM has 576 bytes of system RAM and 576 bytes of parameter RAM, which contains various peripheral registers, parameters, and the buffer descriptors for each of the three serial communication controller (SCC) channels and the serial communication port (SCP) channels. The 24 bit address bus is capable of accessing up to 16 MB of data.
4.2.1.2
SYSTEM MEMORY ADDRESS MAP
The CPU has a 24 bit address bus when combined with UDS*/A0. The address bus is a bi-directional, three state bus capable of addressing 16 MB of data that is configured as 16 bits per word (including the IMP internal address space). Each of the four programmable chip-select lines has two registers that define the starting address of a particular address space and the block size.
4.2.1.3
PROGRAMMABLE READ-ONLY MEMORY
The CPU subsystem has two 512 K x 8 bit programmable read-only memory (PROM) memory devices, which provide a total of 1024 KB. The PROM space is expandable up to 2 MB. The PROM memory devices operate off the 3.3 VDC supply. The CPU chip-select 0 pin (CS0*), is connected to the PROM chip-enable (CE*) pin (signal CSROM*). This special chip-select signal can support bootstrap operation after reset. The interface to the CPU is the 16 bit data bus, and a 19 bit address bus. The address bus is connected to the ADDR<19:1> lines, and the data bus is connected to the DATA<15:0> lines.
4.2.1.4
STATIC RANDOM ACCESS MEMORY
There are two 512 K x 8 bit CMOS static random access memory (SRAM) devices, which provide a total of 1024 KB of data memory. During an SRAM read or write cycle, the chip-enable (CE*) is controlled by the CPU chip-select pin 1 (CS1*, signal name (CSRAM*)). The SRAM space is expandable up to 2 MB. The SRAM operates off the 3.3 VDC supply. The CPU subsystem includes the additional SRAM for video buffer and real-time clock.
4.2.1.5
CONTROL LOGIC
The CPU PWA uses field programmable gate arrays (FPGA), which are high density, high speed, I/O intensive general purpose devices. They are used to implement all the digital control functions, including: memory-map address decoding; memory read-write enable; direct memory access (DMA) request; I/O status signals; chip-select control; motor control; sensor select; and power up/system reset control.
Technical Service Manual |
4 - 3 |
430-10996-001 (Rev. 12/05) |

SECTION 4 THEORY OF OPERATION
4.2.1.6
LCD CONTROLLER
The liquid crystal display (LCD) controller is used to interface the LCD to the CPU. The device displays layered text and graphics, scrolls the display in any direction, and partitions the display into multiple screens. It stores bit-mapped graphic data in external frame buffer memory. The display controller functions include: transferring data from the controlling microprocessor to the buffer memory, reading memory data, converting data to display pixels, and generating timing signals for the buffer memory and LCD panel. The LCD controller accesses 32 KB of frame buffer SRAM (video) via the controller’s video address and data busses (VA<14:0> and VD<7:0>). The LCD controller external clock frequency is 8 MHz. The LCD controller and the display memory are operated off the 3.3 VDC supply. The output signal levels are shifted up to 5 VDC by buffers for interface with the 5 VDC LCD panel.
The interface to the CPU is through the lower 8 bits of the data bus, which is connected to DATA<7:0> lines, address line A1, and LCD chip-select signal CSLCD* (CS2*). This controller is also configured as 8080 family compatible interface device with all the control signals, such as WRLCD* (WR*) and RDLCD* (RD*), generated by the FPGA logic.
4.2.1.7
LCD BACKLIGHT CONTROL
The LCD panel is backlit by a cold cathode fluorescent tube (CCFT) lamp. The CCFT lamp requires 300 Vrms to operate; a current controlled DC-to-AC voltage inverter circuit is used to deliver a current regulated sine wave to the lamp. A switching regulator regulates the CCFT current by monitoring feedback pin 3, and varies its output duty cycle to drive a DC/AC inverter. Intensity control is achieved by superimposing a DC control signal with the feedback signal. The DC control signal is sourced by a voltage divider consisting of a digitally controlled non-volatile potentiometer and three series diodes.
The CPU can adjust LCD backlight intensity by selecting the digitally controlled non-volatile potentiometer and controlling TUBU/D and TUBINC* signals. The potentiometer has a five bit up/down counter with non-volatile memory. It is used to store one of 31 settings of the potentiometer. Each count represents 323 Ω with a range of 323 to 10 KΩ. The current counter value is stored in non-volatile memory after CSTUB* is returned high while the TUBINC* input is also high. The current counter value is not stored if CSTUB* is returned high and TUBINC* is low. The CCFT intensity is directly proportional to the CCFT current, where 0 mArms is minimum intensity and 5 mArms is maximum intensity. The CCFT current is inversely proportional to the counter value.
4.2.1.8
LCD CONTRAST CONTROL
A digitally adjustable LCD bias supply is used to control the LCD contrast over a range of -24 to -8 VDC. It is digitally adjustable in 64 equal steps by an internal digital-to-analog converter (DAC). The CPU provides two signals, LCDADJ (ADJ) and LCDCTL (CTL), to interface with this device. On power up or after a reset, the counter sets the DAC output to the mid-range value. Each rising edge of LCDADJ increments the DAC output. When incremented beyond full scale, the counter rolls over and sets the DAC to the minimum value. Therefore, a single pulse applied to LCDADJ increases the DAC set point by one step, and 63 pulses decrease the set point by one step.
430-10996-001 (Rev. 12/05) |
4 - 4 |
Plum A+® Infusion System |
 Loading...
Loading...