Hospira LifeCare PCA 3 User manual

System
Operating
Manual
Hospira, Inc., Lake Forest, IL 60045, USA
430-04684-002 (Rev. 01/06)
Change History
|
|
Pages |
Title |
Description of Change |
Affected |
430-04684-001 |
Initial Release |
All |
(Rev. 1/05) |
|
|
430-04684-002 |
Second Release |
All |
(Rev. 01/06) |
|
|
430-04684-002 (Rev. 01/06)
LifeCare PCA 3 Infusion System |
i |
Contents
1) DESCRIPTIVE INFORMATION . . . . . . . . . . . . . . . . 1-1
1.1 PRODUCT DESCRIPTION . . . . . . . . . . . . . . . . . . . 1-1 1.2 INDICATIONS FOR USE . . . . . . . . . . . . . . . . . . . . . 1-2 patient selection . . . . . . . . . . . . . . . . . . . . . . . . 1-2 user qualification . . . . . . . . . . . . . . . . . . . . . . . . 1-3 1.3 CONTRAINDICATIONS FOR USE . . . . . . . . . . . . . . . 1-3 1.4 CONVENTIONS . . . . . . . . . . . . . . . . . . . . . . . . . . 1-4 warnings, cautions, and notes . . . . . . . . . . . . . . 1-4
1.5 DEFINITIONS (GENERAL AND CLINICAL) . . . . . . . . . 1-6
1.6 PRECAUTIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-8 artifacts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-8 general . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-9 programming . . . . . . . . . . . . . . . . . . . . . . . . . . 1-10 loading dose/dose limits . . . . . . . . . . . . . . . . . 1-11 operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-12 maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . 1-12 alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-13 epidural administration . . . . . . . . . . . . . . . . . . 1-13 battery operation . . . . . . . . . . . . . . . . . . . . . . . 1-14 sets and accessories . . . . . . . . . . . . . . . . . . . . 1-15
2) PRINCIPLES OF OPERATION . . . . . . . . . . . . . . . . 2-1
2.1 FEATURES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-2 drug recognition . . . . . . . . . . . . . . . . . . . . . . . . 2-2 modes of delivery . . . . . . . . . . . . . . . . . . . . . . . 2-2 programming . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3 battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3 bio medical . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3 options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3 other features . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
2.2 ADMINISTRATION EQUIPMENT . . . . . . . . . . . . . . . 2-4 administration sets . . . . . . . . . . . . . . . . . . . . . . 2-4 2.3 PRINTER KITS . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-5
430-04684-002
ii |
Contents |
3) EQUIPMENT DESCRIPTION . . . . . . . . . . . . . . . . . . 3-1
3.1 COMPONENTS . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-1
3.2 OPERATING BUTTONS & KEYS . . . . . . . . . . . . . . . 3-4
4) BASIC OPERATION . . . . . . . . . . . . . . . . . . . . . . . . 4-1
4.1 GETTING STARTED . . . . . . . . . . . . . . . . . . . . . . . 4-1 unpacking . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-1 connecting the patient pendant . . . . . . . . . . . . 4-1 system self-tests . . . . . . . . . . . . . . . . . . . . . . . . 4-2 data retention . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
4.2 OPERATING THE PCA 3 . . . . . . . . . . . . . . . . . . . 4-3 intravenous PCA administration . . . . . . . . . . . . . 4-3 epidural PCA administration . . . . . . . . . . . . . . . . 4-4 4.3 LOADING VIAL . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-6 4.4 ADJUSTING SETTINGS . . . . . . . . . . . . . . . . . . . . . 4-7 changing alarm volume . . . . . . . . . . . . . . . . . . . 4-8 changing contrast of main display . . . . . . . . . . . 4-9 changing or confirming time and date . . . . . . . . 4-9
4.5 GUIDED START-UP FOR PREFILLED VIALS . . . . . . 4-12
purging the system . . . . . . . . . . . . . . . . . . . . . 4-13 loading dose. . . . . . . . . . . . . . . . . . . . . . . . . . . 4-15
4.6 GUIDED START-UP FOR CUSTOM VIALS . . . . . . . 4-17
5) SELECT MODE . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-1
5.1 MODES OF DELIVERY . . . . . . . . . . . . . . . . . . . . . 5-1 protocols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-1 PCA only . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-1 continuous . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-2 PCA+continuous . . . . . . . . . . . . . . . . . . . . . . . . 5-2
5.2 PROGRAMMING PCA ONLY . . . . . . . . . . . . . . . . . 5-3 5.3 CONTINUOUS MODE . . . . . . . . . . . . . . . . . . . . . . 5-7 5.4 PCA + CONTINUOUS MODE . . . . . . . . . . . . . . . 5-10 5.5 PROTOCOLS . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-16 5.6 DOSE LIMIT (1 OR 4 HOUR) . . . . . . . . . . . . . . . . 5-18
dose limit calculation . . . . . . . . . . . . . . . . . . . . 5-18 programming the 4 (or 1) hr dose limit. . . . . . . 5-20 to program a dose limit . . . . . . . . . . . . . . . . . . 5-20 to program NO dose limit . . . . . . . . . . . . . . . . . 5-21
430-04684-002
LifeCare PCA 3 Infusion System |
iii |
to clear or change dose limit . . . . . . . . . . . . . . 5-22 clearing the history & Rx settings. . . . . . . . . . . 5-23 5.7 USING REVIEW SCREENS . . . . . . . . . . . . . . . . . . 5-24
5.8 CHANGING SETTINGS DURING SETUP . . . . . . . . 5-25 5.9 STOPPING INFUSION OR TURNING PUMP OFF . . . 5-26
5.10 MAKING CHANGES AFTER SETUP . . . . . . . . . . 5-27 to review current settings . . . . . . . . . . . . . . . . 5-27 to change settings . . . . . . . . . . . . . . . . . . . . . . 5-28 to clear shift totals . . . . . . . . . . . . . . . . . . . . . . 5-29 to change a vial . . . . . . . . . . . . . . . . . . . . . . . . 5-30 to add a supplemental loading dose . . . . . . . . 5-32
5.11 CHECKING HISTORY & SETTINGS . . . . . . . . . . . 5-33 5.12 PRINTER SETUP . . . . . . . . . . . . . . . . . . . . . . . . 5-34 5.13 PRINTING EVENT HISTORY LOG . . . . . . . . . . . . 5-39 5.14 DOWNLOADING TO A PC. . . . . . . . . . . . . . . . . . 5-40 5.15 HISTORY AND EVENT LOG . . . . . . . . . . . . . . . . 5-43
6) TROUBLESHOOTING . . . . . . . . . . . . . . . . . . . . . . . 6-1
6.1 STATUS MESSAGES . . . . . . . . . . . . . . . . . . . . . . 6-1
6.2 PUMP ALARM SYSTEM . . . . . . . . . . . . . . . . . . . . . 6-2
6.3 SILENCING AN ALARM . . . . . . . . . . . . . . . . . . . . . 6-3
6.4 ALARMS AND MESSAGES . . . . . . . . . . . . . . . . . . . 6-4
7) MAINTENANCE . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-1
7.1 PUMP STORAGE . . . . . . . . . . . . . . . . . . . . . . . . . 7-1
7.2 CLEANING AND SANITIZING . . . . . . . . . . . . . . . . . 7-1
7.3 BATTERY MAINTENANCE . . . . . . . . . . . . . . . . . . . 7-3
service . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-4
430-04684-002
iv |
Contents |
8) SPECIFICATIONS . . . . . . . . . . . . . . . . . . . . . . . . . . 8-1
8.1 STORED OCCLUSION VOLUME . . . . . . . . . . . . . . . 8-4
8.2 TIME FROM OCCLUSION TO ALARM . . . . . . . . . . . . 8-4
8.3 DELIVERY RATE ACCURACY . . . . . . . . . . . . . . . . . 8-4
trumpet curves . . . . . . . . . . . . . . . . . . . . . . . . . . 8-5
example . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-5
8.4 TRUMPET CURVES . . . . . . . . . . . . . . . . . . . . . . . . 8-7
9) PRESCRIPTION DELIVERY LIMITS . . . . . . . . . . . . 9-1 10) WARRANTY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-1
© Hospira, Inc. |
All Rights Reserved |
This document and the subject matter disclosed herein are proprietary information. Hospira retains all the exclusive rights of dissemination, reproduction, manufacture and sale. Any party using this document accepts it in confidence, and agrees not to duplicate it in whole or in part nor disclose it to others without the written consent of Hospira.
430-04684-002

LifeCare PCA 3 Infusion System |
1- 1 |
1)Descriptive Information
The LifeCare® PCA 3TM Infusion System is the newest Hospira
LifeCare® PCA device. Like its predecessor, the PCA Plus II, the PCA 3 system can be used in a wide range of clinical settings, including but not limited to:
|
|
|
|
|
|
General Floor |
Labor/Delivery/ |
Burn Unit |
|
|
|
Post Partum |
|
|
|
|
|
|
|
|
Medical/Surgical |
Operating Room |
Oncology |
|
|
|
|
|
|
|
Critical Care |
Post Anesthesia |
Pediatrics |
|
|
Units |
Care Unit (PACU) |
|
|
|
|
|
|
|
|
|
|
|
|
The PCA 3 Infusion pump allows clinicians to administer or patients to self-administer, analgesia safely and effectively within clinician programmed limits. The epidural route can be used to provide anesthesia or analgesia.
1.1 Product Description
The primary feature of the PCA 3 is the bar code reader, which is designed to automate drug identification. Other enhancements include new programming features, a numeric keypad to directly enter programming values, and a device weight of less than 12 pounds.
The PCA 3 system includes a microprocessor based infusion device with keypad controls, patient pendant, a bar coded drug vial, and a compatible administration set (see Section 2.3 for list of compatible sets). The pump has a serial port for connection to a computer or printer, and the software is field upgradeable. It is intended to operate on AC power, but an internal battery is provided to maintain operation for short periods of time when AC power is not available.
430-04684-002
1- 2 |
1) Descriptive Information |
The vials are single-use, bar coded and prefilled with a prescription drug by Hospira, or sterile and empty to be customfilled by the hospital pharmacy.
The PCA 3 system is capable of the following modes of delivery:
•PCA ONLY
•CONTINUOUS ONLY
•PCA+CONTINUOUS
The PCA 3 system also provides the ability to store frequently used prescriptions called Protocols. The protocols are only available for Hospira pre-filled vials and must be set up through the service mode by a hospital-designated authority.
1.2 Indications for Use
PCA is a method of pain management that permits patients to treat their pain by self-administering doses of analgesics. PCA can be used to manage all types of pain, but is most commonly used to manage acute pain.
PATIENT SELECTION
Patients selected for use of PCA should be able to understand the relationship between pain, pushing the PCA patient pendant and pain relief, and can physically self-administer a PCA dose using the patient pendant.
430-04684-002

LifeCare PCA 3 Infusion System |
1- 3 |
WARNING
FOR EPIDURAL USE, ADMINISTER ONLY ANESTHETICS/ANALGESICS APPROVED FOR EPIDURAL ADMINISTRATION (AS INDICATED OR ALLOWED BY THE DRUGS’ FDA APPROVED LABELING). EPIDURAL ADMINISTRATION OF DRUGS OTHER THAN THOSE INDICATED FOR EPIDURAL USE COULD RESULT IN SERIOUS INJURY TO THE PATIENT.
USER QUALIFICATIONS
All clinicians should be appropriately trained on programming of the PCA 3 pump prior to use.
The PCA 3 is intended for use at the direction or under the supervision of licensed physicians or certified healthcare professionals. They must be trained in the use of the pump, administration of parenteral and epidural fluids and drugs, and the prevention of related IV complication and precautions to prevent accidental infusion of air. Training should emphasize the assessment and monitoring of patients receiving potent analgesic medications, and the appropriate treatment for possible adverse reactions.
1.3 Contraindications For Use
The PCA 3 should not be used for patient controlled analgesia by patients who do not have the cognitive ability to understand the use of self-administered pain medication nor have the physical capacity to operate the patient pendant, if required.
Drugs not compatible with silicone rubber or PVC plastic, or not stable under infusion conditions, should not be used with this system.
430-04684-002

1- 4 |
1) Descriptive Information |
1.4 Conventions
This section describes the conventions used throughout this manual, as follows:
|
|
|
|
|
|
CONVENTION |
APPLICATION |
EXAMPLE |
|
|
|
|
|
|
|
Italic |
Reference to a |
(See Section 3-1, |
|
|
|
section, figure, or |
Components) |
|
|
|
table |
Primary Only: |
|
|
|
|
|
|
|
|
Function or mode |
Attach an empty |
|
|
|
specific |
container. |
|
|
|
instructions |
|
|
|
|
|
|
|
|
[BRACKETED |
Keys or buttons |
[START/PAUSE] |
|
|
ALL-CAPS] |
on the device are |
or |
|
|
|
displayed in |
|
|
|
|
|
|
|
|
|
[BRACKETED |
|
|
|
|
ALL-CAPS] or |
|
|
|
|
with a graphic. |
|
|
|
|
|
|
|
|
ItalicSmallcaps> |
Softkey Options |
CHOOSE> |
|
|
|
|
|
|
|
Initial Caps |
Screen displays |
Therapy |
|
|
lowercase |
and device labels |
Dose Calculation |
|
|
|
(as appropriate) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Bold |
Emphasis |
...sets are |
|
|
|
|
supplied Sterile |
|
|
|
|
and are for.... |
|
|
|
|
|
|
|
|
|
|
|
WARNINGS, CAUTIONS, AND NOTES
Alert messages used throughout this manual are described below. Pay particular attention to these messages.
430-04684-002

LifeCare PCA 3 Infusion System |
1- 5 |
WARNING
A WARNING MESSAGE CONTAINS SPECIAL SAFETY EMPHASIS AND MUST BE OBSERVED AT ALL TIMES. FAILURE TO OBSERVE A WARNING MESSAGE IS POTENTIALLY LIFE THREATENING.
CAUTION: A CAUTION USUALLY APPEARS IN FRONT OF A PROCEDURE OR STATEMENT. IT CONTAINS INFORMATION THAT COULD PREVENT IRREVERSIBLE PRODUCT DAMAGE OR HARDWARE FAILURE. FAILURE TO OBSERVE A CAUTION COULD RESULT IN SERIOUS PATIENT OR USER INJURY.
NOTE: A Note highlights information that helps explain a concept or procedure.
This symbol directs the user to consult accompanying documents.
NOTE: Figures are rendered as graphic representations to approximate the actual product. Therefore, figures may not exactly reflect the product.
430-04684-002

1- 6 |
1) Descriptive Information |
1.5 Definitions (General and Clinical)
|
|
|
|
|
TERM |
DEFINITION |
|
|
|
|
|
|
1 Hour Dose Limit |
Programmed parameter specifying |
|
|
|
the maximum amount of drug that |
|
|
|
can be administered in a rolling |
|
|
|
(continuously advancing) one hour |
|
|
|
time period. |
|
|
|
|
|
|
4 Hour Dose Limit |
Programmed parameter specifying |
|
|
|
the maximum amount of drug that |
|
|
|
can be administered in a rolling |
|
|
|
(continuously advancing) four hour |
|
|
|
time period. |
|
|
|
|
|
|
Accuracy |
The degree to which the instrument |
|
|
|
is capable of delivering the volume |
|
|
|
of analgesic drug that is displayed |
|
|
|
or targeted to be delivered. |
|
|
|
Accuracy shall be specified as the |
|
|
|
maximum allowable delivery error |
|
|
|
from a targeted or displayed value |
|
|
|
(see product specification, Section |
|
|
|
9). |
|
|
|
|
|
|
Continuous |
Infusion therapy characterized by a |
|
|
|
constant fixed-rate dose. |
|
|
|
|
|
|
Custom Syringe or |
Bar coded Hospira sterile empty |
|
|
Vial |
vial which is custom-filled by a |
|
|
|
licensed pharmacy. |
|
|
|
|
|
|
Default |
Generally refers to the factory |
|
|
|
setting for parameters or options. |
|
|
|
|
|
|
History |
Displays Parameter Settings, Dose |
|
|
|
History and Event Log. Also |
|
|
|
provides access to Print History |
|
|
|
softkey. |
|
|
|
|
|
|
|
|
|
430-04684-002
LifeCare PCA 3 Infusion System |
1- 7 |
|||
|
|
|
|
|
|
TERM |
DEFINITION |
|
|
|
|
|
|
|
|
Lockout Interval |
Programmed time interval |
|
|
|
|
specifying the minimum time that |
|
|
|
|
must pass after a PCA dose or |
|
|
|
|
Loading Dose is administered |
|
|
|
|
before the next PCA dose can be |
|
|
|
|
infused. The bolus requests made |
|
|
|
|
during this period are not delivered. |
|
|
|
|
|
|
|
|
Loading Dose |
An optional dose delivered before |
|
|
|
|
starting normal function of the |
|
|
|
|
pump (or thereafter by unlocking |
|
|
|
|
the door). |
|
|
|
|
|
|
|
|
Occlusion |
Inability of the instrument to infuse |
|
|
|
|
fluid to the patient. Possible causes |
|
|
|
|
of occlusions include kinked |
|
|
|
|
tubing, plugged tubing, etc. |
|
|
|
|
|
|
|
|
Maximum |
The maximum pressure observed in |
|
|
|
Occlusion Pressure |
response to a patient line |
|
|
|
|
occlusion. |
|
|
|
|
|
|
|
|
Patient Pendant |
Hand-held pendant connected to |
|
|
|
|
the instrument that allows the |
|
|
|
|
patient to request a bolus PCA dose |
|
|
|
|
by pressing a button. |
|
|
|
|
|
|
|
|
PCA mode |
Infusion therapy characterized by |
|
|
|
|
bolus doses administered on |
|
|
|
|
patient demand subject to a lockout |
|
|
|
|
interval and, optionally, a 1 or 4 |
|
|
|
|
hour dose limit. |
|
|
|
|
|
|
|
|
PCA 3 Vial |
Bar coded vial compatible with the |
|
|
|
|
PCA 3 instrument that is either |
|
|
|
|
prefilled or custom filled with a |
|
|
|
|
drug. |
|
|
|
|
|
|
|
|
PCA 3 Instrument |
Programmable patient controlled |
|
|
|
|
infusion pump. |
|
|
|
|
|
|
|
|
PCA 3 Set |
Tubing which connects the PCA 3 |
|
|
|
|
Vial to the patient. |
|
|
|
|
|
|
|
|
|
|
|
|
430-04684-002

1- 8 |
1) Descriptive Information |
||
|
|
|
|
|
TERM |
DEFINITION |
|
|
|
|
|
|
Prime |
Manually removing air from the |
|
|
|
syringe and line. |
|
|
|
|
|
|
Purge |
Running the mechanism to remove |
|
|
|
system slack when a new vial/ |
|
|
|
injector is installed. (The system |
|
|
|
must be primed first and |
|
|
|
disconnected from the patient.) |
|
|
|
|
|
|
Warning |
An indication to advise the |
|
|
|
clinician: |
|
|
|
A) of a possible dangerous |
|
|
|
condition such as a low battery |
|
|
|
B) that an attempt has been made to |
|
|
|
use a function in the wrong |
|
|
|
sequence, wrong time, or with the |
|
|
|
wrong values, such as an invalid |
|
|
|
key attempt |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1.6Precautions
•Product damage may occur unless proper care is exercised during the unpacking and setup process. The battery may not be fully charged upon receipt.
ARTIFACTS
•Nonhazardous, low-level electrical potentials are commonly observed when fluids are administered using infusion devices. These potentials are well within accepted safety standards, but may create artifacts on voltage-sensing equipment such as ECG, EMG, and EEG machines. These artifacts vary at a rate that is associated with the infusion rate. If the monitoring machine is not operating correctly or has loose or defective connections to its sensing electrodes, these artifacts may be accentuated so as to simulate actual
430-04684-002

LifeCare PCA 3 Infusion System |
1- 9 |
physiological signals. To determine if the abnormality in the monitoring equipment is caused by the infusion device instead of some other source in the environment, set the infusion device so that it is temporarily not delivering fluid. Disappearance of the abnormality indicates that it was probably caused by the electronic noise generated by the infusion device. Proper setup and maintenance of the monitoring equipment should eliminate the artifact. Refer to the appropriate monitoring equipment system documentation for setup and maintenance instructions.
•The PCA 3 system is designed to operate normally in the presence of most encountered electromagnetic interference (EMI) conditions. In the event of extreme levels of interference, such as those encountered next to an electrosurgical generator, it is possible that the normal operation of a sensor or microcomputer might be disrupted. Even in this event, the outcome would likely be a false alarm or detected system malfunction and would not result in a hazard to patient or clinician.
•Use of radio frequency emitting devices such as cellular telephones and 2-way radios in close proximity of this device may affect its operation.
GENERAL
•Possible explosion hazard exists if used in the presence of flammable anesthetics.
•Potent analgesic medications are used with this device. Refer to drug package insert for precautions and possible adverse reactions.
•Refer to analgesic package enclosure for possible incompatibility with fluid or drug being delivered through the IV line.
•Coupling together of more than one pump into one patient line may significantly affect the infusion rate of at least one of the pumps.
430-04684-002

1- 10 |
1) Descriptive Information |
•Do not use sharp objects such as pens, scissors, or fingernails to press keys. Such objects may damage keys and cause a malfunction.
•Arrange tubing, cords, and cables to minimize the risk of patient strangulation or entanglement.
•Failure to use Hospira vials and Hospira PCA sets with integral anti-siphon valve may cause an inaccurate dose delivery to the patient.
•The system must be primed prior to purging. Remove all air from vial before placing into pump.
•Always close slide clamp on PCA administration set before removing or replacing syringe, and before discontinuing infusion.
•Patient must be disconnected from the PCA set before the purge cycle.
•Vial and injector must be securely locked into the infuser before beginning delivery.
PROGRAMMING
WARNING
FOR CUSTOM SYRINGES, CONFIRM THAT THE DISPLAYED CONCENTRATION (MG/ML) OR (MCG/ ML) EXACTLY MATCHES THE CONCENTRATION VALUE AND DRUG NAME ON THE SYRINGE. IF THEY DO NOT MATCH, UNDER/OVERDOSAGE MAY RESULT.
•In the CONTINUOUS and PCA+CONTINUOUS modes, if a purge is not performed after a syringe change, the pump automatically performs a small system compliance step to remove slack when the [START/PAUSE] key is pressed (with door locked). Although fluid is not normally delivered to the patient during the compliance step, under some conditions up to 0.3 mL of fluid may be delivered. If 0.3 mL of fluid represents a hazard to the patient, the set should be disconnected during this operation.
430-04684-002

LifeCare PCA 3 Infusion System |
1- 11 |
•At flow rates less than 0.5 mL/hr, there may be a significant delay before flow is established if system is not purged.
•Selections are rounded up to the nearest tenth of a digit for mg/mL values or to the nearest digit for mcg/mL values.
LOADING DOSE/DOSE LIMITS
•A loading dose is included in the 4-hour (or 1) dose limit calculation ONLY if administered after the 4-hour dose limit has been programmed. If the loading dose is administered prior to setting the 4-hour dose limit, it will NOT be included in the 4-hour dose limit calculation. The loading dose is always included in the total dose delivered.
•A supplemental “booster” dose can be delivered at any time during setup or operation, even if the 4-hour dose limit is already reached or will be exceeded after delivery.
•Setting a new 4-hour dose limit will not erase the previous 4-hour dose history.
•The concentration can only be changed by removing and re-inserting a custom vial or by turning the pump OFF then ON again. Once the concentration is programmed and confirmed, it cannot be changed without performing one of these two actions. If the concentration is changed, the current settings and consequently the dose limit accumulation are cleared.
•Partial boluses can be the result of interrupting delivery by pressing [START/STOP] (PCA + Cont.), opening the door (PCA Only), loss of power, reaching the dose limit, emptying the vial, or a malfunction alarm.
•If the loading or supplemental “booster” dose causes the 4-hour dose limit to be exceeded, the complete dose will still be delivered. The volume beyond the 4-hour dose limit will count towards the “next” rolling 4-hour dose limit.
430-04684-002

1- 12 |
1) Descriptive Information |
•Always monitor the PCA 3 when delivering medication with the door open.
•Patient Pendant is only to be pressed by the intended patient.
OPERATION
•Perform close assessment and monitoring of patients receiving potent analgesic medication for possible adverse reactions.
•The PCA 3 is not intended to be used for frequent, longterm portable operation. Keep plugged into a properly grounded AC receptacle whenever possible, and reserve battery power for temporary portable operation and emergency backup. If the AC receptacle is in doubt, use battery power.
MAINTENANCE
•Always confirm bar code reader window is clean. Blood, fingerprints, condensation, and other elements may obstruct the view of the bar code reader. Elements on the window (other than scratches) can be cleaned by using one of the recommended cleaning solutions in the
Section 7, Maintenance.
•Window scratches cannot be wiped clean and will probably lead to window replacement.
•To avoid mechanical or electrical damage, do not immerse the pump in any fluids or cleaning solutions.
•Some cleaning and sanitizing compounds may slowly degrade components made from some plastic materials. Using abrasive cleaners or cleaning solutions not recommended by Hospira may result in product damage. Do not use compounds containing combinations of isopropyl alcohol and dimethyl benzyl ammonium chloride.
•Do not sterilize by heat, steam, ETO, or radiation.
•Do not place the PCA 3 in service if it fails the self-test.
430-04684-002

LifeCare PCA 3 Infusion System |
1- 13 |
•Hospira will be responsible for the effect on safety, reliability, and performance of this device only if: adjustments, modifications, or repairs are performed by persons authorized by Hospira; the electrical setup at the point of use complies with appropriate local requirements; and the device is used in accordance with the instructions for use identified in this operating manual.
ALARMS
•If the MALFUNCTION alarm sounds, press the [ON/OFF] key to turn the pump off. Then turn the pump back on. If the malfunction alarm repeats, remove the pump from service.
EPIDURAL ADMINISTRATION
•Recommended use of the epidural route is to provide anesthesia or analgesia for periods up to 96 hours.
•It is strongly recommended that the epidural infusion system be prominently identified as EPIDURAL. Failure to identify the infusion system as epidural could result in incorrect administration of intravenous rather than epidural formulations. In addition, failure to identify the epidural infusion system could result in confusion with other infusion systems delivering concomitant intravenous formulations.
•This device can be used to administer only those anesthetics/analgesics approved for epidural administration (as indicated or allowed by the drugs’ FDA approved labeling). Epidural administration of drugs other than those indicated for epidural use could result in serious injury to the patient.
•For epidural administration, the use of pump sets without Y-sites, and "epidural" stickers indicating ongoing epidural administration are recommended.
•Administration of drugs via the epidural route should be limited to personnel familiar with associated techniques
430-04684-002

1- 14 |
1) Descriptive Information |
and patient management problems. Proper epidural placement of the catheter is essential since catheter migration could result in intravascular or intrathecal administration. Facilities practicing epidural administration must be equipped with resuscitative equipment, oxygen, naloxone, and other resuscitative drugs. Adequate monitoring equipment (e.g., Oximetry) is recommended for continuous monitoring of the patient during epidural administration. Patients must be observed frequently for side effects in a fully-equipped and staffed environment for at least 24 hours following completion of drug administration by the epidural route. DELAYED RESPIRATORY DEPRESSION FOLLOWING CONTINUOUS EPIDURAL ADMINISTRATION OF PRESERVATIVE-FREE MORPHINE SULFATE HAS BEEN REPORTED.
•The epidural space has 58 openings through which fluid can exit. Pressure buildup during administration is transient. However, if a large volume of fluid is administered over a short time period, the pressure will take longer to return to normal. If overdelivery occurs during administration, observe the patient closely for signs of spinal cord compression (disorientation, headache, transient neuralgias) and drug overdose.
BATTERY OPERATION
WARNING
DISCONNECT AC POWER CORD BEFORE REMOVING BATTERY DOOR.
CAUTION: DO NOT OPERATE THE PCA 3 WITH THE BATTERY REMOVED ON PATIENTS. USE OF A PROPERLY MAINTAINED AND CHARGED BATTERY HELPS ENSURE PROPER OPERATION.
•The battery may not be fully charged upon receipt. Connect the PCA 3 to AC power for at least 16 hours.
430-04684-002

LifeCare PCA 3 Infusion System |
1- 15 |
•Use AC power whenever possible. Connect to AC power during storage to ensure a fully charged battery for emergencies.
•Always connect the pump to a properly grounded receptacle unless battery operation is desired. If quality earth grounding source is in doubt, use battery power.
•If the low-battery alarm sounds, connect to AC power immediately.
SETS AND ACCESSORIES
Use Hospira Lifecare PCA Set List 6517 whenever the pump is in CONTINUOUS or PCA+CONTINUOUS modes.
•When using PCA or PCA+CONTINUOUS mode, another fluid line may be attached to the distal backcheck “Y” site. Use Hospira Lifecare PCA Set, List 3559, 6516, or a combination of List 6514 and 6517.
•It is recommended that highly viscous solutions and drugs, colloidal suspensions and emulsions should not be delivered through the inline backcheck valve of the PCA set. Valve functionality may be compromised by the presence of residue.
•Refer to vial and set package inserts for precautions and information on proper handling.
430-04684-002

1- 16 |
1) Descriptive Information |
NOTES
430-04684-002
LifeCare PCA 3 Infusion System |
2- 1 |
2)Principles of Operation
The PCA 3 Infuser is a portable infusion pump that allows a patient to self-administer analgesia within programmed limits as well as providing continuous infusion of desired drug. Generally, a nurse following a physician’s order programs the infuser with operating parameters, which may include the following:
•Loading Dose
•Delivery Mode Setting, i.e., PCA, CONTINUOUS, or PCA+CONTINUOUS
•PCA Dose
•Lockout Interval
•Rate of Continuous Flow
•1 or 4 Hour Dose Limit (factory setting 4-hour)
•Protocols (Hospital configured settings for prefilled vials)
Available operating parameters and their allowed ranges are determined based on the confirmed vial and the delivery mode selected. The loading dose and dose limits are optional. This programmed flexibility allows the physician to tailor an effective pain management program unique to each patient.
The PCA 3 can be programmed to deliver either PCA doses (PCA mode), to deliver only a continuous background infusion with no PCA doses permitted (CONTINUOUS mode), or to deliver a continuous rate and allow PCA doses (PCA+CONTINUOUS mode).
Analgesic drugs may be delivered through the PCA 3 intravenously by any of the three modes mentioned above. In addition, Preservative-Free Morphine Sulfate Injection, USP, or other approved analgesic drugs can be administered epidurally through a recommended Low Priming Volume PCA Set without a Y-adapter. The epidural route can be used to provide analgesia by any of the three modes of infuser operation.
430-04684-002
2- 2 |
2) Principles of Operation |
A “lockout” interval controls the frequency with which a patient may receive a PCA dose of analgesic. If the pump is set in the PCA or PCA+CONTINUOUS mode, the patient may request a bolus of analgesic during therapy by pressing a button on the patient pendant, causing the pump to release the specified bolus of analgesic into the IV line. After a Loading or Supplemental “booster” Dose delivery, the patient cannot receive any additional patient requested boluses until the lockout interval has elapsed, assuming the dose limit has not been exceeded (see Section 5.4 for a detailed description of Dose Limits).
The PCA 3 records therapy settings and up to 400 “events” that may occur during the therapy regimen. Events recorded include opening or closing of the security door, start or stop of continuous infusion, an alarm condition, and so on. All event descriptions are preceded with time of occurrence.
The PCA 3 operates on AC or back-up battery power. It attaches and locks to an IV pole and also has a locking security door to prevent tampering.
The alarm system sounds an audible alarm to alert the user to various conditions or a malfunction (see Section 6.3 for a detailed description of alarm messages).
2.1 Features
DRUG RECOGNITION
•Bar code reader identifies drug name and concentration in the vial (prefilled Hospira vials only)
•Bar code reader identifies custom-filled vials when pharmacy-filled vial is used
MODES OF DELIVERY
•PCA Only
430-04684-002
LifeCare PCA 3 Infusion System |
2- 3 |
•Continuous
•PCA + Continuous
PROGRAMMING
•Keypad with large numbers, decimal point & icons for ease of use
•Prompting alphanumeric display
BATTERY
•8 V Battery
•Long battery life (4 hours) for emergency backup and temporary portable operation
BIO MEDICAL
•Serial Communication
•Upgradability (Field)
•Diagnostics Setup Options
•Alarm History
•Ability to store protocols for Hospira prefilled vials
OPTIONS
•Infusion History
OTHER FEATURES
•Microprocessor control
•Liquid crystal display (LCD) and Light-Emitting Diode (LED) display
•Panel back illumination
•Security Features
•Prefilled and Sterile empty vials
430-04684-002

2- 4 |
2) Principles of Operation |
2.2 Administration Equipment
The following sets and catheters are supplied sterile and are for single use only.
ADMINISTRATION SETS
List 3559: PCA Set, Mini-Bore with Integral AntiSiphon Valve-SL 170 cm. Approximate Priming Volume 2.3 mL. For use in PCA mode via Intravenous route.
List 6514: PCA Extension Set with Backcheck ValveSL 25 cm. Approximate Priming Volume 1.1 mL. For use in conjunction with set 6517 to convert from Continuous to PCA mode via Intravenous route.
List 6516: PCA Set-Long, Mini-Bore with Integral Anti-Siphon Valve-SL 218 cm. Approximate Priming Volume 2.6 mL. For use in PCA mode when extra length needed via Intravenous route.
List 6517: PCA Continuous Infusion Set, Mini-Bore with Integral Anti-Siphon Valve-SL 203 cm. Approximate Priming Volume 1.5 mL.
For use in Continuous and PCA+Continuous modes via Intravenous route.
For use in PCA, Continuous, and PCA+Continuous modes via Epidural route.
430-04684-002

LifeCare PCA 3 Infusion System |
2- 5 |
|
2.3 Printer Kits |
|
|
List |
Complete thermal printer kit includes: |
12406-01: |
printer, connector cable, battery pack, AC |
|
adaptor, & paper pack. |
List |
Connector cable only |
12406-02: |
|
** See current product sales catalog for available drugs **
430-04684-002

2- 6 |
2) Principles of Operation |
NOTES
430-04684-002
LifeCare PCA 3 Infusion System |
3- 1 |
3)Equipment Description
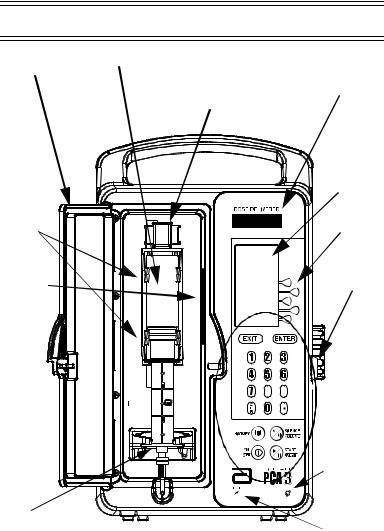
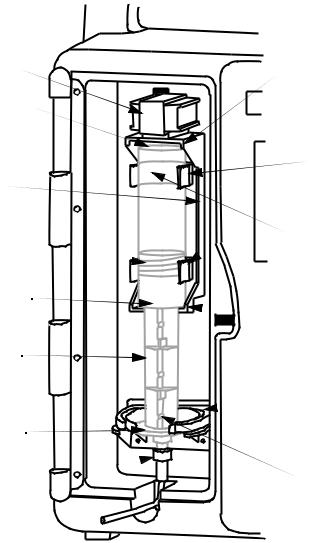
3.1 Components
Security |
Vial |
Door |
|
Vial
Cradle
Clips 
Bar code  Reader Window
Reader Window
Injector 

Vial
Plunger
Cradle |
|
Release |
LED |
Mechanism |
Display |
(Holder) |
|
LCD
Display
Softkeys
Door
 Lock
Lock
Keypad

 Buttons
Buttons
AC
Power
Indicator
Battery
Power
 Indicator
Indicator
Basic Layout of Front Panel and Keypad
430-04684-002
3- 2 |
3) Equipment Description |
Cradle |
|
|
Release |
Upper Vial |
|
Mechanism |
||
Retainer |
||
|
||
Vial Base |
|
|
Bar code |
Vial Cradle |
|
Reader |
||
Clips |
||
Window |
||
|
||
(Vial Bar |
|
|
code must |
Vial Sensor |
|
face) |
||
|
Switch |
|
Vial |
(Top Vial |
|
Cradle |
||
Stopper |
||
Clip) |
||
|
||
Vial Lip |
Lower Vial |
|
|
||
|
Retainer |
|
|
(Middle |
|
Injector |
Bracket) |
|
|
||
|
Injector |
|
Injector |
Flange |
|
Flange (Vial |
Retainer |
|
Plunger) |
|
|
|
Injector |
|
Luer-Lock |
Sensor |
|
Switch |
||
Fitment |
||
(Back of |
||
|
||
|
Retainer) |
Vial Cradle Assembly
430-04684-002
 Loading...
Loading...