Page 1
Instruction Manual
HI 38081
Calcium and
Magnesium
Test Kit for
SPECIFICATIONSSPECIFICATIONS
SPECIFICATIONS
SPECIFICATIONSSPECIFICATIONS
Range > 0 meq/L as Ca & Mg
Smallest Increment 0.6 meq/L for 1.0 mL sample
0.2 meq/L for 3.0 mL sample
Analysis Method Titration
Sample Size 1.0 mL or 3.0 mL
Number of Tests 100 (average)
Case Dimensions 235x175x115 mm (9.2x6.9x4.5")
Shipping Weight 671 g (23.7 oz.)
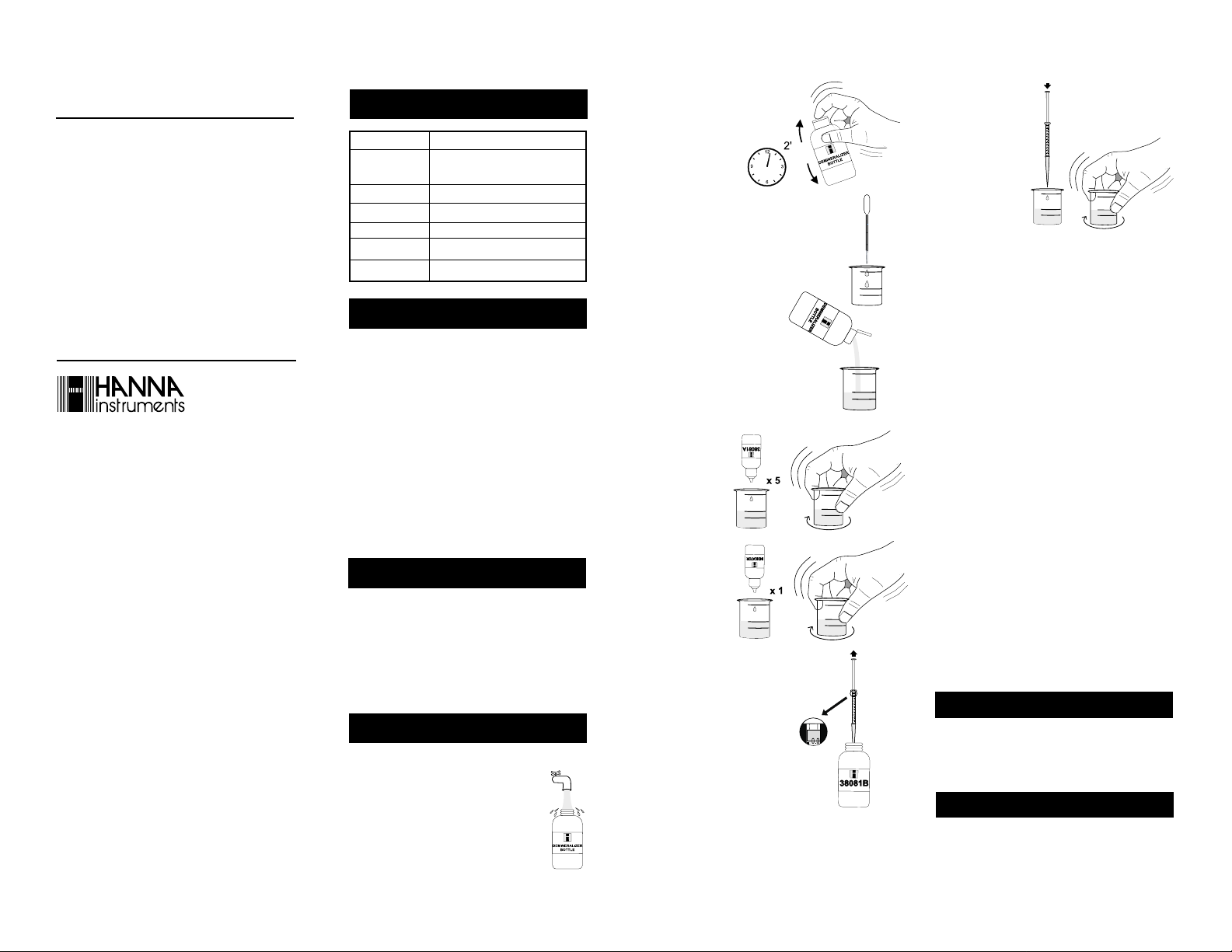
2- Replace the cap
and shake for
least 2 minutes. The
demineralized water
is now ready.
3- Using the 3 mL plastic pipette, transfer 3
mL of your water sample to the 50 mL
plastic vessel.
at
9- As the color
changes from pink
to purple, swirl for
15 seconds after
each additional
drop. Repeat this
until the solution
turns pure blue.
Read off the milliliters of titration solution used from the syringe.
10-Calculate the meq/L of Calcium and Magnesium in your
sample as follows:
Irrigation Water
www.hannainst.com
Dear Customer,
Thank you for choosing a Hanna Product.
Please read the instruction sheet carefully before using the
test kit. It will provide you with the necessary information
for correct use of the kit. If you need additional information,
do not hesitate to e-mail us at tech@hannainst.com.
Remove the chemical test kit from the packing material and
examine it carefully to make sure that no damage has
occurred during shipping. If there is any noticeable damage, notify your Dealer or the nearest Hanna office
immediately.
Each kit is supplied with:
HI 38081A-0 Ca & Mg Reagent, 1 bottle with
•
dropper (30 mL);
HI 38081B-0 EDTA Solution, 2 bottles (2x120 mL)
•
• Calmagite Indicator,
• Demineralizer Bottle with filter cap for about 12 liters
of deionized water (depending on the hardness level
of water to be treated)
•1 calibrated plastic vessel (50 mL);
•1 plastic pipette (1 mL);
•1 plastic pipette (3 mL);
1 syringe (1 mL) with tip.
•
Note: Any damaged or defective item must be returned in
its original packing materials.
1 bottle with dropper (10 mL)
;
SIGNIFICANCE AND USESIGNIFICANCE AND USE
SIGNIFICANCE AND USE
SIGNIFICANCE AND USESIGNIFICANCE AND USE
Calcium presence in natural waters results from passage
through deposits of limestone, dolomite, gypsum and gypsiferous shale. The calcium concentration in water supplies
may extend from 0 to several hundred milligrams per liter,
depending on its source and treatment.
Magnesium is a common constituent of natural waters; in
concentrations greater than 125 mg/L it can cause diuretic
effect. It is also an important contributor to the hardness of
water: when heated, magnesium salts break down forming
incrustation in boilers. Moreover, magnesium is necessary to
plant metabolism since it is an essential constituent of
organic molecules such as chlorophyll.
CHEMICAL REACTIONCHEMICAL REACTION
CHEMICAL REACTION
CHEMICAL REACTIONCHEMICAL REACTION
The Hanna Test Kit determines Calcium and Magnesium in
irrigation water via a titrimetric method: the indicator
chelates with the ions Calcium and Magnesium to form a
red colored complex. As EDTA is added, calcium and magne-
;
;
sium complex with it: the reaction end point is indicated by
a change in color of the indicator from red to blue.
Note: meq/L is milliequivalent per liter.
READ THE ENTIRE INSTRUCTIONS BEFORE USING THE KIT
1- Remove the cap and fill the
Demineralizer Bottle with tap
water.
ISTR38081 02/00 PRINTED IN ITALY
INSTRUCTIONSINSTRUCTIONS
INSTRUCTIONS
INSTRUCTIONSINSTRUCTIONS
4- Flip open the top of the Dem-
ineralizer Bottle cap. Squeeze
the bottle gently to add demineralized water to the plastic
vessel up to the 25 mL mark.
5- Add 5 drops of
HI 38081A-0 Ca
& Mg Reagent
and swirl to mix.
6- Add 1 drop of
Calmagite Indicator and swirl to
mix. If calcium
and magnesium
are present, the
solution will turn
wine red.
7- Take the syringe and push
the plunger completely down
into the syringe. Insert tip
into the HI 38081B-0 EDTA
solution and pull the plunger
out until the lower edge of
the seal is on the 0 mark of
the syringe.
8- Slowly add the titration solution drop by drop, swirling
after each drop.
meq/L (Ca + Mg) = mL of titrant x 20
11- If your sample requires more than 1 mL of titrant to
turn pure blue it requires dilution. Repeat the test by
using the 1 mL pipette to transfer 1 mL of your water
sample to the plastic vessel.
12-Follow the instructions from step 4 to step 9.
13-Calculate the meq/L of Calcium and Magnesium in your
sample as follows:
meq/L (Ca + Mg) = mL of titrant x 60
14-To convert the reading in mg/L (ppm, parts per million)
of CaCO
, multiply the meq/L by 50.
3
15- Rinse all labware with demineralized water after each
analysis and shake dry.
Note: High amounts of copper in your sample will alter the
final end point color. The solution will change from wine
red to purple without turning pure blue. In this case
add drops of titrant until no visible change in color is
obtained.
REFERENCESREFERENCES
REFERENCES
REFERENCESREFERENCES
Adaptation of Standard Methods for the Examination of
Water and Wastewater, 18
HEALTH AND SAFETYHEALTH AND SAFETY
HEALTH AND SAFETY
HEALTH AND SAFETYHEALTH AND SAFETY
The chemicals contained in this kit may be hazardous if
improperly handled. Read Health and Safety Data Sheet
before performing this test.
th
edition, 1992, APA AWWA WEF.
 Loading...
Loading...