Page 1
Instruction Manual
HI 38076
Zinc
Test Kit
with Checker Disc
www.hannainst.com
Dear Customer,
Thank you for choosing a Hanna Product.
Please read the instruction sheet carefully before using the
test kit. It will provide you with the necessary information
for correct use of the kit. If you need additional information,
do not hesitate to e-mail us at tech@hannainst.com.
Remove the chemical test kit from the packing material and
examine it carefully to make sure that no damage has
occurred during shipping. If there is any noticeable damage, notify your Dealer or the nearest Hanna office
immediately.
Each kit is supplied with:
HI 93731A-0 Reagent, packets (100 pcs);
•
HI 93731B-0 Zinc Reagent B (Cyclohexanone), 1
•
bottle (100 mL);
• Demineralizer Bottle with filter cap for about 12 liters
of deionized water (depending on the hardness level
of water to be treated)
1 checker disc (containing the 38076 disc);
•
2 glass vials with caps;
•
• 1 long plastic pipette;
1 plastic pipette (3 mL);
•
1 syringe (1 mL) with tip.
•
Note: Any damaged or defective item must be returned in
its original packing materials.
;
SPECIFICATIONSSPECIFICATIONS
SPECIFICATIONS
SPECIFICATIONSSPECIFICATIONS
Range 0.0 to 4.0 mg/L (ppm) as Zinc
0.0 to 20.0 mg/L (ppm) as Zinc
Smallest Increment 0.1 mg/L [in the 0-4 mg/L range]
0.4 mg/L [in the 0-20 mg/L range]
Analysis Method Colorimetric
Sample Size 7.5 mL and 1.5 mL
Number of Tests 100
Case Dimensions 235x175x115 mm (9.2x6.9x4.5")
Shipping Weight 647 g (22.8 oz.)
SIGNIFICANCE AND USESIGNIFICANCE AND USE
SIGNIFICANCE AND USE
SIGNIFICANCE AND USESIGNIFICANCE AND USE
Zinc is widely used in alloys (brass, bronze and dye-casting
alloys), in galvanizing iron and other metals and as a
fungicide. Zinc is one of the elements that, though in small
quantities, is indispensable for plants life. It is also an
essential growth element in human diet. But in concentration higher than 5 mg/L, it gives a bitter taste to water
and opalescence to alkaline water. Zinc can enter the
domestic water supply from the deterioration of galvanized
iron and dezincification of brass.
Note:
mg/L is equivalent to ppm (parts per million).
CHEMICAL REACTIONCHEMICAL REACTION
CHEMICAL REACTION
CHEMICAL REACTIONCHEMICAL REACTION
Zinc reacts with the zincon reagent to form a brownishgreen complex in a solution buffered at alkaline pH. Since
other metals can form colored complexes with zincon, cyanide is added to complex zinc and any other heavy metal
present. Then, cyclohexanone is added to selectively free zinc
from its cyanide complex so that it can react with zincon to
form the final brown-violet colored product. The amount of
color developed is proportional to the concentration of zinc
present in the aqueous sample.
ISTR38076 04/00 PRINTED IN ITALY
INSTRUCTIONSINSTRUCTIONS
INSTRUCTIONS
INSTRUCTIONSINSTRUCTIONS
READ THE ENTIRE INSTRUCTIONS BEFORE USING THE KIT
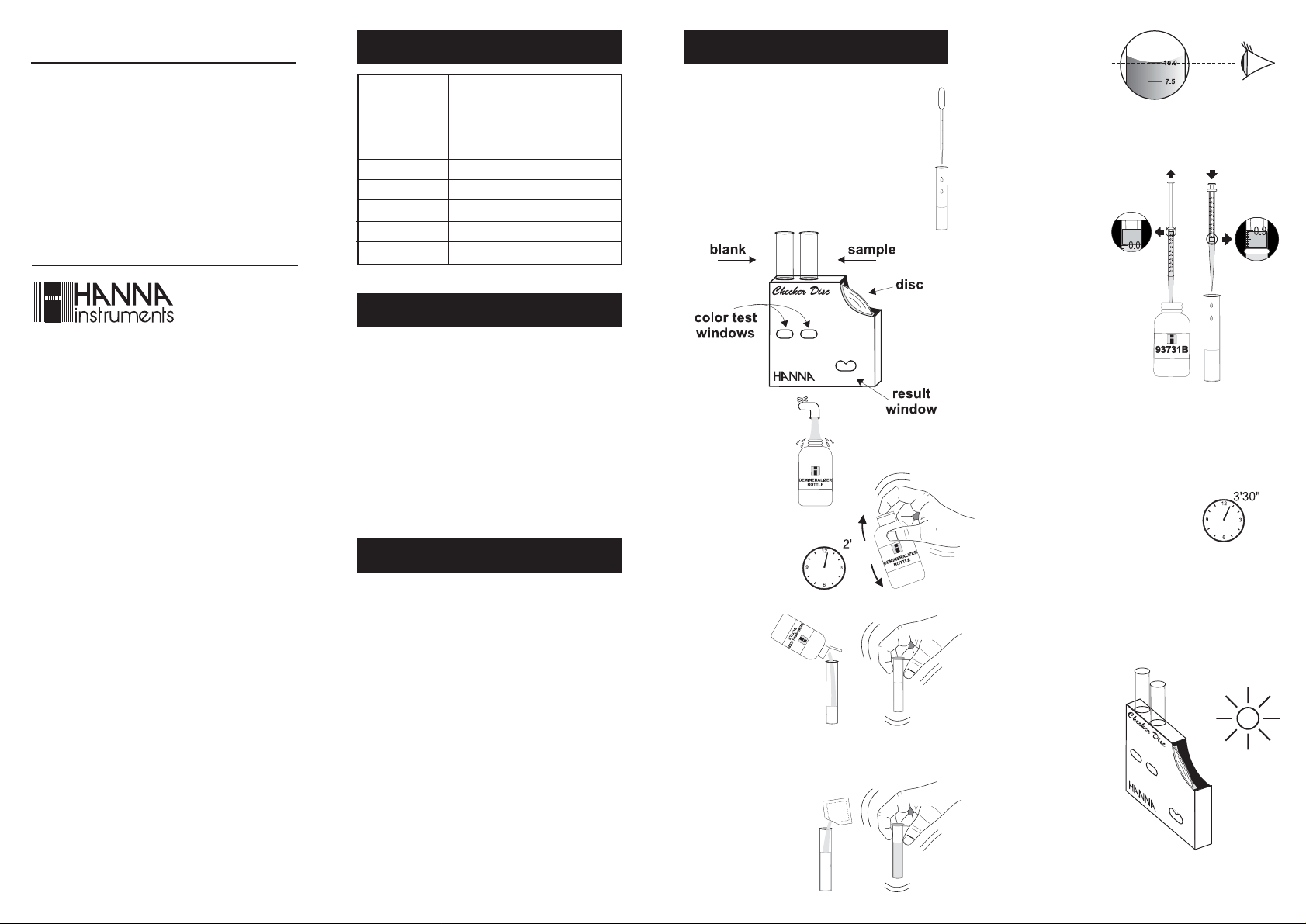
1- Using the 3 mL plastic pipette, fill each
of the two glass vials with 7.5 mL of
sample (up to the mark).
2- Insert one of the vials into the left
hand opening of the checker disc. This
is the blank.
3- Remove the cap
and fill the Demineralizer Bottle
with tap water.
4- Replace the cap and
shake gently for at
least 2 minutes. The
demineralized water
is now ready.
5- Flip open the
top of the Demineralizer Bottle
cap. By gently
squeezing the
bottle, add
demineralized
water to the other vial up to the 20 mL mark. Replace
the cap and shake to mix.
6- Remove the cap, add 1
packet of HI 93731A-0
reagent. Replace the cap
and mix the solution until
the powder has completely dissolved.
7.5 mL
20 mL
7- Using the long
plastic pipette, remove 10 mL of
the orange solution and dispose
of it (the level of the remaining liquid in the vial has to
correspond to the 10 mL mark).
8- Add 1 mL of
HI 93731B-0
reagent to the
vial, using the
syringe.
Note: To measure
exactly 1 mL
of reagent
with the syringe, push
the plunger
completely into
the syringe and insert the tip into reagent bottle.
Pull the plunger out until the lower edge of the seal
is on the 0.0 mL mark of the syringe. Insert the
syringe into the vial and push the reagent out until
the lower edge of the seal is on the 1.0 mL mark.
9- Replace the cap and mix for 15
seconds. Wait for 3 and a half
minutes to allow color to develop.
This is the reacted sample.
Note: the sample is turbid, but this thing does not affect
the measurement.
10-Remove the cap and insert the reacted sample into the
right hand opening of the checker disc.
11-Hold the checker
disc so that a light
source illuminates
the samples from the
back of the windows. It is better to
match the color with
a background uniform and light in
color (e.g. a white
sheet) behind the
checker disc.
Page 2
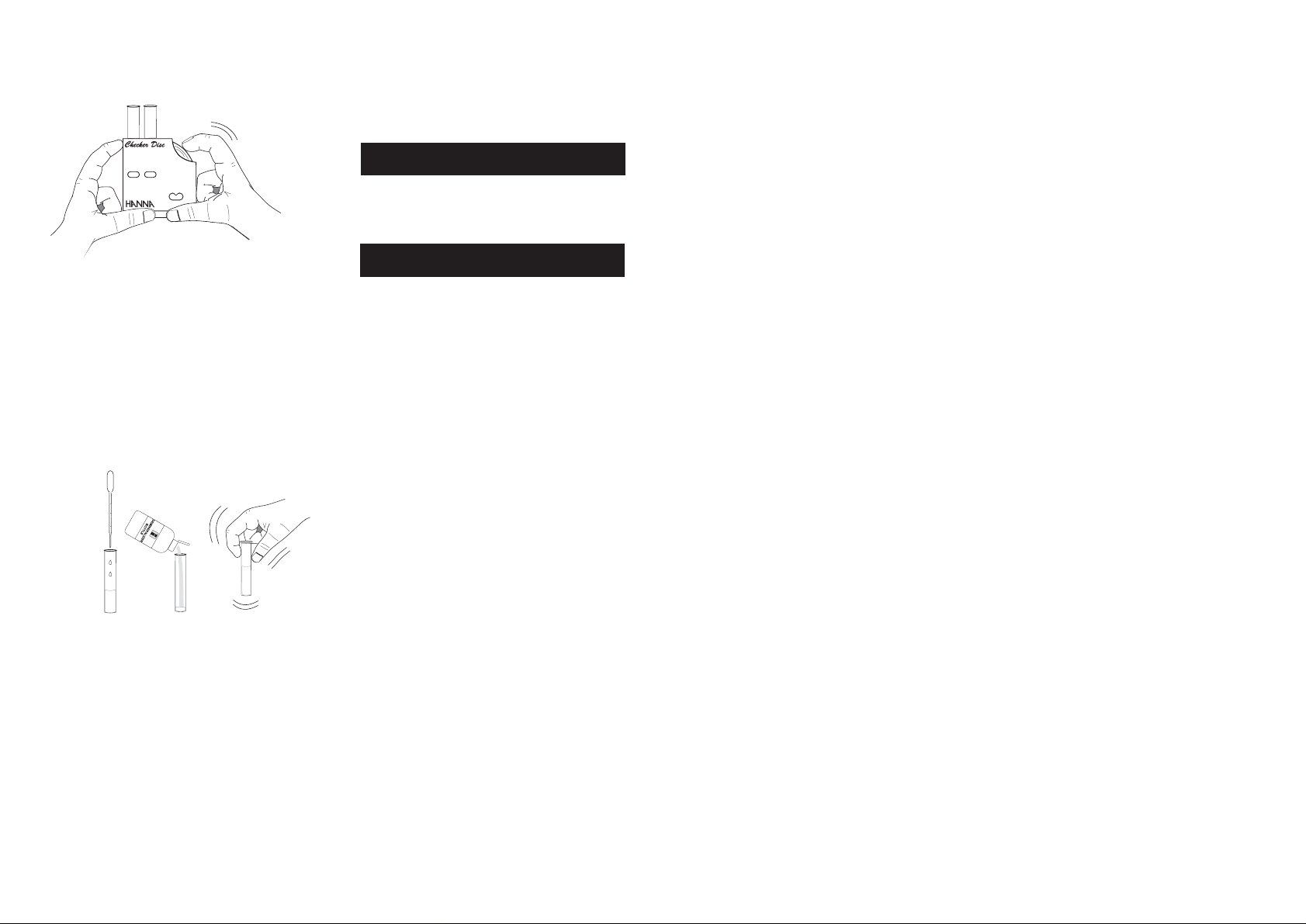
12- Keep the checker disc at a distance of 30-40 cm (12-
16") to match the color. Rotate the disc while looking at
the color test windows and stop when you find the color
match.
13- Read the value in the result window directly in mg/L
(ppm) of Zinc.
14-If the color is too intense to make a color match, then
the original sample needs to be diluted. In this case
perform the test as follows.
15- Using the 3 mL plastic pipette, fill one glass vial with
1.5 mL of sample exactly and add demineralized water
up to the 20 mL mark, replace the cap and swirl to
mix.
pended matter in large amounts should be removed
by prior filtration.
Caution: Ultraviolet radiation may cause fading of colors.
When not in use, keep the disc protected from light,
in a cool and dry place.
REFERENCESREFERENCES
REFERENCES
REFERENCESREFERENCES
Standard Methods for the Examination of Water and
Wastewater, 18th Edition, 1992
APHA/AWWA/WEF.
HEALTH AND SAFETYHEALTH AND SAFETY
HEALTH AND SAFETY
HEALTH AND SAFETYHEALTH AND SAFETY
The chemicals contained in this kit may be hazardous if
improperly handled. Read the relevant Health and Safety
Data Sheet before performing this test.
1.5 mL
16- Prepare the blank as before and insert it into the left
hand opening of the checker disc. Follow the procedure
from step 6 to step 12.
17- Multiply the reading value by 5 to obtain mg/L (ppm)
of Zinc.
18-Discard the reacted sample after measurement, because
the glass might become permanently stained.
For best results: Perform the reading three times and take
the average value (divide by 3 the sum of the three
numbers). Intensely colored samples will make the color
matching determination difficult and they should be
adequately treated before performing the test. Sus-
 Loading...
Loading...