Page 1
Instruction Manual
HI 38058
pH 4.0-10.0
Test Kit
SPECIFICATIONSSPECIFICATIONS
SPECIFICATIONS
SPECIFICATIONSSPECIFICATIONS
Range 4.0 to 10.0 as pH unit
Smallest Increment 0.5 as pH unit
Analysis Method Colorimetric
Sample Size 5 mL
Number of Tests 300
Case Dimensions 165x150x38 mm (6.5x5.9x1.5")
Shipping Weight 215 g (7.6 oz.)
SIGNIFICANCE AND USESIGNIFICANCE AND USE
SIGNIFICANCE AND USE
SIGNIFICANCE AND USESIGNIFICANCE AND USE
INSTRUCTIONSINSTRUCTIONS
INSTRUCTIONS
INSTRUCTIONSINSTRUCTIONS
READ THE ENTIRE INSTRUCTIONS BEFORE USING THE KIT
::
Note
:
Always shake the reagent bottle be-
::
fore use.
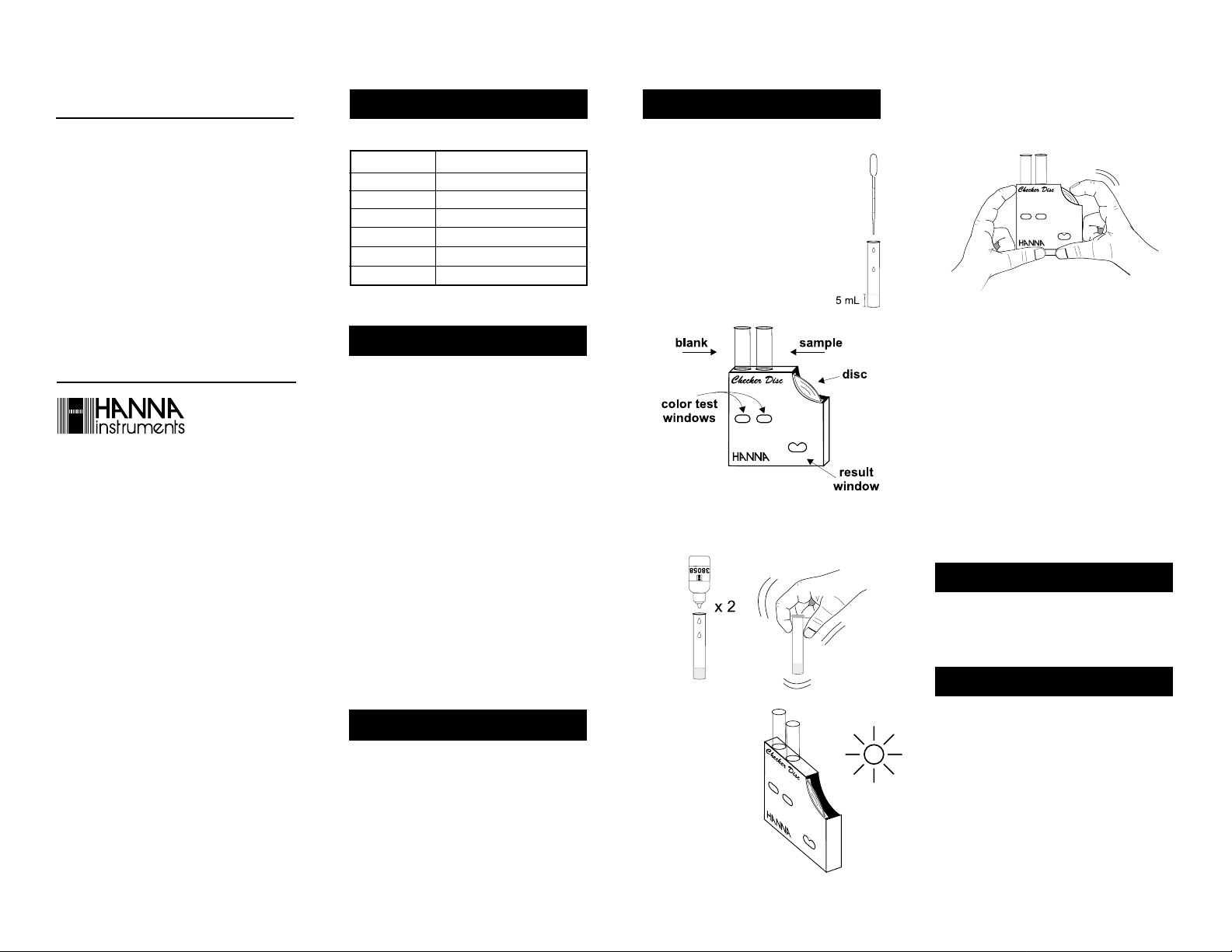
• Using the plastic pipette, fill each glass
vial with 5 mL of sample (up to the
mark).
• Insert one of them into the left hand
opening of the checker disc. This is the
blank.
• Rotate the disc while looking at the color test windows
and stop when you find the color match. Read the
value in the result window directly in pH units.
Note: A concentration of Chlorine above 50 ppm causes
interference inhibiting the development of the color.
In this case require the HI 38058/O Test Kit.
www.hannainst.com
Dear Customer,
Thank you for choosing a Hanna Product.
Please read the instruction sheet carefully before using the
test kit. It will provide you with the necessary information
for correct use of the kit. If you need additional information,
do not hesitate to e-mail us at tech@hannainst.com.
Remove the test kit from the packing material and examine
it carefully to make sure that no damage has occurred
during shipping. If there is any noticeable damage, notify
your Dealer or the nearest Hanna office immediately.
Each kit is supplied with:
HI 38058-0 pH 4.0-10.0 Reagent, 1 bottle with
•
dropper (30 mL);
1 checker disc (containing the 38058 disc);
•
2 glass vials with caps;
•
1 plastic pipette (3 mL).
•
Note: Any damaged or defective item must be returned in
its original packing materials.
pH represents acidity or alkalinity of an aqueous solution
and is proportional to the hydrogen-ion concentration of the
solution. Under neutral conditions water is dissociated into
-
the OH
and H+ ions in equal ratio and hence it has a pH
of 7. When bases or acids are added to a water solution
they ionize, increasing the concentration of OH
respectively. Thus solutions with a pH of 1-3 contain strong
acids, whereas those with a pH of 4-6 contain weak acids.
Weak bases result in solutions of pH 8-10 and strong bases
in pH of 11-13.
Examples of pH value for some liquids:
LiquidLiquid
Liquid
LiquidLiquid
Sea water 7.8-8.2
Gastric juices 1.7
Milk 6.5-7
Soil 6-7 (optimum for crops)
CHEMICAL REACTIONCHEMICAL REACTION
CHEMICAL REACTION
CHEMICAL REACTIONCHEMICAL REACTION
HI 38058-0 reagent reacts in contact with the aqueous
solution changing its color according to the hydrogen-ion
concentration (pH) in the given range.
ISTR38058 02/00 PRINTED IN ITALY
pH ValuepH Value
pH Value
pH ValuepH Value
-
or H+,
• Add to the other glass vial 2 drops of HI 38058-0
reagent. Replace the cap and mix the solution by
gently swirling. This is the reacted sample.
• Remove the cap and insert the reacted sample
into the right hand opening of the checker disc.
• Hold the checker disc so
that a light source illuminates the samples from
the back windows.
For best results: Intensely colored samples will make the
color matching determination difficult and they should
be adequately treated before performing the test.
Suspended matter in large amounts should be removed by prior filtration.
Caution: Ultraviolet radiation may cause fading of colors.
When not in use, keep the disc protected from light,
in a cool and dry place.
REFERENCESREFERENCES
REFERENCES
REFERENCESREFERENCES
Vogel's, Quantitative Chemical Analysis, 5th Ed., Longman
Scientific & Technical.
HEALTH AND SAFETYHEALTH AND SAFETY
HEALTH AND SAFETY
HEALTH AND SAFETYHEALTH AND SAFETY
The chemicals contained in this kit may be hazardous if
improperly handled. Read the relevant Health and Safety
Data Sheet before performing this test.
 Loading...
Loading...