Page 1

HANNA LITERATURE
HI 38050
Hanna publishes a wide range of catalogs and handbooks for
an equally wide range of applications. The reference literature
currently covers areas such as:
• Water Treatment
• Process
• Swimming Pools
• Agriculture
• Food
• Laboratory
• Thermometry
and many others. New reference material is constantly being
added to the library.
For these and other catalogs, handbooks and leaflets contact
your dealer or the Hanna Customer Service Center nearest to
you. To find the Hanna Office in your vicinity, check our home
page at www.hannainst.com.
Nitrate Test Kit
for Soil and
Irrigation Water
Nitrate Test
Handbook
Soil Science
and Management
www.hannainst.com
ISTR38050 01/01 PRINTED IN ITALY
Manufacturers since 1978
Page 2
Index
INTRODUCTION .................................................................................................... 4
THE NITROGEN CYCLE ........................................................................................... 4
15- Separate one side from the other
three to form a cone.
WHY AND WHEN TO TEST FOR NITROGEN.............................................................. 5
HOW TO COLLECT SOIL SAMPLES ............................................................................. 5
FERTILIZATION RECOMMENDATIONS ...................................................................... 6
HOW TO PROGRAM NITROGEN FERTILIZATION........................................................ 7
CONVERSION FACTORS.......................................................................................... 8
WARNING ............................................................................................................ 8
CHEMICAL REACTION............................................................................................. 8
SPECIFICATIONS.................................................................................................... 8
TEST PROCEDURE FOR DETERMINING NITRATE IN IRRIGATION WATER .................... 9
TEST PROCEDURE FOR DETERMINING NITRATE IN SOIL SAMPLES .......................... 10
Calcium Sulfate Extraction ................................................................................ 10
Nitrate Determination ...................................................................................... 11
16- Place the folded filter disc into the funnel and filter the
sample. The extracted sample in the beaker is now ready for
analysis.
NITRATE DETERMINATION
17- Use a pipette to fill each glass vial with 5 mL of the extracted
sample and follow the test procedure as for irrigation water (steps
5 mL
2 to 5).
18- Keep it at a distance of 30-40 cm (12-16") from the eyes to match the color.
Rotate the disc while looking at the color test windows and stop when you find the
color match.
19- Read the value in the result window and multiply it by 2 to obtain mg/L (ppm)
-
of nitrate-nitrogen (N-NO
of nitrate (NO
-
).
3
). Multiply the reading value by 8.86 to obtain mg/L
3
Hanna Instruments reserves the right to modify the design, construction and appearance of its products without advance notice.
2
For best results: Intensely colored samples will make the color matching determination
difficult and they should be adequately treated before performing the test. Suspended
matter in large amounts should be removed by prior filtration.
Caution: Ultraviolet radiation may cause fading of colors. When not in use, keep the
disc protected from light, in a cool and dry place.
Interference: Strong oxidizing and reducing substances; ferric ion (positive interfer-
ence); chloride above 100 ppm (negative interference).
11
Page 3
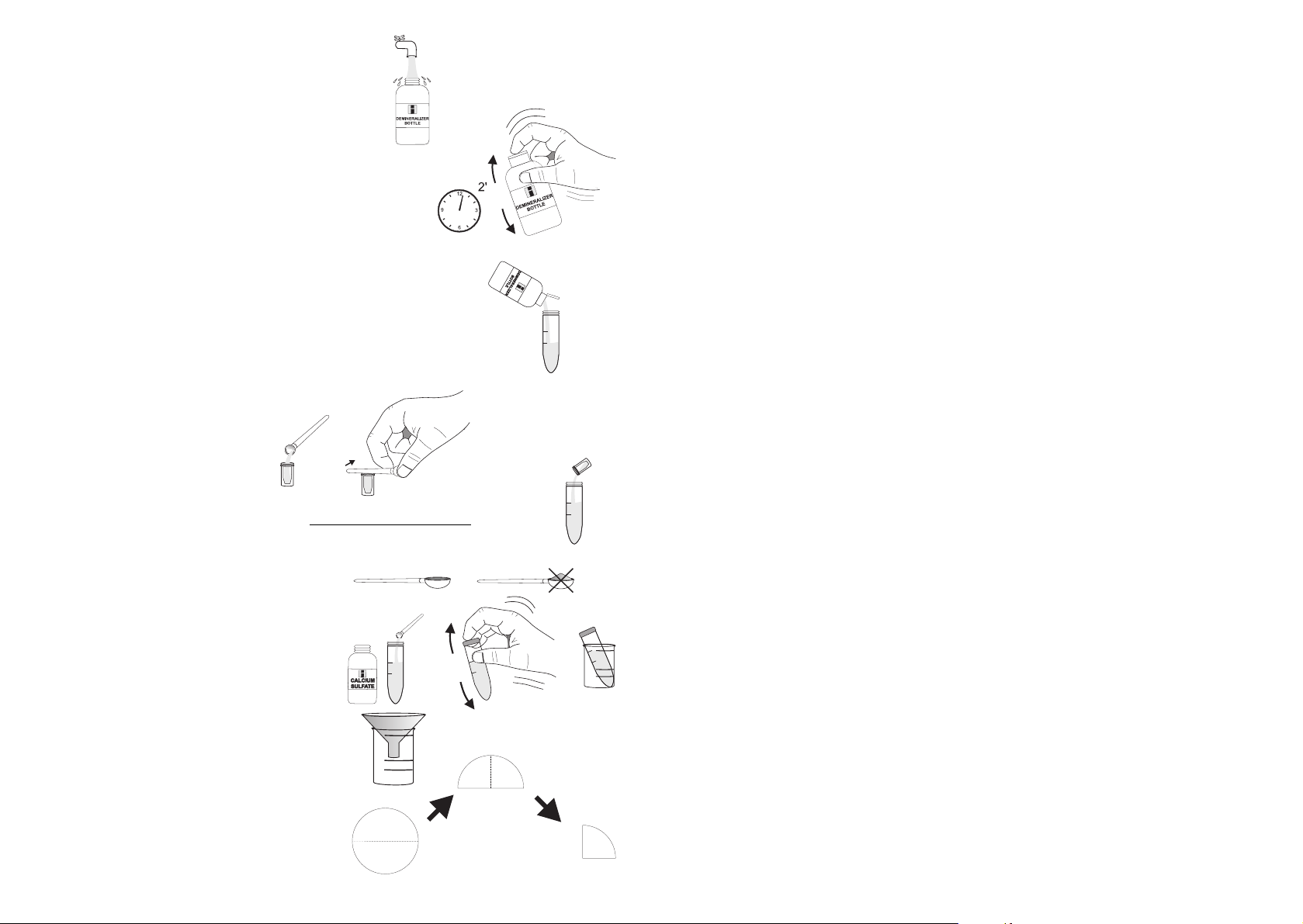
TEST PROCEDURE
FOR DETERMINING
NITRATE IN SOIL
SAMPLES
CALCIUM SULFATE EXTRACTION
7- Remove the cap and fill the Dem-
ineralizer Bottle with tap water.
8- Replace the cap and shake gently for at least
2 minutes. The demineralized water is now
ready.
9- Flip open the top of the Demineralizer Bottle cap.
Squeeze gently the bottle to add demineralized
water to the test tube up to the 20 mL mark.
10- Use the spoon to fill the sample cup with the
sieved soil sample and level the sample in the cup
by discarding the excess soil with the spoon handle.
11- Add to the tube
5 measures of the sample cup of sieved soil
sample.
12- Add 1 level spoon of
Calcium Sulfate. Cap the
tube and mix by shaking it up and down for 1
minute. Place the tube
into one beaker.
Dear Customer,
Thank you for choosing a Hanna Product.
Please read the instruction manual carefully before using the chemical test kit. It will
provide you with the necessary information for correct use of the kit. If you need
additional information, do not hesitate to e-mail us at tech@hannainst.com.
Remove the test kit from the packing material and examine it carefully to make sure
that no damage has occurred during shipping. If there is any noticeable damage,
notify your Dealer or the nearest Hanna office immediately.
Each kit is supplied with:
HI 38050-0 Nitrate Reagent, packets (200 pcs);
•
• 1 checker disc (containing the 38050 disc);
• 2 glass vials with caps.
Extraction Kit:
• Calcium Sulfate, 1 bottle (10 g);
• Demineralizer Bottle with filter cap for about 12 liters of deionized water
(depending on the hardness level of water to be treated)
;
• 1 2-mm soil sieve;
1 plastic test tube (50 mL) with screw cap;
•
• 1 large funnel;
• filter paper discs ∅ 120 mm (100 pcs);
• 1 brush;
• 2 calibrated plastic vessels (50 mL) with cap;
• 1 sample cup (2 g);
1 plastic pipette (3 mL);
•
2 spoons.
•
Note: Any damaged or defective item must be returned in its original packing
materials.
13- Place the funnel on the
top of the other beaker
14- Fold a filter paper disc
twice as shown in the
figure.
.
3
10
Page 4

INTRODUCTION
THE NITROGEN CYCLE
Nitrogen (N) is an indispensable element for plant life. It is present in proteins,
vitamins, chlorophyll, etc. Nitrogen allows the development of the vegetative activity
of the plant, in particular, causes a lengthening of trunks and sprouts and increases
the production of foliage and fruit. It directly increases the crop yield, though the crop
quality depends on other elements.
-
Nitrogen, mostly absorbed by plants as nitrate (NO
), derives from the mineralization
3
of organic matter and the application of fertilizers. Nitrate-nitrogen is not durable in
soil. The large amount required for crop production, makes it necessary to administer
this element in moderate quantities during the crop growth season.
An excess of Nitrogen weakens plants' structure creating an unbalanced relationship
between the green and wooden parts. In addition, the plant becomes less resistant to
diseases. Furthermore excessive nitrogen fertilization can contaminate groundwater
and cause environmental problems.
The Hanna Nitrate Test Kit for Soil and Irrigation Water makes it possible to
determine the need for nitrogen fertilization. It also obtains the best crop response
and avoids over-fertilization.
Nitrogen is the most abundant element present on our planet and can be found in
many different forms. Only a very small part of the total nitrogen is available for
plant growth. The exchanges between available and unavailable nitrogen combine to
form a complex system which is called the nitrogen cycle.
TEST PROCEDURE
FOR DETERMINING
NITRATE IN
IRRIGATION WATER
READ THE ENTIRE INSTRUCTIONS BEFORE USING THE KIT
1- Using the plastic pipette, fill each glass vial with 5 mL of
water sample (up to the mark).
2- Insert one of them into the left hand opening of the
checker disc. This is the blank.
3- Add to the other glass vial
1 packet of HI 38050-0
reagent. Replace the cap,
shake vigorously for 1
minute and wait for 5 minutes. This is the reacted
sample.
5 mL
4- Remove the cap and insert the reacted sample into
the right hand opening of the checker disc.
5- Hold the checker disc so that a light source illumi-
nates the samples from the back of the windows.
6- Keep the checker disc at a dis-
tance of 30-40 cm (12-16") from
the eyes to match the color. Rotate the disc while looking at the
color test windows and stop when
you find the color match. Read
the value in the result window
directly in mg/L (ppm) of nitratenitrogen (N-NO
-
). Multiply the reading by 4.43 to obtain mg/L of nitrate (NO
3
-
).
3
Note: Perform the reading three times and take the average value (divide by 3 the
sum of the three numbers).
4
9
Page 5

CONVERSION
FACTORS
WARNING
1 kg 2.205 lb.
1 ha 2.471 acre
1 kg/ha 0.891 lb./acre
1 ppm (soil) 1 mg/kg
1 ppm (irrigation water) 1 mg/L
1 ppm N 4.43 ppm NO
3
This test gives accurate results for most soil types, nevertheless, some local circumstances can cause erroneous readings. Therefore use this test always with caution.
Whereas an insufficient dose of nutrients decreases the potential crop production, an
excess can have a detrimental effect on the physiology of the plants and the crop
quality. In addition, too much fertilization is unnecessarily costly as well as harmful to
the environment. Hence, only after a technical and economical evaluation, it is
possible to choose the proper quantity of fertilizer to be added.
Note: Legumes (soybean, pea, clover, alfalfa, etc.) are able to take atmospheric nitrogen by a
symbiotic association with Rhizobium bacteria.
A very important source of nitrogen available for plants is the decomposition (mineralization and nitrification) of organic matter, the so called "turnover". However only
part of the organic matter decomposes during the crop growth season. The decomposition rate depends strongly on the local climate, the physical structure and microbiological
activities in the soil, thus it varies from year to year. Other important sources of
nitrogen are fertilization and irrigation when nitrogen compounds are present in the
irrigation water. Even rain and snow can contribute, dissolving the nitrate, nitrite and
ammonia normally present in the atmosphere and carrying them to the soil.
Available nitrate-nitrogen can be lost from the soil in several ways. The most
significant ones are leaching, which occurs during heavy rainfall or where excessive
irrigation is used. Another is assimilation by crops. It is estimated that in natural soils
(woods, forests) about 80% of the absorbed nitrogen is replenished when trees shed
their leaves. In case of crops, the assimilated nitrogen is lost from soil during
harvesting.
CHEMICAL REACTION
SPECIFICATIONS
Nitrate is reduced to nitrite in the presence of Cadmium. The nitrite thus produced
reacts with the reagent to yield an orange compound. The amount of color developed
is proportional to the concentration of nitrate present in the aqueous sample.
Range IW: 0-50 mg/L (ppm) as N-NO
Soil: 0-60 mg/L (ppm) as N-NO
Smallest Increment IW: 1 mg/L (ppm) N-NO
Soil: 2 mg/L (ppm) N-NO
-
3
-
3
-
3
-
3
Analysis Method Colorimetric
Sample Size 5 mL (IW)
10 g of soil (Soil)
Number of Tests 100 (IW), 100 (Soil)
Case Dimensions 235x175x115 mm (9.2x6.9x4.5")
235x175x115 mm (9.2x6.9x4.5")
Shipping Weight 1026 g (36.2 oz.)
Note: IW is Irrigation Water
WHY AND WHEN TO
TEST FOR NITROGEN
HOW TO COLLECT
SOIL SAMPLES
Testing the soil during the crop cycle is a useful tool for next cultivation, in order to
plan fertilization and to know the residues of fertilizers in relation to the crop, tillage
and climate. An analysis can highlight shortages and help in understanding the causes
of an abnormal growth.
The Hanna nitrate-nitrogen test can be performed the whole year round, but testing is
particularly recommended during Spring and Late-spring, when rainfall and temperature-related bursts of microbiological activity often have great influence on the
availability of nitrate-nitrogen.
1) Soil Sample Extraction
2
– Within a large homogeneous area, take 1 or 2 samples per 1000 m
(0.25 acre).
– Even for smaller areas, 2 samples are recommended (the more samples, the better
the end-results, because the end sample is more representative).
– For a small garden or plot, 1 sample is sufficient.
2) Avoid extracting samples from soil presenting obvious anomalies and from border
areas (near ditches and roads).
3) Sample quantity:
Take the same quantity of soil for each sample. For example, use bags with similar
dimensions (1 bag per sample).
4) Depth of extraction:
Sample the top 30 cm (12”) of soil.
5) Mix all the samples together to obtain a homogeneous mixture of soil, discarding
stones and vegetable residues.
8
5
Page 6

6) From this mixture, take the quantity of soil that you need for the analyses.
7) Crumble the large chunks and distribute the soil sample on plastic to air dry it. The
sample dries faster if a fan is used to move air across the sample.
8) Use a small bar to crush the air dried sample and pass it through the 2-mm soil sieve.
Do not store the samples longer than 24 hours in a closed plastic bag. Store the
sample in a cold place and out of direct sunlight, if it can not be dried immediately.
Do not expose the soil to direct sunlight or any heat font.
Table below for the fertilizer recommendations for Corn.
soil N-NO
(ppm) fertilizer soil N-NO
3
3
(ppm) fertilizer
recommendation recommendation
(kg N/ha) (kg N/ha)
<10 100-150 18 32-92
10 120-180 19 21-81
11 109-169 20 10-70
12 98-158 21 9-59
13 87-147 22 0-48
14 76-136 23 0-37
15 65-125 24 0-26
16 54-114 25 0-15
17 43-108 26 0
FERTILIZATION
RECOMMENDATIONS
Before sowing or transferring plants, use a slow-acting fertilizer to enrich the soil for
the long term. Adding organic substances (such as manure and compost) helps to
increase the soil fertility. In case of lack of nitrogen during the crop growth season, use
fertilizers containing nitrate. If necessary add the fertilizer before sprouting or wheat
raising, or as a side dressing while crop is growing. Do not give nitrate at the end of
the plant cycle to crops such as lettuce (where the product is the vegetable part), in
order to avoid its accumulation in the leaves (nitrate is carcinogenic).
The quantity of fertilizer to be added to the soil depends not only on the chemical
state of the soil but also on factors such as present cultivation, local climate, the
physical structure and microbiological activities. If the soil is irrigated, also nitrate
dissolved in the water contributes to the nitrogen fertility (each ppm of a nutrient
dissolved corresponds to 5.0 kg/ha if 50 cm of irrigation water is applied), so does
natural precipitation (about 5-15 kg/ha a year average, up to 50-60 kg/ha in
industrialized areas).
Test results indicate the actual nitrate concentration and allow a fast intervention if the
concentration is insufficient for crop. If the test results are lower than 10 ppm N-NO
(as mg/kg soil) early in the growing season, the nitrate-nitrogen should be considered
deficient and a yield reduction can be expected. A first intervention with a direct
available nitrate fertilizer (about 100 kg N/ha) is recommended.
The exact amount of fertilizer required depends on the type of crop. A nitrogen
concentration between 20 and 25 ppm, for example, is considered as optimal for corn.
Above 26 ppm, addition of more N-fertilizer is not likely to increase yield.
To adjust the nitrogen concentration above 10 ppm of N-NO
in soil, add 11 kg N/ha
3
of side-dressing, for each incremental ppm of nitrate-nitrogen concentration. See the
6
If soil has been previously fertilized (>140 kg N/ha) with slow decomposing
fertilizers (e.g. manure or anhydrous ammonium), then use the lower value of
recommended fertilizer. Take more samples to assure your mixed sample is a
representative one for your field.
HOW TO PROGRAM
NITROGEN
FERTILIZATION
When this test is used for the first time, it is better to perform it during a complete
growth cycle without changing the normal fertilization program. This helps to
familiarize oneself with this test and provides a good reference point in order to
improve the fertilization program. In the subsequent growth cycle, preplant fertilization rate should be reduced by approximately 30%. It is suggested to periodically
check possible nitrogen requirements and to add extra nitrogen if necessary.
Attention should be paid in case of temporarily changes to normal climatic conditions
such as lower temperatures (with a consequent reduction of the turnover of organic
matter) or heavy rainfalls, when nitrate-nitrogen concentrations are expected to be
very low or even absent. After the weather returns to normal, it should be checked that
the nitrate levels are also reinstated.
3
Whenever possible it is recommended to carry out some fertilization experiments on
small ‘strips’ of the field.
When this test kit is used over several years, it becomes a powerful tool to optimize
the nitrogen fertilization program and allows a fast intervention if the nitrogen
concentration becomes insufficient.
7
 Loading...
Loading...