Page 1
Instruction Manual
HI 38035
Total Hardness
and Calcium
Test Kit
www.hannainst.com
Dear Customer,
Thank you for choosing a Hanna Product.
Please read the instruction sheet carefully before using the
test kit. It will provide you with the necessary information
for correct use of the kit. If you need additional information,
do not hesitate to e-mail us at tech@hannainst.com.
Remove the chemical test kit from the packing material and
examine it carefully to make sure that no damage has
occurred during shipping. If there is any noticeable damage, notify your Dealer or the nearest Hanna office
immediately.
Each kit is supplied with:
Buffer Solution pH 10.2±0.2, 1 bottle with dropper
•
(30 mL);
Calmagite Indicator, 1 bottle with dropper (10 mL);
•
EDTA Solution 0-20 gpg range, 1 bottle (100 mL);
•
• HI 38035A-0 Calcium Reagent, 1 bottle with dropper (10 mL);
• HI 38035B-0 Calcium Reagent, 1 bottle with dropper (10 mL);
• HI 38035C-0 Calcium Reagent, 1 bottle with dropper
(15 mL);
• HI 38035D-0 Calcium Reagent, 1 bottle (100 mL);
1 calibrated plastic vessel (50 mL) with cap;
•
1 syringe (1 mL) with two tips.
•
Note: Any damaged or defective item must be returned in
its original packing materials.
SPECIFICATIONSSPECIFICATIONS
SPECIFICATIONS
SPECIFICATIONSSPECIFICATIONS
Range 0 to 20 gpg CaCO
0 to 20 gpg CaCO
Smallest Increment 0.2 gpg CaCO
0.2 gpg CaCO
Analysis Method EDTA titration
Sample Size 25 mL
Number of Tests 100 (Total Hardness), 100 (Calcium)
Case Dimensions 370x270x80 mm (14.6x10.6x3.1")
Shipping Weight 960 g (33.9 oz.)
SIGNIFICANCE AND USESIGNIFICANCE AND USE
SIGNIFICANCE AND USE
SIGNIFICANCE AND USESIGNIFICANCE AND USE
Water hardness has traditionally been defined as the
capacity of water to precipitate soap. The ionic species in
water causing the precipitation were later found to be
primarily calcium and magnesium. At the present time,
water hardness is a quantitative measure of these ions in
the water sample. Now it is also known that certain other
ion species, such as iron, zinc and manganese, contribute to
the overall hardness of water. The measure and subsequent
control of water hardness is essential to prevent scaling and
clogging in water pipes. Calcium concentration in water
depends on the source and on water treatment. Calcium
contributes to the total hardness of water.
The Hanna total hardness and calcium test kit makes
monitoring easy and quick. The compact size provides the
versatility to use the kit anywhere and the design makes
the kit easy to handle.
Note: 1 gpg (grains per gallon) CaCO
ppm CaCO
equivalent to mg/L).
The total hardness level is determined by an EDTA (ethylenediamine-tetraacetic acid) titration. The solution is first adjusted
to a pH of 10 using a buffer solution. The indicator chelates
with metal ions such as magnesium or calcium to form a red
colored complex. As EDTA is added, metal ions complex with it.
After all the free metal ions have been complexed, an excess
EDTA removes the metal ions complexed with the indicator to
form a blue colored solution. This color change from red to
blue is the endpoint of the titration.
ISTR38035 11/00 PRINTED IN ITALY
(where ppm - parts per million - is
3
CHEMICAL REACTIONCHEMICAL REACTION
CHEMICAL REACTION
CHEMICAL REACTIONCHEMICAL REACTION
as total hardness
3
as Ca
3
as total hardness
3
as Ca
3
is equivalent to 17
3
Calcium concentration is determined by an EGTA titration.
Zincon indicator determines a blue color in the solution. The
reaction endpoint is indicated by a change in color from
blue to orange.
INSTRUCTIONSINSTRUCTIONS
INSTRUCTIONS
INSTRUCTIONSINSTRUCTIONS
READ ALL THE INSTRUCTIONS BEFORE USING THE TEST KIT
Determination of Total Hardness
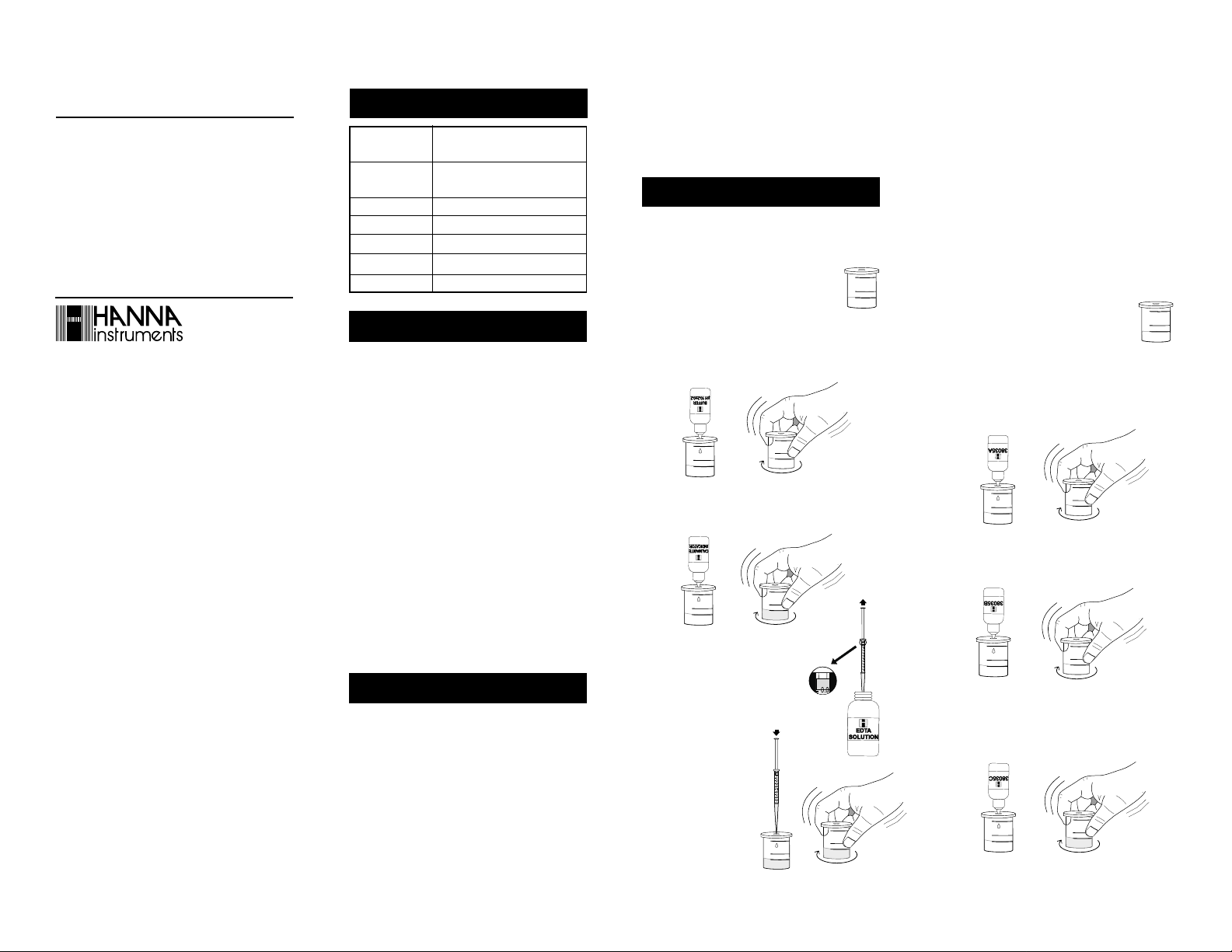
• Remove the cap from the plastic
vessel. Rinse the plastic vessel with
the water sample, fill to the 25 mL
mark and replace the cap.
• Add 5 drops of Buffer Solution through the cap port
and mix carefully swirling the vessel in tight circles.
x 5
• Add 1 drop of Calmagite Indicator through the cap port
and mix as described above. The solution becomes a redviolet color.
x 1
• Take the syringe and push the
plunger completely down into the
syringe. Insert tip into the EDTA
solution gpg range and pull the
plunger out until the lower edge of
the seal is on the 0.0 mL mark of
the syringe.
• Place the syringe tip
into the cap port of
the plastic vessel and
slowly add the titration solution drop by
drop, swirling after each
drop.
25 mL
• Continue adding the EDTA Solution until the solution in
the vessel becomes purple. Then mix for 15 seconds after
each additional drop until the solution in the vessel
turns blue.
• Read off the milliliters of titration solution used from the
syringe and multiply by 20 to obtain the hardness level
of your sample as gpg CaCO
mL of titrant x 20 = gpg CaCO
Determination of Calcium
• Remove the cap from the plastic
vessel. Rinse the plastic vessel with
the water sample, fill to the 25 mL
mark and replace the cap.
• Add 2 drops of HI 38035A-0 Calcium Reagent through
the cap port and mix carefully by swirling the vessel in
tight circles.
x 2
• Add 1 drop of HI 38035B-0 Calcium Reagent through
the cap port and mix as described above.
x 1
• Add 3 drops of HI 38035C-0 Calcium Reagent through
the cap port and mix as described above. The solution
will turn blue.
x 3
.
3
3
25 mL
Page 2
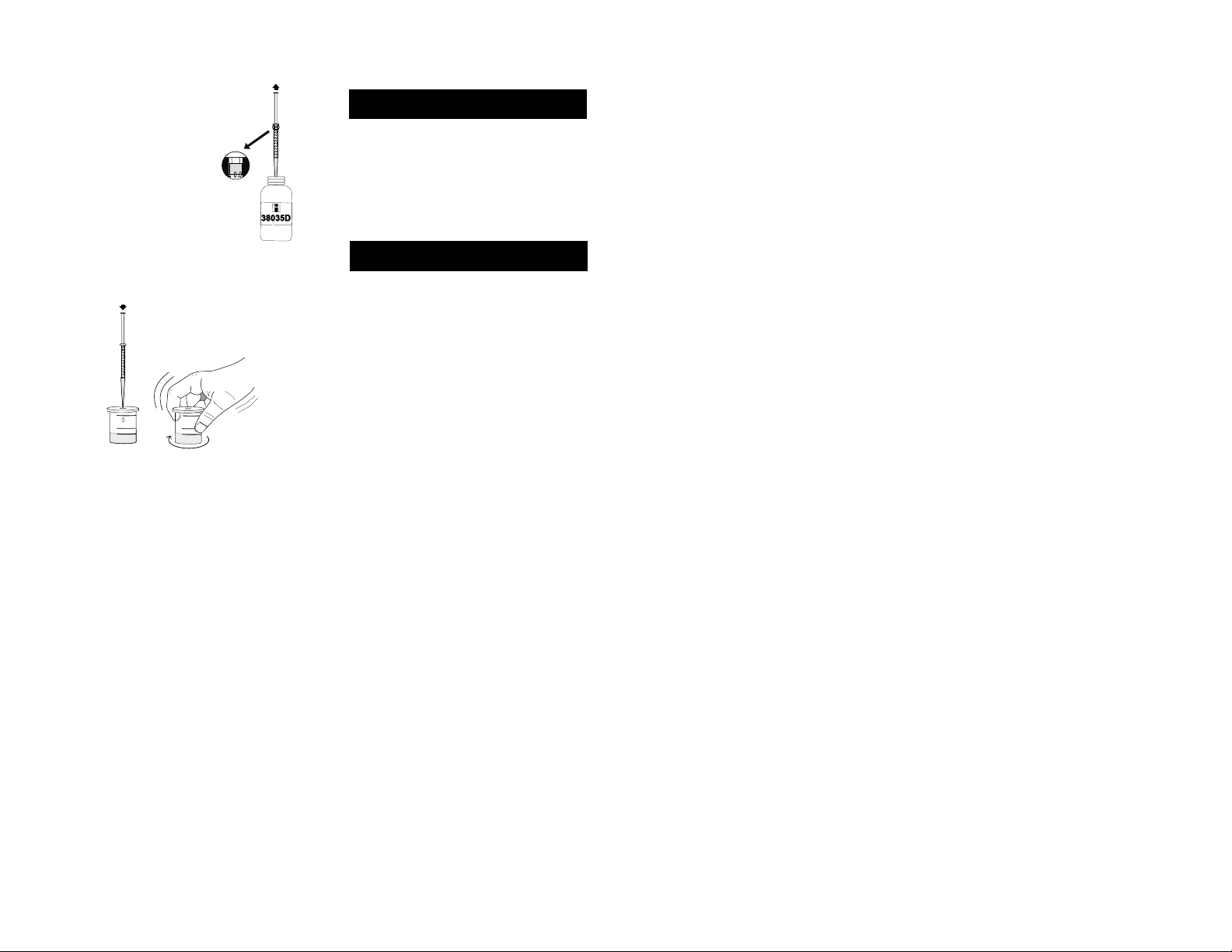
• Take the syringe and push
the plunger completely down
into the syringe. Insert tip into
the HI 38035D-0 Calcium Reagent and pull the plunger
out until the lower edge of the
seal is on the 0.0 mL mark of
the syringe.
• Slowly add the titration solution drop by drop, swirl
after each drop and wait a few seconds.
• Continue adding HI 38035D-0 reagent until the solution in the vessel turns orange. Wait for 30 seconds and
if the solution in the vessel becomes violet, add another
drop of HI 38035D-0 reagent to turn it orange.
REFERENCESREFERENCES
REFERENCES
REFERENCESREFERENCES
Standard Methods for the Examination of Water and Waste-
th
water, 16
Edition, 1985, p. 210-214.
1987 Annual Book of ASTM Standard, vol. 11.01 Water
(1), p. 212-215.
Adaptation of determination of calcium with zincon indicator.
HEALTH AND SAFETYHEALTH AND SAFETY
HEALTH AND SAFETY
HEALTH AND SAFETYHEALTH AND SAFETY
The chemicals contained in this test kit may be hazardous if
improperly handled. Read the relevant Health and Safety
Data Sheets before performing the test.
• Read off the milliliters of titration solution used from the
syringe and multiply by 20 to obtain the calcium
concentration as gpg CaCO
to obtain the calcium concentration as gpg Ca in your
. Multiply the milliliters by 8
3
sample.
Calculate the calcium concentration as:
mL of titrant x 20 = gpg CaCO
3
mL of titrant x 8 = gpg Ca
• To obtain the magnesium concentration subtract calcium concentration from the total hardness:
gpg Mg (as CaCO
= gpg Total Hardness (as CaCO
Interferences: interference may be caused by an excessive
amount of heavy metals (Zn, Co, Cu, Ni, Hg).
3
)=
)- gpg Ca (as CaCO3)
3
 Loading...
Loading...