Haag-Streit OCTOPUS 600 Instructions For Use Manual

1
© HAAG‑STREIT AG, 3098 Koeniz, Switzerland ‑ HS Doc. no. 1500.7220367.04060 – 6. Edition / 2016 – 09
DEUTSCHENGLISHFRANÇAISITALIANOESPAÑOLNEDERLANDS
PORTUGUÊS
SVENSKA
DOC. no. 1500 1500.1400209.04000
INSTRUCTIONS FOR USE
Perimeter
OCTOPUS® 600
6. Edition / 2016 – 09
01-IFU_Octopus600-7220367_04060_eng.indd 1 27.09.2016 08:45:17
2
© HAAG‑STREIT AG, 3098 Koeniz, Switzerland ‑ HS Doc. no. 1500.7220367.04060 – 6. Edition / 2016 – 09
DEUTSCHENGLISH FRANÇAIS ITALIANO ESPAÑOL NEDERLANDS
PORTUGUÊS
SVENSKA
INSTRUCTIONS FOR USE
Perimeter
OCTOPUS® 600
6. Edition / 2016 – 09
Introduction
Thank you for choosing a HAAG-STREIT device. Provided you comply carefully
with the regulations in this instructions for use, we can guarantee the reliable and
unproblematic use of our product.
Purpose of use
The Octopus 600 perimeter is designed for the examination, analysis and documentation of the field of sight, especially the light difference sensitivity and other functions of the human eye.
Contraindication
WARNING!
Certain light stimuli with a high contrast and certain frequencies as presented in the Octopus 600 with the pulsar method can trigger episodes
of photosensitive epilepsy or consciousness disturbances in isolated
cases. This can also occur in patients who have not previously displayed any signs of epilepsy or similar conditions. Should the patient
feel unwell during the examination or if there is any indication of a consciousness disturbance, the examination must be interrupted immediately. A standard white/white (SAP) examination can be performed as
an alternative.
WARNING!
Read the instruction manual carefully before commissioning this product. It contains important information regarding the safety of the user
and patient.
NOTE!
Federal law restricts this device to sale by or on the order of a physician
or licensed practitioner.
01-IFU_Octopus600-7220367_04060_eng.indd 2 27.09.2016 08:45:17
3
© HAAG‑STREIT AG, 3098 Koeniz, Switzerland ‑ HS Doc. no. 1500.7220367.04060 – 6. Edition / 2016 – 09
DEUTSCHENGLISHFRANÇAISITALIANOESPAÑOLNEDERLANDS
PORTUGUÊS
SVENSKA
Contents
1. Safety ................................................................................... 4
1.1 Areas of application of the device ...........................................................................4
1.2 Patient population ...................................................................................................4
1.3 Ambient conditions ..................................................................................................4
1.4 Shipment and unpacking ........................................................................................4
1.5 Installation warnings ..............................................................................................4
1.6 Operation and environment ....................................................................................5
1.7 Disinfection .............................................................................................................5
1.8 Warranty and product liability ..................................................................................5
1.9 Symbols ..................................................................................................................6
2. Introduction .......................................................................... 6
2.1 Device description ...................................................................................................6
2.2 System components ...............................................................................................6
2.3 Device overview ......................................................................................................6
2.4 User interface (14) ..................................................................................................7
2.5 Housing ...................................................................................................................7
2.6 Forehead rest ..........................................................................................................7
2.7 Chin rest (optional) ..................................................................................................7
2.8 Near correction lens ................................................................................................7
2.9 Patient-side cover ..................................................................................................7
2.10 Corrective lenses ....................................................................................................7
2.11 Connections ............................................................................................................8
2.11.1 USB ports ................................................................................................................8
2.11.2 Mains connection ....................................................................................................8
2.11.3 Ethernet port ...........................................................................................................8
2.12 LED background lighting .........................................................................................8
2.13 Fixation control ........................................................................................................8
2.14 Examination data ....................................................................................................8
3. Appliance assembly / installation ...................................... 8
3.1 Transporting the appliance ......................................................................................8
3.2 Connecting the patient response button .................................................................8
3.3 Connecting the electric power supply cable ............................................................9
4. Safesystemcongurationinaccordance
with EN 60601-1 ................................................................. 10
4.1 System versions, OCTOPUS 600 with printer ......................................................10
5. Commissioning ...................................................................11
5.1 Switching on the appliance ...................................................................................11
5.2 Switching off the appliance ...................................................................................11
6. Operation .............................................................................11
6.1 Setting up the patient ...........................................................................................11
7. Software/Helpmenu/Errormessages ...........................11
8. Technical data .....................................................................11
8.1 OCTOPUS 600 .....................................................................................................11
8.2 Infrared illumination ...............................................................................................11
8.3 Dimensions ...........................................................................................................12
8.4 Field of sight .........................................................................................................12
9. Maintenance ....................................................................... 12
9.1 Repairs ..................................................................................................................12
9.2 Cleaning ................................................................................................................12
9.3 Applied parts .........................................................................................................12
A. Appendix ............................................................................ 12
A.1 Accessories / spare parts ......................................................................................12
B. Legalregulations ............................................................... 13
C. Classication ..................................................................... 13
D. Disposal .............................................................................. 13
E. Standards ........................................................................... 13
F. RoHS China ........................................................................ 13
F.1 RoHS declaration ..................................................................................................13
G. Information and manufacturer's declaration
concerningelectromagneticcompatibility
(EMC) .................................................................................. 14
G.1 General .................................................................................................................14
G.2 Table 1: Emitted interference ................................................................................14
G.3 Table 2: Immunity ..................................................................................................15
G.4 Table 3: Immunity (not life-support equipment) .....................................................16
G.5 Table 4: Recommended safe distances (not life-support equipment) .................177
01-IFU_Octopus600-7220367_04060_eng.indd 3 27.09.2016 08:45:17
4
© HAAG‑STREIT AG, 3098 Koeniz, Switzerland ‑ HS Doc. no. 1500.7220367.04060 – 6. Edition / 2016 – 09
DEUTSCHENGLISH FRANÇAIS ITALIANO ESPAÑOL NEDERLANDS
PORTUGUÊS
SVENSKA
1. Safety
DANGER!
Failure to comply with these instructions may result in material damage
or pose a danger to patients or users.
WARNING!
These warnings must absolutely be complied with to guarantee
safe operation of the device and to avoid any danger to users and
to patients.
NOTE!
Important information: please read carefully.
1.1 Areas of application of the device
The users are ophthalmologists, optometrists, opticians, orthoptists or other trained
specialists. The examination is performed in slightly darkened examination rooms.
1.2 Patient population
The patient must be capable of sitting up straight and keeping his head still. He/she
must be physically and mentally able to cooperate well and is mentally capable of
following the examination. Patients must be at least 6 years old.
1.3 Ambient conditions
Transport: Temperature
Air pressure
Relative humidity
from
from
from
−40°C
500 hPa
10%
to
to
to
+70°C
1060 hPa
95%
Storage:
Temperature
Air pressure
Relative humidity
from
from
from
−10°C
700 hPa
10%
to
to
to
+55°C
1060 hPa
95%
Use:
Temperature
Air pressure
Relative humidity
from
from
from
+10°C
800 hPa
30%
to
to
to
+35°C
1060 hPa
90%
Application height < 2,000 m above sea level
1.4 Shipmentandunpacking
• Before you unpack the appliance, check whether the packaging shows traces of
incorrect handling or damage. If this is the case, notify the transport company that
has delivered the goods to you. Unpack the equipment together with a representative of the transport company. Make a report of any damaged parts. This report
must be signed by you and by the representative of the transport company.
• Leave the device in the packaging for a few hours before unpacking it (risk of
condensation).
• Check the appliance for damage after it is unpacked. Return defective applianc-
es in the appropriate packaging.
• Store packaging material carefully, so that it can be used for possible returns or
when moving.
1.5 Installationwarnings
DANGER!
Never use the device in potentially explosive environments where volatile solvents (alcohol, benzine, etc.) and combustible anaesthetics are
in use.
WARNING!
•
Do not modify this equipment without authorization of the manufacturer
.
Installation and repairs may only be performed by trained specialists.
Any third-party device must be connected in compliance with the
EN 60601-1 standard.
Only original HS replacement parts may be used.
The device must not be stacked or placed in close proximity to other
electronic devices.
•
•
•
NOTE!
• The device must be set up in a medical room in such a way that no
direct light falls on it from any side.
• The use of accessories other than than those listed may result in
higher emissions or lower interference immunity of the Octopus 600.
• The software must be installed by trained personnel.
01-IFU_Octopus600-7220367_04060_eng.indd 4 27.09.2016 08:45:18
5
© HAAG‑STREIT AG, 3098 Koeniz, Switzerland ‑ HS Doc. no. 1500.7220367.04060 – 6. Edition / 2016 – 09
DEUTSCHENGLISHFRANÇAISITALIANOESPAÑOLNEDERLANDS
PORTUGUÊS
SVENSKA
1.6 Operation and environment
WARNING!
• To avoid the risk of suffering an electric shock, this device may only
be connected up to the mains with a ground connection.
• The plug, cable and ground connection of the socket must be functioning perfectly.
• Make sure that the appliance is only connected to power supplies as
defined on the type plate. The appliance must be disconnected from
the mains by pulling out the plug before any maintenance and cleaning work is performed.
• Computers and further ancillary devices (printers, etc.) must comply
with the EN 60601-1 standard or be connected through galvanic isolation to external networks (safety isolating transformer).
WARNING!
• The doctor or the operator is obliged to inform the patient about the
safety instructions concerning him and to ensure that these instructions are complied with.
• The examination of the patient, the use of the device and the interpretation of the results may only be conducted by trained and experienced individuals.
• Turning off the eye monitoring functions is not recommended. In all
other cases, the user must monitor the eye personally during the
examination.
• All users must be appropriately trained and familiarised with the
contents of the instructions for use, especially in regard to the safety
information contained therein.
NOTE!
• This appliance must only be operated by qualified and trained personnel. The owner is responsible for their training.
• This appliance may only be used for the purpose described
in these instructions for use.
• The device must be set up in a medical room in such a way that no
direct light falls on it from any side.
• Keep these instructions for use in a place where they are accessible
to those working with the device at all times. Warranty claims can
only be made if the instructions in these instructions for use are
complied with.
• Always remove the dust cover before switching the appliance on. The
device may otherwise become damaged due to overheating. Likewise, make sure that the appliance is switched
off before attaching the dust cover.
• Only original spare parts and original accessories may be used
for repairs. The use of accessories other than than those listed may
result in higher emissions or lower interference immunity of the Octopus 600.
• Turn the device off if it will not be used for an extended period of time.
• Do not expose the device to direct sunlight.
• Protect the device with the dust cover when not in use.
1.7 Disinfection
NOTE!
The device does not need to be disinfected. For more information on
cleaning, please refer to the 'Maintenance' and 'Applied parts' section.
1.8 Warranty and product liability
Haag-Streit products must be used only for the purposes and in the manner des-
cribed in the documents distributed with the product.
The product must be treated as described in the ‘Safety’ chapter. Improper handling can damage the product. This would void all guarantee claims.
Continued use of a product damaged by incorrect handling may lead to personal
injury. In such a case, the manufacturer will not accept any liability.
Haag-Streit does not grant any warranties, either expressed or implied, including
implied warranties of merchantability or fitness for a particular use.
Haag-Streit expressly disclaims liability for incidental or consequential damage
resulting from the use of the product.
This product is covered by a limited warranty granted by your seller.
This product is covered by a limited warranty, which may be reviewed at
www.haag-streit-usa.com.
•
•
•
•
•
•
For US
A only:
•
01-IFU_Octopus600-7220367_04060_eng.indd 5 27.09.2016 08:45:18
6
© HAAG‑STREIT AG, 3098 Koeniz, Switzerland ‑ HS Doc. no. 1500.7220367.04060 – 6. Edition / 2016 – 09
DEUTSCHENGLISH FRANÇAIS ITALIANO ESPAÑOL NEDERLANDS
PORTUGUÊS
SVENSKA
1.9 Symbols
Read the instructions for use
attentively
General warning: Read the ac-
companying documentation
Type B applied part
Notes on disposal, see the
'Disposal' chapter
12
RoHS China
Protective earth (ground)
European certificate of
conformity
Manufacturer
Year of production
Serial number
HS reference number
Test symbol of CSA with ap-
proval for USA
2. Introduction
2.1 Device description
• The Octopus 600 is a screen perimeter for examining the central field of sight
(30°). The device can be employed autonomously, i.e., the examination and control components are integrated in the device.
• Integrated, automatic fixation monitoring increases the reliability of the examina-
tion results.
• The Octopus 600 is employed by clinical users and for research purposes.
• New operating system software and perimetry software can be downloaded and
updated by visiting www.HAAG-STREIT.com.
2.2 System components
The Octopus 600 comprises the following components:
• Octopus 600
• Patient response button (Type B application part)
• Keyboard/mouse (optional)
2.3 Device overview
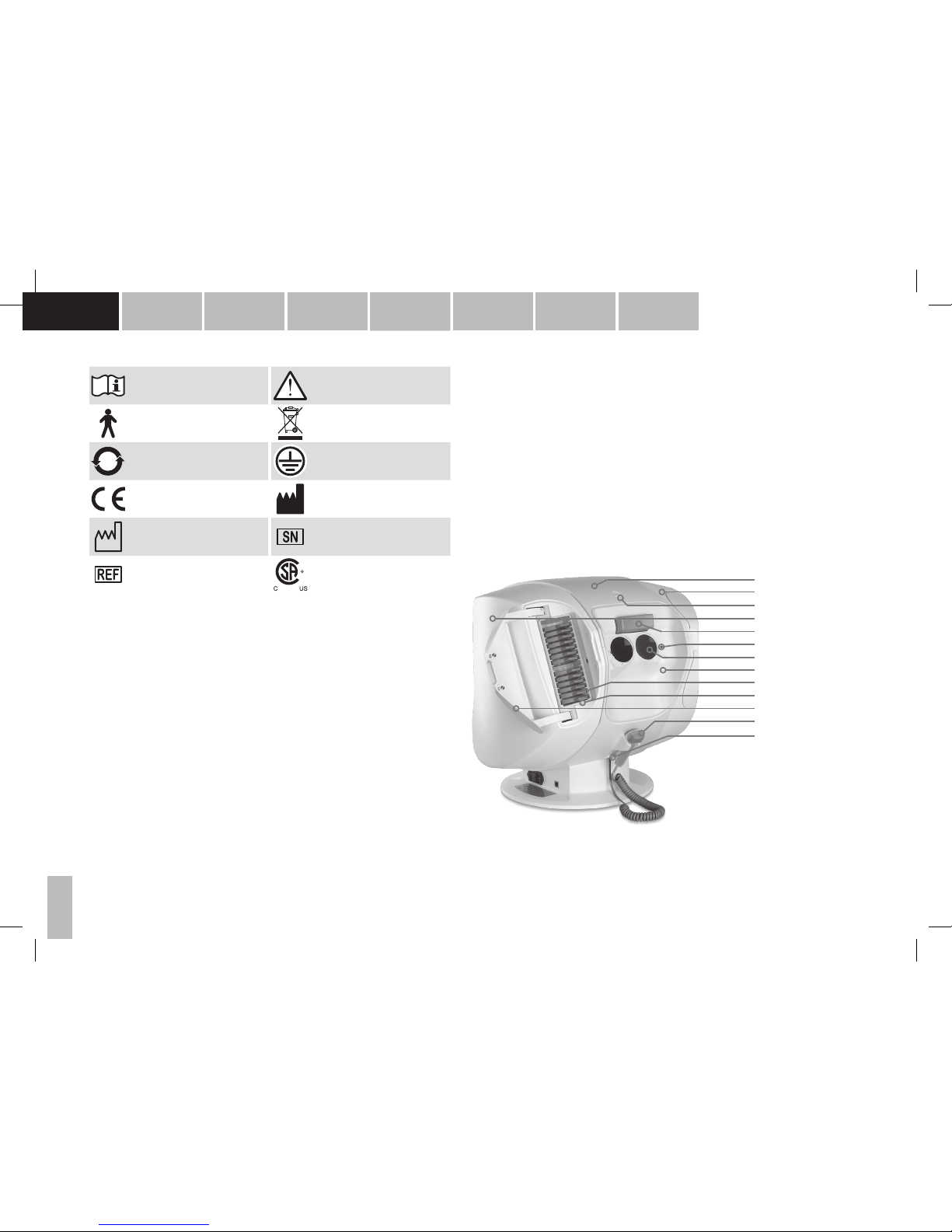
Overview of patient side
1. Upper part of housing
2. Right shell
3. Capacitive button for operating the forehead rest
4. Left shell
5. Forehead rest with integrated sensor for detecting the head position
6. Infrared eye illumination
7. Near correction lens +3.25 dpt
8. Patient-side cover
9. Corrective lenses
10. Corrective lens compartment
11. Automatically closing cover
12. Patient response button
13. Patient response button connection
1
2
3
4
5
6
7
8
9
10
11
12
13
01-IFU_Octopus600-7220367_04060_eng.indd 6 27.09.2016 08:45:20
 Loading...
Loading...