Gendex VisualiX Maintenance manual

VisualiX® eHD
User and Service Manual
Doc# 4519 986 19041 - April 2005

No part of this publication may be reproduced, transmitted,
transcribed or translated into any spoken or computer language
without prior written consent from Gendex Dental Systems.
The content of this manual is subject to modication without
prior notication.

Gendex VisualiXTM eHD
1
User and Service Manual Doc #4519 986 19041 - April 2005
Introduction 3
1.1 Conventions used in the manual............................................................................................3
Safety procedures 5
Standards and regulations
7
3.1 Compliance with regulations .................................................................................................. 7
3.2 Symbols appearing on the Intermediate Electronics (IME) Control Unit plate......8
Product description 9
4.1 Unpacking the VisualiX system components...................................................................10
4.2 System Components.................................................................................................................10
System conguration 13
5.1 Personal Computer requirements........................................................................................13
5.2 Application software.................................................................................................................15
5.3 Compatibility with radiographic generators....................................................................16
Installation 17
6.1 Installation of ActiveX software driver ...............................................................................20
Use 21
7.1 Acquisition of radiographic images ....................................................................................21
7.2 Status Icon ....................................................................................................................................24
7.3 Portability......................................................................................................................................25
7.4 Hygiene..........................................................................................................................................25
Table of contents
1
2
3
4
5
6
7

Gendex VisualiXTM eHD
2
User and Service Manual Doc #4519 986 19041 - April 2005
VisualiXTM and VixWinTM are Trademarks of Gendex Corporation.
Microsoft® and Windows® are registered trademarks of Microsoft®
Corporation.
This manual in English is the original version.
8
9
Maintenance 27
Technical specications
29
Diagnostics
31
Appendix
33
11.1 Printers ..........................................................................................................................................33
11.1.1 Thermal printers .........................................................................................................33
11.1.2 Laser and ink jet printers .........................................................................................33
11.1.3 Sublimation printers .................................................................................................33
11.2 Software ........................................................................................................................................34
11.3 Service............................................................................................................................................34
11.4 Components Code List for VisualiX......................................................................................34
10
11
3
User and Service Manual Doc #4519 986 19041 - April 2005
Introduction
This User Manual contains instructions for safe set-up, use and maintenance of the
Gendex Dental Systems VisualiX eHD system.
It also contains technical specications of the system and basic information on how
the system works.
Please read this manual carefully before starting to use the device, paying particular
attention to warnings, especially safety warnings.
1.1 Conventions used in the manual
This manual features three graphic styles:
Normal: for information which must be read carefully before using the VisualiX system
Detailed notes, identied with the symbol
Safety warnings, identied with the symbol
Gendex is committed to ongoing technical improvement of its products. The information
and gures contained in this User and Service Manual are subject to change without
prior notication.
1

Gendex VisualiXTM eHD
4
User and Service Manual Doc #4519 986 19041 - April 2005
5
User and Service Manual Doc #4519 986 19041 - April 2005
Safety procedures
The device must be installed and used in accordance with the safety regulations and
instructions for use supplied in this User and Service Manual, for the purposes and applications for which it is intended.
Modications and/or additions to the device must be made exclusively by Gendex
personnel or by parties expressly authorized for the purpose by Gendex. Any modications or additions must always comply with standards and generally recognised rules
of good workmanship.
It is up to the user to ensure compliance with all local safety regulations in eect in the
place of installation.
Electrical safety:
The covers on the device may be removed only by qualied, authorised technical
personnel.
The product must be used only in rooms or areas which comply with all laws and
regulations applicable to electrical safety in medical premises, such as CEI standards
regarding use of an additional ground terminal for equipotential connections. This
device must always be disconnected from the power supply before cleaning or
disinfection.
Water and other liquids must not be permitted to penetrate inside the device, where
they could cause short circuit or corrosion. No protection is supplied against liquid
penetration.
The conformity with the IEC standard 601.1.1 and the validity of the CE mark apply
only if the computer is located out of the patient’s reach (at a distance of least 1.5 m
from the patient) and if the computer complies with the IEC 60950 standard.
2
Gendex VisualiXTM eHD
6
User and Service Manual Doc #4519 986 19041 - April 2005
Explosion safety:
This device is not recommended for use in the presence of ammable gases or vapours. Some disinfectants evaporate and form explosive or ammable mixtures. If
disinfectants of this kind are used, it is important to let the vapours disperse before
using the device again.

7
User and Service Manual Doc #4519 986 19041 - April 2005
Standards and regulations
3.1 Compliance with regulations
The VisualiX system complies with the European Community Directive 93/42/EEC regarding medical devices and with the following standards:
• IEC 601.1 (1988), Amendment Nr. 1 (1991), Amendment Nr. 2 (1995)
• IEC 601.1.1 (2000)
• IEC 601.1.2 (2001)
• IEC 601.1.4 (1996), Amendment Nr. 1 (1999)
To ensure compliance, other parts of the system which are electrically wired (computer
and any other optional peripheral devices) must be located out of patient’s reach (at a
distance of not less than 1.5 m from the patient) and comply with the UL/IEC/EN 60950
standard and EC directive 89/336.
In case other parts of the system are non medical devices, further considerations should
be done in accordance with IEC60601-1-1, under the responsibility of the installation
technician.
Compulsory declaration under European Directive 93/42 regarding Medical Devices: In accordance with the requirements of the CE mark, the user must notify the Ministry of Health of
any accidents involving the device and any alterations in the characteristics or performance
of the device, including inadequacy of the instructions provided, which could result in death
or damage to the health of the patient or user.
A copy of this notication must be promptly sent to the manufacturer or a representative
thereof to permit the manufacturer to comply with the requirements of the directive.
3
Gendex VisualiXTM eHD
8
User and Service Manual Doc #4519 986 19041 - April 2005
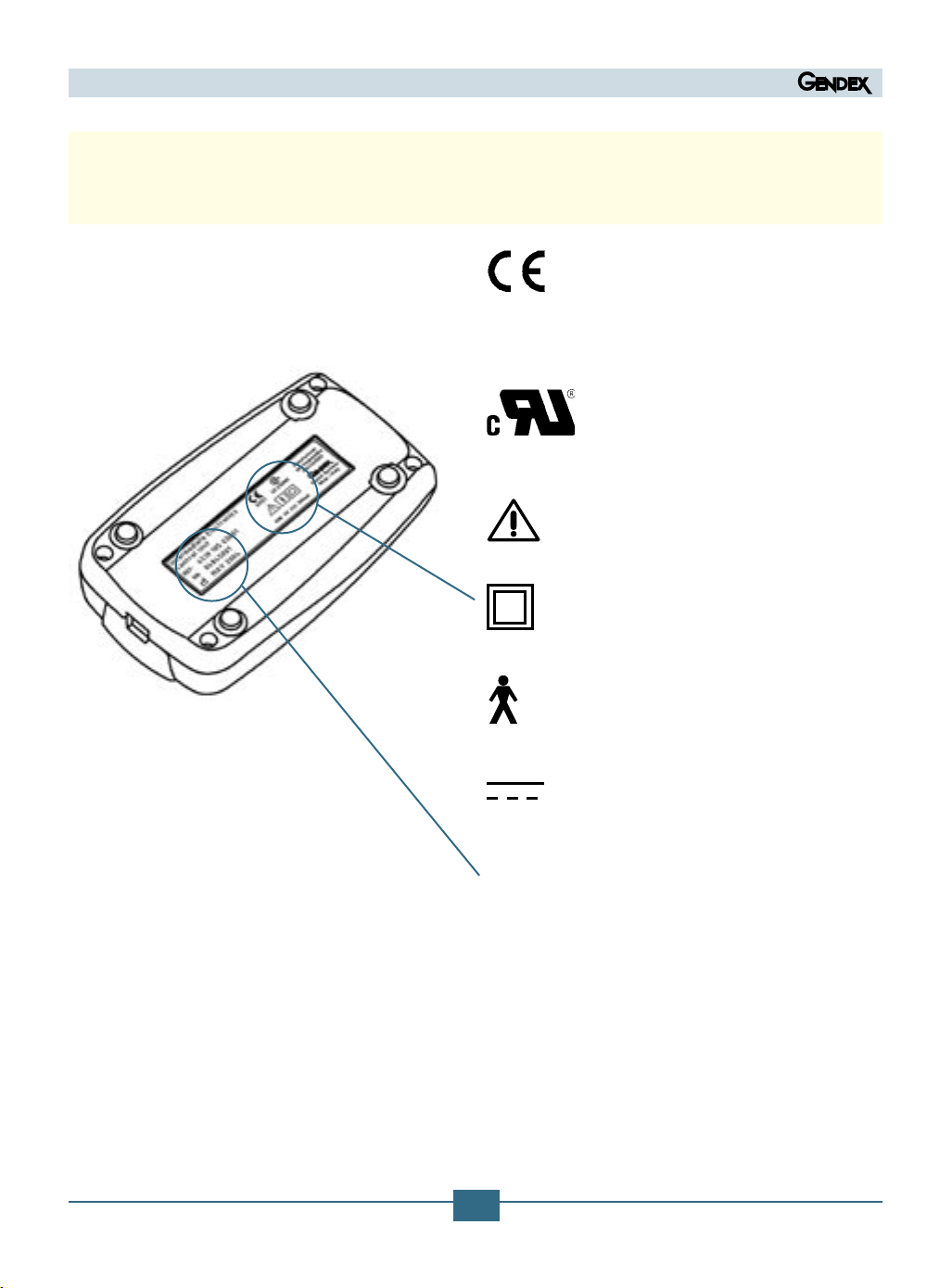
3.2 Symbols appearing on the Intermediate Electronics (IME) Control Unit plate
The symbol CE guarantees the
conformity of the product described
herein to the European Union Directive 93/42/EEC for Medical Devices.
This symbol guarantees the conformity of the product described herein
to the standard UL 60601-1
Please refer to the written
instructions of this manual.
Class II device
(IEC 601.1 - 1988 and Amendments)
BF type device
(IEC 601.1 - 1988 and Amendments)
DC Current
(IEC 601.1 - 1988 and Amendments)
System serial number and sensor
type reference part number
0051
SN

9
User and Service Manual Doc #4519 986 19041 - April 2005
Product description
VisualiX is a direct (lmless) digital X-ray imaging system, conceived specically for
dental radiography in the oral cavity. The system captures X-ray images and makes
them available for display on a Personal Computer (PC) screen.
An X-ray image sensor (CCD) is positioned in the patient’s mouth just like intra-oral lm.
The sensor may be inserted in a special positioning device to facilitate positioning and
alignment with the X-ray beam. The sensor may also be positioned by hand with the
assistance of the patient.
There is no electrical or physical connection between VisualiX and the X-ray generator.
Images are automatically acquired when X-rays are present in a dose which is perceptible to the sensor.
Digital X-ray images are quickly displayed on the screen. Images can be optimized for
viewing via imaging software, stored as image les, and printed out on a suitable printer
if desired. VixWin is one example of a dedicated software that employs a number of
utilities for optimizing viewing and printing of images.
VisualiX must be connected to a PC running on a Microsoft® Windows® operating system
through the standard USB port (Universal Serial Bus). See the “System Conguration”
paragraph for details.
eHD Technology
eHD is the acronym of “Ergonomics and High Denition”: the latest, unsurpassed technology in real-time X-ray imaging, which allows enhanced analysis of radiographic
details using a highly ergonomic system characterised by round corners to facilitate
positioning in the oral cavity.
eHD sensors can be identied by the eHD logo shown below which appears on the top
of the sensor and Intermediate Electronics (IME) box.
4
Gendex VisualiXTM eHD
10
User and Service Manual Doc #4519 986 19041 - April 2005
Gendex VisualiXTM eHD
11
User and Service Manual Doc #4519 986 19041 - April 2005
4.1 Unpacking the VisualiX system components
The Visualix system is carefully inspected and packaged prior to shipment. If the Visualix
system was shipped to you, please remove the contents of the shipping container and
be sure to identify and directly locate each of the system components shown below.
Report any damaged components to the shipping company and any missing
components to your dealer within 24 hours of receiving the shipment.
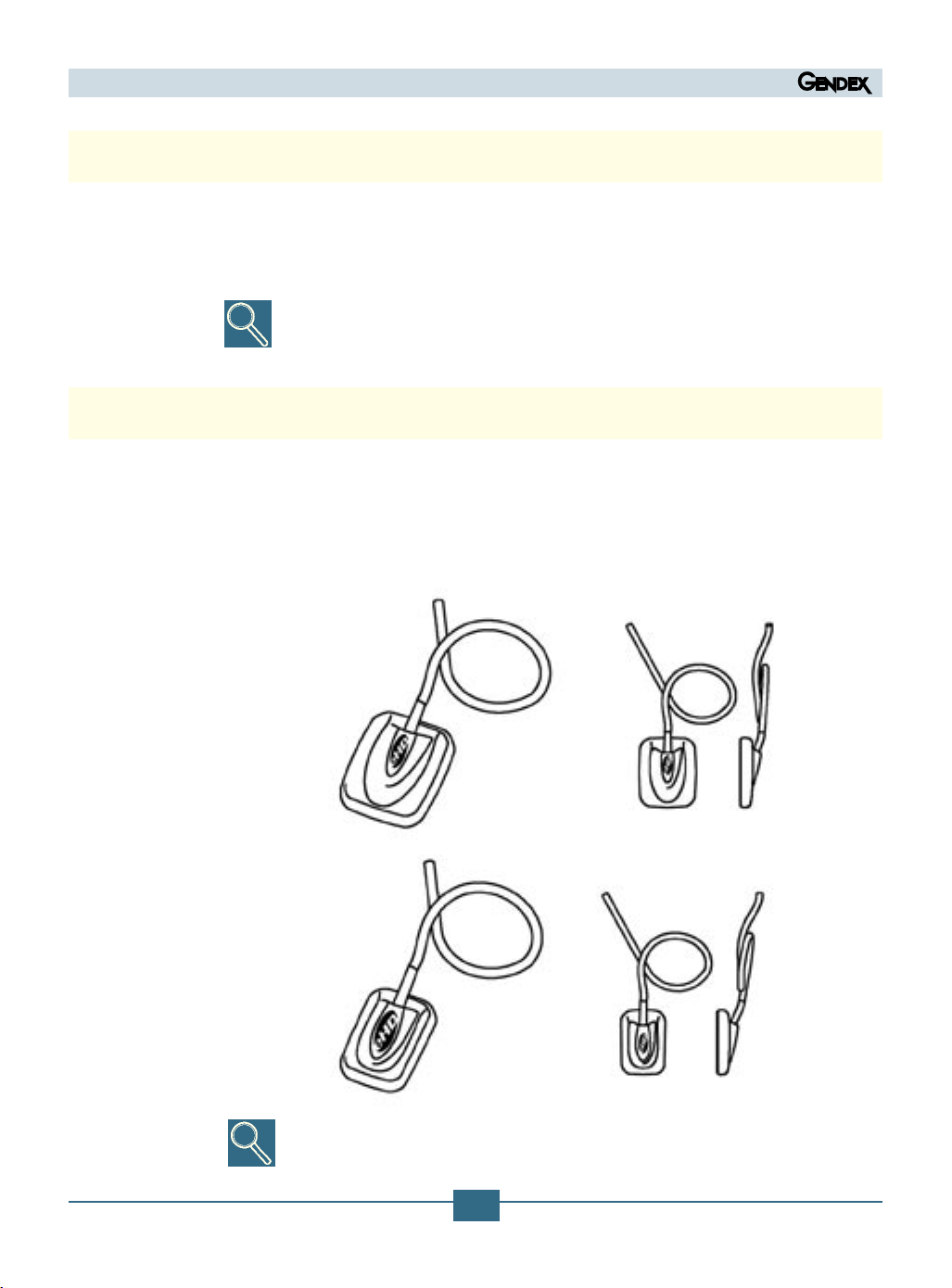
4.2 System Components
1) X-ray image sensor
consisting of a special CCD sensor specically constructed for use in radiography,
enclosed in a hermetically sealed ergonomic capsule, the sensitive surface of which
is covered by a thin layer of a scintillator through which X-ray radiation is converted
into light and then into an electric charge.
The sensor type reference part number appears on the IME label,
see paragraph 3.2
Sensor Large area (Size 2)
Sensor Universal size (Size 1)
 Loading...
Loading...