Page 1

Electrosurgical Analyzer
PN 2202027
April 2007
© 2007 Fluke Corporation, All rights reserved. Printed in USA
All product names are trademarks of their respective companies.
RF303
Operators Manual
Page 2

Warranty and Product Support
Fluke Biomedical warrants this instrument against defects in materials and workmanship for one full year from the date of original purchase. During the warranty
period, we will repair or, at our option, replace at no charge a product that proves
to be defective, provided you return the product, shipping prepaid, to Fluke Biomedical. This warranty does not apply if the product has been damaged by accident or misuse or as the result of service or modification by other than Fluke Biomedical. IN NO EVENT SHALL FLUKE BIOMEDICAL BE LIABLE FOR
CONSEQUENTIAL DAMAGES.
Only serialized products and their accessory items (those products and items bearing a distinct serial number tag) are covered under this one-year warranty.
PHYSICAL DAMAGE CAUSED BY MISUSE OR PHYSICAL ABUSE IS NOT
COVERED UNDER THE WARRANTY. Items such as cables and nonserialized
modules are not covered under this warranty.
Recalibration of instruments is not covered under the warranty.
This warranty gives you specific legal rights, and you may also have other rights
which vary from state to state, province to province, or country to country. This
warranty is limited to repairing the instrument to Fluke Biomedical’s specifications.
Warranty Disclaimer
Should you elect to have your instrument serviced and/or calibrated by someone
other than Fluke Biomedical, please be advised that the original warranty covering
your product becomes void when the tamper-resistant Quality Seal is removed or
broken without proper factory authorization. We strongly recommend, therefore,
that you send your instrument to Fluke Biomedical for factory service and calibration, especially during the original warranty period.
Notices
All Rights Reserved
© Copyright 2007, Fluke Biomedical. No part of this publication may be reproduced, transmitted,
transcribed, stored in a retrieval system, or translated into any language without the written permission
of Fluke Biomedical.
Copyright Release
Fluke Biomedical agrees to a limited copyright release that allows you to reproduce manuals and
other printed materials for use in service training programs and other technical publications. If you
would like other reproductions or distributions, submit a written request to Fluke Biomedical.
Page 3

Unpacking and Inspection
Follow standard receiving practices upon receipt of the instrument. Check the shipping carton for
damage. If damage is found, stop unpacking the instrument. Notify the carrier and ask for an agent to
be present while the instrument is unpacked. There are no special unpacking instructions, but be careful not to damage the instrument when unpacking it. Inspect the instrument for physical damage such
as bent or broken parts, dents, or scratches.
Technical Support
For application support or answers to technical questions, either email techser-
vices@flukebiomedical.com or call 1-800- 648-7952 or 1-425-446-6945.
Claims
Our routine method of shipment is via common carrier, FOB origin. Upon delivery, if physical damage is found, retain all packing materials in their original condition and contact the carrier immediately to file a claim. If the instrument is delivered in good physical condition but does not operate
within specifications, or if there are any other problems not caused by shipping damage, please contact Fluke Biomedical or your local sales representative.
Standard Terms and Conditions
Refunds and Credits
Please note that only serialized products and their accessory items (i.e., products and items
bearing a distinct serial number tag) are eligible for partial refund and/or credit. Nonserialized
parts and accessory items (e.g., cables, carrying cases, auxiliary modules, etc.) are not eligible
for return or refund. Only products returned within 90 days from the date of original purchase are
eligible for refund/credit. In order to receive a partial refund/credit of a product purchase price on a serialized product, the product must not have been damaged by the customer or by the carrier chosen by
the customer to return the goods, and the product must be returned complete (meaning with all manuals, cables, accessories, etc.) and in “as new” and resalable condition. Products not returned within 90
days of purchase, or products which are not in “as new” and resalable condition, are not eligible for
credit return and will be returned to the customer. The Return Procedure (see below) must be followed to assure prompt refund/credit.
Restocking Charges
Products returned within 30 days of original purchase are subject to a minimum restocking fee of 15
%. Products returned in excess of 30 days after purchase, but prior to 90 days, are subject to a minimum restocking fee of 20 %. Additional charges for damage and/or missing parts and accessories will
be applied to all returns.
Return Procedure
All items being returned (including all warranty-claim shipments) must be sent freight-prepaid to our
factory location. When you return an instrument to Fluke Biomedical, we recommend using United
Parcel Service, Federal Express, or Air Parcel Post. We also recommend that you insure your shipment for its actual replacement cost. Fluke Biomedical will not be responsible for lost shipments or
instruments that are received in damaged condition due to improper packaging or handling.
Use the original carton and packaging material for shipment. If they are not available, we recommend
the following guide for repackaging:
Use a double-walled carton of sufficient strength for the weight being shipped.
Use heavy paper or cardboard to protect all instrument surfaces. Use nonabrasive material
around all projecting parts.
Use at least four inches of tightly packed, industry-approved, shock-absorbent ma-
terial around the instrument.
Page 4
Returns for partial refund/credit:
Every product returned for refund/credit must be accompanied by a Return Material Authorization
(RMA) number, obtained from our Order Entry Group at 1-800-648-7952 or 1-425-446-6945.
Repair and calibration:
To find the nearest service center, go to www.flukebiomedical.com/service
In the U.S.A.:
Cleveland Calibration Lab
Tel: 1-800-850-4606
Email: globalcal@flukebiomedical.com
Everett Calibration Lab
Tel: 1-888-99-FLUKE (1-888-993-5853)
Email: service.status@fluke.com
In Europe, Middle East, and Africa:
Eindhoven Calibration Lab
Tel: +31-402-675300
Email: ServiceDesk@fluke.com
In Asia:
Everett Calibration Lab
Tel: +425-446-6945
Email: service.international@fluke.com
, or
Certification
This instrument was thoroughly tested and inspected. It was found to meet Fluke Biomedical’s manufacturing specifications when it was shipped from the factory. Calibration measurements are traceable
to the National Institute of Standards and Technology (NIST). Devices for which there are no NIST
calibration standards are measured against in-house performance standards using accepted test procedures.
WARNING
Unauthorized user modifications or application beyond the published specifications may result in electrical shock hazards or improper operation. Fluke Biomedical will not be responsible for any injuries sustained due to unauthorized equipment modifications.
Restrictions and Liabilities
Information in this document is subject to change and does not represent a commitment by
Fluke Biomedical. Changes made to the information in this document will be incorporated in
new editions of the publication. No responsibility is assumed by Fluke Biomedical for the use
or reliability of software or equipment that is not supplied by Fluke Biomedical, or by its affiliated dealers.
Manufacturing Location
The RF303 Electrosurgical Analyzer is manufactured in Everett, Washington by Fluke Biomedical, 6920 Seaway Blvd., Everett, WA, U.S.A.
Page 5

Table of Contents
1 Introduction and Specifications.............................................. 1-1
Introduction .......................................................................................... 1-3
General Safety Information................................................................... 1-3
Symbols ............................................................................................ 1-4
Warnings and Cautions..................................................................... 1-4
Electromagnetic Interference and Susceptibility............................... 1-5
EC Directive 89/336/EEC EN 50081-1 Emissions....................... 1-6
EC Directive 89/336/EEC EN 50082-1 Immunity........................ 1-6
USA Federal Communications Commission ................................ 1-6
Canadian Department of Communications ................................... 1-6
Control and Interface Panels................................................................. 1-7
Instrument Specifications ..................................................................... 1-9
General Specifications.......................................................................... 1-11
Accessories ........................................................................................... 1-13
2 Installation, Setup, and Maintenance ..................................... 2-1
Installation and Setup............................................................................ 2-3
Power-Up Sequence.......................................................................... 2-3
Operating the Analyzer Using Battery Power................................... 2-5
LED Backlight Display Operation.................................................... 2-7
Ventilation ........................................................................................ 2-7
Connecting Test Leads between the ESU and the Analyzer ............. 2-7
Test Lead Set with Retractable Shrouds ....................................... 2-8
ESU- Dispersive Safety Lead ....................................................... 2-8
ESU Jumper Safety Lead.............................................................. 2-9
ESU CQM Safety Lead................................................................. 2-9
Maintenance.......................................................................................... 2-10
Cleaning............................................................................................ 2-10
Calibration ........................................................................................ 2-10
Replacing Fuses................................................................................ 2-10
3 Operation .................................................................................. 3-1
The Signal Averaging Mode (SAM)..................................................... 3-3
Testing the ESU.................................................................................... 3-3
Key to Panel Diagrams ..................................................................... 3-4
Generator Output Test ...................................................................... 3-4
Basic Contact Quality Monitor (CQM) Test..................................... 3-7
HF Leakage Current Test 1............................................................... 3-10
i
Page 6

RF303
Operators Manual
HF Leakage Current Test 2 .............................................................. 3-13
HF Leakage Current Test 3 .............................................................. 3-16
Connecting an Oscilloscope to the Analyzer........................................ 3-19
Remote Operation via the RS-232 Function......................................... 3-19
Setting Up and Operating the Analyzer in Simplex Mode ............... 3-20
Setting Up and Operating the Analyzer in Duplex Mode................. 3-20
Appendices
A Load Issues................................................................................... A-1
B Interpretation of Fluctuating Readings ......................................... B-1
C Abbreviations ............................................................................... C-1
ii
Page 7

List of Tables
Table Title Page
1-1. Symbols ................................................................................................ 1-4
1-2. Controls and Connections..................................................................... 1-7
1-3. Accessories ........................................................................................... 1-13
1-4. Optional Accessories for the RF303 ..................................................... 1-13
2-1. Power-Up Error Condition Codes......................................................... 2-4
2-2. Battery Operating Status....................................................................... 2-6
2-3. Battery Status Light .............................................................................. 2-6
3-1. Error Responses .................................................................................... 3-22
3-2. Available Duplex Mode Commands..................................................... 3-23
iii
Page 8

RF303
Operators Manual
iv
Page 9

List of Figures
Figure Title Page
1-1. Analyzer Control and Interface Panels ................................................. 1-7
2-1. Fuse Cover............................................................................................ 2-11
3-2. Generator Output Test .......................................................................... 3-7
3-2. Basic CQM Check ................................................................................ 3-9
3-3. HF Leakage Test Number One ............................................................. 3-12
3-4. HF Leakage Test Number Two............................................................. 3-15
3-5. HF Leakage Test Number Three........................................................... 3-18
3-6. Burst Waveform on Oscilloscope Display............................................ 3-19
v
Page 10

RF303
Operators Manual
vi
Page 11

Chapter 1
Introduction and Specifications
Contents Page
Introduction ................................................................................. 1-3
General Safety Information ......................................................... 1-3
Symbols.................................................................................... 1-4
Warnings and Cautions ............................................................ 1-4
Electromagnetic Interference and Susceptibility...................... 1-5
EC Directive 89/336/EEC EN 50081-1 Emissions............... 1-6
EC Directive 89/336/EEC EN 50082-1 Immunity ............... 1-6
USA Federal Communications Commission........................ 1-6
Canadian Department of Communications........................... 1-6
Control and Interface Panels........................................................ 1-7
Instrument Specifications ............................................................ 1-9
General Specifications................................................................. 1-11
Accessories .................................................................................. 1-13
1-1
Page 12

RF303
Operators Manual
1-2
Page 13

Introduction and Specifications
Introduction
1
Introduction
The RF303 Electrosurgical Analyzer, hereafter referred to as the “Analyzer”,
tests electrosurgical units (ESU) for generator output and high frequency (HF)
leakage. It is compatible with both isolated and earth/ground-referenced types
of electrosurgical units. You can test both the high-level monopolar and the
low-level bipolar ESU outputs using this versatile Analyzer.
The Analyzer uses a precision high-voltage capacitive attenuator to sample the
applied ESU signal. You can use this attenuated HF voltage and the selected
test load resistance value to derive the true-rms values of both current and
wattage.
The Analyzer internal test load simulates the range of resistance encountered
during surgical procedures. Additionally, a second, 200 ohm (e) auxiliary test
load resistance is built-in to analyze earth/ground-referenced ESUs as specified
in the International Standard IEC 601-2-2.
The exclusive use of non-conductive, high-impact plastic case material
minimizes extraneous high frequency leakages within the Analyzer.
General Safety Information
This Analyzer complies with safety and technical requirements described in
the following directives:
• UL 3101-1
• CAN/CSA C22.2 No. 1010.1 (1992)
• EC 73/23/EEC EN 61010–1
• ANSI / AAMI HF–18-1986
• IEC 601-2–2
• IEC 1289-1
• IEC 1289-2
A Warning identifies hazardous conditions and actions that could cause
bodily harm or death.
A Caution identifies conditions and actions that could damage the Analyzer,
the equipment under test, or cause permanent loss of data.
1-3
Page 14

RF303
Operators Manual
Symbols
Table 1-1 describes the symbols used in this document.
Table 1-1. Symbols
Symbol Description Symbol Description
W
$
t
Risk of danger. Important
information. See manual.
Conforms to relevant
Canadian and U.S.
standards
Underwriters Laboratories
listed product
Indicates that a terminal is
connected to the chassis
when such a connection is
not apparent.
J Earth ground
X
P
B AC (Alternating Current)
F DC (Direct Current)
~
Hazardous voltage. Risk
of electrical shock.
Conforms to European
Union directives
Do not dispose of this
product as unsorted
municipal waste. Contact
Fluke or a qualified
recycler for disposal.
Warnings and Cautions
When testing electrosurgical units, observe the following to ensure operator
safety and maintain integrity of the high frequency (HF) measurement.
W X Warning
To avoid possible electric shock, burning of the skin, or
personal injury, follow these guidelines:
• Use only test leads supplied with the Analyzer to test the
ESU. These test leads utilize a shrouded 4mm plug to
limit exposure to the high-voltage and high frequency
ESU signal. The retractable ends of these leads are for
use on ESU only.
1-4
Page 15

Introduction and Specifications
General Safety Information
• Do not plug in or remove a test lead from either the ESU
or the Analyzer while the ESU generator is activated (or
keyed). This high frequency ESU signal can be several
thousand volts in amplitude when the output is opencircuited.
• Whenever practical during testing, activate the ESU
generator output using the foot switch supplied with the
ESU.
• No probes or accessories supplied with the analyzer are
intended for handheld use. Set up using the safety test
leads and stand clear when activating the ESU with the
footswitch.
• Place the Analyzer on an insulated, non-conductive work
surface to limit possible HF current paths to earth
ground.
• Routinely inspect test leads for wear and tear. Repair or
replace the test leads to maintain operator safety and
Analyzer performance.
1
Note
To ensure accuracy of measurement, follow these guidelines:
• Whenever practical during testing, place test leads carrying the
ESU signals in parallel, approximately 0.5 meters apart, to limit
capacitive coupling.
• Avoid crossing or tangling test lead cables during use and do not
drape them across conductive, grounded surfaces. Due to their
length, erroneously high HF readings may result.
Electromagnetic Interference and Susceptibility
Like all similar equipment, this equipment generates, uses, and can radiate
radio frequency energy and, if not installed and used in accordance with the
instruction manual, may cause harmful interference to radio communications.
Operation of this equipment in a residential area could cause interference, in
which case the user will be required to correct the interference and bear any
1-5
Page 16

RF303
Operators Manual
costs. The limits established by the following organizations are designed to
provide reasonable protection against harmful interference when the
equipment is operated in a commercial environment.
The Analyzer has been tested by independent testing laboratories and found to
meet the following requirements:
EC Directive 89/336/EEC EN 50081-1 Emissions
Radiated Emissions and Line Conducted Emissions. Verification was to the
limits and methods of EN 55011. The device is classified as EN 55011, Group
A.
EC Directive 89/336/EEC EN 50082-1 Immunity
Electrostatic Discharge Susceptibility, Radiated Susceptibility, and Electrical
Fast Transient/Burst Susceptibility. Verification of compliance was conducted
to the limits and methods of EN 50082-1:1992, IEC 1000-4-2; EN 61000-4-3;
IEC 1000-4-4; EN 61000-4-5; EN 61000-4-6; EN 61000-4-11.
USA Federal Communications Commission
This equipment has been found to comply with the limits for a Class A digital
device, pursuant to Part 15 of the FCC Rules.
Canadian Department of Communications
This digital apparatus does not exceed Class A limits for radio emissions from
digital apparatus set out in the Radio Interference Regulations of the Canadian
Department of Communications.
Le present appareil numerique n'met pas du bruits radioelectriques depassant
les limites applicables aux appareils numerique de la Class A prescrites dans le
Reglement sur le brouillage radioelectrique edicte par le ministere des
Communications du Canada.
1-6
Page 17

Introduction and Specifications
Control and Interface Panels
1
Control and Interface Panels
Figure 1-1 and Table 1-2 describe the controls and interfaces of the Analyzer.
19
9
20
1
2
3
5
4
6
7
8
10
RF-303
ELECTROSURGICAL ANALYZER
11
12
Figure 1-1. Analyzer Control and Interface Panels
Table 1-2. Controls and Connections
13
18
17
16
1514
exz14.eps
Item Name / Description
1 Display
Backlit, numeric, 4-digit LCD
2 Power Indicator (WATTS) Lamp
Displays reading in watts.
3 Current Indicator (mA) Lamp
Displays readings in milliamperes (mA).
1-7
Page 18
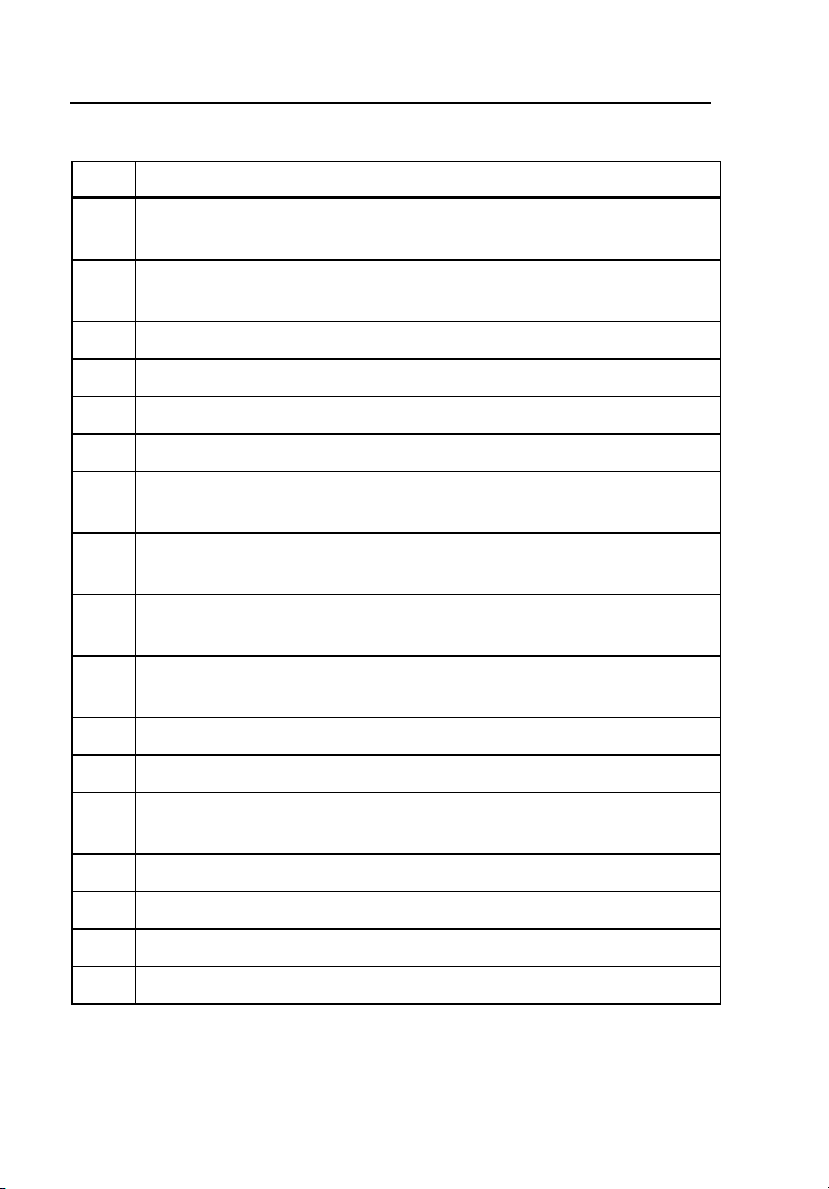
RF303
Operators Manual
Table 1-2 Controls and Connections (cont.)
Item Name / Description
4 Test Load (OHMS) Lamp
Displays resistance in ohms.
5 Mode Selection (MODE SELECT ENT) Pushbutton
Toggles the display between Power (in watts) and Current (in mA).
6 Increase Test Load (OHMS SELECT +) Pushbutton
7 Decrease Test Load (OHMS SELECT -) Pushbutton
8 Carrying Handle
9 Battery Status Lamp
10 Scope Output
BNC connector
11 Measuring Resistance/HF Meter
All measurements made through these inputs.
12 Earth Connections
Provides earth reference for HF Leakage tests.
13 HF Load Resistance
Auxiliary test load for earth/ground-referenced Type BF Tests 2 and 3.
14 Battery Ground / Signal Earth Ground
15 Power Cord Inlet
16 Fuse Cover
Both power mains fuses are located behind this panel.
17 Power On/Off Switch
18 Ventilation Air Outlet
19 Ventilation Air Inlet
20 RS232 Port
1-8
Page 19

Introduction and Specifications
Instrument Specifications
1
Instrument Specifications
Modes of Operation
Line Powered (Battery Charge and Maintenance Charge)
Battery Operation
Offline (Battery Charge and Maintenance Charge)
Displayed Parameters
Power (Watts)
HF Current (milliamperes)
Test Load (ohms)
Test Performed
Generator Output
HF Leakage ........................................................ Performs HF leakage tests to IEC 601
Measurement
Technique ........................................................... Precision high-voltage capacitive
HF Power (watts)
Resolution....................................................... 1 to 400 W / Resolution: 0.1 W
Maximum power input .................................... 400 W rms
Accuracy..................................................... ± 5 % of reading or ± 3 watts,
HF Current
Range ......................................................... 30 to 2500 mA rms, Resolution: 1 mA
Accuracy..................................................... ± 2.5 % of reading or ± 15 mA,
Bandwidth/System Response
Bandwidth of rms converter circuit (1 % accuracy)
Flat response.................................................. 10 kHz to 10 MHz
-3 dB points..................................................... 1 kHz to 20 MHz
System Response (measurement circuitry and selected test load):
-3 dB points..................................................... 1 kHz to 10 MHz @ 300 e
2-2, 1289-2, ANSI/AAMI standards:
Type BF Test 1 – Earth referenced
monopolar output Type CF/Bipolar –
Isolated monopolar or bipolar output
attenuator samples applied ESU
signal. This directly measured HF
voltage and the selected test load
resistance value utilized to derive the
true-rms values of both current and
wattage readings.
whichever is greater
whichever is greater.
1-9
Page 20

RF303
Operators Manual
Test Load Section
Main Test Load
Selections........................................................15
Selection range ...............................................50 to 750 e
Step size..........................................................50 e
Duty Cycle .......................................................50 % @ 400 W (maximum 30 seconds
Resonance impedance variation ..................... ± 0.5 dB maximum (<10 MHz)
Accuracy (DC to 500 kHz)...................................± 4 % of selected value measured at
Auxiliary Leakage Test Load Fixed:
200 e
Accuracy..........................................................± 4 %
Power rating ....................................................225 W
Input Capacitance (nominal)
Active to dispersive .........................................30 pF
Active or Dispersive to Earth ground...............40 pF
Battery
Type: Sealed lead-acid
Voltage ................................................................12 volts nominal
Capacity ..............................................................2.2 A H
Field serviceable .................................................No
Typical time between recharges..........................2-hour minimum
Battery cycles ......................................................200
Recharging ..........................................................Instrument has internal, automatic
Auxiliary Contact Quality Monitor
Testing Feature ...................................................The main test load section is used to
Display
Type ....................................................................LCD, 7-segment
Display size .........................................................Four full digits
Overall display size .............................................2.0” x 0.75”
ON during any one-minute period)
calibration to: ± 1 % (across the entire
operating temperature range)
charger that is activated when unit is
plugged into wall with power cord. No
external charger required.
perform a simple Auxiliary Contact
Quality Monitor Testing Feature
(CQM) operational check.
1-10
Page 21

Introduction and Specifications
General Specifications
Front-Panel Controls / Pushbuttons
Measurement Select (1)
Load Select:
Increment test load (+) one step
Decrement test load (-) one step
Designations:
Generator output-active (1)
Generator output-dispersive (2)
Auxiliary HF leakage load (2)
Connector type ................................................... 4-mm (0.160”) diameter safety sockets
Input voltage limit................................................ 10,000 V peak
Input current limit ................................................ 3 amperes rms
Installation category............................................ II
Side Input connection
Designation......................................................... Signal reference
Oscilloscope Output
Transformer coupled output
Scale Factor........................................................ uncalibrated
Connector type ................................................... BNC
Calibration Period
Calibration recommended every 12 months.
1
General Specifications
Temperature Range
Operating: ........................................................... 15 to 35 °C
Storage ............................................................... 0 to 50 °C
Humidity Range
90 % non-condensing
Altitude
To 2,000 meters
Ventilation
Internal fan with variable speed control
Over temperature detector
Magnetic tachometer sensor to detect blocked fan motor
1-11
Page 22

RF303
Operators Manual
Power Requirements
Universal input switching supply (12 V dc output)
Operating Voltages:
Specified..........................................................115 V ac/230 V ac
Maximum range...............................................83 to 264 V ac
Operating frequencies:
Specified..........................................................50 Hz/60 Hz
Maximum range...............................................47 to 63 Hz
Maximum input requirement............................60 VA
Fusing
External (user-replaceable)
Quantity ...........................................................2
250 V, 3.15 A, Type F, L1 and L2
Case construction
High-impact plastic, UL94-V0
Weight
5.6 kg (14.15 lb)
Dimensions
15.24 cm H x 34.24 cm W x 30.48 cm D (6.00 in. H x 13.48 in. W x 12.00 in. D)
Intended Use
Indoor
Installation category II
Pollution degree 2
Portable equipment
Sound levels less than 65 db A
1-12
Page 23

Introduction and Specifications
Accessories
1
Accessories
Table 1-3 lists the accessories provided with the Analyzer and their
corresponding Fluke part numbers. Table 1-4 lists optional accessories and
their part numbers.
Table 1-3. Accessories Provided with the Analyzer
Accessory Fluke Part #
Analyzer Operaters Manual 2202027
Analyzer Accessory Kit
Includes: (quantity)
Fuse: type F, 3.15 A, 250 V (2) 2183792
Suregrip large alligator clip set (2/set) 1610159
Test lead set with retractable sheaths (2/set) 1903307
ESU – Dispersive safety lead (1) 2772171
ESU – CQM safety lead (1) 2772180
ESU – Jumper safety lead (2) 2772209
Ground pin adapter (1) 2242165
Table 1-4. Optional Accessories for the Analyzer
Accessory Fluke Part #
Watertight protective carrying case 2248587
Serial cable 2204472
2202009
1-13
Page 24

RF303
Operators Manual
1-14 2-1
Page 25

Chapter 2
Installation, Setup, and Maintenance
Contents Page
Installation and Setup .................................................................. 2-3
Power-Up Sequence................................................................. 2-3
Operating the Analyzer Using Battery Power.......................... 2-5
LED Backlight Display Operation ........................................... 2-7
Ventilation................................................................................ 2-7
Connecting Test Leads between the ESU and the Analyzer .... 2-7
Test Lead Set with Retractable Shrouds............................... 2-8
ESU- Dispersive Safety Lead............................................... 2-8
ESU Jumper Safety Lead...................................................... 2-9
ESU CQM Safety Lead ........................................................ 2-9
Maintenance................................................................................. 2-10
Cleaning ................................................................................... 2-10
Calibration................................................................................ 2-10
Replacing Fuses ....................................................................... 2-10
Page 26

RF303
Operators Manual
2-2
Page 27

Installation, Setup, and Maintenance
Installation and Setup
2
Installation and Setup
The Analyzer has a universal power supply and automatically operates with
applied main voltages having a maximum range of 83-264 V ac. You do not
need to adjust voltage via jumpers or programming tabs to operate the
Analyzer.
Set up the Analyzer for operation by attaching the supplied power cord to the
power cord inlet located on the right panel of the Analyzer case. If necessary,
please refer to “Instrument Familiarity” to help you identify the power cord
inlet.
Power-Up Sequence
After plugging the Analyzer into a correctly rated earth-ground outlet, locate
the power switch on the right panel. Move the switch to the ON position
(marked with the
sequence:
1. The three front LED indicator lamps illuminate, and you see this
information momentarily appear in the display:
– symbol). Next, you should observe the following power-up
2. Next the installed version of the Analyzer’s firmware briefly appears in
the display. Here’s an example:
2-3
exz01.eps
Page 28

RF303
Operators Manual
Note
The letter E, followed by a number indicates an error condition; the
Analyzer has encountered a problem during its internal self-test. See
“Power-Up Error Condition Codes”, below.
3. The instrument will briefly display several random numbers that are
associated with the rms output offset during initialization.
4. The display then reverts to a Power Save Mode, where the display is
blanked out, and the backlight is dim. The battery and mode LEDs remain
ON. The Analyzer advances to its default test load condition of 300 ohms,
ready to measure ESU power in watts.
Power-Up Error Condition Codes
Table 2-1 shows all error condition code numbers that can appear in the
display at power-up and the condition indicated by the code.
Table 2-1. Power-Up Error Condition Codes
Code Number Error Condition Indicated
E 03 EPROM checksum wrong
exz02.eps
2-4
E 04 RAM bad
E 05 EEPROM checksum wrong
E 06 ac-to-dc power converter bad
E 07 RMS converter bad
E 08 Peak detectors bad
E 09 Temperature sensors bad
Page 29

Installation, Setup, and Maintenance
Installation and Setup
2
Operating the Analyzer Using Battery Power
You can operate the Analyzer using either ac power or dc power. When you
power on the Analyzer with the supplied ac power cord installed and
connected to an appropriate ac power source, the Analyzer operates on ac
power. When you power on the Analyzer with the ac power cord disconnected,
the Analyzer automatically operates on battery (dc) power.
Note
The battery is a sealed lead-acid type. Do not attempt to replace the
battery. Return the Analyzer to a service center for replacement of the
battery.
The Analyzer contains an internal ac/dc converter. During ac power operation,
this converter assists in charging the battery, if it is not already fully charged.
If the battery is fully charged, it is held in a maintenance float mode.
If you remove the ac power cord while the Analyzer is powered on, the
Analyzer automatically resets in a battery-powered mode. The Analyzer can
operate on a fully-charged battery for approximately two hours. Battery age,
previous battery cycles, and Analyzer operating conditions all affect the actual
length of time that the Analyzer operates on battery power.
When the battery nears the end of its discharge cycle, the unit automatically
turns off to protect the battery from damage. This action is preceded by a
warning interval of about 5 minutes, indicated by a flashing red light of the
battery status indicator.
When the ac power cord is connected between the Analyzer and an ac power
source, and the Analyzer is not powered on, the battery charger is active.
However, instrument controls and measurement circuits do not receive power.
See Table 2-2, below for a summary of battery operating status under various
conditions.
2-5
Page 30

RF303
Operators Manual
Table 2-2. Battery Operating Status
Power Source to
Analyzer
Connected to ac
Power Source?
No On Battery Discharging
No Off No power Open circuit,
Yes Off No power Charging
Yes On AC Charging
On/Off Switch
Setting
Analyzer
Instrument
Controls and
Measurement
Circuits
Battery Status
minimal leakage
Battery Status Light
The battery status indicator light is labeled BATTERY STATUS. Table 2-3
shows status light displays and their corresponding conditions.
Table 2-3. Battery Status Light
Light Battery Condition
Flashing green Battery is being qualified (checked) with medium
charge or receiving full charge. Voltage is OK.
Steady green Maintenance charge voltage OK or battery
operation with voltage OK.
Flashing red and green Automatic shut-down is pending, battery is nearly
exhausted.
Flashing red Automatic shut-down is imminent.
Steady red When plugged in; indicates that the battery is not
charging and needs replacement by the Fluke
Biomedical Service Center.
Note
The Analyzer should be charged overnight, when the battery is fully
drained.
2-6
Page 31

Installation, Setup, and Maintenance
Installation and Setup
2
LED Backlight Display Operation
The Analyzer’s display lights when you select a load or when a test
measurement is received by the instrument.
Ventilation
The Analyzer requires proper ventilation so that it does not overheat during
operation. Always ensure that the two ventilation ports, located on each side of
the Analyzer, are not blocked during use. Maintain at least four inches (10.2
centimeters) of clear space around each of these ports.
An internal fan located immediately behind the grille on the left side port
supplies forced-air ventilation. Sensors measure the temperature of the load
resistors, and the fan accelerates as the load temperature increases. Fan rotation
is monitored also. If the load temperature is excessive or if the fan is not
functioning, an error condition is indicated on the display, and the audio
transducer emits a beeping sound. The error display codes are:
E 01: Temperature too high.
E 02: Fan not operating.
W Caution
Permanent damage to the Analyzer can occur if you
continue to apply a high-level ESU signal after the alert
has been activated.
Connecting Test Leads between the ESU and the Analyzer
A complete set of test leads is supplied with the Analyzer. Use these test leads
to connect the ESU generator output to the Analyzer and to configure the
Analyzer to conduct a specific ESU test. Safe connection to the Analyzer is
facilitated by shrouded safety plugs.
2-7
Page 32

RF303
Operators Manual
W X Warning
To avoid possible electric shock, burning of the skin, or
personal injury, follow these guidelines: Retractable end of
test leads are for use on ESU only. No probes or
accessories supplied with the Analyzer are intended for
handheld use. Set up using the safety test leads and stand
clear when activating the ESU with the footswitch.
The following is a description of the supplied test leads.
Test Lead Set with Retractable Shrouds
Part # 1903307
Color: One red and one black
Quantity: Two/set
One end of each test lead has a fixed shroud 4mm banana plug to connect with
the Analyzer. The other end has a retractable shroud 4mm banana plug
compatible with the active electrode/bipolar jacks on most ESU panels. A red
safety alligator clip is included for direct connection to an actual active
electrode element if preferred.
A removable black clamp facilitates firm attachment to a ground reference
point. This lead is important for making leakage measurements in the battery
mode of operation.
ESU- Dispersive Safety Lead
Part # 2772171
Color: Clear with blue connectors
Quantity: One
One end of the test lead has a single fixed-shroud 4mm banana plug to connect
with the Analyzer. The other end has a CQM style two-pin connector that
plugs directly into the neutral (or dispersive) electrode jack on most ESU
panels.
2-8
Page 33

Installation, Setup, and Maintenance
Installation and Setup
Note
The mechanical pin is removed from the CQM connector to disable
CQM during ESU generator output tests.
ESU Jumper Safety Lead
Part # 2772209
Color: Black
Quantity: Two
Both ends of these two short jumpers have fixed shroud 4mm banana plugs
and are used to configure the Analyzer for the IEC Type BF Tests 1 and 2,
which utilize the auxiliary HF load resistance (200e). Both jumpers are
required to conduct these tests.
ESU CQM Safety Lead
Part # 2772180
Color: Clear with blue and yellow connectors
2
Quantity: One
One end of the test lead has two fixed shroud 4mm banana plugs to connect
across the Analyzer test load. The other end has a CQM style 2PIN connector
that plugs directly into the neutral (or dispersive) electrode jack on most ESU
panels.
Note
The mechanical pin is intact to enable the CQM function during CQM
testing. Do not use this lead for output testing.
2-9
Page 34

RF303
Operators Manual
Maintenance
Follow the recommendations below to keep the Analyzer in good working
condition.
Cleaning
Moisten a clean cloth with a mild solution of detergent and water only and
wipe the Analyzer clean.
W Caution
To avoid damaging the Analyzer, use only dilute, mild
detergent for cleaning.
Calibration
Calibrate the Analyzer every 12 months.
Replacing Fuses
The Analyzer contains two operator-replaceable fuses, rated as Type F, 3.15 A,
and 250 V. These fuses are in the two main supply lines.
You can replace one or both of these fuses by opening the fuse cover located
on the right panel. Refer to Figure 2-1 for a diagram of the fuse cover
assembly. If necessary, see “Instrument Familiarity”, to identify the fuse cover.
2-10
Page 35

Installation, Setup, and Maintenance
Maintenance
Figure 2-1. Fuse Cover
2
exz16.eps
W X Warning
To avoid severe electrical shock, disconnect the power
source before replacing fuses.
1. Make sure that the power switch is in the OFF position (O) and disconnect
the power cord. The Analyzer is now safely powered down.
2. Pry the left side of the fuse cover up from the power inlet assembly, using
a small flat-blade screwdriver. The fuse cover latches on the right side and
remains attached to the Analyzer.
3. Remove a fuse carrier with your fingers, lifting it from the power inlet
housing.
Note
To avoid damage to the Analyzer, do not use any sharp instrument to
lift a fuse carrier.
4. Replace the fuse in the carrier with one of the same amperage and voltage
ratings.
2-11
Page 36

RF303
Operators Manual
5. Reinstall the fuse carrier into the power inlet module, ensuring that the
arrows on the ends of the fuse carriers point up.
6. Close the fuse cover until it snaps into place.
7. Reconnect the power cord, and power-up the Analyzer, if desired.
If you have replaced these fuses and the Analyzer still does not work properly,
contact the Fluke Biomedical Technical Assistance Center at 800-648-7952.
2-12
Page 37

Chapter 3
Operation
Contents Page
The Signal Averaging Mode (SAM)............................................ 3-3
Testing the ESU........................................................................... 3-3
Key to Panel Diagrams............................................................. 3-4
Generator Output Test.............................................................. 3-4
Basic Contact Quality Monitor (CQM) Test............................ 3-7
HF Leakage Current Test 1 ...................................................... 3-10
HF Leakage Current Test 2 ...................................................... 3-13
HF Leakage Current Test 3 ...................................................... 3-16
Connecting an Oscilloscope to the Analyzer ............................... 3-19
Remote Operation via the RS-232 Function................................ 3-19
Setting Up and Operating the Analyzer in Simplex Mode....... 3-20
Setting Up and Operating the Analyzer in Duplex Mode ........ 3-20
3-1
Page 38

RF303
Operators Manual
3-2
Page 39

Operation
The Signal Averaging Mode (SAM)
3
The Signal Averaging Mode (SAM)
Upon power-up, the Analyzer defaults to the instantaneous algorithm for
power output measurement, which shows fluctuating ESU output if it exists.
You can select the desired SAM by depressing the MODE SELECT and OHM SELECT keys simultaneously. Each time you initiate this key
sequence, the Analyzer increments to the next mode. Starting at the default
mode (“F 0”), the next mode entered is the one-second signal-averaging mode
(“F 1” appears on the display). Initiating the key sequence again selects the
two-second signal averaging mode (“F 2” appears on the display). Initiating
the key sequence again brings the unit back to the default instantaneous mode
(“F 0” appears on the display), and so on.
Testing the ESU
The sections that follow describe the specific ESU tests you can perform using
the Analyzer. Test procedures prompt you to manually connect test leads in the
required configuration.
W X Warning
To avoid possible electric shock, burning of the skin, or
personal injury, follow these guidelines: Retractable end
of test leads are for use on ESU only. No probes or
accessories supplied with the analyzer are intended for
handheld use. Set up using the safety test leads and stand
clear when activating the ESU with the footswitch.
The Analyzer does not internally configure the test leads. You can view
functional-block diagrams for each test at the end of each test procedure
section in this chapter. Additional information about test leads is given in
“Installation.”
3-3
Page 40

RF303
Operators Manual
Note
The Analyzer can test a wide range of electrosurgical units for basic
operation and performance. It is compatible with both isolated and
earth–ground referenced outputs, and with both monopolar and
bipolar outputs. If you have any questions about testing an ESU, you
can review “Specifications” and the technical service manual for the
ESU you want to test. In addition, you can contact our technical
assistance center at 800-648-7952.
Key to Panel Diagrams
The Analyzer uses lights to indicate whether the value displayed in the
window represents watts, mA, or ohms. The diagrams in this section resemble
the Analyzer panel. The key is as follows:
○ Analyzer panel light is off
● Analyzer panel light is on
Generator Output Test
The Analyzer provides an effective method of attaching a resistive load to the
ESU under test and displays the output directly in either watts or HF current of
the applied ESU signal. See Figure 3-1 for a diagram of the generator output
test.
Test Procedures
This test involves:
• connecting the ESU you want to test to the Analyzer
• selecting a test load resistance
• measuring the ESU’s power output
• measuring the ESU’s current output
• ending the test
To connect the ESU to the Analyzer for a monopolar output test:
1. Connect the ESU Active electrode to the yellow jack (marked) active,
using one of the test leads from the set, Part # 1903307.
3-4
Page 41

Operation
Testing the ESU
3
2. Connect the ESU Neutral/Dispersive electrode to the left blue jack
dispersive, using the test lead, Part # 2772171.
3. Continue to load selection.
To connect the ESU to the Analyzer for a bipolar output test:
1. Connect the ESU Bipolar-1 electrode to the yellow jack (marked) active,
using one of the test leads from the set, Part # 1903307.
2. Connect the ESU Bipolar-2 electrode to the left blue jack dispersive, using
the other test lead from the set, Part # 1903307.
3. Continue to load selection.
To select the desired test load resistance:
1. Press the OHMS SELECT increase (+) or decrease (-) buttons until the
test load value you want appears in the display. After approximately one
second, the display returns to a blank screen. Below is an example of a test
load value as it appears in the display.
WATTS
mA
OHMS
ENT
MODE SELECT
OHMS SELECT
exz03.eps
Note
The default (or initial power-up) test load selection is 300 ohms.
3-5
Page 42

RF303
Operators Manual
To measure the ESU’s power output:
1. Activate the ESU.
2. If the WATTS indicator lamp on the panel is lit, go to Step 3. If it is not
lit, press the MODE SELECT (ENT) button to select WATTS and go to
Step 3.
3. View the power measurement on the display. Here is an example:
WATTS
mA
OHMS
ENT
MODE SELECT
OHMS SELECT
exz04.eps
To measure the ESU’s current output:
1. While the ESU is still activated, press the MODE SELECT (ENT) button
until the milliamperes (mA) indicator lamp lights.
2. View the ESU’s current output (in mA). Below is an example.
WATTS
mA
OHMS
ENT
MODE SELECT
OHMS SELECT
To end the generator output test:
Deactivate the ESU.
W Caution
To avoid damage to the Analyzer and the ESU under test,
do not exceed the Analyzer’s duty cycle, which is 30
seconds ON during any continuous one-minute period.
3-6
exz05.eps
Page 43

Operation
M
3
exz06.eps
Testing the ESU
ESU RF303
YEL
BLU
BLU
HF
Meter
Supply
Mains
Earth
ESU Active
Active
Dispersive
CQM
Figure 3-1. Generator Output Test
ESU Dispersive
Main
Test
Load
Basic Contact Quality Monitor (CQM) Test
This test of the contact quality monitor uses the Analyzer’s test load to
simulate a patient’s skin resistance in contact with the dual element
neutral/dispersive electrode pad.
Note
Perform this test on electrosurgical units without energizing the
generator output. Do not connect the ESU active electrode to the
Analyzer during this test.
The CQM (REM) test is intended for the neutral/dispersive electrode of
monopolar ESUs equipped with a contact quality monitor. The
neutral/dispersive electrode is actually two separate pads attached to the
patient’s skin. The CQM (REM) circuit issues an alarm only if the patient
loses contact with one or both of the two pads. See Figure 3-2 for a diagram at
the end of this section.
3-7
Page 44

RF303
Operators Manual
Test Procedures
The procedures involved with this test include:
• connecting the ESU neutral/dispersive electrode to the Analyzer
• selecting a 50-ohm test load resistance
• increasing load resistance on the Analyzer to observe the ESU’s
visual or audio alarm
To connect the ESU’s neutral/dispersive electrode to the Analyzer:
1. Connect one of the two color-coded banana plugs (test lead Part #
2772180) to the Analyzer’s yellow jack active.
2. Connect the other banana plug to the Analyzer’s left blue jack dispersive.
3. Connect the 2PIN CQM (REM) connector on the other end of the test lead
to the neutral/dispersive electrode of the ESU.
To select a 50-ohm test load resistance setting:
1. Press the OHMS SELECT increase (+) or decrease (-) buttons as needed
until the test load value 50 appears in the display. After approximately one
(1) second, the display returns to a blank screen. Below is an example of a
test load value as it appears in the display.
3-8
ENT
MODE SELECT
WATTS
mA
OHMS
OHMS SELECT
exz12.eps
Page 45

Operation
Testing the ESU
3
To observe the ESU’s visual or audible alarm:
1. With the 50-ohm test load attached to the ESU CQM (REM) input, it
should sense this level of resistance in the "pass zone." Depending upon
the ESU device manufacturer, this pass zone can extend from 135 to 250
ohms. Test load settings over the pass zone activate the CQM alarm.
Note
Refer to the technical support/service manual of the ESU you are
testing for the recommended check-out procedure for the ESU CQM
feature.
2. Increase the test load from 50 ohms until the CQM alarm sounds. The
value at that point is the CQM alarm resistance.
Supply
Mains
Earth
ESU
ACTIVE
DISPERSIVE
CQM
Figure 3-2. Basic CQM Check
X No connection
ESU CQM
Basic CQM Check
(Contact Quality Monitor)
RF303
BLU
Main
Test
Load
YEL
exz13.eps
3-9
Page 46

RF303
Operators Manual
HF Leakage Current Test 1
The HF leakage current test number one is an IEC isolated output/bipolar, CFtype leakage current test. It shows you the amount of open-circuit high
frequency current leakage from a single isolated electrode to earth/ground
through a 200-ohm resistive test load. See Figure 3-3 for a diagram of this test
at the end of this section.
Note
This high frequency leakage current test is intended only for isolated
output electrosurgical units. If you test the active electrode of an
earth-referenced Type BF ESU in the manner described in this
section, you will be measuring the full output of the generator, not a
high frequency leakage current. While conducting this test
inappropriately will not damage the Analyzer, the resulting high
frequency leakage current measurement will not be valid for an
earth/ground referenced ESU.
Test Procedures
The procedures involved with this test include:
• connecting the ESU electrode you want to test to the Analyzer
• selecting a 200-ohm test load resistance
• measuring the ESU’s HF leakage current
• ending the test
To connect the ESU electrode to the Analyzer:
1. If you are testing the ESU’s active or single bipolar electrode, connect the
electrode to the yellow jack active, using one of the test leads from the set,
Part # 1903307, or
If you are testing the ESU’s neutral/dispersive electrode, connect that
electrode to the yellow jack active, using the test lead Part # 2772171.
2. Connect the jumper between the right blue jack dispersive and the left
green jack ground earth reference, using test lead Part # 2772209.
3-10
Page 47

Operation
Testing the ESU
3
3. If operating under battery power, attach a test lead from the set (Part #
1903307) from the battery ground on the side panel to the ground earth
reference.
To select a 200-ohm test load resistance setting:
1. Press the OHMS SELECT increase (+) or decrease (-) buttons as needed
until the test load value 200 appears in the display. After approximately
one second, the display returns to a blank screen. Below is an example of
a test load value as it appears in the display.
WATTS
mA
OHMS
ENT
MODE SELECT
OHMS SELECT
exz07.eps
To measure the ESU’s signal HF leakage:
1. If the mA indicator lamp on the panel is lit, go to Step 2. If it is not lit,
press the MODE SELECT (ENT) button to select mA and go to Step 2.
2. Activate the ESU and view the signal HF leakage measurement in
milliamperes on the display. Here is an example.
WATTS
mA
OHMS
ENT
MODE SELECT
OHMS SELECT
exz18.eps
To end HF leakage test number one:
Deactivate the ESU to end the HF leakage test.
3-11
Page 48

RF303
Operators Manual
W Caution
To avoid damage to the Analyzer and the ESU under test,
do not exceed the Analyzer’s duty cycle, which is 30
seconds ON during any continuous one-minute period.
HF Leakage Test 1
OPEN CIRCUIT TEST
CONNECT ONE HF LEAD ONLY
ESU RF303
ESU
Active
SUPPLY
MAINS
EARTH
ACTIVE
DISPERSIVE
CQM
F
TYPE CF
WHILE IN BATTERY OPERATION MODE:
Use Lead from side battery ground banana
jack to earth reference point.
OR
ESU
Dispersive
Jumper
YEL
MAIN
TEST
LOAD
BLU
GRN
GROUND EARTH
REFERENCE
Figure 3-3. HF Leakage Test Number One
METER
M
HF
exz11.eps
3-12
Page 49

Operation
Testing the ESU
3
HF Leakage Current Test 2
The HF leakage test number two is an IEC earth-referenced, BF-type leakage
current test. You perform the test by connecting a 200-ohm resistive test load
from the ESU’s active electrode to earth/ground on the Analyzer. High
frequency leakage current is then measured through a second 200-ohm
resistive load. You can view the resulting measurement on the Analyzer’s
display. See Figure 3-4 for a diagram of this test at the end of this section.
Test Procedures
The procedures involved with this test include:
• connecting the ESU you want to test to the Analyzer
• selecting a 200-ohm test load resistance
• measuring the ESU’s HF leakage current
• ending the test
To connect the ESU to the Analyzer:
1. Connect the ESU Active electrode to the right gray jack aux HF leakage,
using one of the test leads from the set, Part # 1903307.
2. Connect the ESU Neutral/Dispersive electrode to the left blue jack
dispersive, using test lead Part # 2772171.
3. Connect a jumper between the yellow jack active and the left green jack
ground earth reference, using test lead Part # 2772209.
4. Connect a jumper between the right blue jack dispersive and the left gray
jack aux HF leakage, using test lead Part # 2772209.
5. If operating under battery power, attach a test lead from the set (Part #
1903307) from the battery ground on the side panel to the ground earth
reference.
To select a 200-ohm test load resistance setting:
1. Press the OHMS SELECT increase (+) or decrease (-) buttons repeatedly
until the test load value 200 appears in the display. After approximately
one second, the display returns to a blank screen. Below is an example of
a test load value as it appears in the display.
3-13
Page 50

RF303
Operators Manual
WATTS
mA
OHMS
ENT
MODE SELECT
OHMS SELECT
exz07.eps
To measure the ESU’s signal HF leakage:
1. If the mA indicator lamp on the panel is lit, go to Step 2. If it is not lit,
press the MODE SELECT (ENT) button to select mA and go to Step 2.
2. Activate the ESU and view the signal HF leakage measurement in
milliamperes on the display. Here is an example.
WATTS
mA
OHMS
ENT
MODE SELECT
OHMS SELECT
exz18.eps
To end HF leakage test number two:
Deactivate the ESU to end the HF leakage test.
W Caution
To avoid damage to the Analyzer and the ESU under test,
do not exceed the Analyzer’s duty cycle, which is 30
seconds ON during any continuous one-minute period.
3-14
Page 51

Operation
Testing the ESU
Earth Reference Leakage Type BF Test 2
(Load between electrodes)
ESU
RF303
3
SUPPLY
MAINS
EARTH
Active Lead
ACTIVE
Jumper
DISPERSIVE
CQM
ESU
Dispersive
Jumper
TYPE BF
WHILE IN BATTERY OPERATION MODE:
Use Lead from side battery ground banana
jack to earth reference point.
Figure 3-4. HF Leakage Test Number Two
GRA
GRA
BLU
BLU
MAIN
TEST
LOAD
YEL
GRN
HF
METER
M
GROUND
EARTH
REFERENCE
exz08.eps
3-15
Page 52

RF303
Operators Manual
HF Leakage Current Test 3
The HF leakage test number three is an IEC earth-referenced, BF-type leakage
current test. You perform the test by connecting a 200-ohm resistive test load
from the ESU’s active electrode to earth/ground on the Analyzer. Then you
connect a second 200-ohm resistive load from the ESU’s neutral/dispersive
electrode to earth/ground on the Analyzer. The Analyzer then displays the high
frequency current leakage from the ESU you’re testing. See Figure 3-5 for a
diagram of this test at the end of this section.
Test Procedures
The procedures involved with this test include:
• connecting the ESU you want to test to the Analyzer
• selecting a 200-ohm test load resistance
• measuring the ESU’s HF leakage current
• ending the test
To connect the ESU to the Analyzer:
1. Connect the ESU Active electrode to the right gray jack aux HF leakage,
using one of the test leads from the set, Part # 1903307.
2. Connect the ESU Neutral/Dispersive electrode to yellow jack active, using
test lead Part # 2772171.
3. Connect a jumper between the right blue jack dispersive and the left green
jack ground earth reference, using test lead Part # 2772209.
4. Connect a jumper between the right green jack ground earth reference and
the left gray jack aux HF leakage, using test lead Part # 2772209.
5. If operating under battery power, attach a test lead from the set (Part #
1903307) from the battery ground on the side panel to the ground earth
reference.
To select a 200-ohm test load resistance setting:
1. Press the OHMS SELECT increase (+) or decrease (-) buttons as needed
until the test load value 200 appears in the display. After approximately
one second, the display returns to a blank screen. Below is an example of
a test load value as it appears in the display.
3-16
Page 53

Operation
Testing the ESU
WATTS
mA
OHMS
ENT
MODE SELECT
OHMS SELECT
3
exz07.eps
To measure the ESU’s signal HF leakage:
1. If the mA indicator lamp on the panel is lit, go to Step 2. If it is not lit,
press the MODE SELECT (ENT) button to select mA and go to Step 2.
2. Activate the ESU and view the signal HF leakage measurement on the
display. The measurement will be in milliamperes. Here is an example.
WATTS
mA
OHMS
ENT
MODE SELECT
OHMS SELECT
exz18.eps
To end HF leakage test number three:
Deactivate the ESU to end the HF leakage test.
W Caution
To avoid damage to the Analyzer and the ESU under test,
do not exceed the Analyzer’s duty cycle, which is 30
seconds ON during any continuous one-minute period.
3-17
Page 54

RF303
Operators Manual
Earth Reference Leakage Type BF Test 3
(Load from active electrode to earth)
ESU
RF303
SUPPLY
MAINS
EARTH
ESU Active
ACTIVE
Jumper
DISPERSIVE
CQM
ESU
Dispersive
Jumper
TYPE BF
WHILE IN BATTERY OPERATION MODE:
Use Lead from side battery ground banana
jack to earth reference point.
Figure 3-5. HF Leakage Test Number Three
GRA
GRA
GRN
YEL
MAIN
TEST
LOAD
BLU
GRN
HF
METER
M
GROUND
EARTH
REFERENCE
exz10.eps
3-18
Page 55

Operation
Connecting an Oscilloscope to the Analyzer
3
Connecting an Oscilloscope to the Analyzer
You can connect an oscilloscope to the Analyzer. Connect a standard coaxial
cable to the scope out BNC connector located on the top left of the panel.
Adjust your oscilloscope to view the applied waveform. Refer to Figure 3-6 to
see a typical burst waveform.
Figure 3-6. Burst Waveform on Oscilloscope Display
exz17.bmp
Remote Operation via the RS-232 Function
You can operate the Analyzer locally or remotely. Local mode is the default;
you manually control the Analyzer by pressing the buttons on the panel. Set
the desired test load and indicate whether to measure in watts or milliamperes.
All test load and measurement values appear on the display.
Two remote modes are also available: Simplex Mode (unidirectional
communication) and Duplex Mode (bi-directional communication).
3-19
Page 56

RF303
Operators Manual
Setting Up and Operating the Analyzer in Simplex Mode
In Simplex Mode, you control operation of the Analyzer from the panel. By
pressing the MODE SELECT (ENT) key, you can transmit a fixed data string
to a host device that includes the ESU readings (in watts), HF current (in
milliamps), and test load value (in ohms). The Analyzer sends data to a host
device at a fixed baud rate. The Analyzer cannot receive commands from a
remote terminal or computer in Simplex Mode.
To set up and operate in Simplex Mode, follow these steps:
1. Connect the Analyzer to the computer with a serial interface cable, Part #
2238659.
2. Set the computer to receive data at a baud rate of 2400 with 8 data bits, no
parity, and 1 stop bit.
3. With the Analyzer power turned off, press and hold both the + and -
OHMS SELECT keys located on the panel.
4. Power-up the Analyzer, and then release both keys when the double beep
is heard.
5. Set up the Analyzer to conduct the desired ESU measurement.
6. Press and release the MODE SELECT key to transmit the data while a
measurement is displayed.
7. Data transfers in the format:
Watts =XXXX, I=YYYY, Load =ZZZ.
8. To exit Simplex Mode, power down the Analyzer.
Setting Up and Operating the Analyzer in Duplex Mode
In Duplex Mode, you control operation of the Analyzer from a remote terminal
or computer by issuing specific remote commands that control all features of
the Analyzer. You can view test data received as a result of sending a remote
command on the host controller's display. In Duplex Mode, all panel keys are
"locked out" to allow all functions to be under full direction of the host
controller.
To set up and operate in Duplex Mode, follow these steps:
1. Connect the Analyzer to the computer with a serial interface cable, Part #
2238659.
3-20
Page 57

Operation
Remote Operation via the RS-232 Function
2. Set the computer to communicate with a baud rate of 2400 with 8 data
bits, no parity, and 1 stop bit.
Note
Serial port flow control uses neither XON/XOFF signals nor
hardware flow control (RTS/CTS).
3. Power-up the Analyzer without pressing any keys.
4. To set the Analyzer in Remote Mode, send the GOTOREMOTE
command. After the command is received by the Analyzer, four horizontal
bars appear on the display. Since the controlling computer always receives
a return value, a response, the asterisk will be displayed on the monitor.
5. Control the Analyzer with the desired commands.
6. To exit Remote Mode and return to Local Mode, send the GOTOLOCAL
command.
Observe the following while operating in Duplex Mode:
• Commands must be terminated by a carriage return/ line feed (CR)
(LF).
3
• Setup commands always end with the colon character and a
parameter: (plus the parameter) For example, the setup command
SETLOAD requires the colon followed by the load value (in ohms):
SETLOAD: 50
• Multiple commands (or parameters) are separated by commas. All
possible command (parameter) choices are shown. Unless otherwise
stated, the default parameters are listed first.
• As mentioned earlier, every command returns a value as a response.
The return value may be a string of alphanumeric characters.
Commands that do not require any special return value will show the
star character * simply to indicate that the command has been
executed.
• After the Analyzer receives a command, it will ignore all other
commands until after it has responded to the first command.
• If you do not enter a command and just press the Enter key (carriage
return) on the computer keyboard, the Analyzer will return the ?
(question mark) character.
3-21
Page 58

RF303
Operators Manual
• All responses are uppercase, followed by CR and LF.
• Measurement readings from the Analyzer are returned identically as
they would appear on the Analyzer's display; that is, as numbers, with
or without a decimal point, and followed by any appropriate unit of
measurement.
• Errors always return a response beginning with: ?ERR=XX (plus the
description). Table 3-1 lists each of the error responses.
Table 3-1. Error Responses
Error Response Explanation
?ERR=01,BAD OR MISSING
PARAMETERS
?ERR=02,ILLEGAL NUMBER OF
PARAMETERS
?ERR=03,PARAMETER OUT OF
RANGE OR SYNTAX ERROR
?ERR=04,COMMAND NOT
AVAILABLE FROM THIS MODE
?ERR=05, DEACTIVATE ESU
BEFORE ADJUSTING
?ERR=06,ACCESS DENIED The command is a calibration
The received command requires
parameters, and there were none, or
they were not recognized.
The command did not include the
correct number of parameters.
One or more of the parameters were
out of the correct range, or there was
an error in the syntax of the
command.
The Model Analyzer is not in the
special sub mode within the remote
that is required for the command.
There is an ESU signal present at the
meter inputs that is not allowed for
the command to be executed.
command and the calibration jumper
is not connected
Table 3-2 describes various commands available in Duplex Mode.
3-22
Page 59

Operation
Remote Operation via the RS-232 Function
Table 3-2. Available Duplex Mode Commands
3
Command Description Parameters
Required
GOTOREMOTE
GOTOLOCAL
IDENT
VER
SETMODE:{parm}
Places the Analyzer in
the duplex remote
mode.
Returns the Analyzer to
normal (local mode)
operation / exits duplex
remote mode.
Identifies the Analyzer
model and firmware
version number.
Identifies the firmware
version number.
Changes the format of
the Analyzer response
to the RDMETER
command. Separate the
command from the
desired mode
(parameter) using a
colon. Refer to
RDMETER below.
Example:
SETMODE:OUTPUT
None *
None
None
None
OUTPUT (for
measuring
generator
output).
RFLKG (for
measuring
HF leakage).
RARF (for
conducting
CQM test).
Return Value
(Response)
*
Model Analyzer name
and the version of the
firmware.
Example: MODEL
Analyzer VERSION
X.XX
Where X.XX is the
version number.
Firmware version
number.
Example: VERSION
X.XX
Where: X.XX is the
version number.
*
3-23
Page 60

RF303
Operators Manual
Table 3-2. Available Duplex Mode Commands (cont.)
Command Description Parameters
Required
RDMODE Prompts the Analyzer to
transmit the currently
selected mode.
EXITMODE Prompts the Analyzer to
exit the currently
selected mode.
Note: You must exit the
current mode before
entering another.
SETLOAD:{parm} Sets the measurement
test load resistance from
50-750 ohms in 50 ohm
increments. Not
available in RFLKG
mode.
RDLOAD Resistance value of the
selected test load
appears in the display.
None NONE
None *
Values of 50750 in
increments of
50
None RRRR (ohms)
Return Value
(Response)
OUTPUT
RFLKG
RARF
*
Where RRRR is the
load value in ohms.
Formatted to four
digits with leading
spaces as required.
RDPOWER Prompts an Analyzer
power measurement in
watts. Not available in
RFLKG and RARF
modes.
None PPP.P (w)
3-24
Page 61

Operation
Remote Operation via the RS-232 Function
Table 3-2. Available Duplex Mode Commands (cont.)
3
Command Description Parameters
Required
RDCURRENT Prompts an Analyzer
current measurement in
milliamperes. Not
available in RARF
mode.
RDMETER Prompts the Analyzer to
measure the applied
signal of the ESU.
Prompts the Analyzer to
return all measurements
of the applied signal of
the ESU.
{MODE=NONE}
None AAAA (mA)
None
None I=AAAA(mA),
Return Value
(Response)
Where AAAA is
current in
milliamperes.
CF=XX.X,
KVPP=XXX,
PWR=PPP.P(w)
Where AAAA is the
Current reading in
milliamps, XX.X is the
Crest Factor, XXX is
the peak-to-peak
voltage in kVolts and
PPP.P is the power in
watts.
3-25
Page 62

RF303
Operators Manual
Table 3-2. Available Duplex Mode Commands (cont.)
Command Description Parameters
Required
Prompts the Analyzer to
return all measurements
of the applied signal of
the ESU.
{MODE=OUTPUT}
Prompts the Analyzer to
measure the applied
signal current of the
ESU.
{MODE=RFLKG}
Prompts the Analyzer to
return the current test
load.
{MODE=RARF}
None I=AAAA(mA),
None I=AAAA(mA)
None REM RESISTANCE=
Return Value
(Response)
CF=XX.X,
KVPP=XXX,
PWR=PPP.P(w)
Where AAAA is the
Current reading in
milliamps, XX.X is the
Crest Factor, XXX is
the peak-to-peak
voltage in kVolts and
PPP.P is the power in
watts
Where AAAA is the
Current reading in
milliamps.
RRR
Where RRR is the
load value in ohms.
3-26
Page 63

Appendices
Appendix Title Page
A Load Issues .................................................................................. A-1
B Interpretation of Fluctuating Readings ........................................ B-1
C Abbreviations............................................................................... C-1
Page 64

Page 65

Appendix A
Load Issues
Load Issues
The load resistors typically used in ESU analyzers are not ideal; they have
some reactive components that are frequency dependent. The RF303 Analyzer
derives applied power by measuring the voltage across the set load and
calculating the power (V2/R). Most other ESU analyzers on the market derive
the applied power by measuring the current flowing through a set load and
calculating the power (I2*R).
At fundamental frequencies below 500 kHz and regardless of the load setting,
the two methods of measurement are comparable. Above 500 kHz, and at the
extremes of the loads, the readings displayed by the two methods differ on
opposite sides of the expected value.
As an example, when testing the Conmed Excalibur Electrosurgical Unit in the
monopolar output with the Analyzer load set to 50 ohms, the set value on the
Conmed correlates with the displayed value on the Analyzer. When in the
bipolar mode, the Analyzer displays higher than expected values – up to 35%
higher. The same test performed on some current measuring analyzers
produces lower than expected values. This discrepancy is caused by the
difference in fundamental frequencies between the monopolar and bipolar
modes. The Conmed operates at 500 kHz in monopolar mode and 1 MHz in
bipolar mode. When comparing readings measured with a V2/R device to
those measured with an I2*R device, the total measurement difference is
usually larger than 35%, because of different methods of deriving power.
This difference does not mean that the Analyzer is malfunctioning or is in
error. Rather, it reflects the different results that the two measurement
techniques produce when the load deviates from the nominal value used in the
power calculations.
Note
Most ESU manufacturers use the current measuring technique to
calibrate production units.
A-1
Page 66

RF303
Operators Manual
A-2
Page 67

Appendix B
Interpretation of Fluctuating
Readings
Interpretation of Fluctuating Readings
The RF303 Analyzer incorporates an accurate digital measurement system that
operates in three modes. The first measurement mode (default mode) utilizes a
relatively short sampling time and does not filter or average ESU output. The
two selectable measurement modes utilize longer sampling times that average
ESU output. When using the default measurement mode, you may observe
some ESU output readings that fluctuate plus or minus 10% or more,
depending on the unit under test. This is normal operation and is not indicative
of a problem with the Analyzer.
When fluctuating readings are observed, take note and determine if this is
normal behavior for the ESU under test, or if this behavior is a sign of a
problem.
• Older ESUs may have unstable output due to old technology or
design. This is typical and is acceptable, based on the manufacturer’s
limits.
• Many newer-generation ESUs utilize instantaneous feedback loops,
which constantly adjust the output and can cause an oscillating effect.
This result is also satisfactory and is considered normal for these
devices.
• In some cases, fluctuating ESU power output is evidence of a
problem. Fluctuating output on some ESUs may indicate the
weakening of output from power transistors or other ESU ailments
and is not acceptable.
If an ESU normally has fluctuating output, then operating the Analyzer in one
of the Signal Averaging Modes (SAM) may be desirable. SAM significantly
reduces fluctuating readings on the display of the Analyzer making it easy to
read an average value.
B-1
Page 68

RF303
Operators Manual
B-2
Page 69

Appendix C
Abbreviations
Abbreviations
The following list includes abbreviations used in this document.
A ampere
ANSI American National Standards Institute
AAMI
BLU blue (color)
BPM beats per minute
dB decibel
°C degrees Celsius (centigrade)
CQM Contact Quality Monitor
DMM digital multimeter
EEPROM electrically erasable PROM
ECG electrocardiograph or electrocardiogram
ESU Electrosurgery Unit
EUT equipment under test
°F degrees Fahrenheit
GRA gray (color)
GRN green (color)
Hz hertz
Association for the Advancement of Medical
Instrumentation
C-1
Page 70

RF303
Operators Manual
in inch
K kilo (103)
kg kilogram
kHz kilohertz
ke kilohm
lb pound
LED light-emitting diode
LCD liquid crystal display
6
M meg(a) (10
MHz megahertz
Me megohm
m meter
m milli (10
mA milliampere
)
-3
)
mm millimeter
mV millivolt
p-p peak-to-peak
REM Return Electrode Monitor
s second
YEL yellow (color)
-6
µ micro (10
)
µA microampere
µV microvolt
e ohm
C-2
 Loading...
Loading...