Page 1
Instruction Manual Chloride Electrode
EUTECH INSTRUMENTS PTE LTD.
INSTRUCTION MANUAL
CHLORIDE ION ELECTRODE
1
Page 2

Instruction Manual Chloride Electrode
TABLE OF CONTENTS
GENERAL INSTRUCTIONS ..........................................................................................................3
Introduction..............................................................................................................................................4
Required Equipment.................................................................................................................................4
Required Solutions................................................................................................................................... 4
GENERAL PREPARATION............................................................................................................5
Electrode Preparation...............................................................................................................................5
Electrode Slope Check (with pH/mV meter) ...........................................................................................5
Electrode Slope Check (with ion meter)..................................................................................................6
MEASUREMENT..............................................................................................................................6
Measuring Hints....................................................................................................................................... 6
Sample Requirements...............................................................................................................................7
Units of Measurement..............................................................................................................................7
MEASUREMENT PROCEDURE ................................................................................................... 7
Direct Measurement.................................................................................................................................7
Direct Measurement of Chloride (using a pH/mV meter)........................................................................7
Direct Measurement of Chloride (using a ion meter)...............................................................................9
Low Level Chloride Determination (using a pH/mV meter).................................................................10
Low Level Chloride Determination (using an ion meter)......................................................................11
ELECTRODE CHARACTERISTICS........................................................................................... 11
Reproducibility.......................................................................................................................................11
Interferences...........................................................................................................................................11
Removal of Various Interferences with CISA .......................................................................................12
Complexation.........................................................................................................................................12
Temperature Influences..........................................................................................................................13
Electrode Response................................................................................................................................13
Limits of Detection ................................................................................................................................15
pH Effects ..............................................................................................................................................15
Electrode Life.........................................................................................................................................15
Electrode Storage...................................................................................................................................15
ELECTRODE THEORY................................................................................................................16
Electrode Operation ...............................................................................................................................16
TROUBLESHOOTING GUIDE....................................................................................................17
Meter......................................................................................................................................................17
Glassware...............................................................................................................................................17
Electrodes...............................................................................................................................................17
Standards and Reagents..........................................................................................................................18
Sample....................................................................................................................................................18
Technique...............................................................................................................................................18
TROUBLESHOOTING HINTS.....................................................................................................19
SPECIFICATIONS..........................................................................................................................21
ORDERING INFORMATION.......................................................................................................21
2
Page 3

Instruction Manual Chloride Electrode
GENERAL INSTRUCTIONS ............................................................ Error! Bookmark not defined.
Introduction............................................................................................................Error! Bookmark not defined.
Required Equipment...............................................................................................Error! Bookmark not defined.
Required Solutions.................................................................................................Error! Bookmark not defined.
GENERAL PREPARATION.........................................................ERROR! BOOKMARK NOT DEFINED.
Electrode Preparation.......................................................................................ERROR! BOOKMARK NOT DEFINED.
Electrode Slope Check (with pH/mV meter)....................................................E
Electrode Slope Check (with ion meter) ..........................................................E
RROR! BOOKMARK NOT DEFINED.
RROR! BOOKMARK NOT DEFINED.
MEASUREMENT.............................................................................................. Error! Bookmark not defined.
Measuring Hints...............................................................................................ERROR! BOOKMARK NOT DEFINED.
Sample Requirements.......................................................................................E
Units of Measurement......................................................................................E
RROR! BOOKMARK NOT DEFINED.
RROR! BOOKMARK NOT DEFINED.
MEASUREMENT PROCEDURE ..................................................... Error! Bookmark not defined.
Direct Measurement.........................................................................................ERROR! BOOKMARK NOT DEFINED.
Direct Measurement of Silver (using a pH/mV meter)....................................E
Direct Measurement of Silver (using an ion meter).........................................E
Direct Measurement of Sulfide (using a pH/mV meter)..................................E
Direct Measurement of Sulfide (using an ion meter).......................................E
Low Level Silver Determinations (using a pH/mV meter)..............................E
RROR! BOOKMARK NOT DEFINED.
RROR! BOOKMARK NOT DEFINED.
RROR! BOOKMARK NOT DEFINED.
RROR! BOOKMARK NOT DEFINED.
RROR! BOOKMARK NOT DEFINED.
TITRATION......................................................................................... Error! Bookmark not defined.
Titration of Sulfide...........................................................................................ERROR! BOOKMARK NOT DEFINED.
Titration of Silver.............................................................................................E
Low Level Chloride Titration .......................................................................... E
Indicator Titration ............................................................................................E
RROR! BOOKMARK NOT DEFINED.
RROR! BOOKMARK NOT DEFINED.
RROR! BOOKMARK NOT DEFINED.
ELECTRODE CHARACTERISTICS............................................... Error! Bookmark not defined.
Reproducibility.................................................................................................ERROR! BOOKMARK NOT DEFINED.
Interference ......................................................................................................E
Complexation...................................................................................................E
Temperature Influences....................................................................................E
Electrode Response..........................................................................................E
Limits of Detection...........................................................................................E
pH Effects.........................................................................................................E
Electrode Life...................................................................................................E
Electrode Storage .............................................................................................E
RROR! BOOKMARK NOT DEFINED.
RROR! BOOKMARK NOT DEFINED.
RROR! BOOKMARK NOT DEFINED.
RROR! BOOKMARK NOT DEFINED.
RROR! BOOKMARK NOT DEFINED.
RROR! BOOKMARK NOT DEFINED.
RROR! BOOKMARK NOT DEFINED.
RROR! BOOKMARK NOT DEFINED.
ELECTRODE THEORY.................................................................... Error! Bookmark not defined.
Electrode Operation..........................................................................................ERROR! BOOKMARK NOT DEFINED.
TROUBLESHOOTING GUIDE........................................................ Error! Bookmark not defined.
Meter................................................................................................................ERROR! BOOKMARK NOT DEFINED.
Glassware.........................................................................................................E
Electrodes.........................................................................................................E
Standards and Reagents....................................................................................E
Sample..............................................................................................................E
Technique.........................................................................................................E
RROR! BOOKMARK NOT DEFINED.
RROR! BOOKMARK NOT DEFINED.
RROR! BOOKMARK NOT DEFINED.
RROR! BOOKMARK NOT DEFINED.
RROR! BOOKMARK NOT DEFINED.
TROUBLESHOOTING HINTS......................................ERROR! BOOKMARK NOT DEFINED.
SPECIFICATIONS.............................................................................. Error! Bookmark not defined.
ORDERING INFORMATION........................................ERROR! BOOKMARK NOT DEFINED.
3
Page 4

Instruction Manual Chloride Electrode
EUTECH INSTRUMENTS PTE LTD.
CHLORIDE ION ELECTRODE
INSTRUCTION MANUAL
GENERAL INSTRUCTIONS
Introduction
Eutech Instruments Chloride Ion Electrode is used to measure chloride ions in aqueous solutions
quickly, simply, accurately, and economically.
Required Equipment
1. A pH/mV meter or an ion meter, either line operated or portable.
2. Semi-logarithmic 4-cycle graph paper for preparing calibration curves when using the
meter in the mV mode.
3. A magnetic stirrer.
4. Eutech Chloride Ion Combination Epoxy-body Electrode, Code no. EC-CLO-03.
5. Polishing Paper, Code no. EC-MIS-PP, to polish dirty or etched electrode membranes.
Required Solutions
1. Deionized or distilled water for solution and standard preparation.
2. Eutech Ionic Strength Adjuster (ISA), 5M NaNO
solution from your own laboratory stock, half fill a 1,000 ml volumetric flask with distilled
water and add 425 grams of reagent-grade sodium nitrate, NaNO
dissolve the solid. Fill the flask to the mark with distilled water, cap, and invert the flask
several times to mix the contents. ISA is added at the rate of 2 ml of ISA to each 100 ml of
standard or sample to adjust the ionic strength to about 0.1M.
3. Eutech Electrode Filling Solution, 1M/0.1M KNO3/KCl, Code no. EC-RE-018, for the ECCLO-03 chloride combination epoxy electrode. Eutech Electrode Filling Solution, 1M
KNO3 Code No. EC-RE-013, for the ECR-04-HC double junction reference electrode or
the EC-CLO-02 chloride combination glass electrode.
4. Eutech Chloride Standard, 0.1M NaCl, Code no. EC-SCS-CL1-BT. To prepare this
solution from your own laboratory stock, add 5.84 grams of reagent-grade sodium
chloride, NaCl, to a one liter volumetric flask about half-full of distilled water. Swirl the
flask to dissolve the solid. Fill to the mark with distilled water, cap, and upend the flask
several times to mix the solution.
, Code no. EC-ISA-CL1. To prepare this
3
. Swirl the flask to
3
4
Page 5

Instruction Manual Chloride Electrode
5. Eutech Chloride Standard, 1,000 ppm Cl-1, Code no. EC-SCS-CL2-BT. To prepare this
solution from your own laboratory stock, add 1.65 grams of reagent-grade sodium
chloride, NaCl, to a one liter volumetric flask about half-full of distilled water. Swirl the
flask to dissolve the solid. Fill to the mark with distilled water, cap, and upend the flask
several times to mix the solution.
6. Eutech Chloride Standard, 100 ppm Cl-1, Code no. EC-SCS-CL3-BT. To prepare this
solution from your own laboratory stock, add 0.165 grams of reagent-grade sodium
chloride, NaCl, to a one liter volumetric flask about half-full of distilled water. Swirl the
flask to dissolve the solid. Fill to the mark with distilled water, cap, and upend the flask
several times to mix the solution.
GENERAL PREPARATION
Electrode Preparation
Remove the rubber caps covering the electrode tips and the rubber insert covering the filling hole of
the reference electrode. Fill the combination electrode or the reference electrode with the filling
solution shipped with the electrode to a level just below the fill hole. No preparation is required
with a sealed reference electrode. Connect the electrodes to the proper terminals of the meter as
recommended by the meter manufacturer.
Electrode Slope Check (with pH/mV meter)
(Check electrodes each day)
1. To a 150 ml beaker, add 100 ml of distilled water and 2 ml of ISA. Place the beaker on a
magnetic stirrer and begin stirring at a constant rate. After assuring that the meter is in the
mV mode, lower the electrode tips into the solution.
2. Using a pipet, add 1 ml of 0.1M or 1,000 ppm chloride standard to the beaker. When the
reading has stabilized, record the millivolt reading.
3. Using a pipet, add 10 ml of the same chloride standard used above to the beaker. When the
reading has stabilized, record the millivolt reading.
4. Determine the difference between the two readings. A difference of -57±3 mV indicates
correct electrode operation, assuming the solution temperature is between 20o and 25oC.
See the TROUBLESHOOTING section if the potential change is not within this range.
Slope is defined as the change in potential observed when the concentration changes by a factor of
10.
5
Page 6

Instruction Manual Chloride Electrode
Electrode Slope Check (with ion meter)
(Check electrodes each day)
1. Prepare standard chloride solutions whose concentrations vary by tenfold. Use either the
0.1M or 1,000 ppm chloride standard. Use the serial dilution method for this preparation.
2. To a 150 ml beaker, add 100 ml of the lower value standard and 2 ml of ISA. Place the
beaker on the magnetic stirrer and begin stirring at a constant rate. Lower the electrode tips
into the solution. Assure that the meter is in the concentration mode.
3. Adjust the meter to the concentration of the standard and fix the value in the memory
according to the meter manufacturer's instructions.
4. Rinse the electrodes with distilled water and blot dry.
5. To another 150 ml beaker, add 100 ml of the higher value standard and 2 ml of ISA. Place
the beaker on the magnetic stirrer and begin stirring at a constant rate. Lower the electrode
tips into the solution.
6. Adjust the meter to the concentration of the standard and fix the value in the memory.
7. Read the electrode slope according to the meter manufacturer's instructions. Correct
electrode operation is indicated by a slope of 90-100%. See the TROUBLESHOOTING
section if the slope is not within this range.
MEASUREMENT
Measuring Hints
All samples and standards should be at the same temperature for precise measurement. A difference
o
C in temperature will result in about a 2% measurement error.
of 1
Constant, but not violent, stirring is necessary for accurate measurement. Magnetic stirrers can
generate sufficient heat to change the solution temperature. To counteract this effect, place a piece
of insulating material, such as styrofoam sheet, between the stirrer and beaker.
Always rinse the electrodes with distilled water and blot dry between measurements. Use a clean,
dry tissue to prevent cross-contamination.
For samples with high ionic strength, prepare standards whose composition is similar to the sample.
Always check to see that the membrane is free from air bubbles after immersion into the standard or
sample.
6
Page 7

Instruction Manual Chloride Electrode
Sample Requirements
All samples must be aqueous and not contain organics which can dissolve the epoxy electrode body
and/or the cement bonding the sensing crystal to the electrode body. Infrequent measurements in
solutions containing methanol, ethanol, benzene, and acetonitrile are permitted. Highly polar
solvents slowly attack the electrode. Please check with Eutech Instruments Pte Ltd. before using the
electrode in other organic solvents.
The temperature of the standard solutions and of the sample solutions should be the same and
o
below 50
C.
Interferences should be absent. If they are present, use the procedures found in the
Interferences
and Electrode Response sections to remove them.
The pH range for the chloride ion electrode is 2-12. Neutralize samples outside this range with acid
or base to bring them in range.
Units of Measurement
Chloride concentrations are measured in units of parts per million, equivalents per liter, moles per
liter, or any other convenient concentration unit. Table 1 indicates some of the concentration units.
TABLE 1: Concentration Unit Conversion Factors
ppm Cl-1 moles/liter Cl
-1
354.50 1.0X10-2
35.45 1.0X10-3
3.55 1.0X10-4
MEASUREMENT PROCEDURE
Direct Measurement
Direct measurement is a simple procedure for measuring a large number of samples. A single meter
reading is all that is required for each sample. The ionic strength of samples and standards should
be made the same by adjustment with ISA for all chloride solution. The temperature of both sample
solution and of standard solutions should be the same.
Direct Measurement of Chloride (using a pH/mV meter)
1. By serial dilution of the 0.1M or 1,000 ppm standards, prepare 10-2, 10-3, and 10-4M or 100
and 10 ppm chloride standards. Add 2 ml of ISA per 100 ml of standard. Prepare standards
with a composition similar to the samples if samples have an ionic strength above 0.1M.
-4
2. Place the most dilute solution (1.0x10
M or 10 ppm) on the magnetic stirrer and begin
stirring at a constant rate. After assuring that the meter is in the mV mode, lower the
electrode tips into the solution. When the reading has stabilized, record the mV reading.
7
Page 8
Instruction Manual Chloride Electrode
3. Place the mid-range solution (1.0x10-3M or 100 ppm) on the magnetic stirrer and begin
stirring. After rinsing the electrodes with distilled water, blot dry and immerse the
electrode tips in the solution. When the reading has stabilized, record the mV reading.
4. Place the most concentrated solution (1.0x10-2M or 1,000 ppm) on the magnetic stirrer and
begin stirring. After rinsing the electrodes with distilled water, blot dry and immerse the
electrode tips in the solution. When the reading has stabilized, record the mV reading.
5. Using the semi-logarithmic graph paper, plot the mV reading (linear axis) against the
concentration (log axis). Extrapolate the curve down to about 1.0X10-5M or 1 ppm. A
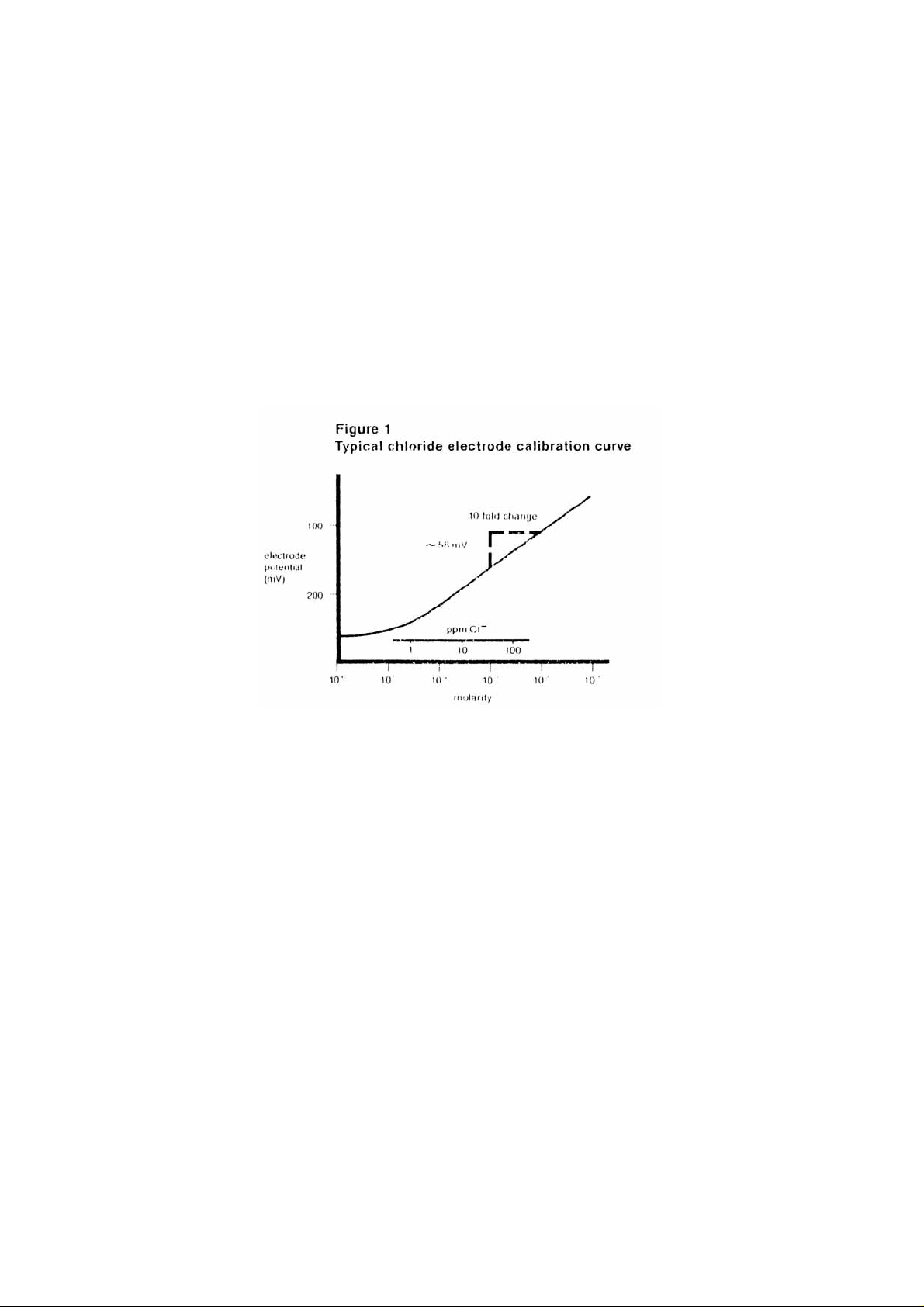
typical calibration curve can be found in Figure 1.
A calibration curve is constructed on semi-logarithmic paper when using a
pH/mV meter in the millivolt mode. The measured electrode potential in
mV (linear axis) is plotted against the standard concentration (log axis). In
the linear region of the curve, only three standards are necessary to
determine a calibration curve. In the non-linear region, additional points
must be measured. The direct measurement procedures given are for the
linear portion of the curve. The non-linear portion of the curve requires the
use of low level procedures.
6. To a clean, dry 150 ml beaker, add 100 ml of sample and 2 ml of ISA. Place the beaker on
the magnetic stirrer and begin stirring. Place the electrode tips in the solution. When the
reading has stabilized, record the mV reading. Determine the concentration directly from
the calibration curve.
7. The calibration should be checked every two hours. Assuming no change in ambient
temperature, place the electrode tips in the mid-range standard. After the reading has
stabilized, compare it to the original reading recorded in Step 3 above. A reading differing
by more than 0.5 mV or a change in the ambient temperature will necessitate the repetition
of Step 2-5 above. A new calibration curve should be prepared daily.
8
Page 9

Instruction Manual Chloride Electrode
Direct Measurement of Chloride (using a ion meter)
1. By serial dilution of the 0.1M or 1,000 ppm chloride standard, prepare two chloride
standards whose concentration is near the expected sample concentration. Measure out 100
ml of each standard into individual 150 ml beakers and add 2 ml of ISA to each.
2. Place the more dilute solution on the magnetic stirrer and begin stirring at a constant rate.
Assure that the meter is in the concentration mode.
3. Lower the electrode tips into the solution.
4. Adjust the meter to the concentration of the chloride standard and fix the value in the
memory according to the meter manufacturer's instructions after stabilization of the
reading.
5. Rinse the electrodes with distilled water and blot dry.
6. Place the more concentrated solution on the magnetic stirrer and begin stirring at a
constant rate.
7. Lower the electrode tips into the solution.
8. Adjust the meter to the concentration of the chloride standard and fix the value in the
memory according to the meter manufacturer's instructions after stabilization of the
reading.
9. For low level measurements, place the rinsed, dried electrodes into the solution containing
100 ml of distilled water and 2 ml of ISA. After stabilization, fix the blank value in the
meter according to the meter manufacturer's instructions.
10. Place 100 ml of the sample and 2 ml of ISA in a 150 ml beaker, place it on the magnetic
stirrer, and begin stirring.
11. Immerse the electrode tips in the solution and wait for the reading to stabilize. Read the
concentration directly from the meter display.
12. The calibration should be checked every two hours. Assuming no change in ambient
temperature, place the electrode tips in the first chloride standard. After the reading has
stabilized, compare it to the original reading in Step 4 above. A reading differing by more
than 0.5 mV or a change in the ambient temperature will necessitate the repetition of Steps
2-8 (9) above. The meter should be re-calibrated daily.
9
Page 10

Instruction Manual Chloride Electrode
Low Level Chloride Determination (using a pH/mV meter)
Use the following low level chloride measurement procedure in the non-linear portion of the
calibration curve. (See Figure 1). This procedure is recommended for solutions containing less than
1.0X10-4M.
1. Using 20 ml of standard ISA, dilute to 100 ml with distilled water. This low level ISA
(1.0M NaNO3) is added at the rate of 1 ml low level ISA to each 100 ml of sample. The
-2
background ionic strength will be 1.0X10
M.
2. Dilute 10 ml of 0.1M standard to 100 ml to prepare a 1.0X10
-2
M standard solution for
measurements in moles per liter. Use the 1,000 ppm standard for measurements in ppm.
Standards should be prepared fresh daily.
3. Add 1 ml of the low level ISA to a 100 ml volumetric flask and fill to the mark with
distilled water. Pour this solution into a 150 ml beaker and place the beaker on the
magnetic stirrer. Begin stirring at a constant rate.
4. Place the electrode tips in the solution. Assure that the meter is in the mV mode.
5. Add increments of the 1.0X10-2M or 1,000 ppm standard as given in Table 2 below.
6. After the reading has stabilized, record the mV reading after each addition.
TABLE 2: Step-wise Calibration for Low Level Chloride Measurements
Added Concentration
Step Pipet Volume (ml) M ppm
1 A 0.1 1.0X10-5 1.0
-5
2 A 0.1 2.0X10
3 A 0.2 4.0X10
2.0
-5
4.0
4 A 0.2 6.0X10-5 6.0
-5
5 A 0.4 9.9X10
6 B 2.0 2.9X10
9.9
-4
29.0
7 B 2.0 4.8X10-4 48.0
Pipet A = 1 ml graduated pipet
Pipet B = 2 ml pipet
Solutions: additions of 1,000 ppm or 1.0X10-2M standard to 100 ml
of ISA as prepared in Step 3 above.
7. On a semi-logarithmic graph paper, plot the millivolt reading (linear axis) against the
concentration (log axis) as in Figure 1.
8. Rinse the electrodes in distilled water and blot dry.
10
Page 11

Instruction Manual Chloride Electrode
9. Measure out 100 ml of the sample into a 150 ml beaker, add 1 ml of low level ISA, and
place the beaker on the magnetic stirrer. Begin stirring. Lower the electrode tips into the
solution. After the reading has stabilized, record the mV reading and determine the
concentration from the low level calibration curve.
10. Prepare a new low level calibration curve daily. Check the calibration curve every two
hours by repeating Steps 2-7.
Low Level Chloride Determination (using an ion meter)
Follow the procedure given for normal chloride determinations using an ion meter and the blank
correction procedure.
ELECTRODE CHARACTERISTICS
Reproducibility
Electrode measurements reproducible to ±2% can be obtained if the electrode is calibrated every
hour. Factors like temperature fluctuations, drift, and noise limit reproducibility. Reproducibility is
independent of concentration within the electrode's operating range.
Interferences
A surface layer of silver metal may be formed by strongly reducing solutions. A layer of silver salt
may be deposited on the membrane if high levels of ions forming very insoluble salts are present in
the sample. Performance may be restored by polishing. See the section Electrode Response for
proper polishing procedure.
Though measurements can be made in solutions containing oxidizing agents such as MnO
-1
4
mercury ions must not be present in the samples.
The maximum allowable ratio of interfering ion to chloride ion is given in Table 3. This ratio is
expressed as the ratio of the interfering ion molarity to the chloride molarity. Readings will be in
error if this ratio is exceeded. Neither accuracy of the measurement nor surface of the electrode
membrane will be affected if the ratio is less than that listed in the table.
TABLE 3: Maximum Allowable Ratio of Interfering Ion to Chloride Ion
Interferences Chloride Ion (M)
-1
OH
NH
S2O
Br
S
I
CN
(1)
80
(2)
1.2X10
3
-2
(2)
1.0X10
3
-1
(3)
3.0X10
-2
(4)
1.0X10
-1
(3)
5.0X10-7
-1
(4)
2.0X10
-1
-2
-3
-6
-7
,
11
Page 12

Instruction Manual Chloride Electrode
1. Acidify with 1M HNO3 to pH 4 to remove hydroxide interference.
2. These substances represent complexing species whose maximum level can be exceeded
without electrode damage. Value shown represents a 1% error.
3. Add CISA to solutions containing mixed halides to remove interferences. See the
procedure below.
4. Add CISA or a solution of Ni+2 to remove sulfide or cyanide interferences.
Removal of Various Interferences with CISA
CISA is an oxidizing agent which will oxidize up to a 100-fold excess of CN-1 over Cl-1, 100 ppm
NH3, 100 ppm Br-1 or I-1, or 500 ppm S-2. Chloride measurement interferences may be removed by
using CISA. The reagents used to prepare CISA are strong oxidizing agents and should be handled
in a fume hood.
To prepare CISA, add approximately 800 ml of distilled water to a 1 liter volumetric flask. Add
15.1 grams of NaBrO3 to the flask and swirl to dissolve the solid. Slowly add 75 ml of concentrated
nitric acid (70% w/w or 15.9N), mix, and dilute to the mark with distilled water.
To use CISA, mix equal amounts of CISA and sample. Solutions should be allowed to stand for ten
minutes before measuring. Since chloride will be oxidized upon prolonged standing, all standards
or samples mixed with CISA should be discarded after measuring. A fresh mixture of CISA and
standard should be prepared for each calibration. After adding CISA, follow the procedures for
direct measurement.
Complexation
Total concentration (Ct) consists of free ions (Cf) and complexed or bound ions (Cc) in solution:
Ct = Cf + Cc
Since the electrode only responds to free ions, any complexing agent in the solution reduces the
measured concentration of ions.
12
Page 13

Instruction Manual Chloride Electrode
Chloride ions complex with some metal ions. Table 4 lists the levels of complexing metals causing
a 10% error at 1.0X10-4M chloride.
TABLE 4: Levels of Complexing Agents Causing a 10% Error at 1.0X10-4M Chloride
Ion Concentration
Bi
Cd
Mn
Pb
Sn
Tl
+3
4.0X10-4M (80 ppm)
+2
2.0X10-3M (200 ppm)
+2
2.0X10-2M (1100 ppm)
+2
2.0X10-3M (400 ppm)
+2
6.0X10-3M (700 ppm)
+3
4.0X10-5M (8 ppm)
Temperature Influences
Samples and standards should be within ±1C of each other, since electrode potentials are influenced
by changes in temperature. Because of the solubility equilibrium on which the electrode depends,
the absolute potential of the reference electrode changes slowly with temperature. The slope of the
electrode, as indicated by the factor "S" in the Nernst equation, also varies with temperature. Table
5 gives values for the "S" factor in the Nernst equation for the chloride ion.
TABLE 5: Temperature vs. Values for the Electrode Slope
Temp. (oC) "S"
0 54.2
10 56.2
20 58.2
25 59.2
30 60.1
40 62.1
50 64.1
If changes in temperature occur, the electrodes should be re-calibrated. The temperature range for
the chloride ion electrode is 0o-80oC, provided that temperature equilibrium has occurred. If the
temperature varies substantially from room temperature, equilibrium times up to one hour are
recommended.
Electrode Response
Plotting the electrode mV potential against the chloride concentration on semi-logarithmic paper
results in a straight line with a slope of about 56 mV per decade. (Refer to Figure 1.)
13
Page 14

Instruction Manual Chloride Electrode
The time needed to reach 99% of the stable electrode potential reading, the electrode response time,
varies from several seconds in highly concentrated solutions to several minutes near the detection
limit. (Refer to Figure 2.)
A drifting potential reading or a decrease in electrode slope may mean that the electrode membrane
needs polishing.
To polish the membrane:
1. If using polishing paper, cut off a 1-2" piece and place it face up on the lab bench.
2. Put a few drops of distilled or deionized water in the center of the paper.
3. Holding the paper (cotton) steady with one hand, bring the membrane of the electrode
down perpendicular to the paper and, with a slight swirling motion, gently polish the tip of
the electrode against the surface of the polishing paper (cotton) for a few seconds.
4. Rinse the electrode surface with distilled or deionized water and soak the electrode tip in
standard solution for about five minutes before use.
5. If using jeweller's rouge, place a cotton ball on the table top and flatten it using the bottom
of a beaker.
6. Put 1-2 drops of distilled or deionized water in the center of the cotton pad. Add a small
amount of jeweller's rouge to the damp cotton.
7. Continue with Steps 3 and 4 above.
14
Page 15

Instruction Manual Chloride Electrode
Limits of Detection
The upper limit of detection in pure sodium chloride solutions is 1M. In the presence of other ions,
the upper limit of detection is above 1.0x10-1M chloride, but two factors influence this upper limit.
Both the possibility of a liquid junction potential developing at the reference electrode and the salt
extraction effect influence this upper limit. Some salts may extract into the electrode membrane at
high salt concentrations, causing deviation from the theoretical response. Either dilute samples
between 1M and 1.0x10-1M or calibrate the electrode at 4 or 5 intermediate points.
The lower limit of detection is influenced by the slight water solubility of the electrode pellet.
Refer to Figure 1 for a comparison of the theoretical response to the actual response at low levels of
chloride. Chloride measurements below 10-4M CL-1 should employ low level procedures.
pH Effects
Hydroxide ion interferes with measurements of low levels of chloride although the electrode can be
used over a reasonable pH range. Table 3 should be used to determine the minimum pH at which
low level chloride measurements can be made without more than a 10% error due to hydroxide ion
interference.
Electrode Life
A chloride ion electrode will last for six months in normal laboratory use. On-line measurements
might shorten operational lifetime to several months. In time, the response time will increase and
the calibration slope will decrease to the point calibration is difficult and electrode replacement is
required.
Electrode Storage
The electrode may be stored for short periods of time in 1.0x10-2M chloride solution. For longer
storage (longer than two weeks), rinse and dry the sensing pellet and cover the membrane tip with
any protective cap shipped with the electrode. The reference portion of the combination electrode
(or the outer chamber of the reference electrode) should be drained of filling solution, if refillable,
and the rubber insert placed over the filling hole.
15
Page 16

Instruction Manual Chloride Electrode
ELECTRODE THEORY
Electrode Operation
A chloride ion electrode is composed of a glass or an epoxy body and a silver chloride/silver sulfide
membrane. When the membrane is in contact with a solution containing chloride ions, an electrode
potential develops across the membrane. This electrode potential is measured against a constant
reference potential, using a pH/mV meter or an ion meter. The level of chloride ions, corresponding
to the measured potential, is described by the Nernst equation:
E = E
- S log X
o
where:
E = measured electrode potential
Eo = reference potential (a constant)
S = electrode slope (~56 mV/decade)
X = level of chloride ions in solution
The activity, X, represents the effective concentration of the ions in solution. The activity is related
to the free ion concentration, Cf, by the activity coefficient, γ , by:
X = γ Cf
Activity coefficients vary, depending on total ionic strength, I, defined as:
I = ½Σ CxZ
2
x
where:
Cx = concentration of ion X
Zx = charge of ion X
Σ = sum of all of the types of ions in the solution
In the case of high and constant ionic strength relative to the sensed ion concentration, the activity
coefficient, γ , is constant and the activity, X, is directly proportional to the concentration.
To adjust the background ionic strength to a high and constant value, ionic strength adjuster (ISA)
is added to samples and standards. The recommended ISA for chloride is NaNO3. Solutions other
than this may be used as ionic strength adjusters as long as ions that they contain do not interfere
with the electrode's response to chloride ions. Samples with high ionic strength (greater than 0.1M)
do not need ISA added and standards for these solutions should be prepared with a composition
similar to the samples.
The reference electrode must also be considered. When two solutions of different composition are
brought into contact with one another, liquid junction potentials arise. Millivolt potentials occur
from the inter-diffusion of ions in the two solutions. Electrode charge will be carried unequally
across the solution boundary resulting in a potential difference between the two solutions, since
ions diffuse at different rates. When making measurements, it is important to remember that this
potential be the same when the reference is in the standardizing solution as well as in the sample
solution or the change in liquid junction potential will appear as an error in the measured electrode
potential.
16
Page 17

Instruction Manual Chloride Electrode
The composition of the liquid junction filling solution in the reference electrode is most important.
The speed with which the positive and negative ions in the filling solution diffuse into the sample
should be equitransferent. No junction potential can result if the rate at which positive and negative
charge carried into the sample is equal.
Strongly acidic (pH = 0-2) and strongly basic (pH = 12-14) solutions are particularly troublesome
to measure. The high mobility of hydrogen and hydroxide ions in samples make it impossible to
mask their effect on the junction potential with any concentration of an equitransferent salt. One
must either calibrate the electrodes in the same pH range as the sample or use a known increment
method for ion measurement.
TROUBLESHOOTING GUIDE
The goal of troubleshooting is the isolation of a problem through checking each of the system
components in turn: the meter, the glassware, the electrodes, the standards and reagents, the sample,
and the technique.
Meter
The meter may be checked by following the check-out procedure in the instrument instruction
manual.
Glassware
Clean glassware is essential for good measurement. Be sure to wash the glassware well with a mild
detergent and rinse very well with distilled or deionized water. Clean glassware will drain without
leaving water droplets behind.
Electrodes
The electrode may be checked by using the procedure found in the sections entitled Electrode
Slope Check.
1. Be sure to use distilled or deionized water when following the procedures given in
Electrode Slope Check.
2. If the electrode fails to respond as expected, see the sections
Electrode Response. Repeat the slope check.
Measuring Hints and
3. If the electrodes still fail to respond as expected, substitute another chloride ion electrode
that is known to be in good working order for the questionable electrode. If the problem
persists and you are using an electrode pair, try the same routine with a working reference
electrode.
4. If the problem persists, the reagent may be of poor quality, interferences in the sample may
be present or the technique may be faulty. (See
Technique sections below.)
Standards and Reagents, Sample, and
5. If another electrode is not available for test purposes, or if the electrode in use is suspect,
review the instruction manual and be sure to:
- Clean and rinse the electrodes thoroughly.
- Prepare the electrodes properly.
17
Page 18

Instruction Manual Chloride Electrode
- Use the proper filling solution.
- Adjust the pH and the ionic strength of the solution by the use of the proper ISA.
- Measure correctly and accurately.
- Review TROUBLESHOOTING HINTS.
Standards and Reagents
Whenever problems arise with the measuring procedure that has been used successfully in the past,
be sure to check the standard and reagent solutions. If in doubt about the credibility of any of the
solutions, prepare them again. Errors may result from contamination of the ISA, incorrect dilution
of standards, poor quality distilled/deionized water, or a simple mathematical miscalculation.
Sample
Look for possible interferences, complexing agents, or substances which could affect the response
or physically damage the sensing electrode (or the reference electrode) if the electrodes work
perfectly in the standard, but not in the sample.
Try to determine the composition of the samples prior to testing to eliminate a problem before it
starts. (See Measuring Hints, Sample Requirements, and Interferences.)
Technique
Be sure that the electrode's limit of detection has not been exceeded. Be sure that the analysis
method is clearly understood and is compatible with the sample.
Refer to the instruction manual again. Reread sections on GENERAL PREPARATION and
ELECTRODE CHARACTERISTICS.
If trouble still persists, call Eutech Instruments Pte Ltd. at (65) 6778-6876 and ask for the Customer
Services Department.
18
Page 19

Instruction Manual Chloride Electrode
TROUBLESHOOTING HINTS
Symptom Possible Causes Next Step
Out of Range defective meter check meter with shorting
Reading strap (see meter
instruction manual)
defective electrode check electrode operation
electrodes not plug electrode into position
plugged in properly and reset meter
reference electrode clean junction
junction clogged
static electricity wipe plastic meter face with
detergent solution
meter or stirrer ground meter or stirrer
not grounded
Noisy or Unstable defective meter check meter with
Readings (readings shorting strap
continuously or
rapidly changing) air bubble on membrane remove bubble by
re-dipping electrode
ISA not used use recommended ISA
meter or stirrer ground meter or
not grounded stirrer
defective electrode replace electrode
electrode exposed soak electrode in
to interferences chloride standard
Drift (reading samples and standards allow solutions to come to
slowly changing at different temperatures room temperature before
in one direction) measurement
incorrect reference use recommended
filling solution filling solution in outer
of reference electrode
membrane dirty or etched polish membrane
19
Page 20

Instruction Manual Chloride Electrode
Low Slope or standards contaminated prepare fresh standards
No Slope or incorrectly made
ISA not used use recommended ISA
standard used as ISA use ISA
"Incorrect Answer" incorrect scaling plot millivolts on the linear
(but calibration of semi-log paper axis. On the log axis, be
curve is good) sure concentration numbers
within each decade are
increasing with increasing
concentration
incorrect sign be sure to note sign of
millivolt number correctly
incorrect standards prepare fresh standards
wrong units used apply correct conversion factor:
1.0x10-3M = 35.5 ppm as Cl-
complexing agents remove complexing agent
20
Page 21

Instruction Manual Chloride Electrode
SPECIFICATIONS
Concentration Range: 1M to 5.0X10-5M (35,500 to 1.8 ppm)
pH Range: 2 to 12
Temperature Range: 0o-80oC (80 to 100oC intermittent use)
Resistance: <1 Mohm
Reproducibility: +/-2%
Interferences See section on Interferences
Size: 110 mm length; 12 mm diameter; 1 m cable length
Storage: Store in chloride solution
ORDERING INFORMATION
CODE/NO. DESCRIPTION
EC-CLO-03 Chloride Ion Combination Electrode, epoxy body
EC-SCS-CL1-BT Chloride Standard, 0.1M NaCl
EC-SCS-CL2-BT Chloride Standard, 1,000 ppm NaCl
EC-SCS-CL3-BT Chloride Standard, 100 ppm NaCl
EC-ISA-CL1-BT Chloride Ionic Strength Adjuster (ISA), 5 M NaNO3
EC-MIS-PP Polishing Paper for the Chloride Ion Electrodes
21
 Loading...
Loading...