EUTECH INSTRUMENTS AMMONIA GAS-SENSING ELECTRODE Instruction Manual

Instruction Manual Ammonia Gas-sensing Electrode
1
EUTECH INSTRUMENTS PTE LTD.
AMMONIA GAS-SENSING ELECTRODE
INSTRUCTION MANUAL
GENERAL INSTRUCTIONS
Introduction
Eutech Instruments Ammonia Gas-Sensing Electrode is used to quickly, simply, accurately, and
economically measure dissolved ammonia in aqueous solutions. It can also be used to measure the
ammonium ion after conversion to ammonia or organic nitrogen from Kjeldahl digestion of the
sample. The measurement is not affected by sample color or turbidity and samples do not need to be
distilled. Interference from anions, cations, and dissolved species, other than volatile amines, do not
occur. With a flow-through cap, the electrode can be used in flow-through applications.
Required Equipment
1. A pH/mV meter or an ion meter, either line operated or portable.
2. Semi-logarithmic 4-cycle graph paper for preparing calibration curves when using the
meter in the mV mode.
3. A magnetic stirrer.
4. Eutech Ammonia Gas-sensing Electrode, Code No. EC-NH3-01.
Required Solutions
1. Deionized or distilled water for solution preparation.
2. Eutech Ammonia Standard, 0.1M NH
4
Cl, Code No. EC-SCS-AA1-BT. To prepare this
solution from your own laboratory stock, half fill a one liter volumetric flask with distilled
water and add 5.35 grams of reagent-grade NH4Cl. Swirl the flask gently to dissolve the
solid. Fill the flask to the mark with distilled water, cap, and upend several times to mix
the solution.
3. Eutech Ammonia Standard, 1,000 ppm NH
3
as N, Code No. EC-SCS-AA2-BT. To prepare
this solution from your own laboratory stock, half fill a one liter volumetric flask with
distilled water and add 3.82 grams of reagent-grade NH4Cl. Swirl the flask gently to
dissolve the solid. Fill the flask to the mark with distilled water, cap, and upend several
times to mix the solution.
4. Eutech Ammonia Standard, 100 ppm NH3 as N, Code No. EC-SCS-AA3-BT. To prepare
this solution from your own laboratory stock, half fill a one liter volumetric flask with
distilled water and add 0.382 grams of reagent-grade NH4Cl. Swirl the flask gently to
dissolve the solid. Fill the flask to the mark with distilled water, cap, and upend several
times to mix the solution.

Instruction Manual Ammonia Gas-sensing Electrode
2
5. Eutech Ionic Strength Adjuster (ISA) Solution, 10 M NaOH, Code No. EC-ISA-AA1-BT.
To prepare this solution from your own laboratory stock, half fill a 1,000 ml beaker with
distilled water, add 400 grams of reagent-grade NaOH (sodium hydroxide). Swirl the flask
gently under a hood to dissolve the solid. Allow to cool and fill to the mark with distilled
water. Stir the solution and store in a plastic bottle.
6. Eutech Ionic Strength Adjuster (ISA) Solution, 5M NaOH/.05M Disodium EDTA/10%
Methanol with Color Indicator, Code No. EC-ISA-AA2-BT. To prepare this solution from
your own laboratory stock, half fill a 1,000 ml beaker with distilled water and add 200
grams of reagent-grade NaOH. Stir the solution to dissolve the pellets, add 18.61 grams of
disodium EDTA, and stir the solution again until all solids have dissolved. Allow solution
to cool. In a separate 150 ml beaker, add a tiny amount (10-20 mg) of thymolphthalein to
100 ml of methanol and stir to dissolve. Pour the methanol solution into the 1,000 ml
beaker and stir to blend. The solution should turn a dark blue. Fill to the 1,000 ml mark
with distilled water and stir to blend.
GENERAL PREPARATION
Electrode Preparation
This electrode is shipped dry. Before using, unscrew the large cap (See Figure 5), and remove the
inner glass electrode from the outer body. Fill the outer body with 2 to 3 ml of internal filling
solution. Place the inner glass electrode into the outer body, and screw on the large cap until finger
tight. Place the assembled electrode in an electrode holder with a 20o angle from the vertical to
avoid trapping air bubbles at the bottom of the electrode.
Checking Membrane
A small hole of any size on the membrane or breakage of the membrane causes failure of the
electrode. It is recommended to check the membrane on every newly assembled electrode.
1. Connect a newly assembled electrode to a pH/mV meter.
2. Lower the electrode tip in distilled water.
3. Record the reading after stirring the distilled water for about 15 minutes.
4. Add proper ISA solution (see
Required Solutions) to the distilled water. A drastic change
in the reading in a negative direction indicates damage of the membrane.
Changing Membrane (see Figure 4)
1. Unscrew the top cap from the outer body and remove the inner glass body from the epoxy
outer body. Carefully place the glass body aside.
2. Unscrew the bottom cap from the outer body. Remove the old membrane from around the
threads and electrode tip opening.
3. Using the tweezers provided, grab a new piece of white membrane material by the edge
and remove from the separator paper. Then, with the hand not holding the tweezers, hold

Instruction Manual Ammonia Gas-sensing Electrode
3
the electrode body up at the threads with thumb and forefinger. Place one edge of the
membrane against the threads and hold in place with your thumb. Stretch new membrane
lengthwise across the electrode opening so that it smoothly covers the opening. Place the
other edge of the membrane against the threads and hold in place with your forefinger.
4. Place the bottom cap gently over the membrane onto the threads and screw the bottom cap
on until finger-tight. Check that the membrane is free of wrinkles and holes or else repeat
the above steps.
5. Using the syringe provided, fill the outer body with approximately 2 ml of inner filling
solution. Place glass inner body into epoxy outer body containing the internal filling
solution and screw on the upper cap until finger-tight.
Connecting the Electrode to the Meter
Connect the electrode to the meter according to meter manufacturer's instructions. No external
reference electrode is required. To prevent air entrapment, mount the electrode at a 20
o
angle from
the vertical.
Electrode Slope Check (with pH/mV meter)
(check electrodes each day)
1. To a clean, dry, 150 ml beaker, add 100 ml of distilled water and 1 ml of ISA. Place the
beaker on a magnetic stirrer and begin stirring at a constant rate. After assuring that the
meter is in the millivolt mode, lower the electrode tip into the solution. Remove air bubbles
by redipping probe.
2. Using a pipet, add 1 ml of 0.1M or 1,000 ppm standard into the solution. When the reading
has stabilized, record the mV value.
3. Using a pipet, add 10 ml of the same ammonia standard used above to the beaker. When
the reading has stabilized, record the mV value.
4. Determine the difference between the two readings. The electrode is operating correctly if
a difference of 56±3 mV is found, assuming the solution temperature is between 20
o
and
25
o
C. See the TROUBLESHOOTING section if the potential change is not within this range.
Slope is defined as the change in potential observed when the concentration changes by a factor of
10.
Electrode Slope Check (with ion meter)
(check electrodes each day)
1. Prepare standard ammonia solutions whose concentrations vary by tenfold. Use either the
0.1M or 1,000 ppm ammonia standard. Use the serial dilution method for this preparation.
2. To a 150 ml beaker, add 100 ml of the lower value standard and 1 ml of ISA. Place the
beaker on the magnetic stirrer and begin stirring at a constant rate. Lower the electrode tips
into the solution. Assure that the meter is in the concentration mode.
Instruction Manual Ammonia Gas-sensing Electrode
4
3. Adjust the meter to the concentration of the standard and fix the value in the memory
according to the meter manufacturer's instructions.
4. Rinse the electrodes with distilled water and blot dry.
5. To another 150 ml beaker, add 100 ml of the higher value standard and 2 ml of ISA. Place
the beaker on the magnetic stirrer and begin stirring at a constant rate. Lower the electrode
tips into the solution.
6. Adjust the meter to the concentration of the standard and fix the value in the memory.
7. Read the electrode slope according to the meter manufacturer's instructions. Correct
electrode operation is indicated by a slope of 90-100%. See TROUBLESHOOTING section
if the slope is not within this range.
MEASUREMENT
Measuring Hints
Samples should be measured immediately after collection. Samples should be stored according to
the directions given in Sample Storage if immediate measurement is not possible.
The ratio of a surface area to volume in the beaker should be minimized. Beakers containing the
samples or the standard should be kept covered between measurements.
The ammonia ISA, 10M NaOH, should be added just before measurement. All samples and
standards should be at the same temperature for precise measurement. A difference of 1oC in
temperature will result in approximately a 2% error. All samples must be aqueous.
Always rinse the electrode with distilled water and blot dry between measurements. Use a clean,
dry tissue to prevent cross-contamination.
Constant, but not violent, stirring is necessary for accurate measurement. Magnetic stirrers can
generate sufficient heat to change solution temperature. To counteract this effect, place a piece of
insulating material, such as gauze or styrofoam, between the beaker and the magnetic stirrer.
Always check to see that the membrane is free from air bubbles after immersion into standard or
sample.
Sample Storage
Samples should be measured immediately after preparation or collection, if possible. Wait only long
enough for temperature equilibration between the sample and the electrode. If stirring a 100 ml
basic solution in a 150 ml beaker, the rate of ammonia loss at room temperature is about 50% in six
hours. The loss of C0
2
increases with increasing temperature.
If the samples cannot be measured immediately, add 0.5 ml of 1M HCl to each liter of sample to
make them slightly acidic (pH 6) and store in tightly capped vessels. Prior to measurement, add
10M NaOH to make the samples slightly basic.
Instruction Manual Ammonia Gas-sensing Electrode
5
Sample Requirements
Sodium hydroxide buffer must be added to standards and samples before measurement. When 10M
NaOH is added, all standards and samples should be in the range of pH 11 to 14. In this range, all
ammonium species are converted to ammonia. Adding the 10M NaOH adjusts the total level of
dissolved species below 1M. If the total level is greater than 1M, the sample should be diluted
before measurement. For further explanation, see the section entitled Effect of Dissolved Species.
Units of Measurement
Measurement of ammonia can be expressed in units of moles/liter, ppm as nitrogen, ppm as
ammonia, or other convenient concentration unit. Table 1 lists conversion units.
TABLE 1: Concentration Unit Conversion Factors
moles/liter ppm N ppm NH3
10
-
2
140.0 170.0
10
-
3
14.0 17.0
10
-
4
1.4 1.7
MEASUREMENT PROCEDURE
Direct Measurement
Direct measurement is a simple procedure for measuring a large number of samples. A single meter
reading is all that is required for each sample. The ionic strength of samples and standards should
be made the same by adjustment with ISA for all ammonia solutions. The temperature of both
sample solutions and standard solutions should be the same.
Direct Measurement of Ammonia (using a pH/mV meter)
1. By serial dilution, prepare three standard solutions from the 0.1M or 1,000 ppm stock
standard. The resultant concentrations should be 10
-
2
M, 10
-
3
M, and 10
-
4
M or 1,000, 100
and 10 ppm ammonia standards. Add 1 ml of ISA per 100 ml of standard. Prepare
standards with a composition similar to the samples if the samples have an ionic strength
above 0.1M.
2. Place the most dilute solution (10
-
4
M or 10 ppm) on the magnetic stirrer and begin stirring
at a constant rate. After assuring that the meter is in the mV mode, lower the electrode tip
into the solution. After the reading has stabilized, record the mV value.
3. Place the mid-range solution (10
-
3
M or 100 ppm) on the magnetic stirrer and begin stirring
at a constant rate. After rinsing the electrode with distilled water, blot dry, and immerse the
electrode tip in the solution. When the reading has stabilized, record the mV value.
4. Place the most concentrated solution (10
-
2
M or 1,000 ppm) on the magnetic stirrer and
begin stirring at a constant rate. After rinsing the electrode with distilled water, blot dry,
and immerse the electrode tip in the solution. When the reading has stabilized, record the
mV value.
Instruction Manual Ammonia Gas-sensing Electrode
6
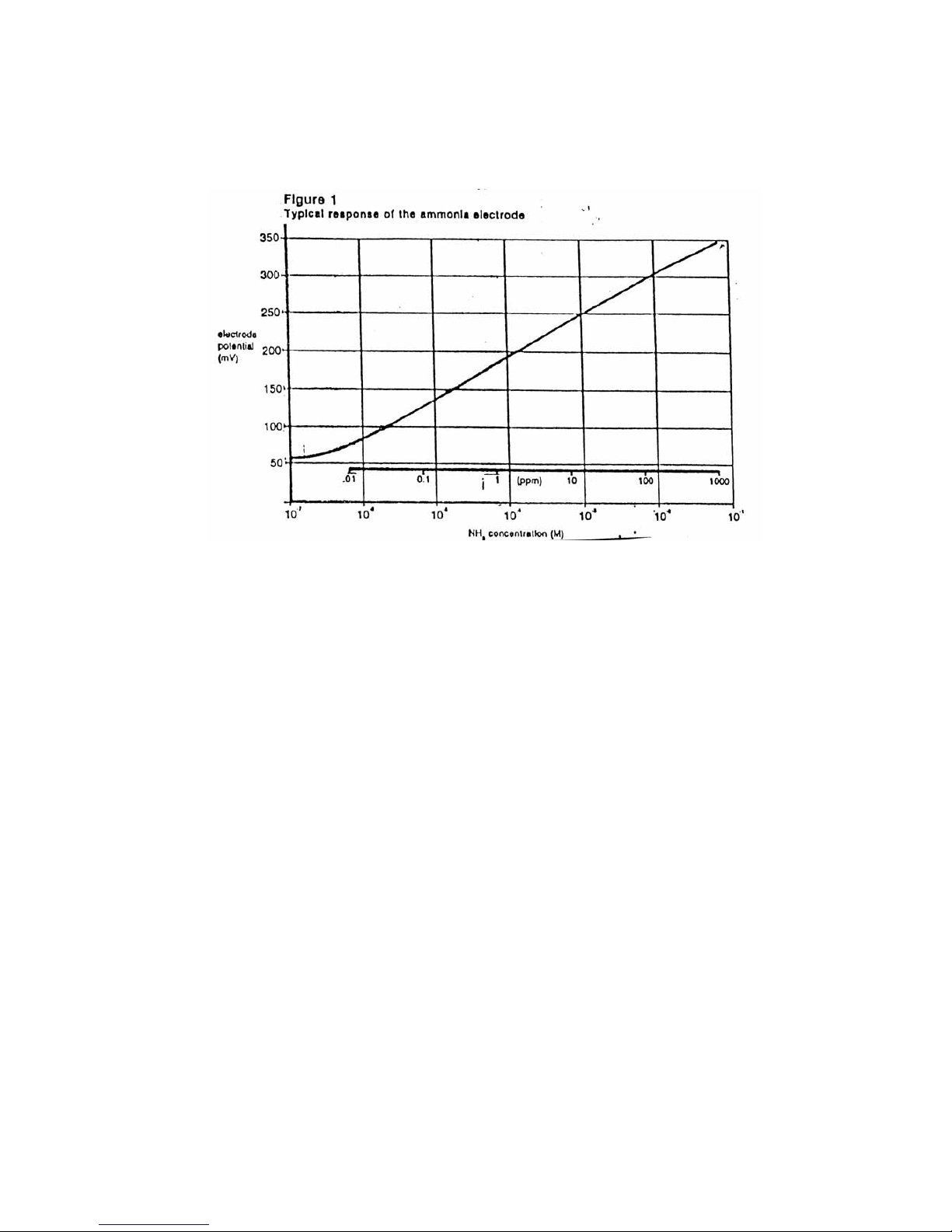
5. Using the semi-logarithmic graph paper, plot the mV reading (linear axis) against the
concentration (log axis). Extrapolate the calibration curve down to about 1.0 x 10
-
5
M. A
typical calibration curve can be found in Figure 1.
A calibration curve is constructed on semi-logarithmic paper when using the pH/mV
meter in the millivolt mode. The measured electrode potential in mV (linear axis) is
plotted against the standard concentration (log axis). In the linear region of the curve,
only three standards are necessary to determine a calibration curve. In the non-linear
region, additional points must be measured. The direct measurement procedures
given are for the linear portion of the curve. The non-linear portion of the curve
requires the use of low level procedures.
6. To a clean, dry 150 ml beaker, add 100 ml of sample and 1 ml of ISA. Place the beaker on
the magnetic stirrer and begin stirring at a constant rate. Rinse the electrodes with distilled
water, blot dry, and lower the electrode tip into the solution. When the reading has
stabilized, record mV reading. Using the calibration curve, determine sample
concentration.
7. The calibration should be checked every two hours. Assuming no change in ambient
temperature, place the electrode tips in the mid-range standard. After the reading has
stabilized, compare it to the original reading recorded in Step 3 above. A reading differing
by more than 0.5 mV or a change in the ambient temperature will necessitate the repetition
of steps 2-5 above. A new calibration curve should be prepared daily.
Direct Measurement of Ammonia (using an ion meter)
1. By serial dilution of the 0.1M or 1,000 ppm ammonia standard, prepare two ammonia
standards whose concentration is near the expected sample concentration. Add 1 ml of ISA
to each 100 ml of standard.

Instruction Manual Ammonia Gas-sensing Electrode
7
2. Place the more dilute solution on the magnetic stirrer and begin stirring at a constant rate.
Assure that the meter is in the concentration mode.
3. Lower the electrode tip into the solution.
4. Adjust the meter to the concentration of the ammonia standard and fix the value in the
memory according to the meter manufacturer's instructions after stabilization of the
reading.
5. Rinse the electrode with distilled water and blot dry.
6. Place the most concentrated solution on the magnetic stirrer and begin stirring at a constant
rate.
7. Lower the electrode tip into the solution. Adjust the meter to the concentration of the
ammonia standard and fix the value in the memory according to the meter manufacturer's
instructions after stabilization of the reading.
8. For low level measurements, place the rinsed, dried electrodes into a solution containing
100 ml of distilled water and 1 ml of ISA. After stabilization, fix the blank value in the
meter according to the meter manufacturer's instructions.
9. Place 100 ml of the sample and 1 ml of ISA in a 150 ml beaker. Place the beaker on the
magnetic stirrer and begin stirring.
10. Lower the electrode tip in solution. When the reading has stabilized, read the concentration
directly from the meter display.
11. The calibration should be checked every 2 hours. Assuming no change in ambient
temperature, place the electrode tip in the first ammonia standard. After the reading has
stabilized, compare it to the original reading in step 3 above. A reading differing by more
than 0.5 units or a change in ambient temperature will necessitate the repetition of steps 28 above. The meter should be re-calibrated daily.
Low Level Ammonia Determination (using a pH/mV meter)
As the concentration of ammonia decreases, the rate of ammonia diffusion through the membrane is
slow, the rate of equilibrium between the ammonium in the internal filling solution and ammonia is
slow, and thus the response time increases. If the internal filling solution is diluted with ammoniafree distilled water (1:10), response at low levels can improve. Measurements can be speeded up by
first placing the electrode tip in an ammonia-free pH 4 buffer, then into the sample. Always keep
standards and samples covered. Work with large solution volumes to minimize surface-area-tovolume ratio, thereby avoiding ammonia absorption from air. Allow 5-10 minutes for a stable
reading in the pH 4 buffer or a low-level solution.
Use the following low level ammonia measurement procedure in the non-linear portion of the
calibration curve. (See Figure 1.) This procedure is used for ammonia samples containing less than
1.0x10
-
5
M ammonia.
 Loading...
Loading...