Page 1

Generator 300 System
User Manual
*
*Formerly known as
ULTRACISION®HARMONIC SCALPEL
®
Page 2

Page 3

GEN04
Table of Contents
Chapter 1 – General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Indications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Chapter 2 – Principles of Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
System Components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Generator 300 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Hand Piece . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Instrument . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Power Levels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Controls, Indicators, and Connections . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Unpacking Instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Chapter 3 – System Setup and Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
System Startup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
System Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
System Shutdown . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Chapter 4 – Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Audible Indicators and Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Error Codes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Chapter 5 – Cleaning and Disinfection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Generator and Cart Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Foot Switch Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Chapter 6 – Safety and Function Testing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Safety Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Function Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Chapter 7 – Warnings and Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
System Warnings and Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Instrument Warnings and Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
Chapter 8 – System Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Chapter 9 – Warranty . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Chapter 10 – Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Page 4

Page 5

Please read all information carefully.
Failure to properly follow the instructions may lead to serious surgical consequences.
Important: The HARMONIC™ Generator 300 System User Manual is designed to provide instructions for use of the HARMONIC
Generator 300, Foot Switch, and Cart (see Chapter 8 – System Specifications for applicable product codes). This manual is not a
reference to surgical techniques.
Note: Refer to package inserts provided separately for information about the Hand Piece, Hand Switching Adaptor, Adaptors,
Test Tip and Instruments prior to using the system.
Indications
The HARMONIC System is indicated for soft tissue incisions when bleeding control and minimal thermal
injury are desired. The HARMONIC System instruments can be used as an adjunct to or substitute for
electrosurgery, lasers, and steel scalpels.
Contraindications
• The instruments are not indicated for incising bone.
• The instruments are not intended for contraceptive tubal occlusion.
Chapter 1 – General Information
1
GEN04
Page 6

User Manual
2
GEN04
Page 7

The HARMONIC System utilizes ultrasonic energy to enable hemostatic cutting and/or coagulation of soft
tissue. The system consists of an ultrasonic generator, a foot switch, an optional hand-switching adaptor, a
hand piece, and a variety of open and minimally invasive instruments.
Important: The HARMONIC Generator 300 System User Manual is designed to provide instructions for use of
the HARMONIC Generator 300, Foot Switch, and Cart (see Chapter 8 – System Specifications for applicable
product codes). This manual is not a reference to surgical techniques.
Note: Refer to package inserts provided separately for information about the Hand Piece, Hand Switching
Adaptor, Adaptors, Test Tip and Instruments prior to using the system.
System Components
Generator 300
The generator supplies the hand piece with electrical energy and facilitates selection of power levels, system
monitoring, and system diagnostics.
Power is delivered by activating the foot switch or hand switch.
Hand Piece
The hand piece contains an acoustic transducer that converts the electrical energy supplied by the generator
to mechanical motion. The transducer is connected to an amplifier which amplifies the motion produced by
the transducer and relays it to the instrument.
Instrument
The mechanical motion from the hand piece advances to the instrument, transmitting ultrasonic energy which
enables hemostatic cutting and/or coagulation of tissue.
Note: Throughout this manual “instrument(s)” refers to HARMONIC blades, ball coagulators, or coagulating
shears.
Power Levels
The generator delivers two power levels: minimum (MIN) and maximum (MAX). The minimum power
level may be adjusted by the user from Level 1 to 5. The maximum power level is always Level 5. With all
instruments except the ball coagulator, use a higher generator power level for greater tissue cutting speed and
a lower generator power level for greater coagulation. For the ball coagulator, higher generator power levels
will provide greater coagulation. The amount of energy delivered to the tissue and resultant tissue effects are
a function of many factors including the power level selected, instrument characteristics, grip force (when
applicable), tissue tension, tissue type, pathology, and surgical technique.
Note: Refer to the instruments’ package inserts for additional power level information, including
recommended starting power levels.
Chapter 2 – Principles of Operation
3GEN04
Page 8

User Manual
4
GEN04
Controls, Indicators, and Connections
Fig. 2-1 Front Panel
1 READY When this indicator is green, the system is ready for activation.
2 STANDBY Push this button to toggle between Standby and Ready modes. In Standby mode,
this button, and the STANDBY icon, light up and all power is removed from the
hand piece. Both the foot switch and hand switch are disabled. Upon power-up, the
system defaults to Standby mode enabled.
3 INCREASE/ Push this button to increase or decrease the minimum (MIN) power
DECREASE POWER setting to the desired level (from 1 to 5). The level chosen will be shown on the
LEVEL graphic display. The power level may be adjusted when the generator is in Ready or
Standby mode.
4 POWER This switch controls the main electrical power to the generator.
5 VOLUME Turn this knob to adjust the volume of the activation tones. A tone will sound
indicating the volume level selected.
6MIN Indicates the user-settable minimum power level setting. When this power level is
activated (by foot switch or hand switch), the “MIN” indicator will flash. The
system defaults to “MIN” power level 3. Refer to the instruments’ package inserts
for the recommended minimum power level.
7 MAX Indicates the maximum power level setting. This setting is always “5”. When this
power level is activated (by foot switch or hand switch), the “MAX” indicator
will flash.
8 ALARM INDICATOR This red indicator appears only if a system alarm occurs in response to a component
or generator problem.
Page 9
9 HAND PIECE This receptacle is used to connect the hand piece to the generator.
RECEPTACLE
10 HAND ACTIVATION When the indicator is green, hand activation on the hand switching adaptor is
enabled. To disable the Hand Activation mode, depress the button. Upon power-up,
the system defaults to Hand Activation mode disabled.
Note: If the foot switch is installed, the foot switch is always enabled.
11 TEST Depressing this button initiates the Test mode. This mode is used during
troubleshooting. The generator will emit a tone when the Test mode is active and
“TEST IN PROGRESS” will appear on the display.
12 GRAPHIC DISPLAY In Ready or Standby modes, this display indicates the minimum (user-settable
level 1 to 5) and maximum (level 5) power levels. If a system or component
problem exists, error codes will appear on this display.
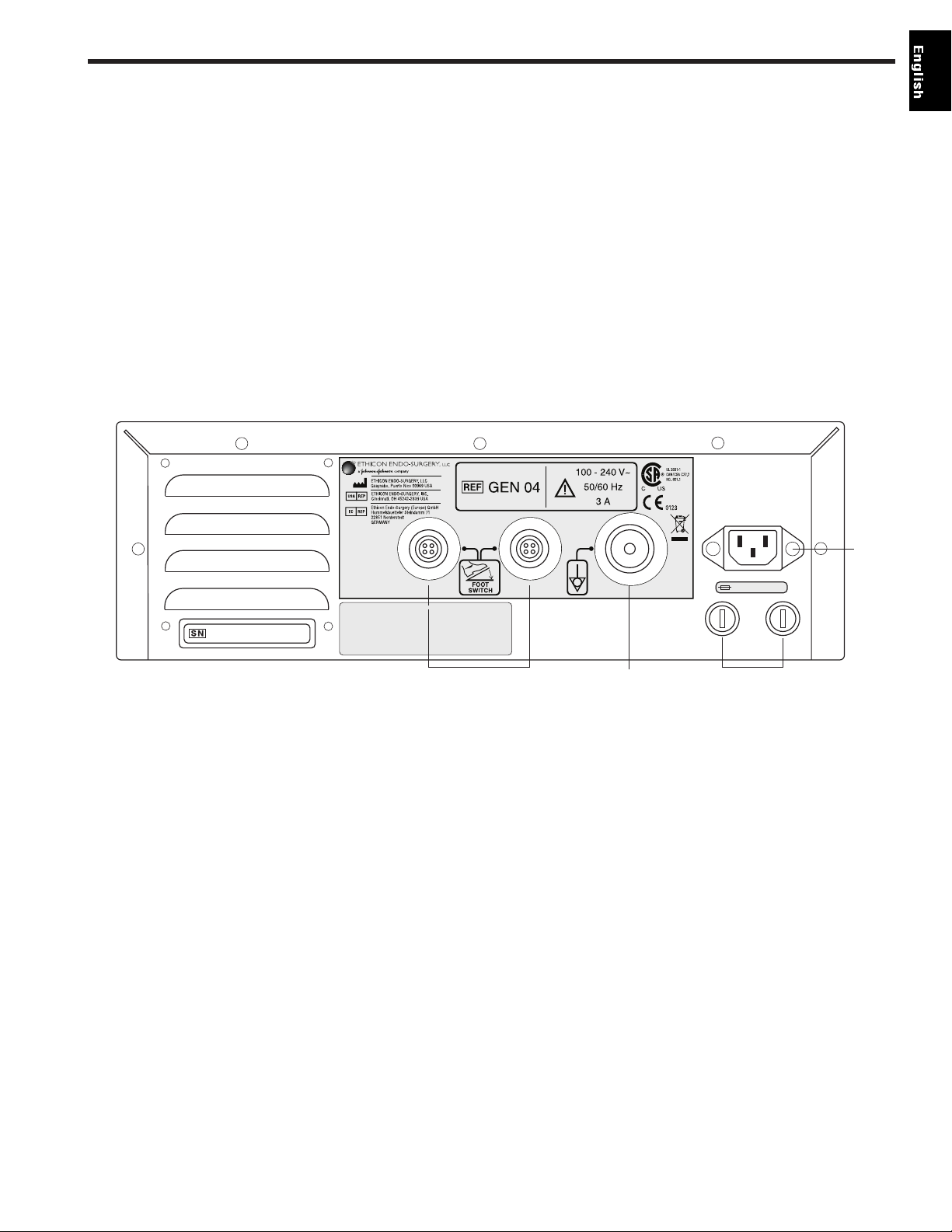
Fig. 2-2 Back Panel
13 FOOT SWITCH Identical receptacles allow connection of up to two foot switches for
RECEPTACLES user convenience. If only one foot switch is used, connect to either receptacle.
14 POTENTIAL This terminal provides a means for connection to a Potential Equalization
EQUALIZATION Conductor.
TERMINAL
15 FUSES See the H
ARMONIC Generator 300 Service Manual for additional information.
16 POWER CORD This receptacle is used to attach the power cord to the generator. For power cord
RECEPTACLE requirements, see Chapter 8 – System Specifications.
AUDIBLE SIGNALS The generator delivers audible tones to signal activation and alarm states. The user
may choose from three activation tone pitches. See Chapter 3 – System Setup and
Operation for tone selection information. Upon power-up, the system defaults to the
last tone chosen (the mid-pitch tone is factory-set).
Chapter 2 – Principles of Operation
5GEN04
T3.15H 250V
13 14 15
16
6,678,621
6,908,472
Covered by one or more of the following US Patents
Page 10
GEN04
User Manual
6
Unpacking Instructions
The HARMONIC Generator 300 System includes several components that are purchased separately. Upon
receiving the ordered components, check for visible shipping damage. If damage is seen, contact your
Ethicon Endo-Surgery representative.
System components may include the following parts (for product codes, see Chapter 8 – System
Specifications):
Generator 300 – includes the generator, power cord, user manual, and service manual.
Note: The User Manual includes a troubleshooting guide (see back pocket of manual binder). Remove the
self-adhesive guide’s backing and adhere the guide to the top panel of the generator. Placement guides for the
Troubleshooting Guide are found on the generator’s top panel.
Foot Switch – includes the foot switch and detachable cable assembly.
Note: The foot switch is required if the system will be used with coagulating shears or instruments without
the hand switching adaptor. Since the generator has receptacles for two foot switches, two foot switches may
have been shipped.
Cart – the cart is optional. It is designed to hold one HARMONIC Generator. The cart requires assembly;
instructions are included with the cart.
Page 11

System Startup
Warning: Products manufactured or distributed by companies other than Ethicon Endo-Surgery may not be
compatible with the HARMONIC System. Use of such products may lead to unanticipated results and possible
injury to the user or patient.
Caution: The HARMONIC system includes components that are shipped non-sterile (e.g. hand piece, hand
switching adaptor, adaptors, and blade wrench). Sterilize products as required before beginning system setup.
Refer to individual package inserts for cleaning and sterilization instructions.
1 Confirm that the generator power switch is off during setup.
2 Secure the generator on its cart or on another suitable fixture. To secure the generator on its
cart, place the generator’s rubber feet into the corresponding holes on the cart. Push down on
the generator’s top panel.
Caution: To prevent overheating during use, ensure that the air vents found on the
generator’s bottom and back panels are not blocked and that they allow adequate clearance
from obstructions to allow air to flow freely through the generator enclosure. Avoid placing
the generator on a soft surface.
Warning: The HARMONIC system must be operated within the required ambient operating
conditions. Refer to Chapter 8 – System Specifications for requirements.
Caution: Do not simultaneously touch the patient and generator.
3 Connect the line cord into the AC inlet located on the generator’s rear panel and into an
appropriately-grounded outlet. If the power cord is wrapped around the cart handle, it must
be completely removed from the cart handle prior to plugging it into the power outlet.
Warning: Verify that the outlet voltage correctly corresponds to the generator’s
requirements (see Chapter 8 – System Specifications). Connection to an improper power
supply may result in damage to the generator and risk of shock or fire hazard.
4a. Attach the foot switch cable to the foot switch:
Note: Although installation of the foot switch is optional when using the hand switching
adaptor, installing the foot switch is recommended in case its use is needed during the
procedure.
• Confirm that the connector and receptacle are dry and clean.
• Orient the slot on the foot switch cable’s larger connector at 12 o’clock.
• Seat the connector in the foot switch receptacle.
• Turn the connector collar clockwise until tight. Ensure the collar is finger-tight to
prevent inadvertent activation because of fluid ingress.
b. Connect the foot switch cable’s smaller connector to the foot switch receptacle on the rear
panel of the generator.
• Confirm that the connector and receptacle are dry and clean.
• Align the red dot on the foot switch 4-pin connector with the red dot on the 4-pin
receptacle on the generator back panel.
Note: The generator has two identical foot switch receptacles. If one foot switch is used,
either receptacle may be used.
Repeat steps 4a and 4b if a second foot switch will be used.
Chapter 3 – System Setup and Operation
7GEN04
Page 12

5 Connect the instrument and adaptor (or hand switching adaptor), if required, to the hand
piece following instructions in their package inserts.
Note: The hand switching adaptor must be at room temperature to function properly. Do not
immerse in water to cool rapidly. After steam sterilization, allow hand switching adaptor to
air cool for at least 15 minutes prior to use.
6 Connect the hand piece connector to the receptacle on the front panel. Align the white dot on
the connector with the white dot on the generator. Ensure the hand piece connector is clean
and dry before connecting the hand piece to the generator. Fully insert the hand piece
connector to assure complete, proper connection to the generator.
7 Turn the generator power switch on and observe the power-up sequence. During power-up,
the following indicators on the front panel will briefly illuminate:
• READY, STANDBY, MIN, MAX, TEST, ATTENTION, HAND ACTIVATION
The system will run its start-up sequence and display the software version. An audible tone
will sound during the initiation sequence.
Note: The entire power-up initiation sequence should not exceed ten seconds.
If the start-up sequence deviates from the description above, contact qualified service personnel
following hospital protocol.
When the initiation sequence is complete, the system will go to Standby. However, if the system
detects a faulty generator or incorrect hand piece, a fault will appear on the graphic display (the
power levels will not be visible) and an audible alarm will sound. Refer to Chapter 4 –
Troubleshooting or the Troubleshooting Guide.
8 Power Levels: Upon startup, the generator defaults to power level 3 (MIN) and 5 (MAX).
The minimum (MIN) power level is user-settable from power levels 1 to 5. To adjust the
power level, depress the up/down arrow button to the left of the minimum power level
display. Set the power level based on surgeon preference and/or recommendations provided
in the instrument’s package insert (for more information, see Power Levels section in
Chapter 2 – Principles of Operation).
9 Audible tones: The generator has three activation tone sets from which to choose (the mid-
pitch tone is factory set). To choose another tone:
a. Switch power off.
b. Switch power on. Then immediately depress and hold both the STANDBY and HAND
ACTIVATION buttons. When the graphic shown in Figure 3-1 appears on the display,
release the STANDBY and HAND ACTIVATION buttons.
c. While in the Tone Selection mode, the generator will automatically sequence through the
available tone pitches. To select a tone, depress any button on the control panel. The
generator will return to Standby mode. The tone chosen will be saved until it is changed
again by accessing the Tone Selection mode.
Fig. 3-1 Tone Selection Display
Adjust tone volume by turning the knob on the lower left corner of the control panel. A
tone will sound to indicate the volume level selected. For safety reasons, the tone may not
be disabled.
User Manual
8
GEN04
Page 13

System Operation
Important: The HARMONIC Generator 300 System User Manual is designed to provide instructions
for use of the HARMONIC Generator 300, Foot Switch, and Cart (see Chapter 8 – System
Specifications for applicable product codes). This manual is not a reference to surgical techniques.
Note: Refer to package inserts provided separately for information about the Hand Piece, Hand
Switching Adaptor, Adaptors, Test Tip and Instruments prior to using the system.
After completing system setup, the system may be operated.
1 Place the generator in Ready mode by depressing the STANDBY button.
2 System check and activation:
Each time the generator is activated after exiting Standby, hold the instrument in the air (if
coagulating shears are used, open the clamp arm) and depress the MIN or MAX power level
on the foot switch or hand switching adaptor. “TEST IN PROGRESS” will appear on the
graphic display and a rapid two-tone pulse will sound while the test is occurring. During this
five-second period, a system check is being performed.
• If the system is operating properly, the activation tone corresponding to the power
level activated will be heard when the check is complete. Stop activation, position
the instrument on tissue, and resume activation.
• If the system is not operating properly, an error code will appear (refer to Chapter 4
– Troubleshooting or the Troubleshooting Guide).
Warning: To avoid user or patient injury, ensure that the instrument is clear of other
instruments, drapes, the patient or other objects during the system check. Safety measures
(in accordance with hospital protocol) taken in the presence of aerosols should be in effect
during the system check.
Note: The foot switch or hand switch must be depressed until the system check is completed.
If the switch is released prematurely, the check will reinitiate at the next activation.
Note: The system check will also be performed whenever the hand piece is removed and
replaced or TEST is pressed.
Note: The hand activation button on the generator control panel must be illuminated for the
hand switches to be active. To deactivate the hand switches, depress the hand activation
button (if the hand activation button is not illuminated, hand switches will be inactive).
Note: If the hand switch will not turn off during operation, depress the button corresponding
to the power level opposite that being activated to turn it off - an alarm will sound. Press the
HAND ACTIVATION button to disable the hand switching adaptor. Place the generator in
Standby, and replace the hand switch; or, continue using the foot switch after deactivating
the hand switch.
If the foot switch will not turn off during operation, depress the pedal corresponding to the
power level opposite that being activated to turn it off - an alarm will sound. Release the
pedal to silence the alarm. Place the generator in Standby, and replace the foot switch.
Chapter 3 – System Setup and Operation
9GEN04
Page 14

3 If the system senses a generator, hand piece, or instrument fault during use, an audible alarm
(tone with long pulses) will sound and a visual alarm indicator will appear on the control
panel. (Refer to Chapter 4 – Troubleshooting or the Troubleshooting Guide to resolve the
problem.)
Warning: Place the generator in Standby mode before removing or replacing an instrument,
hand switching adaptor or hand piece or when the system is not in use.
System Shutdown
1 Turn the generator power switch off and remove power cord from outlet.
2 Disconnect the hand piece, instrument, and adaptor or hand switching adaptor (if used) and
process them as indicated in their respective package inserts.
3 Clean the generator and cart and disinfect the foot switch(es) following hospital protocol (for
recommendations, refer to Chapter 5 – Cleaning and Disinfection).
4 Store foot switch(es) on the cart shelves provided. Each shelf will hold one foot switch.
5 Wrap foot switch cable(s) and the power cord on the cart’s back handle for storage.
User Manual
10
GEN04
Page 15

The Generator 300 System supports a series of alarms and error codes to help in the identification and
troubleshooting of component problems. These guides are meant as an adjunct to, but not a substitute for,
clinical judgment and observations.
Audible Indicators and Alarms
Tone Possible Cause and Corrective Action
No tone when system is activated. Confirm foot switch is fully connected (if hand switch is not
being used).
Confirm foot switch is not faulty.
If hand switch is used, confirm it is connected and not faulty.
Confirm hand activation is enabled if hand switching adaptor is
being used.
Activation (brief pulses) System is being activated or is in Test mode. System is operating
properly. MIN and MAX power have unique tones.
Alert (three-tone sequence) Activation is attempted while generator is in Standby mode. Push the
STANDBY button to return the generator to Ready mode.
Two or more foot or hand switches are recognized by the generator as
being activated simultaneously. Reactivate using only one switch.
Constant tone 1) Instrument is in contact with too much tissue. Reduce the amount of
tissue in contact with the instrument. If tone persists, carefully remove
any tissue that has collected in the distal end of the instrument shaft.
2) Hand piece and/or blade fault. Press TEST to identify source of fault.
Prolonged solid tone during Hand piece and/or blade fault. Press TEST to identify source of fault.
activation (exceeds 10 seconds)
Alarm (two-tone sequence) A component or system problem has occurred. Refer to the Error
Codes section in this chapter or the Troubleshooting Guide.
Note: This alarm will activate for three seconds, then will silence itself
for 30 seconds. This cycle will continue until the error is resolved or
the main power switch is turned off.
Error Codes
The generator will recognize specific faults in five areas: generator, hand piece, instrument, foot switch or
hand switch. When a fault is identified, an alarm will sound, the alarm indicator will appear on the generator
control panel, and the source of the problem will appear on the graphic display (the power levels will not be
displayed). See Fig. 4-1 below for an example. Follow the procedures outlined below (or in the
Troubleshooting Guide) to resolve the problem.
Fig. 4-1 Alarm Indicator (example)
Chapter 4 – Troubleshooting
11GEN04
Page 16

GEN04
User Manual
12
Error Code 1: Generator Error Code 1 indicates either there is a functional problem with the
generator or the front panel button(s) were activated during power-up
sequence.
Cycle the power OFF then ON. If error persists, power off system and
contact service.
Error Code 2: Generator Error Code 2 indicates that the generator is overheating.
Temperature
1 Power off system. Remove any obstructions blocking the air
vents on the generator’s bottom and back panels. If there is no
apparent obstruction or external heat source, contact service.
2 Power on the system and wait for up to 30 minutes for generator
error to clear.
3 If error code persists, contact service.
Error Code 3: Hand Piece Error Code 3 indicates a problem with the hand piece.
1 Confirm that the hand piece connector is fully inserted and
properly oriented – white dot on handpiece is aligned with white
dot on front panel. If the error code does not clear within three
seconds after the hand piece is properly connected, press TEST.
2 The instrument may not be tightened properly or tissue may
have collected in the distal end of the instrument shaft. Tighten
instrument using blade wrench and carefully remove tissue from
distal end of instrument sheath. Press STANDBY to clear error
code and return to Ready mode. Activate system. If the pre-run
test is running, ensure instrument is in air. If using shears, ensure
jaws are open and not in contact with any objects during prerun test.
Note: Inspect the blade wrench hub for cracks or wear before use. If
damage is seen, replace the blade wrench. Before use after
autoclaving, cool the blade wrench at room temperature for at least 45
minutes or soak it in room temperature sterile water for 5 minutes.
3 If the error persists, install a test tip to isolate the problem. Press
TEST button.
- If system indicates a hand piece error, hand piece is bad or
test tip is bad. Replace hand piece or install a new test tip
and press TEST.
- If no error occurs with test tip attached, replace instrument.
4 Press STANDBY to return to Ready mode. Activate system.
Error Code 4: Hand Piece Error Code 4 indicates that the hand piece has exceeded its
Temperature specified operating temperature. For immediate recovery, use another
hand piece; or, follow the steps below to determine the cause of the
error condition and alternate recovery methods.
Page 17

The following are possible causes of an increase in hand piece
temperature. To correct, complete the appropriate steps below and
allow the hand piece to cool before resuming operation.
1 The hand piece is still warm from recent steam sterilization.
Allow the hand piece to cool at room temperature for at least 45
minutes or soak it in room-temperature sterile water for 5
minutes before resuming operation.
Note: The hand switching adaptor (HSA07) should not be submerged
for rapid cooling purposes. This may render the hand switching
adaptor inoperable for an extended period of time. After steam
sterilization, allow the hand switching adaptor to air cool at least
15 minutes prior to use.
2 The instrument may not be tightened properly or tissue may
have collected in the distal end of the instrument shaft. Tighten
instrument using blade wrench and carefully remove tissue from
distal end of instrument sheath. Press STANDBY to clear error
code and return to Ready mode. Activate system. If the pre-run
test is running, ensure instrument is in air. If using shears, ensure
jaws are open and not in contact with any objects during prerun test.
Note: Inspect the blade wrench hub for cracks or wear before use. If
damage is seen, replace the blade wrench. Before use after
autoclaving, cool the blade wrench at room temperature for at least 45
minutes or soak it in room temperature sterile water for 5 minutes.
3 If the error persists, install a test tip to isolate the problem. Press
TEST button.
- If system indicates a hand piece error, hand piece is bad or
test tip is bad. Replace hand piece or install a new test tip
and press TEST.
- If no error occurs with test tip attached, replace instrument.
4 Press STANDBY to return to Ready mode. Activate system.
5 If the hand piece does not show evidence of overheating and
troubleshooting steps 1-4 above do not appear to resolve the
problem, perform the following:
a. Leave the hand piece at room temperature for 24 hours or
more.
b. Remove any test tip or instrument from the hand piece.
c. With the generator turned off, plug the hand piece into any
Generator 300.
d. Power up the generator in Biomed mode.
- Press and hold down the STANDBY button and down
arrow key.
- Wait for a steady display – approximately 10 seconds.
- If a “Generator” error occurs, then one of the buttons was
not properly held down. If this happens, repeat the power up
procedure in Biomed mode.
Chapter 4 – Troubleshooting
13GEN04
Page 18

e. Record the “XDUCER CAPACITANCE” value.
- Press the STANDBY button, if necessary, until
it illuminates.
- Use the increase/decrease arrow keys to get to
“Page 2 of 21”.
- Record the number opposite “XDUCER CAPACITANCE”.
- Press the STANDBY button until the Standby light
turns off.
- Leave the hand piece plugged into the generator. Do not
remove hand piece during entire procedure. Do not activate
the MIN or MAX activation buttons on the foot switch or
hand switch if either is attached.
- After a period of time that exceeds 30 or more minutes,
press the STANDBY button until the STANDBY light
is illuminated.
- Again, read the “XDUCER CAPACITANCE” on
Page2of21.
- If the number has changed, the update was successful.
- If the number has not changed, then this update attempt did
not succeed. Power down the generator and repeat Step 5.
Note: As the hand piece ages, the generator performs measurements
and updates a key hand piece parameter. This function is performed
when the internal temperature of the hand piece is stable at room
temperature. Certain usage patterns may prevent this update from
occurring and subsequently make the hand piece diagnostics more
sensitive to temperature. The steps above will cause an update of the
hand piece parameter and return the system to designed sensitivity.
Warning: To avoid user or patient injury, ensure that the instrument is
clear of other instruments, drapes, the patient or other objects before
pressing TEST. Safety measures (in accordance with hospital protocol)
taken in the presence of aerosols should be in effect while in Test mode.
Note: Do not run the Test mode while an electrosurgical generator is
being activated in the room. Interference from the electrosurgical
generator may affect test results.
Error Code 5: Instrument Error Code 5 indicates a problem with the instrument.
1 The instrument may not be tightened properly or tissue may
have collected in the distal end of the instrument shaft. Tighten
instrument using blade wrench and carefully remove tissue from
distal end of instrument sheath. Press STANDBY to clear error
code and return to Ready mode. Activate system. If the pre-run
test is running, ensure instrument is in air. If using shears,
ensure jaws are open and not in contact with any objects
during pre-run test.
User Manual
14
GEN04
Page 19

Note: Inspect the blade wrench hub for cracks or wear before use.
If damage is seen, replace the blade wrench. Before use after
autoclaving, cool the blade wrench at room temperature for at least
45 minutes or soak it in room temperature sterile water for 5 minutes.
2 If the error persists, install a test tip to isolate the problem. Press
TEST button.
- If system indicates a hand piece error, hand piece is bad or
test tip is bad. Replace hand piece or install a new test tip
and press TEST.
- If no error occurs with test tip attached, replace instrument.
3 Press STANDBY to return to Ready mode. Activate system.
Note: Inspect the blade wrench hub for cracks or wear before use.
If damage is seen, replace the blade wrench. Before use after
autoclaving, cool the blade wrench at room temperature for at least
45 minutes or soak it in room temperature sterile water for 5 minutes.
Warning: To avoid user or patient injury, ensure that the instrument
is clear of other instruments, drapes, the patient, or other interference
before pressing TEST. Safety measures (in accordance with hospital
protocol) taken in the presence of aerosols should be in effect while
in Test mode.
Note: Do not run the Test mode while an electrosurgical generator is
being activated in the room. Interference from the electrosurgical
generator may affect test results.
Error Code 6: Foot Switch Error Code 6 indicates a foot switch pedal is stuck in the ON position.
Confirm generator receptacle, foot switch receptacles and cable
connectors are clean and dry or replace the foot switch.
Note: If the error persists, replace foot switch.
Error Code 7: Hand Switch Error Code 7 indicates the hand switch is stuck in the ON position.
Confirm contacts in the distal end of hand piece and in the proximal
end of the hand switching adaptor are dry or replace the hand
switching adaptor.
Note: If the error persists, replace hand switch.
Chapter 4 – Troubleshooting
15GEN04
Page 20

User Manual
16
GEN04
Page 21

Generator and Cart Cleaning
Clean generator and cart following hospital protocol. Before cleaning, turn the generator main power off and
unplug the power cord from the grounded electrical outlet.
Warning: Spilling or spraying fluids on or into the generator or immersing the generator may result in
damage to the generator and risk of shock or fire hazard.
Proceed with cleaning as follows:
1 Prepare a neutral pH detergent or neutral pH enzymatic detergent according to the detergent
manufacturer’s directions.
2 Use a soft, clean cloth lightly moistened with the cleaning solution to manually clean all surfaces
(including the generator’s display).
3 Rinse thoroughly using a soft, clean cloth lightly moistened with warm tap water.
4 Dry with a clean, soft cloth.
Foot Switch Cleaning
The foot switch and cable should be cleaned after each use as follows:
1 Disconnect the foot switch from the generator.
2 Prepare a neutral pH enzymatic detergent according to the detergent manufacturer’s directions.
3 With the cable securely attached to the foot switch, soak the foot switch and cable in the detergent
solution for two minutes.
Note: Keep the foot switch cable connector that connects to the generator dry at all times to prevent
inadvertent activation.
4 After soaking, use a soft-bristled brush to manually clean the foot switch and cable keeping them
immersed in the detergent solution.
5 Thoroughly rinse the foot switch and cable – with the cable securely attached to the foot switch – with
warm, running tap water for at least one minute.
6 Dry all surfaces with a clean, soft cloth.
Chapter 5 – Cleaning and Disinfection
17GEN04
Page 22

User Manual
18
GEN04
Page 23

Test hand piece, generator, and foot switch for safety and function according to hospital protocol. Refer to
individual package inserts for safety and function testing for other multi-patient use components.
Safety Test
Generator: A qualified hospital technician should perform a leakage current test.
Foot Switch: Examine the foot pedals, cable connectors, and cable for cracks or other damage and replace
if damaged.
Other Components: Examine the components by following the instructions in their individual package
inserts.
Function Test
1 Perform complete instrument preparation and hand piece attachment as described in Chapter 3 –
System Setup and Operation. Attach the test tip rather than an instrument.
2 Verify that the orange STANDBY indicator is illuminated.
3 Push the STANDBY button to leave Standby mode and enter Ready mode.
4 Verify that the green READY indicator is illuminated.
5 Verify that MIN Power Level 3 and MAX Power Level 5 are displayed.
6 Push the Increase and Decrease Power Level button up and down to confirm the MIN Power Level
changes from 1 to 5.
7 Turn the generator off. Wait five seconds, then turn the generator back on. Wait ten seconds, then
confirm MIN Power Level 3 and MAX Power Level 5 are displayed. Confirm the generator is not
being activated unexpectedly.
8 Place the generator in Ready mode by depressing the STANDBY button. Hold the hand piece so that
the distal portion is in the air and step on the MAX foot switch pedal (before activation begins, a fivesecond system check will be performed – “TEST IN PROGRESS” will appear on the display). Verify
that the MAX Power Level indicator on the control panel flashes and that the MAX activation tone is
heard.
Warning: To avoid user injury, ensure that the test tip is clear of tissue, other instruments, or other
objects before activating the system.
9 Hold the hand piece so that the distal portion is in air and step on the MIN foot switch pedal. Verify
that the MIN Power Level indicator on the control panel flashes and that the MIN activation tone is
heard.
Warning: To avoid user injury, ensure that the test tip is clear of tissue, other instruments, or other
objects before activating the system.
Calibration
Refer to the Generator 300 System Service Manual for system calibration information.
Chapter 6 – Safety and Function Testing
19GEN04
Page 24

User Manual
20
GEN04
Page 25

System Warnings and Precautions
• Minimally invasive procedures should be performed only by persons having adequate training and
familiarity with minimally invasive techniques. Consult medical literature relative to techniques,
complications, and hazards prior to performance of any minimally invasive procedure.
• Minimally invasive instruments may vary from manufacturer to manufacturer. When minimally invasive
instruments and accessories from different manufacturers are employed together in a procedure, verify
compatibility prior to initiation of the procedure.
• A thorough understanding of the principles and techniques involved in laser, electrosurgical, and ultrasonic
procedures is essential to avoid shock and burn hazards to both patient and medical personnel and damage
to the device or other medical instruments. Ensure that electrical insulation or grounding is not
compromised. Do not immerse electrosurgical instruments in liquid unless the instruments are designed and
labeled to be immersed.
• Safe and effective ultrasonic surgery is dependent not only upon equipment design, but also, to a large
extent, upon factors under control of the operator. It is important that the instructions supplied with this
equipment be read, understood, and followed in order to enhance safety and effectiveness.
• As with all energy sources (Electrosurgery, Laser, or Ultrasound), there are concerns about the carcinogenic
and infectious potential of the by-products, such as tissue smoke plume and aerosols. Appropriate measures
such as protective eyewear, filtration masks, and effective smoke evacuation equipment should be used in
both open and laparoscopic procedures.
• To avoid user or patient injury in the event that accidental activation occurs, the HARMONIC instrument
blades should not be in contact with the patient, drapes, or flammable materials while not in use. During
prolonged activation in tissue, the instrument blade, clamp arm and distal end of the shaft may become hot.
Avoid unintended blade contact with tissue, drapes, surgical gowns, or other unintended sites after
activation.
• To avoid user or patient injury, the HARMONIC Generator should not be used prior to biomedical evaluation
if it shows signs of damage or is suspected of being dropped or having fluids spilled on it.
• After removing the instrument, examine the tissue for hemostasis. If hemostasis is not present, appropriate
techniques should be used to achieve hemostasis.
• Products manufactured or distributed by companies other than Ethicon Endo-Surgery may not be
compatible with the HARMONIC system. Use of such products may lead to unanticipated results and possible
injury to the user or patient.
• The HARMONIC system, including the hand piece, is not Magnetic Resonance safe and is not Magnetic
Resonance compatible.
• To reduce the risk of interference, electrosurgical systems and the HARMONIC system should be plugged into
separate electrical power circuits. Locate the HARMONIC system, including the hand piece cable, at least 3 ft.
(approximately 1 m) from electrosurgical systems and their hand piece (e.g., pencil) cables.
• Equipment not suitable for use in the presence of a flammable anesthetic mixture with air or with oxygen or
nitrous oxide. It is possible to create sparks by hitting other metal instruments. Sparks may ignite
flammable gases such as bowel gas.
• The HARMONIC system must be operated within the required ambient operating conditions. Refer to Chapter
8 – System Specifications for requirements.
• To prevent overheating during use, ensure that the air vents found on the generator’s bottom and back
panels are not blocked and that they allow adequate clearance from obstructions to allow air to flow freely
through the generator enclosure. Avoid placing the generator on a soft surface.
• Verify that the outlet voltage correctly corresponds to the generator’s requirements (see Chapter 8 – System
Specifications). Connection to an improper power supply may result in damage to the generator and risk of
shock or fire hazard.
• The HARMONIC system includes components that are shipped non-sterile (e.g. hand piece, hand switching
adaptor, adaptors, and blade wrench). Sterilize products as required before beginning system setup. Refer to
individual package inserts for cleaning and sterilization instructions.
• To avoid user or patient injury, ensure that the instrument is clear of other instruments, drapes, the patient,
or other objects before pressing TEST and during the system check. Safety measures (in accordance with
hospital protocol) taken in the presence of aerosols should be in effect during the system check and while in
Test mode.
Chapter 7 – Warnings and Precautions
21GEN04
Page 26

• To avoid user injury, ensure that the test tip is clear of tissue, other instruments, or other objects before
activating the system.
• Do not simultaneously touch the patient and generator.
• Place the generator in the Standby mode before removing or replacing an instrument, hand switching
adaptor or hand piece or when system is not in use.
• Spilling or spraying fluids on or into the generator or immersing the generator may result in damage to the
generator and risk of shock or fire hazard.
• In case of system failure, ensure the availability of the appropriate backup equipment relevant to the
specific procedure.
Instrument Warnings and Precautions
Blades
All blades have an intermittent operation of 15 second on/off intervals, unless the duty cycle is explicitly
specified otherwise in the individual instrument package inserts.
Coagulating Shears
During prolonged activation in tissue, the instrument blade, clamp arm, and the distal 7 cm of the shaft
may become hot. Avoid unintended contact with tissue, drapes, surgical gowns, or other unintended sites
at all times.
Note: Refer to individual package inserts for additional warnings and precautions.
User Manual
22
GEN04
Page 27

Product Codes Required Components for System Operation:
GEN04: Generator 300
HP054/HP055: Hand Piece (includes HST02 Test Tip and
TLB01 Blade Wrench)
1
Instruments and Adaptors:
Contact your Ethicon Endo-Surgery representative for information
about instruments available for use with this system. Some
instruments may require use of an adaptor.
Optional Components:
FSW01: Foot Switch
2
HSA07: Hand Switch
1,2
CRT01: Cart
1
Refer to separate product insert supplied with this component.
2
At a minimum, either the foot switch or the hand switch is required
to operate the generator. When the hand switch is used, availability of
the foot switch is recommended.
Degree of Protection
Against Electric Shock Type CF Applied Part
Class of Protection
Against Electric Shock Class I
Safety Standards EN 60601-1
Degree of Protection
Against Harmful Ingress
of Water Generator: Ordinary equipment
Footswitch: IPX8
Safety Classification UL 2601-1
CSA C22.2 601.1
EN 60601-1
Mains Input Voltage: 100-240 VAC
Frequency: 50/60 Hz
Current Consumption: 3 amp
Ambient Operating
Conditions Temperature 18˚C to 23˚C
Humidity: 10-90% non-condensing
Atmospheric Pressure Range: 700hPa-1060hPa
Transport and Storage
Conditions Temperature: -35˚C to +54˚C
Humidity: 10-95% non-condensing
Atmospheric Pressure Range: 700hPa-1060hPa
Chapter 8 – System Specifications
23GEN04
Page 28

Date of Manufacture The date of manufacture may be determined by viewing the serial
number on the rear panel of the generator. The fourth and fifth
characters indicate the year of manufacture as follows:
GN401 = year 2001
GN402 = year 2002
GN403 = year 2003
GN404 = year 2004
GN405 = year 2005
GN406 = year 2006
GN407 = year 2007
Power Cord North American removable power cord set with the
following characteristics:
Plug Style: NEMA 5-15 (clear) North American Hospital Grade
Receptacle: IEC 60320 C13 with straight non-angled cord entry
Cord Length: 4.6 meters nominal
Current Rating: 13A
Voltage Rating: 125 VAC minimum
Wiring Code: North American
Cordage Description: SJT (UL) or SJT (CSA)
Conductors: 16 AWG 3C
Agency Approvals Required: UL and CSA
International removable power cord set with the following
characteristics:
Plug Style: as needed by particular country requirements
Receptacle: IEC 60320 C13 with straight non-angled cord entry
Cord Length: 2.44 - 4.6 meters nominal
Current Rating: 10A
Minimum conductor size cross-sectional area: 1.0mm2copper
Voltage Rating: 250 VAC minimum
Wiring: international
Cordage Type: HAR
Item to have certification by at least one of the following agencies:
VDE,ASTA, SEMKO, KEMA, LCIE, DFT, IMQ, SEV
Duty Cycle Duty Cycle is determined by hand piece and instrument in use. For
duty cycle information, refer to applicable instrument(s) and hand
piece inserts and/or Chapter 7 – Warnings and Precautions.
Weight Generator: 7.48 kg nominal
(unpacked)
Cart: 42.0 kg nominal
Overall Dimensions Generator 300 (HxWxD): 5.3" (13 cm) x 14.5" (37 cm) x 15.2" (39 cm)
Cart (HxWxD): 37.3" (95 cm) x 17.7" (45 cm) x 27.6" (70 cm),
including handle
Disposal Some internal components of the generator, foot switch and foot
switch cable contain lead. Disposal should be performed according
to local requirements and regulations.
User Manual
24
GEN04
Page 29

GEN04 25
Chapter 9 - Warranty
This warranty and the rights and obligations hereunder shall be construed under and governed by the
laws of the State of Ohio, U.S.A.
Ethicon Endo-Surgery warrants this product to be free from defects in material and workmanship under
normal use and preventive maintenance for the respective warranty period shown below. Ethicon EndoSurgery’s obligation under this warranty is limited to the repair or replacement, at its option, of any
product, or part thereof, which has been returned to Ethicon Endo-Surgery or its Distributor within the
applicable time period shown below and which examination disclosed, to Ethicon Endo-Surgery’s
satisfaction, to be defective. This warranty does not apply to any product, or part thereof, that has been: (1)
adversely affected due to use with devices manufactured or distributed by parties not authorized by
Ethicon Endo-Surgery (2) repaired or altered outside Ethicon Endo-Surgery’s factory in a way so as to, in
Ethicon Endo-Surgery’s judgement, affect its stability or reliability, (3) subjected to improper use,
negligence or accident, or (4) used other than in accordance with the design and use parameters,
instructions and guidelines for the product or with functional, operational or environmental standards for
similar products generally accepted in the industry.
Ethicon Endo-Surgery’s products are warranted for the following periods after delivery to the
original purchaser:
Hand Pieces Nine (9) Months, Parts and Labor
Generators One (1) Year, Parts and Labor
Carts One (1) Year, Parts and Labor
Foot Switches and Cables One (1) Year, Parts and Labor
Sterilization Tray One (1) Year, Parts and Labor
UNLESS SUPERCEDED BY APPLICABLE LOCAL LAW, THIS WARRANTY IS IN LIEU
OF ALL OTHER WARRANTIES, EXPRESS OR IMPLIED, INCLUDING THE WARRANTIES
OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE, AND OF ALL
OTHER OBLIGATIONS OR LIABILITIES ON THE PART OF ETHICON ENDO-SURGERY
AND IS A PURCHASER’S EXCLUSIVE REMEDY. IN NO EVENT SHALL ETHICON ENDOSURGERY BE LIABLE FOR SPECIAL, INCIDENTAL OR CONSEQUENTIAL DAMAGES
INCLUDING, WITHOUT LIMITATION, DAMAGES RESULTING FROM LOSS OF USE,
PROFITS, BUSINESS OR GOODWILL, OTHER THAN AS EXPRESSLY PROVIDED BY A
SPECIFIC LAW. Ethicon Endo-Surgery neither assumes nor authorizes any other person to assume
for it any other liability in connection with the sale or use of any of Ethicon Endo-Surgery products.
There are no warranties that extend beyond the terms hereof.
Ethicon Endo-Surgery reserves the right to make changes to products built and/or sold by them at any
time without incurring any obligation to make the same or similar changes on products previously built
and/or sold by them.
Page 30

User Manual
26
GEN04
Page 31

On
Off
Type CF Applied Part
Lot
Temperature
Relative Humidity
Attention - Consult Accompanying
Documents/See Instructions For Use
Non-Sterile
Date of Manufacture
Fragile
This end up
Keep dry
Increase/Decrease
Serial Number
Equipotential
Chapter 10 – Symbols
27GEN04
Fuse
Safe working load
Test
Hand Activation
Volume
Minimum
Maximum
Ready
Standby
Reorder Number
Caution: Federal (USA) law restricts this device
to sale by or on the order of a physician.
ON/OFF Time for Intermittent Operation.
Refer to the individual package insert and/or
Chapter 7 – Warnings and Precautions for
additional specifications.
Foot switch
Category AP (Anaesthetic Proof) Equipment
Page 32

Manufacturer
Authorized Representative in the European Community
Authorized Representative in the USA
Electrical and electronic equipment. Return waste to a collection system or treatment and recycling facilities.
Applicable in the EU. Follow decontamination instructions before returning waste.
User Manual
28
GEN04
Page 33

Page 34

Page 35

Page 36

32
GEN04
REF
GEN04
Ethicon Endo-Surgery (Europe) GmbH
Hummelsbuetteler Steindamm 71
22851 Norderstedt
GERMANY
Johnson & Johnson AG
CH-8957 Spreitenbach
SWITZERLAND
ETHICON ENDO-SURGERY, INC.
Cincinnati, OH 45242-2839 USA
1-800-USE-ENDO
P40361P05
P40361P05
 Loading...
Loading...