Emerson CA-3000, 3200, CA-3200, CM-3000, CM-3200 User Manual
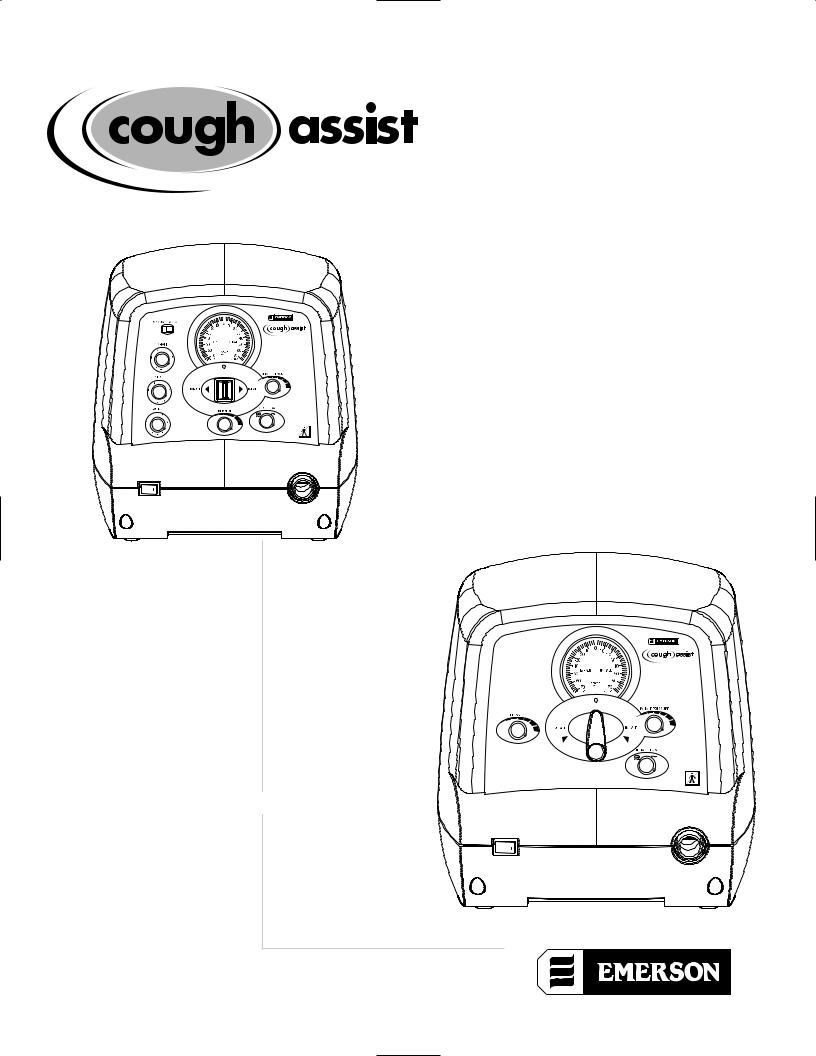
™ USER’S GUIDE
MODELS CA-3000 & CA-3200
o
MODELS CM-3000 & CM-3200
o
MEDICAL PRODUCT INNOVATIONS SINCE 1928
T A B L E O F C O N T E N T S
S E C T I O N |
1 |
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . |
|
2 |
Warnings and Cautions . . . . . . . . . . . . . . . . . . |
|
3 |
Specifications . . . . . . . . . . . . . . . . . . . . . . . . . |
|
4 |
Controls, Connectors and Visual Indicators . . . . |
|
5 |
Operating Procedure . . . . . . . . . . . . . . . . . . . . |
|
6 |
Cleaning and Disinfection . . . . . . . . . . . . . . . . |
|
7 |
Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . |
|
8 |
Preventive Maintenance and Troubleshooting Guide |
|
9 |
Warranty and Service . . . . . . . . . . . . . . . . . . . |
1
2
3
6
7
10
14
15
16
18
SECTION 1
I N T R O D U C T I O N
The Emerson CoughAssist™ Mechanical In-Exsufflator (MI-E) assists patients in clearing retained bronchopulmonary secretions by gradually applying a positive pressure to the airway, then rapidly shifting to a negative pressure. This rapid shift in pressure, via a facemask, mouthpiece or an endotracheal or tracheostomy tube, produces a high expiratory flow rate from the lungs, simulating a cough, a technique referred to as “mechanical insufflation-exsufflation.” The automatic CoughAssist MI-E (CA-3000, CA-3200) has timing mechanisms to automate the inspiratory and expiratory cycles as well as a manual control. The manual CoughAssist MI-E (CM-3000, CM-3200) uses a manually operated valve to shift from positive to negative pressure and back.
Those who might benefit from the use of the CoughAssist MI-E include any patient with an ineffective cough due to muscular dystrophy, myasthenia gravis, poliomyelitis, or other neurologic disorder with some paralysis of the respiratory muscles, such as spinal cord injury. It may also be used to treat ineffective cough due to other bronchopulmonary diseases, such as emphysema, cystic fibrosis and bronchiectasis. It is effective for both trached and non-invasively ventilated patients.
VIndications for Use: Any patient unable to cough or clear secretions effectively due to reduced peak cough expiratory flow (less than 2 to 3 liters per second), resulting from high spinal cord injuries, neuro-muscular deficits or severe fatigue associated with intrinsic lung disease, is a candidate for this device.
VContraindications: Any patient with a history of bullous emphysema, known susceptibility to pneumothorax or pnuemo-mediastinum, or known to have had any recent barotrauma, should be carefully considered before use.
2
SECTION 2
W A R N I N G S A N D C A U T I O N S
E Q U I P M E N T C L A S S I F I C AT I O N
Per IEC 60601-1, Medical Electrical Equipment, General Requirements for Safety, the
CoughAssist MI-E is classified as follows:
Class 1 Equipment: Equipment in which protection against electric shock does not rely on basic insulation only, but includes a grounding pin on the power cord. For ground reliability always plug the power cord into an AC grounded outlet.
Type BF Equipment: Type B piece of equipment with an F-Type applied part.
A Type B piece of equipment is one that provides a particular degree of protection against electric shock, particularly regarding allowable leakage current and reliability of the protective earth connection (grounding).
F-Type applied part is one that extends from the patient into the equipment and is isolated from all other parts of the equipment.
Water Ingress: This device does not have protection against ingress of water.
Disinfection: With the exception of the patient circuit, this device can be disinfected using 70% isopropyl alcohol or equivalent. (See Section 6: Cleaning and Disinfection.)
Flammable Anesthetics: This device is not suitable for use in the presence of a flammable anesthetic mixture with air, or in the presence of a flammable anesthetic mixture with oxygen or nitrous oxide.
Intermittent Operation: This device is designed for Intermittent Operation Only and not for continuous use. The device should not be cycled continuously for more than 5 minutes. After such time, the unit should either be turned off or left idling with the blower on for at least 5 minutes.
3

I M P O R TA N T S A F E G U A R D S |
|
|
|
|
D E F I N I T I O N S |
|
|
|
|
Throughout this guide the following definitions apply: |
|
|
|
|
• Warning/DANGER: A condition that could cause electrocution or injury to a user |
|
|
|
|
|
or operator if instructions are not followed. |
|
|
|
• CAUTIONS: A condition that could cause damage to equipment or cause inaccurate |
|
|
|
|
|
function. |
|
|
|
W A R N I N G S |
|
|
|
|
V |
Patients known to have cardiac instability should be monitored for pulse and oxygen |
|
|
|
|
|
|
|
|
|
saturation very closely. |
|
|
|
V |
Soreness and/or pain in the chest from a pulled muscle may occur in patients |
|
|
|
|
|
|
|
|
|
using the CoughAssist MI-E for the first time if the positive pressure used exceeds |
|
|
|
|
pressures, which the patient normally receives during Positive Pressure Therapy.1 |
|
|
|
|
Such patients should start at a lower positive pressure during treatment, and gradually |
|
|
|
|
(over several days, or as tolerated) increase the positive pressure used. |
|
|
|
V |
Do not use in the presence of flammable anesthetics. |
|
|
|
|
|
|
|
|
V |
Connection should be made to a grounded outlet only. |
|
|
|
|
|
|
|
|
V |
Do not place or store the device where it can fall or be pulled into a tub or sink. |
|
|
|
|
|
|
|
|
V |
If the device comes into contact with water, unplug the unit. |
|
|
|
|
|
|
|
|
V |
Never operate the CoughAssist MI-E if it has a damaged cord or plug, is not work- |
|
|
|
|
|
|
|
|
|
ing properly, or has been dropped, damaged or immersed in water. |
|
|
|
|
|
|
|
|
1 Positive Pressure Therapy includes the use of a volume ventilator, nasal or mask ventilation or CPAP (Continuous |
4 |
|||
|
|
|
||
Positive Airway Pressure), or IPPB (Intermittent Positive Pressure Breathing). |
|
|
|
|
|
|
|
|
|

W A R N I N G S A N D C A U T I O N S
VReplace fuses only with ones having the same ratings for blow characteristics, current and voltage.
VDo not remove the cover; there are no serviceable parts inside the unit. Refer all service to authorized personnel.
VAlways check time and pressure settings before each treatment.
C A U T I O N S
•Federal Law (USA) restricts this device to use by, or at the direction of a physician.
•Position the CoughAssist MI-E so that the air intake ports on the side and rear of the unit are not blocked.
•Never operate the device unless a bacterial/viral filter is attached to the patient circuit.
•Always use a new filter when using the device on a new patient.
•This device is designed for Intermittent Operation Only and not for continuous use. The device should not be cycled continuously for more than 5 minutes. After such time, the unit should either be turned off or left idling with the blower on for at least 5 minutes.
•Turn the unit off when not in use.
•Keep the cord away from heated surfaces.
•Do not sterilize with ethylene oxide gas or steam sterilize the pump or pump housing.
•This device should only be used by trained personnel.
5
 Loading...
Loading...