Donjoy Iceman CLASSIC3, Iceman Classic, Iceman CLEAR3 User Manual

IceMan® CLEAR3
IceMan® CLASSIC &
IceMan® CLASSIC3
Cold Therapy Units
Instructions for Use

TABLE OF CONTENTS
English |
. . . |
. . . . . . . . . . . . . . . |
. . . . . . . . . . . |
. . . |
. |
. . . . .2. . . . . . |
Español . |
. . . |
. . . . . . . . . . . . . . . . . . . |
. . . . . . . . . . . . . . |
. . . . |
. |
. . . . . 17 |
Français |
.. . . |
. . . . . . . . . . . . . . . . . . . |
. . . . . . . . . . . . . . |
. . . . |
. |
. . . . . 33 |
1

THIS DEVICE CAN BE COLD ENOUGH TO CAUSE SERIOUS INJURY. SERIOUS ADVERSE REACTIONS AND SAFETY HAZARDS MAY OCCUR WHEN USING THIS DEVICE.
INDICATIONS FOR USE
When to use the IceMan®
The intended use of the IceMan® CLEAR3, IceMan® CLASSIC, and IceMan® CLASSIC3 is for the temporary reduction of swelling and pain after surgery or injury.. This is a non-sterile prescription device for single patient use to provide localized external application of cold therapy.. This device may not be used for any other purpose..
OPERATING PRINCIPLE
The DonJoy® IceMan® devices provide cold therapy by flowing cold water, from an ice bath through an applied therapy pad..
INTENDED USER PROFILE
The intended user should be a licensed medical professional, the patient, the patient’s caretaker, or a family member providing assistance.. The user should be able to:
·Read, understand and be physically capable to perform all the directions, warnings and cautions provided in the information for use..
2

CONTRAINDICATIONS
When not to use the IceMan® DO NOT use this device on patients with Raynaud’s phenomenon or other vasospastic conditions; Buerger’s disease; cold allergy or hypersensitivity; cryoglobulinemia; paroxysmal cold hemoglobinuria or other cold agglutinin disorders; pheochromocytoma; sickle cell anemia or history of cold injury..
LIMITATIONS ON SPECIAL PATIENT POPULATIONS
Limit the use of the IceMan® with these patients Limit the use of this device with patients who are unresponsive, incapacitated, have altered mental status or altered pain perception.. Post-surgical patients under sedation or on analgesics or anesthetics, as well as patients taking hypnotics, anxiolytics, or antidepressants, must be monitored frequently during use of this device.. These patients may not be able to perceive pain, burning, numbness, tingling or decreased sensation and may be susceptible to injury.. Discontinue cold therapy immediately at the first sign of cold injury..
3

WARNINGS & PRECAUTIONS
 WARNING
WARNING
•THIS DEVICE CAN BE COLD ENOUGH TO CAUSE SERIOUS INJURY. SERIOUS ADVERSE REACTIONS AND SAFETY HAZARDS MAY OCCUR WHEN USING THIS DEVICE.
•Read and understand all warnings and Instructions for Use
before using this device..
•For IceMan® CLEAR3 or IceMan® CLASSIC3 (WITHOUT thermometer and temperature control) DO NOT use this device without a prescription from a physician.. Rx only.. Your prescrip-
tion must state how long and how often the device should be used and the length of breaks between uses.. DO NOT use this device if a prescription has not been provided to you or if you do not understand the prescription.. Use of this device without a prescription or failure to follow the prescription may result in serious injury, including tissue necrosis..
•For IceMan® CLASSIC (WITH thermometer and temperature control) DO NOT use this device without a prescription from a physician.. Rx only.. Your prescription must state a
temperature, how long and how often the device should be used and the length of breaks between uses.. DO NOT use this device if a prescription has not been provided to you or if you do not understand the prescription.. Use of this device without a prescription or failure to follow the prescription may result in serious injury, including tissue necrosis..
•This device can be cold enough to cause serious injury, including
tissue necrosis.. You must be able to check your skin condition under the cold pad frequently (at least every hour).. DO NOT use this device if you cannot check your skin condition frequently (at least every hour).. Check for increased pain, burning, numbness, tingling, increased redness, discoloration, itching, increased swelling, blisters, irritation or other changes in skin condition under the cold pad or around the treatment area.. If you experience any of these conditions, immediately discontinue use of this device and contact your physician..
•This device is intended only for single patient use.. Secondary use can cause serious injury, including infection..
•Application of the cold pads directly on the skin may result in serious injury, including tissue necrosis.. DO NOT let any part of the cold pad touch your skin.. ALWAYS use with a barrier between your skin and the cold pad..
4

WARNINGS & PRECAUTIONS (con’t)
•The barrier between your skin and the cold pad may develop moisture during use, which may create colder temperatures on the skin.. Temperatures that are too cold may result in serious injury, including tissue necrosis.. ALWAYS check for moisture on the barrier between your skin and the cold pad.. If moisture is present on the barrier, immediately discontinue use of this device..
•Poor connections between hoses may cause leaking, which may result in serious injury, including infection and tissue necrosis.. ALWAYS listen for a “snap” or “click” when connecting the IceMan® cold therapy unit hose to the cold pad hose.. Use only IceMan® cold pads..
•Use of the IceMan® with wet hands or in a wet location may result in electrical shock and serious injury.. DO NOT handle transformer or power cord with wet hands or in a wet location.. The power supply unit is the mains power disconnect.. Do not position the equipment to make access to the disconnect difficult.. Only connect equipment to the power supply provided for this product..
•DO NOT use the IceMan® near flammable anesthetics or oxygen enriched environment, which may result in explosion and serious injury..
•Keep power cord, hose, small parts, and packaging materials away from children and animals.. These items pose a risk for suffocation or strangulation..
•It could be unsafe to use accessories, detachable parts and materials, or interconnect to other equipment not described in these instructions, or otherwise modify the equipment..
•Care must be taken when operating this device adjacent to other equipment.. Potential electromagnetic or other interference could occur to this or other equipment.. Try to minimize this interference by not using other electronic equipment in conjunction with this device..
•To avoid the risk of electrical shock, do not disassemble the IceMan®.. If device is not functioning properly, please contact DonJoy product support..
PRECAUTIONS
When to exercise special care when prescribing the IceMan®
Exercise special care prescribing this device for the following patients: those with arthritic conditions; peripheral vascular disease; children under the age of 12; those with decreased skin sensitivity; poor circulation, or compromised local circulation; hypercoagulation disorders; diabetes or neuropathies..
5

PATIENT INFORMATION
Directions for use of the IceMan®
•A physician must prescribe treatment to be rendered by this device, which must state a temperature (for the IceMan® CLASSIC), how long and how often the device should be used and the length of breaks between uses.. You must follow the individual prescription provided to you by your physician..
•This device can be cold enough to cause serious injury, including tissue necrosis.. You must be able to check your skin condition under the cold pad.. DO NOT use if you cannot check your skin condition frequently (at least every hour).. People are sensitive to cold in diverse ways and may react differently to cold treatment..
•Check for increased pain, burning, numbness, tingling, increased redness, discoloration, itching, increased swelling, blisters, irritation or other changes in skin condition under the cold pad or around the
treatment area.. If you experience any of these conditions, immediately discontinue use of this device and contact your physician..
•Inform your physician if any of the following apply to you: arthritic conditions; peripheral vascular disease; under the age of 12; decreased skin sensitivity; poor circulation or compromised local circulation; hypercoagulation disorders; diabetes or neuropathies..
•Check for moisture on the barrier between your skin and cold pad. If moisture is present on the barrier, immediately discontinue use of this device..
•Do not cast or bandage over IceMan® cold pads.
•Use only approved IceMan® cold therapy pads with the IceMan® units.
•To ensure a reliable connection between the IceMan® and IceMan® cold pad, “snap” or “click” hoses together into place so that the fit is tight and snug.. Monitor hose connections during use..
•This device is intended for single patient use.
•Follow all precautions necessary to avoid electrical shock, fire, burns, or other personal injury from electrical power by using the device indoors, with dry hands, and in a dry location.. Keep all electrical connections away from water..
•Never use this device if the power cord or plug is damaged.
•The IceMan® is non-sterile and is not intended to be sterilized.. Do not attempt to sterilize the unit by any means..
•Rx only.
6
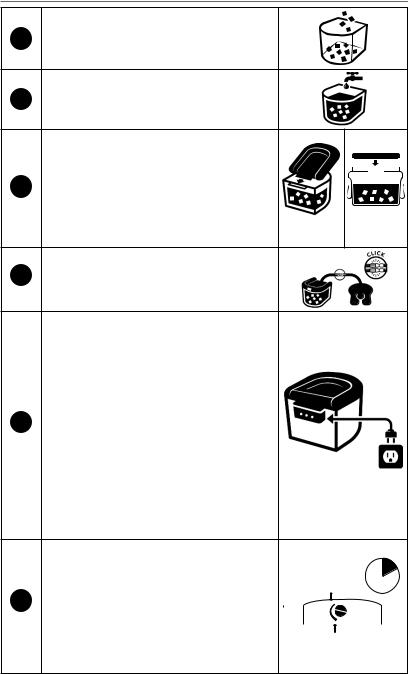
OPERATING INSTRUCTIONS
1 Add ice to fill line inside the device..
2Add cold water to fill line..
3a. CLEAR3 Lid – Place lid on the device
making sure the lip inserts into the groove.. Then press the lid down to close and secure..
3 |
3b. CLASSIC and CLASSIC3 Lid – With the |
|
|
|
handle down, place lid on device making sure |
|
|
|
the label is facing up.. Secure the lid by raising |
|
|
|
the handle, which will enage the lid locking |
3a |
3b |
|
mechanism.. |
Connect the IceMan® hose to the cold pad
4hose.. To ensure a reliable connection, “snap” or “click” hoses together into place so that the fit is tight and snug..
To turn the device on, insert cord into connection on the back of the device and plug power supply into the wall outlet.. (To turn off the device unplug it..) WARNING! When
applying the cold pad, DO NOT let any part of the cold pad touch your skin.. Always use
5with a barrier between your skin and the cold
pad.. Apply cold pad to patient.. Refer to application instructions provided with cold pad..
Check for moisture on the barrier between the patient’s skin and cold pad.. If moisture is present on the barrier, immediately discontinue use of this device..
|
For IceMan® CLASSIC |
|
|
|
|
|
|
Set temperature control on the hand console |
10 |
||||
|
|
|
|
|
||
6 |
starting at the white dot.. Allow 10 minutes |
minimum cold |
||||
after the cold pad is placed on the patient for |
|
|
|
|
||
|
|
|
|
|||
|
the temperature to stabilize.. Then adjust the |
|
|
|
|
|
|
|
|
|
|
|
|
|
temperature to the range prescribed by the |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
cold |
||
|
physician.. |
maximum |
||||
|
|
|
|
|
||
DO NOT use if you do not have a prescription..
7

STORAGE & CLEANING INSTRUCTIONS
•Unplug the power supply from the electrical outlet..
•TO AVOID DANGER OF ELECTRICAL SHOCK, DO NOT UNPLUG THE POWER SUPPLY WITH WET HANDS.
•Disconnect pad from hose..
•Drain cooler and wipe dry.
•Drain pad by holding so that the hose is hanging downward.
•Press in the buttons at the end of the hose and allow all water to drain out of the pad..
•If cleaning is necessary: wipe down device and hand wash pads and wraps with mild soap and warm water.. Air dry..
ENVIRONMENTAL & SERVICE LIMITS
•Operating Temperature Range: 5 C - 40 C
•Operating Relative Humidity Range: 15% - 90%
•Storage and Transportation Temperature: -25 C - 70 C
•Storage and Transportation Relative Humidity Range: up to 90%
•Atmospheric Pressure Range: 700 hPa - 1060 hPa
•Shelf Life: 10 Years
•Service Life: 400 Operating Hours
POWER SUPPLY
•To order a replacement power supply contact DJO Global Customer
Support..
•DonJoy® IceMan® CLASSIC & CLEAR3 Power Supply: DJO P/N 25-4882
•DonJoy® IceMan® CLASSIC3 Power Supply:DJO P/N 25-4041
WARRANTY
DJO, LLC will repair or replace all or part of the unit and its accessories for material or workmanship defects for a period of six months from the date of sale.
8
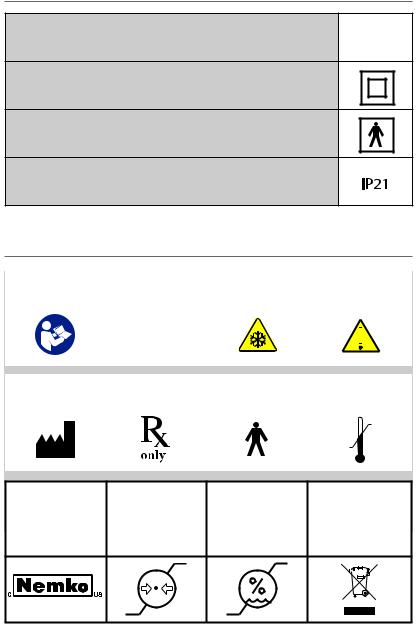
SAFETY CLASSIFICATIONS
Mode of Operation – Continuous Operation
External Electrical Power Source – Class II Equipment
Degree of Protection Against Electric Shock – Type BF Equipment
International Protection Marking Code which indicates that the device has been tested to Standard IEC 60529 for ingress protection..
LEGEND / SYMBOL DESCRIPTION
Attention / Read |
Class II Equip- |
Cold Temperature |
Warning Sign |
|||||
Manual |
|
ment |
||||||
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Manufacturer |
Rx Only |
Type BF Equip- |
Temperature |
||
|
ment |
Limits |
|||
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This device must |
||||||||
|
|
Atmospheric |
be separated from |
||||||||
Safety Mark |
Humidity Limits |
household waste |
|||||||||
Pressure Limits |
|||||||||||
|
|
and recycled as |
|||||||||
|
|
|
|||||||||
|
|
|
electronic waste |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
60601
Blue – Action Required by User
Yellow – Proceed With Caution
Orange – Warning
9

TROUBLESHOOTING
Pump will not turn on
•Check all electrical connections and make sure the wall plug has power.
Cold pad will not cool down/Cold pad pressure is low
•Make sure trapped air is out of cold pad once power is applied.
•Make sure cooler is filled with ice and water.
•Check all hose connections and make sure cold pad is wrapped properly to allow water to flow.
No water flow
•Check water level. Add water if necessary.
•Check and clean filter cap located under pump assembly.
•Cold pad and hose are wrapped too tightly or the hose is kinked. Unwrap and rewrap the cold pad with cold therapy unit running, making sure water is circulating freely throughout the cold pad and the hose is not kinked.
•Make sure connection between the cold therapy unit hose and cold pad hose is properly connected.
Water leak at connector
•Connector between cold therapy unit hose and cold pad hose is not properly connected. Stop machine, disconnect hose, and reconnect hose listening for a “snap” or “click”, and restart the unit.
•Check barrier to ensure it is dry. Replace with dry barrier, if it is wet.
•Check o-rings.
WEBSITE
For more details please refer DJO Global website: djoglobal.com
PRODUCT SUPPORT
Contact manufacturer if assistance is needed in setting up, using or maintaining the equipment or to report unexpected operation or events..
For product support call +1-888-405-3251 or +1-760-727-1280.
10

ACCESSORIES
Contact DJO Global product support at the above number or visit www..djoglobal..com for information and availability of any of the following accessories:
•Cold Pads*
•Cold Wraps*
•Dressings*
* Sterile options available.
11

COMPLIANCE STATEMENTS
ELECTROMAGNETIC COMPATIBILITY (EMC)
Iceman has been tested and found to comply with the electromagnetic compatibility (EMC) limits for medical devices to IEC 60601-1-2.. These limits are designed to provide reasonable protection against harmful interference in a typical medical installation..
Caution: Medical electrical equipment requires special precautions regarding EMC and must be installed and operated according to these instructions.. It is possible that high levels of radiated or conducted radio-frequency electromagnetic interference (EMI) from portable and mobile RF communications equipment or other strong or nearby radio-frequency sources, could result in performance disruption of the system.. Evidence of disruption may include equipment ceasing to operate, or other incorrect functioning.. If this occurs, survey the site of disruption, and take the following actions to eliminate the source(s)..
•Turn equipment in the vicinity off and on to isolate disruptive equipment..
•Relocate or reorient interfering equipment.
•Increase distance between interfering equipment and your system.
•Manage use of frequencies close to the system frequencies.
•Remove devices that are highly susceptible to EMI.
•Lower power from internal sources within the facility control (such as paging systems)..
•Label devices susceptible to EMI..
•Educate clinical staff to recognize potential EMI-related problems.
•Eliminate or reduce EMI with technical solutions (such as shielding).
•Restrict use of personal communicators (cell phones, computers) in areas with devices susceptible to EMI..
•Share relevant EMI information with others, particularly when evaluating new equipment purchases which may generate EMI..
•Purchase medical devices that comply with IEC 60601-1-2 EMC Standards (3V/meter EMI immunity, limit interference level to 0..0014 V/meter)..
12

ELECTROMAGNETIC COMPATIBILITY (EMC) TABLES – RF EMISSIONS CLASS B
Guidance and Manufacturer’s Declaration – Electromagnetic Emissions
Iceman is intended for use in the electromagnetic environment specified below. The customer or the user of Iceman should assure that it is used in such an environment.
Emissions Tests |
Compliance |
Electromagnetic Environment |
|
Guidance |
|||
|
|
||
|
|
|
|
RF Emissions CISPR 11 |
Group 1 |
Iceman uses RF energy only for its |
|
|
|
internal function. Therefore, its RF |
|
|
|
emissions are very low and are not likely |
|
|
|
to cause any interference in nearby |
|
|
|
electronic equipment. |
|
|
|
|
|
RF Emissions CISPR 11 |
Class B |
Iceman is suitable for use in all |
|
|
|
||
|
|
establishments, including domestic |
|
Harmonic Emissions |
Class A |
||
establishments and those directly |
|||
IEC 61000-3-2 |
|
||
|
connected to the public low-voltage |
||
|
|
||
Voltage Fluctuations |
Complies |
power supply network that supplies |
|
buildings used for domestic purposes. |
|||
IEC 61000-3-3 |
|
||
|
|
||
|
|
|
13

ELECTROMAGNETIC COMPATIBILITY (EMC)
TABLES – RF EMISSIONS CLASS B (con’t)
Guidance and Manufacturer’s Declaration – Electromagnetic Immunity
Iceman is intended for use in the electromagnetic environment specified below. The customer or the user of Iceman should assure that it is used in such an environment.
|
IEC 60601 Test |
Compliance |
Electromagnetic |
|
Immunity Test |
Environment |
|||
Level |
Level |
|||
|
Guidance |
|||
|
|
|
||
|
|
|
|
|
Electrostatic |
±8 kV contact |
±8 kV contact |
Floors should be |
|
Discharge (ESD) |
|
|
wood, concrete or |
|
|
|
|
ceramic tile. If floors |
|
IEC 61000-4-2 |
±15 kV air |
±15 kV air |
are covered with |
|
synthetic material, |
||||
|
|
|
||
|
|
|
the relative humidity |
|
|
|
|
should be at least |
|
|
|
|
30%. |
|
|
|
|
|
|
Electrical Fast |
±2 kV for power |
±2 kV for power |
Mains power quality |
|
Transient/Burst |
supply lines ±1 KV |
supply lines ±1 kV |
should be that of a |
|
|
typical commercial |
|||
IEC 61000-4-4 |
for input/ |
for input/ |
or hospital |
|
environment. |
||||
|
output lines |
output lines |
||
|
|
|||
|
|
|
|
|
Surge |
±1 KV differential |
±1 kV differential |
Mains power quality |
|
|
mode ±2 kV |
mode ±2 kV |
should be that of a |
|
IEC 61000-4-5 |
typical commercial |
|||
|
common mode |
common mode |
or hospital |
|
|
environment. |
|||
|
|
|
||
|
|
|
|
|
Voltage dips, short |
Voltage dips of |
Voltage dips of |
Mains power quality |
|
interruptions and |
>95% for 0.5 cycles |
>95% for 0.5 cycles |
should be that of a |
|
voltage variations |
typical commercial |
|||
on power supply |
30% for 25 cycles |
30% for 25 cycles |
or hospital |
|
environment. If the |
||||
input lines |
|
|
||
60% for 5 cycles |
60% for 5 cycles |
user of the Cold |
||
|
||||
|
>95% for 250 |
>95% for 250 cycles |
Therapy Unit |
|
|
requires continued |
|||
|
cycles (5s) |
(5s) |
operation during |
|
IEC 61000-4-11 |
|
|
power mains |
|
|
|
interruptions, it is |
||
|
|
|
||
|
|
|
recommended that |
|
|
|
|
Iceman be powered |
|
|
|
|
from an |
|
|
|
|
uninterrupted power |
|
|
|
|
supply or a battery. |
|
|
|
|
|
|
Power Frequency |
Inductive loop at |
Inductive loop at |
Power frequency |
|
(50/60Hz) |
50 Hz and 60 Hz, |
50 Hz and 60 Hz, |
magnetic fields |
|
Magnetic Fields |
to 30 amps (rms) |
to 30 amps (rms) |
should be at levels |
|
|
per meter |
per meter |
characteristic of a |
|
|
typical location in a |
|||
IEC 61000-4-8 |
|
|
||
|
|
typical commercial |
||
|
|
|
or hospital |
|
|
|
|
environment. |
|
|
|
|
|
NOTE: UT is the ac mains voltage prior to application of the test level.
14
 Loading...
Loading...