Page 1

Cosmed does not assume the liability for interpretation mistakes of this
documentation or for casual or consequential damages in connection with the
provision, representation or use of this documentation.
No parts of this manual may be reproduced or transmitted in any form without
the express permission of COSMED Srl.
COSMED Software can be installed only in one device.
Excel is a registered trademark of Microsoft Corporation.
DBIII is a registered trademark of Bordland International Inc.
Lotus 123 is a registered trademark of Lotus Development Corporation .
microQuark User manual, V Edition
September 2003
Copyright © 2001 COSMED
Copyright © 2003 COSMED
COSMED Srl - Italy
http://www.cosmed.it
Part N. C02268-02-91
Page 2

Table of contents
Getting started 7
Important notices.................................................................... 8
Intended use............................................................................................ 8
Warnings................................................................................................. 8
Contraindication ................................................................... 11
Contraindications for the Spirometer tests............................................ 11
Absolute contraindications .............................................................11
Relative contraindications .............................................................. 11
Contraindications for Bronchial provocation tests................................ 11
Absolute contraindications .............................................................12
Relative contraindications .............................................................. 12
Environmental condition of use ............................................ 13
Safety and conformity ........................................................... 14
Safety.............................................................................................. 14
EMC ............................................................................................... 14
Quality Assurance...........................................................................14
Medical Device Directive (CE mark) ............................................. 14
Keynotes................................................................................ 15
Typographic keynotes........................................................................... 15
Graphic keynotes .................................................................................. 15
Systems Overview ................................................................. 16
Before starting ...................................................................... 17
Checking the packing contents .............................................................17
microQuark standard packaging ..................................................... 17
Warranty registration ........................................................... 18
Register the product via software ...................................................18
How to contact COSMED.............................................................. 18
Complain, feedback and suggestions.............................................. 18
PC configuration required .................................................... 19
Technical features ................................................................. 20
Measurements 21
Measured parameters .......................................................... 22
2 - microQuark User Manual
Page 3
FVC - Forced Vital Capacity................................................................ 22
VC/IVC - Slow Vital Capacity and Ventilatory pattern ....................... 23
MVV - Maximum Voluntary Ventilation............................................. 23
Bronchoprovocation Response....................................................... 23
Installation 25
Prepare the microQuark .......................................................26
Software installation .............................................................27
Installing the software .......................................................................... 27
Run the software................................................................................... 27
PC port configuration........................................................................... 27
Software main features .........................................................28
Display ................................................................................................. 28
Tool bar................................................................................................ 28
Show/hide the toolbar..................................................................... 28
Dialog windows.................................................................................... 28
Use of the keyboard ....................................................................... 28
Use of the mouse............................................................................ 28
Scroll bars............................................................................................. 29
On-line help.......................................................................................... 29
Software version................................................................................... 29
Calibration 31
The calibration program .......................................................32
Running the Calibration program......................................................... 32
Log file................................................................................................. 32
Turbine calibration ................................................................33
Checking the system signals .................................................35
The control panel.................................................................................. 35
Using the control panel ................................................................. 35
Database Management 37
Settings ..................................................................................38
Graphs............................................................................................ 38
Serial port....................................................................................... 38
Units of measurements ................................................................... 39
Using extra fields ........................................................................... 39
Index - 3
Page 4

Customise the fields........................................................................ 39
Patient's database ................................................................ 40
Patient Card .................................................................................... 40
Visit Card .......................................................................................41
Test Card ........................................................................................41
Import/export a Tests card .................................................................... 41
Diagnosis Database............................................................................... 41
Archive maintenance ............................................................ 43
Reorganise the archive.......................................................................... 43
Delete the archive ................................................................................. 43
Backup and restore................................................................................43
Backup............................................................................................ 43
Restore............................................................................................ 44
Spirometry 45
Setting spirometry options ................................................... 46
Spirometry ............................................................................................ 46
Automatic Interpretation.................................................................46
Quality control................................................................................ 47
Parameters manager.............................................................................. 47
Predicted values manager .....................................................................48
Predicteds set.................................................................................. 48
Set the current predicted................................................................. 49
Formula definition.......................................................................... 49
Page set-up............................................................................................ 50
Spirometry tests .................................................................... 52
Recommendations for spirometry tests................................................. 52
Forced Vital Capacity (pre) ................................................... 53
Perform a FVC (pre) test.......................................................................53
Test encouragement.............................................................................. 54
Perform the FVC test with the encouragement............................... 54
Slow Vital Capacity................................................................ 55
Perform a SVC test ............................................................................... 55
Maximum Voluntary Ventilation .......................................... 57
Perform a MVV test..............................................................................57
Bronchial Provocation Test ................................................... 58
Bronchodilator test................................................................................58
4 - microQuark User Manual
Page 5
Methacholine and Histamine Bronchial provocation Tests.................. 58
Perform the test .................................................................................... 59
Bronchial Provocation protocols Database........................................... 60
Enter a new Bronchial provocation protocol in the
archive............................................................................................ 61
Viewing results ......................................................................62
Tests of the current patient ............................................................. 62
Delete a test.................................................................................... 62
Printing results.......................................................................63
Printing Reports.................................................................................... 63
Printing the active window................................................................... 64
Printing a series of reports.................................................................... 64
Electronic reports (*.pdf)...................................................................... 64
Export data ........................................................................................... 65
Export a test ................................................................................... 65
System maintenance 67
System maintenance..............................................................68
Cleaning and disinfection..................................................................... 68
Cleaning the turbine flowmeter...................................................... 69
Precautions during the cleaning of the turbine ............................... 69
Suggested disinfection solutions .................................................... 70
Inspections............................................................................................ 70
Appendix 71
Service - Warranty.................................................................72
Warranty and limitation of liability...................................................... 72
Return goods policy for warranty or non warranty repair..................... 73
Repair Service Policy........................................................................... 73
Privacy Information ...............................................................75
Personal data treatment and purposes................................................... 75
How your personal data are treated ...................................................... 75
The consent is optional, but….............................................................. 75
Holder of the treatment......................................................................... 75
Customer rights .................................................................................... 76
Converting factors configuration ..........................................77
ATS 94 recommendations......................................................78
Index - 5
Page 6

ATS recommendations ......................................................................... 78
Predicted values.................................................................... 79
ERS93................................................................................................... 79
Reference Adult:............................................................................. 79
Reference Paediatric:...................................................................... 79
KNUDSON 83...................................................................................... 79
Reference Adult/ Paediatric:........................................................... 79
ITS (White race) ................................................................................... 79
Reference Adult/ Paediatric:........................................................... 79
ITS (Black race).................................................................................... 79
Reference Adult/ Paediatric:........................................................... 79
LAM ..................................................................................................... 80
Reference Adult/ Paediatric:........................................................... 80
Multicéntrico de Barcelona................................................................... 80
Reference Adult/ Paediatric:........................................................... 80
NHANES III......................................................................................... 80
Reference Adult/ Paediatric:........................................................... 80
Automatic diagnosis (algorithm) ..........................................................80
Quality Control Messages..................................................................... 81
References ............................................................................. 82
6 - microQuark User Manual
Page 7
Getting started
Page 8

Important notices
Intended use
microQuark is an electrical medical device designed to perform
pulmonary function tests. It is to be used by physicians or by
trained personnel on a physician responsibility.
Caution: Federal law restricts this device to sale by or on the
order of a physician.
This equipment has been conceived with the aim of providing an
auxiliary instrument allowing:
• the formulation of lung pathology diagnosis;
• important studies concerning human physiology;
• the collection of important information in sport medicine.
No responsibility attaches COSMED Srl for any accident
happened after a wrong use of the device, such as:
• use by non qualified people;
• non respect of the device intended use;
• non respect of the hereunder reported precautions and
instructions.
Warnings
The device, the program algorithms and the presentation of
measured data have been developed according to the
specifications of ATS (American Thoracic Society) and ERS
(European Respiratory Society). Other international references
have been followed when these were not available. All
bibliography references are reported in Appendix.
The present handbook has been developed with respect of the
European Medical Device Directive requirements which sort
microQuark within Class II a.
It is recommended to read carefully the following precautions
before putting the device into operation.
The precautions reported below are of fundamental importance to
assure the safety of all COSMED equipment users.
1. This user manual is to be considered as a part of the medical
device and should always be kept on hand.
2. Safety, measure accuracy and precision can be assured only:
8 - microQuark User Manual
Page 9

• using the accessories described in the manual or given
with the device. Actually non recommended accessories can
affect safety unfavourable. Before using non recommended
accessories it is necessary to get in touch with the
manufacturer;
• ordinary equipment maintenance, inspections,
disinfection and cleaning are performed in the way and with
the frequency described;
• any modification or fixing is carried out by qualified
personnel;
• the environmental conditions and the electrical plants
where the device operates are in compliance with the
specifications of the manual and the present regulations
concerning electrical plants. In particular grounding
reliability and leakage current suppression can only be
assured when the device three – wire receptacle is connected
to a yellow - green return connected to earth ground.
Attempting to defeat the proper connection of the ground
wire is dangerous for users and equipment.
3. Before powering the system, check the power cables and the
plugs. Damaged electrical parts must be replaced
immediately by authorised personnel.
4. Cleaning residue, particulates, and other contaminates
(including pieces of torn or broken components) in the
breathing circuit pose a safety risk to the patient during
testing procedures. Aspiration of contaminates can
potentially be life-threatening. You must follow all the
cleaning procedures in System Maintenance, and you must
thoroughly inspect the components after cleaning and before
each patient test.
5. This device is not suitable for use in presence of flammable
anaesthetics. It is not an AP nor an APG device (according to
the EN 60 601-1 definitions).
6. Keep the device away from heat and flame source,
flammable or inflammable liquids or gases and explosive
atmospheres.
7. In accordance with their intended use microQuark is not to
be handled together with other medical devices unless it is
clearly declared by the manufacturer itself.
Chapter 1 - Getting started - 9
Page 10
8. It is recommended to use a computer with electromagnetic
compatibility CE marking and with low radiation emission
displays.
9. It is necessary to make the PC, connected to the
microQuark,
compliant with EN 60601-1 by means of an isolation
transformer.
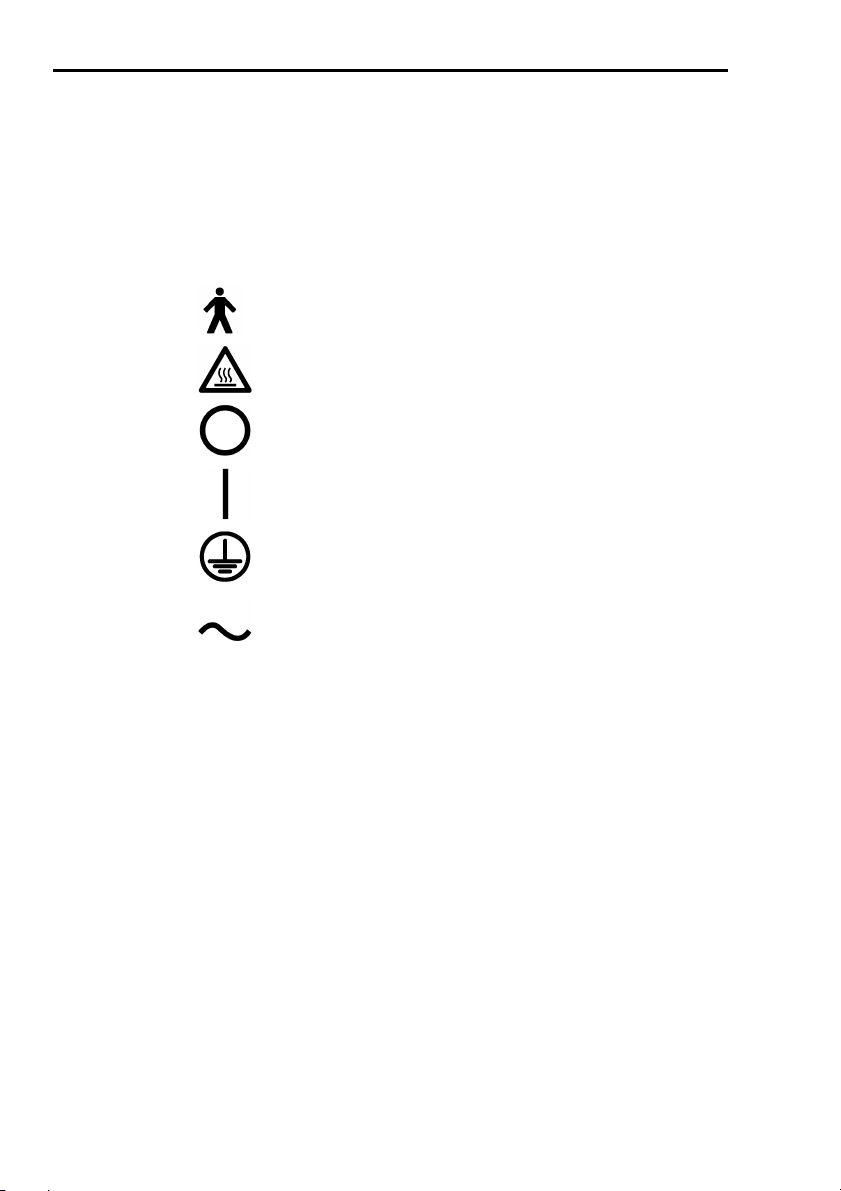
10. Graphical symbols used in accordance to present
specifications are described here below:
Equipment type B (EN60601-1)
Danger: high temperature
OFF
ON
Protective earth ground
Alternating current
10 - microQuark User Manual
Page 11

Contraindication
The physical strain to execute the respiratory manoeuvre is
contraindicated in case of some symptoms or pathology. The
following list is not complete and must be considered as a piece
of mere information.
Contraindications for the Spirometer tests
Absolute contraindications
For FVC, VC and MVV tests:
• Post-operating state from thoracic surgery
For FVC tests:
• Severe instability of the airways (such as a destructive
bronchial emphysema)
• Bronchial non-specific marked hypersensitivity
• Serious problems for the gas exchange (total or partial
respiratory insufficiency)
Relative contraindications
For FVC tests:
• spontaneous post-pneumothorax state
• arterial-venous aneurysm
• strong arterial hypertension
• pregnancy with complications at the 3
For MVV test:
• hyperventilation syndrome
rd
month.
Contraindications for Bronchial provocation tests
The bronchial provocation tests must be executed according to
the doctor’s discretion. There are not data that reveal specific
contraindication for the bronchial provocation test through
inhalation.
The modern standard processes have been revealing secure in
several clinical studies. However it is recommendable to respect
the following contraindications:
Chapter 1 - Getting started - 11
Page 12

Absolute contraindications
• Serious bronchial obstruction (FEV1 in adults)
• Recent myocardium infarct
• Recent vascular-cerebral accident
• Known arterial aneurysm
• Incapacity for understanding the provocation test procedures
and its implications.
Relative contraindications
• Bronchial obstruction caused by the respiratory manoeuvre.
• Moderate or serious bronchial obstruction. For ex. Predicted
value FEV1 less than 1.51 in men and predicted value FEV1
in women less than 1.21.
• Recent infection in the superior air tracts
• During the asthmatic re-acuting
• Hypertension
• Pregnancy
• A pharmacology treatment epilepsy
12 - microQuark User Manual
Page 13

Environmental condition of use
COSMED units have been conceived for operating in medically
utilised rooms without potential explosion hazards.
The units should not be installed in vicinity of x-ray equipment,
motors or transformers with high installed power rating since
electric or magnetic interferences may falsify the result of
measurements or make them impossible. Due to this the vicinity
of power lines is to be avoided as well.
Cosmed equipment are not AP not APG devices (according to
EN 60601-1): they are not suitable for use in presence of
flammable anaesthetic mixtures with air, oxygen or nitrogen
protoxide.
If not otherwise stated in the shipping documents, Cosmed
equipment have been conceived for operating under normal
environmental temperatures and conditions [IEC 601-1(1988)/EN
60 601-1 (1990)].
• Temperature range 10°C (50°F) and 40°C (104°F).
• Relative humidity range 20% to 80%
• Atmospheric Pressure range 700 to 1060 mBar
• Avoid to use it in presence of noxious fumes or dusty
environment and near heat sources.
• Do not place near heat sources.
• Cardiopulmonary resuscitation emergency equipment
accessible.
• Adequate floor space to assure access to the patient during
exercise testing.
• Adequate ventilation in the room.
Chapter 1 - Getting started - 13
Page 14

Safety and conformity
Safety
IEC 601-1 (1988) /EN 60 601-1 (1990);
Find reported below the complete classification of the device:
• Class I type B device
• Protection against water penetration: IP00, ordinary
equipment unprotected against water penetration
• Non sterile device
• Device not suitable in the presence of flammable
anaesthetics;
• Continuous functioning equipment;
EMC
The system meets the EMC Directive 89/336
EN 60601-1-2
EN 55011 Class B (emission), IEC 1000-4-2, IEC 1000-4-3, IEC
1000-4-4
Quality Assurance
UNI ISO 9001 (Registration n° 387 Cermet)
Medical Device Directive (CE mark)
MDD 93/42/EEC (Notified Body 0476).
Class IIa
14 - microQuark User Manual
Page 15
Keynotes
Here are the keynotes used to make the manual easier to read.
Typographic keynotes
These are the typographic keynotes used in the manual.
Style Description
Bold indicates a control or a key to be pressed.
“Italic” indicates a messages shown by the firmware.
Graphic keynotes
These are the graphic keynotes used in the manual.
Illustration Description
shows the button to click in the software to
activate the related feature.
Chapter 1 - Getting started - 15
Page 16

Systems Overview
microQuark is an instrument designed for lung function
screening; the core of the system is the “intelligent” flowmeter
that, connected through the serial port (RS232), turns any
Personal Computer (laptop or desktop) in a complete spirometric
lab.
The system is composed by the turbine flowmeter, the
measurement and data elaboration device (lightweight and
ergonomic), the communication cable and by the Software pack.
16 - microQuark User Manual
Page 17

Before starting
Before operating the microQuark system we strongly recommend
to check the equipment and register you as a customer.
Checking the packing contents
Make sure that the package contains the items listed below. In
case of missing or damaged parts, please contact Cosmed
technical assistance.
microQuark standard packaging
Code Qty Description
C00960-01-04 1 microQuark unit
C02235-01-05 1 Turbine
A 662 100 001 2 Nose clips
C01739-02-35 1 PC Software
C00137-01-20 20 Paediatric paper mouthpieces
C00136-01-20 20 Adult paper mouthpieces
C00063-01-20 1 Conic mouthpiece
C00214-01-20 1 Paediatric adapter
A 362 300 004 1 Serial cable RS232 USB Power
C00067-02-94 1 Registration card
C02268-02-91 1 User manual
C01999-02-DC 1 Conformity declaration
Chapter 1 - Getting started - 17
Page 18

Warranty registration
Before using the system, please take a moment to fill in the
registration form and the warranty and return them to COSMED,
by doing this you are eligible to the customers assistance service.
For further information, please refer to the enclosed registration
and warranty form. If the form is not enclosed in the packaging,
please contact directly COSMED.
Register the product via software
Together with the PC software, a registration software is
supplied. With this software it is possible to fill in an electronic
form with the customer information.
1. To run the software, double click on the icon Registration or
select Registration… from ? menu.
2. Type the requested information and click Send… to send the
form via e-mail to COSMED.
How to contact COSMED
For any information you may need, please contact the
manufacturer directly at the following address:
COSMED S.r.l.
Via dei Piani di Monte Savello, 37
P.O. Box n. 3
00040 - Pavona di Albano
Rome - ITALY
Voice: +39 (06) 931.5492
Fax: +39 (06) 931.4580
email: customersupport@cosmed.it
Internet: http: //www.cosmed.it
Complain, feedback and suggestions
If you have any complain, feedback information or suggestion,
please inform us at complain@cosmed.it.
18 - microQuark User Manual
Page 19

PC configuration required
• Pentium 133 MHz.
• Windows 95, 98, XP.
• 16 Mb RAM .
• 3.5 drive.
• VGA, SVGA monitor.
• USB port.
• Serial Port RS 232 available.
• Any Mouse and Printer compatible with the MS Windows™
operative system.
• PC conform to European Directive 89/336 EMC
Chapter 1 - Getting started - 19
Page 20

Technical features
Flowmeter Bidirectional digital turbine
Flow Range: 0.03 - 20 l/s
Volume Range: 12 l
Accuracy: ± 3% or 50 ml
Resistance @12 l/s: < 0.7 cmH2O/l/sec
Mouthpieces: Ø 31 and Ø22 mm
Serial port: RS232C
Dimensions: 150 x 45 x 53 mm
Weight: 77g
20 - microQuark User Manual
Page 21
Measurements
Page 22

Measured parameters
FVC - Forced Vital Capacity
Symbol UM Parameter
FVC l Forced Expiratory Vital Capacity
FEV1 l Forced Expiratory Volume in 1 sec
FEV1/FVC% % FEV1 as a percentage of FVC
PEF l/sec Peak Expiratory Flow
FEV0.5 l Forced Expiratory Volume in 0.5 sec
FEV6 l Forced Expiratory Volume in 6 sec
FEV1/FEV6 % FEV1 as a percentage of FEV6
FEV6/FVC% % FEV6 as a percentage of FVC
Best FVC l Best Forced Expiratory Vital Capacity
Best FEV1 l Best Forced Expiratory Volume in 1 sec
Best PEF l/sec Best Peak Expiratory Flow
Vmax25% l/sec Expiratory Flow @25% of the FVC
Vmax50% l/sec Expiratory Flow @50% of the FVC
Vmax75% l/sec Expiratory Flow @75% of the FVC
FEF25-75% l/sec Mid-exp flow between 25-75%FVC
FET100% sec Forced expiratory time
FEV2 l Forced Expiratory Volume in 2 sec
FEV3 l Forced Expiratory Volume in 3 sec
FEV2/FVC% % FEV2 as a percentage of FVC
FEV3/FVC% % FEV3 as a percentage of FVC
FEV1/VC% % Tiffenau index
FEF50-75% l/sec Mid-exp flow between 50-75%FVC
FEF75-85% l/sec Mid-exp flow between 75-85%FVC
FEF0.2-1.2% l/sec Mid-exp flow between 0.2 l - 1.2 l
FiVC L Inspiratory Forced Vital Capacity
FiF25-75% l/sec Forced mid-inspiratory flow
FiV1 l/sec Forced Inspiratory Volume in 1 sec
PIF l/sec Peak Inspiratory Flow
VEXT ml Extrapolated Volume (back extrapolation)
PEFT msec Time to PEF (10% - 90%)
22 - microQuark User Manual
Page 23

VC/IVC - Slow Vital Capacity and Ventilatory pattern
Symbol UM Parameter
EVC l Expiratory Vital Capacity
IVC l Inspiratory Vital Capacity
ERV l Expiratory Reserve Volume
IRV l Inspiratory Reserve Volume
IC l Inspiratory Capacity
VE l/min Expiratory Minute Ventilation
Vt l Tidal Volume
Rf 1/min Respiratory Frequency
Ti sec Duration of Inspiration
Te sec Duration of Expiration
Ttot sec Duration of Total breathing cycle
Ti/Ttot —- Ti/Ttot ratio
Vt/ti l/sec Vt/ti ratio
MVV - Maximum Voluntary Ventilation
Symbol UM Parameter
MVV l/min Maximum Voluntary Ventilation
MVt l Tidal Volume (during MVV)
MRf 1/min Maximum Respiratory frequency
MVVt sec MVV duration time
Bronchoprovocation Response
Symbol UM Parameter
FallFEV1 % Fall in FEV1 from baseline or post diluent
FallVmax50% % Fall in Vmax50% from ref.
P10 —- Provocative dose causing FEV1 to fall 10%
P15 —- Provocative dose causing FEV1 to fall 15%
P20 —- Provocative dose causing FEV1 to fall 20%
Chapter 2 - Measurements - 23
Page 24

24 - microQuark User Manual
Page 25

Installation
Page 26

Prepare the microQuark
Power supply is provided by PC through the keyboard/mouse
PS2 plug. The unit is turned on/off automatically by the PC.
1. Insert the turbine and be sure to push the turbine up to touch
the end of the holder.
2. Connect the microQuark to the PC, through the RS232 (data)
and USB (power supply) port, as shown in the following
picture.
26 - microQuark User Manual
Page 27

Software installation
Installing the software
1. Select Run... from Windows Start menu.
2. In the Command line box, type a:\install (assuming the disk
is in drive A:).
3. Click on OK (or press ENTER key).
4. The program will load up a dialog box and ask for a
directory where to be installed.
5. The installation software will present a dialog box in which
you have to select the device. Select microQuark.
6. When the installation is over, the program will advise you
with a message indicating that the installation has been
successfully completed, click on End.
Notice: The software is copy-protected. Install the software from
the original disk.
Run the software
1. In the Windows Start menu, open the Program Group in
which the software was installed.
2. Click the Quark PFT icon.
PC port configuration
The first time the software is used, it is necessary to configure the
communication port with the PC (USB, COM1, COM2,…).
For further details, see the chapter Database management.
Chapter 3 - Installation - 27
Page 28

Software main features
Display
The program may contain several windows. The active window
is highlighted with a different colour of the caption. Some
functions of the program are "active window" sensitive (Print,
right key of the mouse).
Tool bar
Many of the functions that may be selected from the menu can be
activated more rapidly by clicking with the mouse on the
corresponding icon in the tool bar.
Positioning the mouse cursor on one of the buttons of the toolbar
(if the option Hints is enabled), the description of the
corresponding function is shown in a label.
Show/hide the toolbar
Select Toolbar from Options menu in order to show or hide the
toolbar.
Dialog windows
The typical operating environment of Microsoft Windows is the
Dialog box. This window is provided with a series of fields in
which input the information.
Use of the keyboard
• To move the cursor among fields, press the Tab key until
you reach the desired field.
• Press the Enter key to confirm the information input on the
dialog box or press the Esc key to cancel changes.
Use of the mouse
• To move the cursor among fields, move the mouse on the
desired field and left-click.
• Click on the OK button with the Left button of the mouse to
confirm the information input on the dialog box or click on
Cancel button to cancel changes.
28 - microQuark User Manual
Page 29

Scroll bars
Some windows are provided with scroll bars that help to see data
exceeding the window space available.
• To move the scroll bar row by row click the scroll arrows at
• To move the scroll bar page by page click on the grey area at
On-line help
COSMED Help is a complete on-line reference tool that you can
use at any time. Help is especially useful when you need
information quickly or when the user manual is not available.
Help contains a description of each command and dialog box,
and explains many procedures for accomplishing common tasks.
To get the Help on line, press the F1 key.
Software version
To know the software version and the serial number of the
software, select About Quark PFT… from Help menu.
the end of the scroll bars
both sides of the scroll fields
Chapter 3 - Installation - 29
Page 30

30 - microQuark User Manual
Page 31

Calibration
Page 32

The calibration program
Running the Calibration program
Start the program and choose Calibration from the Test Menu.
The software runs the Calibration software and the main menu
changes accordingly.
Log file
The program creates and updates as default the calibration log
file, containing the conditions and the results of all the
calibrations performed by the user.
To access the file select File/Report File... from the calibration
program.
32 - microQuark User Manual
Page 33

Turbine calibration
microQuark is calibrated by COSMED. ATS recommends a daily
calibration of the turbine. However if it is correctly maintained,
turbine retains its precision for longer periods. We advice to
calibrate the turbine daily to detect malfunctioning.
Note: if you are using a slow PC, we recommend to set an higher
refresh time.
In order to calibrate the turbine:
1. Connect the turbine flowmeter to the calibration syringe.
2. Select Calibration from Test menu.
3. Select Reference values from the Calibration menu and
enter the syringe volume, if different to the displayed one.
4. Select Turbine from the Calibration menu.
5. When the Calibration Turbine dialog box appears with the
syringe piston initially pushed all the way in, move the
piston in and out for 5 inspiratory strokes and 5 expiratory
strokes in order to get the first values appearing on the
screen. Then move the syringe piston for other 10 strokes
(IN and EX).
6. At each of the 10 steps the software displays the results of
the manoeuvre and the percentage error in the reading.
Chapter 4 - Calibration - 33
Page 34

7. At the end of this operation, the software displays the new
calibration factors. Press OK to store the new value.
The 3 litres calibration syringe can be purchased directly from
COSMED (P/N: C00600-01-11).
Note: If a bacterial filter is used for the tests, do use it also
during the turbine calibration.
34 - microQuark User Manual
Page 35

Checking the system signals
The control panel
The Control Panel, which can be activated from the
Calibration/Control panel… menu item, is a useful tool to
check the main hardware functions of microQuark.
By using the controls on Control Panel you are able to read the
signals acquired by the system both as voltages and processed
data.
Using the control panel
edit parameters
(name, unit,…)
mV / real values
display
Signal refresh
time
Select all
channels
Deselect all
channels
Chapter 4 - Calibration - 35
Page 36

36 - microQuark User Manual
Page 37

Database
Management
Page 38

Settings
The software allows to configure some options selecting
Configure from the Option menu.
Graphs
All the graphs visualised and/or printed can be customised in
colours and appearance.
1. Select the desired colours of the curves (5 curves max can be
overlapped on the same graph).
2. Enable or disable the Grid option.
3. Enable or disable the Show Info Test option.
Serial port
You must select the serial port RS 232 that will be used to
connect the microQuark with the PC.
To select the serial port, click on the proper COM button (the
selected port must be different from the mouse one).
38 - microQuark User Manual
Page 39

Units of measurements
It is possible to configure the units of measurements, weight and
height, for printing and viewing.
To select the units of measurement click on cm/Kg or in/lb
according to the desired format.
Using extra fields
The Patient’s database is organised in 3 different cards (Patient
card, Visit Card and Test card.) where it is possible to store the
information about patients and visits .
Besides the standard information, it is possible to customise some
fields (user free fields), entering and labelling measurements
coming from other devices.
The customisable free fields are:
• 3 fields in the Patient Card (Patient’s information)
• 3 fields in the Visit Card (information about the visits)
• 3 fields (2 numeric) in the Test card information about Test)
Customise the fields
In the group User free fields type the desired text in the 9 fields
available.
Chapter 5 - Database Management - 39
Page 40

p
Patient's database
The Patients database consists of a Patient Card, a Visit Card and
a Test Card in which are listed all tests performed by the patient.
Select Archive Navigator from the File menu or press the
button by side.
Note: after having
deleted a record
(patient, visit or
test), it is
recommended to
reorganize the
archive in order to
free disk s
ace.
Patient Card
It collects all the information of a patient (first name, last name,
date of birth) which remain the same for each visit. For each
patient there is only one Patient Card, which is created the first
time the Patient performs a test.
To move within the database use the following buttons:
Move to the first patient in the archive
Move to the previous patient in the archive
Move to the next patient in the archive
Move to the last patient in the archive
Find a patient in the archive
Enter a new patient in the archive
Delete current patient from the archive
Edit the current patient card
40 - microQuark User Manual
Page 41

Visit Card
It collects all information relative to the visit (diagnosis, visit
description...) and to the patient information subject to change
between one visit and another (height, weight, smoke). Each
patient can be related to several Visit Cards provided they have
been created in different days. Before carrying out any
spirometric test it is necessary to create a new Visit Card or to
open the today’s Visit Card.
To move within the database use the following buttons:
Move to the first visit in the archive
Move to the previous visit in the archive
Move to the next visit in the archive
Move to the last visit in the archive
Find a visit in the archive
Enter a new visit card in the archive
Delete current visit card from the archive
Edit the current visit card
Test Card
It contains all the information about the test.
To move within the database use the following buttons:
Delete current test from the archive.
Edit the current test
Import/export a Tests card
This function allows to import /export a test card with the
respective visit and patient card.
1. Select the patient.
2. Choose the tests to be exported and press the key by side. All
data will be imported/exported in the XPO file format
(Cosmed proprietary).
Diagnosis Database
The program allows to manage a diagnosis database, whose
records are composed by a diagnosis ID code and a string of text.
Chapter 5 - Database Management - 41
Page 42

The report of the visits can be done either by typing the desired
text in the field “Diagnosis” of the Visit Card or, more quickly,
retrieving from the diagnosis database the desired one.
If you want to insert, modify or delete a diagnosis from the
database select Database Diagnosis... from the File menu.
42 - microQuark User Manual
Page 43

Archive maintenance
The software allows to manage files selecting Archive from the
File menu.
It is advisable to perform the archive reorganisation every month,
in order to free space on the hard disk and/or to correct possible
errors present within the database.
It is possible also that your have no more hard disk space. So,
you have to delete all the data. In this case, it is useful to perform
the initialising.
Reorganise the archive
1. Select Reorganize archive from the File menu.
2. Wait for the end of the operation before performing any
other function.
Delete the archive
1. Select Initialize Archive from the File menu.
2. Wait for the end of the operation before performing any
other function.
Backup and restore
It is strongly recommended to backup files, a warning message
will be displayed monthly. This function allows the user to
restore the data if the PC or the HD will not work anymore.
Backup
1. Select Backup archive from the File menu.
2. Selecting the destination path with the Browse key or press
New to create a new directory. Press OK to confirm.
Chapter 5 - Database Management - 43
Page 44

3. In the dialog box it will appear an estimate of the number of
floppy you need in order to back up the archives. Press OK.
Restore
1. Select Restore archive from the File menu.
2. On the Restore dialog box specify the drive source and press
OK, a dialog box will appear indicating all data of the
backup processed.
44 - microQuark User Manual
Page 45

Spirometry
Page 46

Setting spirometry options
The software allows to configure some options selecting
Configure from the Option menu.
Spirometry
Automatic Interpretation
microQuark has the function of interpreting each test performed
by a patient visualising an automatic diagnosis. The algorithm
has been calculated basing on “Lung Function Testing: selection
of reference values and interpretative strategies, A.R.R.D. 144/
1991:1202-1218”. The automatic diagnosis is calculated at the
end of the FVC if:
• the automatic diagnosis option is enabled.
• the patient’s anthropometric data allow the calculation of the
LLN (Lower Limit of Normal range).
• at least one FVC test has been performed.
To enable/disable the automatic diagnosis:
1. Click on Enable Automatic Interpretation.
2. Select the LLN (Lower Limit of Normal Range) criteria
among the ATS (LLN=Pred-0.674*SD), ERS (LLN=Pred-
1.647*SD) or 80%Pred (LLN=Pred*0.8) specifications.
46 - microQuark User Manual
Page 47

Quality control
microQuark allows a quality test control. The calculation has
been carried out referring to “Spirometry in the Lung Health
Study: Methods and Quality Control, A.R.R.D. 1991; 143:12151223”. The messages concerning the quality control are shown at
the end of the test.
To enable/disable the quality control, click on Enable Quality
Control checkbox.
Parameters manager
The program allows to calculate a huge number of parameters; it
is advisable, in order to simplify the analysis of the results, to
view, to print and to sort the desired parameters only. Select the
menu item Options/Parameters...
View
Move the parameters to view into the Selected parameters list.
Print
Move the parameters to print into the Selected parameters list.
Sort
Drag the parameter up or down with the mouse.
Customise
Add, modify and delete custom parameters.
If it is necessary to restore the default parameters press the button
in the left corner of the window to initialise the parameters
database.
Chapter 6 - Spirometry - 47
Page 48

Predicted values manager
The program contains a preset of predicted equations, but the
user is allowed to customise its own predicted sets. Select
Predicteds... from Options menu.
The window is divided into two forms: Predicteds set and
Formula definition.
Predicteds set
This form allows the user to manage the set of predicteds. The
following information define a set:
Name: identifies the set and cannot be duplicated;
Description: free field;
Age: the adult predicteds start since this age.
To enter a new set of predicteds click on the New button. The
field Name must be filled and must be unique. To stop without
saving click on the Cancel button. To save the set, click on the
Save button.
To delete a set of predicteds click on the Delete button. If a set is
deleted, also the associated formulae are deleted.
It is possible to generate a new set of predicteds with the same
attributes and the same formulae of the selected one. To do this
click on the Copy... button and specify a new Name.
To import a set of predicteds click on the Import... button and
select a file of Predicteds files type.
To export a set of predicteds click on the Export... button.
48 - microQuark User Manual
Page 49

In the list Set current predicteds choose the current predicteds
for printing and viewing.
Set the current predicted
microQuark allows to calculate the predicted values according to
the following configurable sets:
Adults Paediatrics
ERS 93 Zapletal
Knudson83 Knudson83
ITS white ITS white
ITS black ITS black
LAM LAM
MC Barcelona MC Barcelona
Nhanes III Nhanes III
Select the desired choice in the group Predicted.
Formula definition
This form allows the user to manage the formulae associated to a
set of predicteds.
Select the set of predicteds from the list Predicteds set.
To insert a new parameter click on the New... button.
The parameter formulae can be:
• calculated according to the predicteds in the list Use the
predicteds formulae;
Chapter 6 - Spirometry - 49
Page 50

Page set-up
• customised by the user with the option ...or the customised
formulae.
The Delete button deletes the selected parameter.
The Copy button stores the selected parameter in memory.
The Paste button inserts a new parameter from the one copied. If
the name is not unique, the user is asked whether to specify a
new name or to replace the existing parameter.
Select Page Setup... from the File menu.
Header All the printouts carried out by the
program are preceded by 3 rows of
customisable header (usually they contain
the name and the address of the Hospital
using the spirometer).
Data Patient and visit information are printed
below the header. These data are reported
on 3 columns and 5 rows. the user may
configure the disposition, change and
eventually cancel the fields, as he prefers.
Margins Configures the print margins from the
borders of the paper. The unit of measure
is decided in Units of measurements.
Footer Configures information at the bottom of
the page.
50 - microQuark User Manual
Page 51

Printed file name Defines the automatic name to be
asssigned to the pdf file, if the report will
be printed in this format.
In the example it has been set to create a
filename composed by <date of the test>
followed by <last name> and <first
name>.
Chapter 6 - Spirometry - 51
Page 52

Spirometry tests
Once completed the phases of the introduction of the patient’s
data and the visit data, it is possible to carry out the spirometric
Note: Read
carefully the
contraindications
in Chapter 1.
Recommendations for spirometry tests
tests.
microQuark allows to perform the following tests:
Key Test
FVC pre Forced Vital Capacity
FVC post Forced Vital Capacity after bronchial stimulation
SVC Slow Vital Capacity
MVV Maximum Voluntary Ventilation
Before performing any test make sure that:
1. microQuark is properly connected to your PC and the
selected serial port (COM1, COM2) corresponds to the one
effectively use.
2. The name shown on the status bar corresponds to the patient
who is to carrying out the tests.
3. The today’s visit card exists.
• The patient should wear the nose clips
• The turbine has been recently calibrated (ATS recommends a
daily calibration)
• The paper mouthpiece or the antibacterial filter is properly
connected to the flowmeter through the corresponding
adapter
For hygienic reasons, we strongly recommend the use of a
bacterial filter.
If a kid must perform the test it is recommended to enable the
encouragement function which shows exactly the manoeuvre of
the FVC test.
52 - microQuark User Manual
Page 53

Forced Vital Capacity (pre)
FVC is a reference test to verify obstructive (airflow limitations)
and restrictive disorders (lung volume limitations). To achieve
good test results it is fundamental a good manoeuvre (quality
control messages, real time plots …)
The main parameters measured during FVC tests are:
FVC Forced Vital Capacity
FEV1 Forced Expiratory Volume in 1 second
FEV1/FVC% FEV1 as a percentage of FVC
PEF Peak Expiratory Flow
FEF25-75% Forced mid-Expiratory Flow
The two representative plots are the Flow/Volume and
Volume/Time loops.
By comparing FVC, FEV1 and FEV1/FVC% values the software
allows an automatic interpretation concerning the levels of
obstructive and/or restrictive disorders.
Perform a FVC (pre) test
1. Select Forced Vital Capacity pre from the Test menu and
wait for the green led is prompted on the right side of the
screen.
2. Explain the manoeuvre to the patient and press the F2 key.
3. Wait some seconds and perform the test.
4. After having performed the test, press F3 or wait for the
automatic end (5 seconds without flow), so that the software
displays the F/V and V/t graphs, the main parameters, and
the predicteds values.
5. In order to visualise the F/V and V/t graph and the main
parameters press the following buttons:
view Flow Volume graph
view Volume Time graph
view data of the test
6. Press Alt+F3 to stop the acquisition discarding the results.
7. Repeat the test until it is correctly performed (ATS
recommends 3 times).
Chapter 6 - Spirometry - 53
Page 54

8. Press Exit to visualise the test list carried out during current
session together with the results of the main parameters.
9. Select the test that you want to save (the Best Test according
to the ATS criteria is highlighted as default) and press OK.
Test encouragement
During FVC manoeuvre you might experience some lack of
collaboration with kids or with other patients. In this case you
may find a good help in using the encouragement software tool.
Perform the FVC test with the encouragement
1. Select Encouragement from View menu.
2. Perform the test as explained in the previous paragraph.
54 - microQuark User Manual
Page 55

Slow Vital Capacity
Important test for assessing COPD (chronic obstructive
pulmonary disease) patients affected by this disease might
present a the Slow Vital Capacity could be higher than the Forced
one (FVC).
The main parameters measured during SVC tests are:
EVC Expiratory Slow Vital Capacity
IVC Inspiratory Slow Vital Capacity
ERV Expiratory Reserve Volume
IRV Inspiratory Reserve Volume
If the inspiratory/expiratory maximal manoeuvre is preceded by a
some breaths at tidal volume the software allows to measure the
Respiratory Pattern, represented by the following parameters:
VE Ventilation per minute
Vt Tidal volume
Rf Respiratory frequency
Ttot Breath time
Ti/Ttot Inspiratory time/Ttot
Vt/Ti Vt/Ti
Perform a SVC test
1. Select Slow Vital Capacity from the Test menu and wait
for the green led is prompted on the right side of the screen.
2. Press F2 and instruct the Patient to breath normally until the
message “carry out…” is prompted; then ask to perform a
Slow Vital Capacity (deep inhalation, maximal slow
expiration and deep inhalation again).
3. Press F3 or wait for automatic interruption (5 seconds
without flow) in order to visualise the V/t graph together
with the main parameters compared to the predicted values
4. To visualise the V/t graph and the main parameters press the
follow buttons:
view Volume Time graph
view data of the test
5. Press Alt+F3 to stop the acquisition discarding the results.
Chapter 6 - Spirometry - 55
Page 56

6. Repeat the test until it is correctly performed (ATS
recommends 3 times).
7. Press Exit to visualise the test list carried out during current
session together with the results of the main parameters.
8. Select the test that you want to save (the Best Test according
to the ATS criteria is highlighted by default) and press OK.
The reference for the ERV calculation is displayed on the V/T
graph.
56 - microQuark User Manual
Page 57

Maximum Voluntary Ventilation
Test for assessing the maximum ventilatory capacity. In the past,
it was commonly performed during routine PF tests, however its
clinical use declined over the years. Today MVV test is most
commonly performed as part of the exercise tolerance tests,
where it is used as an index of maximum ventilatory capacity.
Test consists in breathing in and out deeply and rapidly for 12, 15
seconds. The expired volume during this short period is then
extrapolated
The most important measured parameter is the following:
MVV Maximum Voluntary Ventilation
Perform a MVV test
1. Select Maximum Voluntary Ventilation from the test
menu and wait for the green led is prompted on the right side
of the screen.
2. Press F2 and make the Patient breath as much deeply and
rapidly as possible for at least 12 seconds.
3. Press F3 or wait for automatic interruption (5 seconds
without flow) in order to visualise the V/t graph together
with the main parameters compared to the predicted values
4. To visualise the V/t graph and the main parameters press the
follow buttons:
view Volume Time graph
view data of the test
5. Press Alt+F3 to stop the acquisition discarding the results.
6. Repeat the test until it is correctly performed (ATS
recommends 3 times).
7. Press Exit to visualise the test list carried out during current
session together with the results of the main parameters.
8. Select the test that you want to save (the Best Test according
to the ATS criteria is highlighted as default) and press OK.
Chapter 6 - Spirometry - 57
Page 58

Bronchial Provocation Test
Bronchodilator test
Note: Read
carefully the
contraindications
in Chapter 1.
Bronchodilators are administered routinely in the PFT laboratory
to determine whether airflow obstruction is reversible.
Bronchodilators increase airway calibre by relaxing airway
smooth muscle.
The test consists of comparing results between the reference FVC
(FVC PRE) and the FVC POST performed after the
administration of the drug. Increasing value of 13-15% of FEV1,
respect to the basal value (FVC Pre) is considered as a reversible
condition.
Main parameters are the following:
DFEV1%pre Change of FEV1 as a percentage of test PRE
DFVC%pre Change of FVC as a percentage of test PRE
DPEF%pre Change of PEF as a percentage of test PRE
Some authors states that the above mentioned parameters are too
dependent from the FVC Pre, hence latest reference (ERS93, [A
comparison of six different ways of expressing the
bronchodilating response in asthma and COPD; reproducibility
and dependence of pre bronchodilator FEV1: E. Dompeling, C.P.
van Schayck et Al; ERJ 1992, 5, 975-981]) recommend the
following parameters:
DFEV1%pred Change of FVC as percentage of predicted value
DFEV1%poss Change of FEV1 as percentage of possible value
Methacholine and Histamine Bronchial provocation Tests
The most common indication for performing methacholine and
histamine bronchial challenges is to diagnose hyperresponsive
airways. Some patients demonstrate normal baseline pulmonary
function despite complaints of “tightness” wheezing, cough, and
a little or not response to bronchodilator. Other patients
demonstrate spirometric improvement after use of bronchodilator
have diurnal variation in peak flows. In this groups aerosolised
bronchial challenges are used to confirm a diagnosis of Asthma.
We can summarise the use of the test as follows:
1. Diagnose asthma
2. Confirm a diagnosis of asthma
58 - microQuark User Manual
Page 59

3. Document the severity of hyperresponsivness
4. Follow changes in hyperresponsivness
When patients with hyperresponsive airways inhale certain
pharmacologic agents (i.e. Methacholine or histamine) the
airways respond by constricting.
Test consists of executing repeated FVC following the
pharmacologic agents inhalation according to an established
protocol. The fall of the FEV1 parameter is used to calculate the
bronchial hyperresponsivness. The most important parameter is
the PD20 that is amount of drug (mg/ml) that causes a reduction
of 20% of the FEV1 respect the basal value (without drug).
Main parameters are:
P10 Dose that causes a 10% fall of FEV1.
P15 Dose that causes a 15% fall of FEV1.
P20 Dose that causes a 20% fall of FEV1.
The representative plot is the Dose/response curve, showing the
percentage variation of FEV1 versus the Drug dose in
logarithmic scale.
The program assumes as the baseline test the best FVC pre
carried out during the today’s visit. You can change the reference
pre test editing the Post test.
The name of the drug, its quantity and its unit of measurement,
can be typed immediately before any FVC post manoeuvre
(manual protocol) or can be stored in a database of
bronchoprovocation (File/Bronchial Provocation protocols
Database…).
Perform the test
(During 1st step only) select Protocol... from the Test menu and
choose the name of the bronchoprovocation protocol that you are
going to use (manual protocol if you want to type the
information about the agent before any manoeuvre)
1. Select FVC post from the Test menu.
2. Select an existing protocol or click on “manual protocol”,
and wait the green leds turned on.
3. Press F2, or the button by side, to start the test.
4. Press F3, or the button by side, to achieve the test.
Chapter 6 - Spirometry - 59
Page 60

5. In order to visualise the V/t graph and the main parameters
press the follow buttons:
view Flow Volume graph
view data of the test
view bronchial provocation response
6. Press Alt+F3 to stop the acquisition discarding the results.
7. Repeat the test until it is correctly performed (ATS
recommends 3 times).
8. Press Exit to visualise the test list carried out during current
session together with the results of the main parameters.
9. Select the test that you want to save (the Best Test according
to the ATS criteria is highlighted as default) and press OK.
Bronchial Provocation protocols Database
The response to a bronchoprovocator is usually assessed in terms
of change in the FEV1, vital capacity or airways resistance on the
basis of serial measurements (FVC manoeuvres) in which the
results of the initial test constitute the reference values. The
international literature proposes several standardised protocols in
order to address the methodological issues of the various
available techniques.
The possibility to store a bronchoprovocation protocol in a
database is useful to simplify and automate the sequence of
operations that the Physician need to execute during the
bronchoprovocation tests.
The typical sequence of activities to carry out a
bronchoprovocation test are:
1. Typing and storing a bronchoprovocation protocol in the
database (usually only once).
2. Selection of protocol among the list of the ones already
present in the database before carrying out the FVC post
tests (the selection of “manual protocol” allows to execute
the test fully manually).
3. Performing the Post tests.
60 - microQuark User Manual
Page 61

Enter a new Bronchial provocation protocol in the archive
1. Select Bronchoprov. protocols database from the File
menu.
2. Type the Protocol name, the Bronchoprovocator name and
the unit of measurement in the proper input fields.
3. If the bronchoprovocator has a cumulative effect select the
cumulative check button.
4. Enter the quantities for each step and press the button
.
Chapter 6 - Spirometry - 61
Page 62

Viewing results
All the visualisation functions refer to the test carried out by the
Current Patient, whose name is indicated on the left-side of the
status bar.
To view tests results:
1. Select the Patients from the File menu
2. Select the patient corresponding to the test you want to view.
3. Select in the list box of the tests up to 5 tests of the kind
(FVC, VC/IVC, or MVV) and press OK.
To switch between graph and or data use the following buttons
on the toolbar:
view Flow Volume graph (F5)
view Volume Time graph (F6)
view data of the test (F7)
view bronchial provocation response.
If you need more than one visualisation meantime use the New
Window function from the Window menu.
If you need to display a list of visits:
• Select Visits list... from the File menu.
• Type the name of the Company and/or the time interval
desired or simply confirm for the complete list.
Tests of the current patient
If a current patient has been selected you can quickly view his
tests selecting Test current patient... from the View menu.
Delete a test
1. Select Patients from the File menu or press the button by
side.
2. Select the test that you want to eliminate from the list of the
tests referred to the Current Patient and press Delete.
62 - microQuark User Manual
Page 63

Printing results
You can print out in three different ways:
• printing the Report
• printing the Active Window
• printing a series of reports
Printing Reports
To print a report of the current visit, select Print report… from
File menu. The software will choose automatically the best
performed test.
The standard Report is composed by 1, 2 or 3 pages depending if
you wish to printout the FVC data and the graphs together on the
first page or if you wish to printout the bronchoprovocation
response.
• Selecting the option One page (no ATS) the report will
contain, on one page, the F/V and V/t graphs of the best test,
overlapped on the FVC Post, the patient data, the notes, the
diagnosis and the test results.
• Otherwise the report will contain two pages, the first with
the patient data, the graphs and the diagnosis, and the second
one with the measured parameters, according to the ATS
recommendations.
• The 3
Chapter 6 - Spirometry - 63
rd
page will contain the bronchoprovocation response.
Page 64

Select the desired options:
FVC graph Prints the F/V and V/t curves for the best
One page (no ATS) Prints data and graphs on the first page.
Response Prints the bronchoprovocator response.
FVC post Prints data and graphs for the Post FVC test
Preview Views a report preview on the screen.
Printing the active window
This printout function is only enabled when the active window
(title bar highlighted) is one of the following objects:
• Any kind of Graph.
• Numeric data
• List of visit
To print the active window select Print Active window from
File menu.
Printing a series of reports
Sometimes it is useful to printout automatically a series of reports
(all tests carried out with the employees, all tests carried out in
the today's session).
To print out proceed as follows:
1. Select Visit List from the File menu
2. Set the criteria of the visits to be added in the list (from,
to,...)
3. Select Print Report from the File menu.
FVC test.
(the test can be selected among the test
performed in the current visit).
Electronic reports (*.pdf)
If an Adobe PDF writer “Printer Driver” is installed and set as
the default printer, it is possible to store the printout report
automatically in any location of the HD or eventually LAN paths
according to a customizable filename format.
It is possible to define the created filename format selecting
File/Page Set up... (see Page set-up).
64 - microQuark User Manual
Page 65

Export data
With this function you can export the test data in 4 different
formats:
• *.txt (ASCII)
• *.xls (Microsoft Excel)
• *.wk1 (Lotus 123)
• *.xpo (Cosmed)
Export a test
1. Select Export tests from the File menu.
2. Select the test to export from the list box and press OK.
3. Type the name and the format of the file in the dialog Save
as. If the ASCII format is selected, the Text button in the
dialog box Save as allows you to configure the separators for
character based files.
With the *.xpo Cosmed file format it is possible to import
data from another Quark archive. Press OK to confirm.
4. Select the folder for the export and type the file name. Press
OK to confirm. A status bar will show the file creation.
Chapter 6 - Spirometry - 65
Page 66

66 - microQuark User Manual
Page 67

System
maintenance
Page 68

System maintenance
All service operations which are not specified in this user manual
should be performed by qualified personnel in accordance with
the service handbook (to be required to the manufacturer).
All materials used in the construction of the microQuark are non
toxic and pose no safety risk to the patient or operator.
Prior to the device cleaning, disinfection and inspection it is
necessary to switch off the device and to disconnect adapters
from the supply mains.
In order to guarantee the highest accuracy of measurements we
recommend you to disinfect the turbine periodically.
Cleaning and disinfection
Cleaning and disinfecting instructions are of fundamental
importance to control infections and assure patient safety. In fact
aspiration of residue, particles and contaminated agents are life –
threatening.
In this handbook we strongly recommend you to follow the rules
worked out by ATS and ERS (see: ”Lung Volume Equipment
and Infection Control” – ERS/ATS WORKSHOP REPORT
SERIES, European Respiratory Journal 1997; 10: 1928 – 1932),
which are summarised and adapted for COSMED products as
follows:
• Accessible internal as well as external surfaces of equipment
exposed to expired gas should be washed and disinfected
prior to testing of subsequent patients.
• Disinfecting should ideally be performed by heat
sterilisation, but gas or liquid sterilisation can be used if the
equipment is well cleaned first (no droplets of saliva/sputum
remain).
• Disposable gloves should be worn when handling
mouthpieces, when cleaning equipment exposed to saliva or
sputum and especially when drawing blood.
• Laboratory staff should wash hands prior to testing of each
patient.
• Adopt particular precautions when testing patients with
recognised high – risk communicable diseases (e.g.
tuberculosis, multidrug – resistant staphylococcus). In these
68 - microQuark User Manual
Page 69

cases, the clinical need for such testing should justify the
risks.
During the disinfection:
• do not use alcohol or other liquids containing gluteraldehyde
on the exterior surfaces of the equipment. Actually they can
damage polycarbonates plastics and may produce unhealthy
substances.
• do not use abrasive powders or glass cleaners containing
alcohol or ammonia on the plexiglas components of the
equipment
• do not steam autoclave any parts of the equipment unless it
is clearly specified.
• do not immerse the optoelectronic reader.
Warning: Do not
use alcoholic
solutions for the
turbine,
otherwise there
can be damages
to the plastic
material.
Cleaning the turbine flowmeter
It is necessary to disinfect periodically the turbine for sanitary
measures or/and for the correct device function.
As disinfecting solution it is suggested Sodium hypochlorite 5%
(bleach).
The disinfecting procedure is easy and may be effected every
time the user needs, keeping attention to some precautions:
1. Take out the turbine.
2. Dip it in a disinfectant solution (non alcoholic based) for
about 1 hour.
3. Rinse the turbine in a vessel, filled of clean water, shaking
gently to remove the disinfectant (do not clean the turbine by
putting it under running water!).
4. Let it dry to air.
5. After cleaning the turbine, check if the turbine propeller
rotates freely even with a low speed air flow.
6. Connect the turbine to the reader.
Precautions during the cleaning of the turbine
1. Do not expose the turbine to high heat and do not put it
under running water.
2. Do not ever dip the optoelectronic reader in any kind of
solution, the liquid infiltration would damage the internal
circuit.
3. Do not use alcoholic solutions to clean the turbine.
Chapter 7 - System maintenance - 69
Page 70

Inspections
Suggested disinfection solutions
Helipur H Plus Braun Melsungen AG
Gigasept FF Schulke & Mayr GmbH
Dismozon pur Bode Chemie GmbH
TETA-S Fresenius AG
CIDEX Johnson & Johnson
The equipment requires easy inspections to be carried out in
order to assure a proper electrical and mechanical safety level in
the years.
These inspections are highly recommended after a rough use of
the equipment or after a period of storage in unfavourable
environmental conditions.
Referring to the electrical safety, it is important to check the
conditions of insulation materials of cables, plugs and any other
visible part by means of simple inspection, when the equipment
is switched off and adapters (or electrical feeders) are
disconnected from the supply mains.
Extract the turbine from the unit and verify, by inspection, that
the turbine axis fits correctly its seats and the blade is strongly
fastened on the axis itself (it can be useful to shake slightly the
turbine in order to note any anomalous movement).
Check if there are any torn or broken components in the
breathing circuits: remember that they can create safety risk to
patients during tests.
70 - microQuark User Manual
Page 71

Appendix
Page 72

Service - Warranty
Warranty and limitation of liability
COSMED provides a one (1) year limited warranty from the date
of the original sale of COSMED products. All COSMED
products are guaranteed to be free from defect upon shipment.
COSMED’s liability for products covered by this warranty is
limited exclusively to replacement, repair, or issuance of a credit
for the cost of a defective product, at the sole discretion of
COSMED. COSMED shall not be liable under the foregoing
warranty unless (i) COSMED is promptly notified in writing by
Buyer upon discovery of defect; (ii) the defective product is
returned to COSMED, transportation charges prepaid by Buyer,
(iii) the defective product is received by COSMED no later than
four weeks after the last day of the one (1) year limited warranty
period; and (iv) COSMED’s examination of the defective product
establishes, to COSMED’s exclusive satisfaction, that such defect
was not caused by misuse, neglect, improper installation,
unauthorised repair or alteration, or accident. If the product is
manufactured by a third-party, COSMED shall make available
for the Buyer’s benefit only those warranties which COSMED
has received from the third-party manufacturer(s). COSMED
hereby specifically disclaims any and all warranties and/or
liabilities arising from defect(s) and/or damage(s) to and/or
caused by products manufactured by third-party manufacturers.
Buyer must obtain written authorisation from COSMED prior to
the repair or alteration of COSMED products(s). Failure of
Buyer to obtain such written authorisation shall void this
warranty.
COSMED hereby specifically disclaims any and all other
warranties of any kind, whether express or implied, in fact or by
law, including, but without limitation, any and all warranties of
merchantability and/or fitness for a particular purpose.
COSMED shall not be liable for special, indirect and/or
consequential damages, nor for damages of any kind arising from
the use of any COSMED’s products, whether said products are
used alone or in combination with other products or substances.
Determination of the suitability of any of COSMED’s product(s)
furnished hereunder for the use contemplated by Buyer is the sole
risk and responsibility of Buyer, and COSMED has no
responsibility in connection therewith. Buyer assumes all risks
72 - microQuark User Manual
Page 73

and liabilities for loss, damage or injury to persons or property of
Buyer or others arising out of the use or possession of
COSMED’s products.
The limited warranty as herein above set forth shall not be
enlarged, diminished, modified or affected by, and no obligation
or liability shall arise or grow out of, the renderings of technical
advice or service by COSMED, its agents or employees in
connection with Buyer’s order or use of the product(s) furnished
hereunder.
Return goods policy for warranty or non warranty repair
Goods shipped to COSMED for repair are subject to the
following conditions:
1. Goods may only be returned after your receipt of a Service
Return Number (SRN) from COSMED S.r.l.
2. Place your SRN report and Packing List outside the package.
3. Goods returned must be shipped with freight and insurance
charges prepaid. Collect shipments will not be accepted.
4. The following list of goods are not eligible for return unless
proven defective.
- Special order items
- Expendable products
- Goods held over 30 days from COSMED’s invoice date.
- Used goods not in original shipping containers.
- Goods which have been altered or abused in any way.
5. The following parts are not covered by warranty:
- consumables
- fragile glass or plastic parts
- rechargeable batteries
- damages due to use of the device not conforming to the
indication reported in this manual
Repair Service Policy
Goods returned to seller for Non-Warranty repair will be subject
to conditions 1, 2, 3, 4.
The returned goods need to re-enter COSMED together with the
customs documents (Pro-forma Invoice and Customs Paper) as
requested by the Italian law.
• The shipment has to be qualified as a Temporary Export.
Chapter 8 - Appendix - 73
Page 74

• All the goods returned to COSMED without the customs
papers will not be accepted.
For European Community members:
Pro-Forma invoice complete with:
• Number
• Description of the goods
• Quantity
• Serial Number
• Value in €
• Number of parcel
• Gross weight
• Net weight
• Reason for resent (i.e. Resent for repair)
In case you should send the system for repair please contact the
nearest service centre or contact COSMED at the following
address:
COSMED S.r.l.
Via dei Piani di Monte Savello 37
P.O. Box 3
00040 Pavona di Albano - Rome, Italy
tel. +39 (06) 9315492
fax +39 (06) 9314580
E-mail: customersupport@cosmed.it
For USA customers only please contact:
COSMED USA Inc
1808 North Halsted Street
Chicago, IL 60614 USA
Phone: +1 (312) 642-7222
Fax: +1 (312) 642 7212
email: usa.sales@cosmed.it
To ensure that you receive efficient technical assistance, please
specify as precisely as possible the nature of the problem as it is
specified on the assistance information form.
We advise you to save the original packaging. You may need it in
case to ship the unit to a technical assistance centre.
74 - microQuark User Manual
Page 75

Privacy Information
Dear Customer,
we inform you that your personal data are gathered and will be
used by Cosmed Srl in conformity with the requirements of the
Italian privacy law (Decreto Legislativo 196/2003). We believe it
is important for you to know how we treat your personal data.
Personal data treatment and purposes
We request and process your personal data:
a. to place an order, register a product, request a service,
answer a survey, enter a contest, correspond with us (all of
the above, in the following: “service”) and, if necessary, to
supply the Competent Authorities with the required
information;
b. in order to define your commercial profile;
c. in order to use your commercial profile for own marketing
and advertising purposes;
d. for accounting purposes, including e-mailing of commercial
invoices;
e. for providing your information to selected business partners
(also abroad), in order to supply the service;
How your personal data are treated
Your personal data will be stored in electronic format, and
protected at the best from destruction, loss (even accidental), not
authorized accesses, not allowed treatment or use not in
conformity with the purposes above listed.
The consent is optional, but…
If you deny the consent, we regret we cannot supply the service.
Holder of the treatment
The holder of the treatment is Cosmed Srl, Via dei Piani di
Monte Savello 37, Pavona di Albano Laziale (RM). The
responsible of the personal data treatment is indicated in the
documentation stored by Cosmed Srl itself.
Chapter 8 - Appendix - 75
Page 76

Customer rights
In accordance with art.7 of the Law, you can:
a. obtain confirmation of the existence of your personal data
b. obtain:
c. deny your consent to the treatment of your personal data;
These rights can be exercised directly requesting in writing to the
holder of the treatment.
and their communication in intelligible form;
• updating, correction or integration of your data;
• deletion or transformation in anonymous form of your
personal data;
76 - microQuark User Manual
Page 77

Converting factors configuration
You can edit the parameters shown in Control Panel by selecting
Control Panel from the Calibration menu in the calibration
program, then pressing the button by side.
You might configure the following options:
Name: identify the parameter
Unit of meas.: unit of measurement
Base line and Gain: factors used to convert the acquired raw
data (mV) into the final format according to
Y=(mV-BL)*Gain. The value entered for
gain must be multiplied by 1000 (for
Gain=1, enter 1000).
Precision: the number of decimals shown as 0
Chapter 8 - Appendix - 77
Page 78

ATS 94 recommendations
Reference: “Standardization of Spirometry: 1994 Update”
“American J. Respiratory Critical Care Medicine”, Vol. 152,
1107-1136; 1995.
ATS recommendations
Volume range: 8l (BTPS)
Flow range: ±14 l/sec
Volume accuracy: ±3% or < 50ml
Flow accuracy: ±5% or < 200ml/sec
Flowmeter resistance: <1.5 cmH2O da 0 a 14 l/sec
Reproducibility: the 2 largest of 3 acceptable FEV1 and FVC
values should be within 5% or 150 ml.
The end of test: no change in volume for 1 second with at least 6
seconds of collected volume.
Accumulation time: the maximum time allowed for volume
accumulation during the VC manoeuvre should be at least 30
seconds and at least 15 seconds during the FVC.
The spirometer should be store at least 8 FVC maneuvres.
FEV1 should be calculated by using the “back extrapolation”
method to detect the start of the test, extrapolated volume must
not be higher then 5% FVC or 150ml.
The graphic resolution of the printed report must be as in the
following:
Volume: 10 mm/l
Flow: 5 mm/l/sec
Time: 20 mm/sec
F/V ratio: 2:1
The total number of error (FVC e FEV1 >±3.5%, FEF25-75%
>5.5%) during the measurement of the 24 standard waveforms
must be lower than 4.
78 - microQuark User Manual
Page 79

Predicted values
ERS93
Reference Adult:
Standardized Lung Function Testing: Official Statement of the
European Respiratory Society, The European Respiratory Journal
Volume 6, Supplement 16, March 1993.
Reference Paediatric:
Compilation of reference values for lung function measurements
in children: Ph. H. Quanjer, J. Stocks, G.Polgar, M. Wise, J.
Karlberg, G. Borsboom; ERJ 1989, 2, Supp.4,184s-261s.
KNUDSON 83
Reference Adult/ Paediatric:
Changes in the Normal Maximal Expiratory Flow-Volume Curve
with Growth and Anging: J. Knudson, D. Lebowitz, J. Holdberg,
B. Burrows; ARRD 1983; 127:725-734
Note: SD@FEV1/FVC e FEV1/VC da ERS93
ITS (White race)
Reference Adult/ Paediatric:
Intermountain Thoracic Society: Clinical Pulmonary Function
Testing, second edition (1984) pp 101, 144
Note: SD@FEV1/FVC e FEV1/VC da ERS93
ITS (Black race)
Reference Adult/ Paediatric:
Intermountain Thoracic Society: Clinical Pulmonary Function
Testing, second edition (1984) pp 101, 144
Note: SD@FEV1/FVC e FEV1/VC da ERS93
Chapter 8 - Appendix - 79
Page 80

LAM
Reference Adult/ Paediatric:
A survey of ventilatory capacity in Chinese subjects in Hong
Kong: Lam Kwok-Kwong, Pang Shing et Al. Annals of Human
Biology, 1982, vol. 9, No. 5, 459-472.
Note: SD@FEV1/FVC e FEV1/VC da ERS93
Multicéntrico de Barcelona
Reference Adult/ Paediatric:
Spirometric reference values from a Mediterranean population: J.
Roca, J. Sanchis, A. Agusti-Vidal, F. Segarra, D. Navajas. R.
Rodriguez-Roisin, P. Casan, S. Sans. Bull. Eur. Physiopathol.
Respir. 1986, 22, 217-224.
NHANES III
Reference Adult/ Paediatric:
Spirometric reference values from a sample of the general US
population: John L. Hankinson, John. R. Odencrantz and
Kathleen B. Fedan. Am J Respir Critr Care Med 1999, 159,
1798-187.
Automatic diagnosis (algorithm)
Reference: “Lung Function Testing: selection of reference
values and interpretative strategies”, A.R.R.D., 144/ 1991:1202-
1218.
LLN=Pred-0.674*SD (ATS, 50° percentile)
LLN=Pred-1.647*SD (ERS, 95° percentile)
LLN=Pred*0.8 (80%Pred)
Message interpretation Criterion
Normal Spirometry FVC and FEV1/FVC > LLN
Obstructive abnormality
(it may be physiological) % Pred FEV1 >= 100
Obstructive abn.: mild % Pred FEV1 < 100 and >= 70
Obstructive abn.: moderate % Pred FEV1 < 70 and >= 60
Obstructive abn.: mod. severe % Pred FEV1 < 60 and >= 50
80 - microQuark User Manual
Page 81

Obstructive abn.: severe % Pred FEV1 < 50 and >= 34
Obstructive abn.: very severe % Pred FEV1 < 34
Restrictive abn.: mild FVC<LLN and %Pred FVC>=70
Restrictive abn.: moderate % Pred FVC < 70 and >= 60
Restrictive abn.: mod. severe % Pred FVC < 60 and >= 50
Restrictive abn.: severe % Pred FVC < 50 and >= 34
Restrictive abn.: very severe % Pred FVC < 34
Quality Control Messages
Reference: Spirometry in the Lung Health Study: Methods and
Quality Control, ARRD 1991; 143:1215-1223.
Message Criterion
Start faster VEXT >5% of the FVC and >150ml
Blast out harder PEFT >120 msec
Avoid coughing 50% drop in the flow in first second
Blow out longer FET100% <6 sec.
Blow out more air flow >0.2l/s within 20 ml of FVC
Blow out harder dPEF<10%
Take a deeper breath dFVC<200ml and 5% best FVC
Blow out faster dFEV1<200ml and 5% FEV1
That was a good test No errors
FVC reproducible diff. 2 max FVC within 0.2 l
FEV1 reproducible diff. 2 max FEV1 within 0.2 l
PEF reproducible diff. 2 max PEF within 10 %
MVV time too short MVV time less than 12 sec
Chapter 8 - Appendix - 81
Page 82

References
ATS ’94: “Standardization of Spirometry: 1994 Update”,
American J. Respiratory Critical Care Medicine, Vol. 152, 11071136; 1995
ERS ’93: “Standardized Lung Function Testing: Official
Statement of the European Respiratory Society”, The European
Respiratory Journal Volume 6, Supplement 16, March ”
“Standardization of Spirometry: 1987 Update”, American
Review of Respiratory Disease, Vol. 136, 1285-1289; 1987
Lung function", J.E. Cotes, Blackwell scientific publications
"Guidelines for Clinical Exercises Testing Laboratories", I.L.
Pina, G.J. Balady, P. Hanson, A.J. Labovitz, D.W. Madonna, J.
Myers. American Heart Association. 1995; 91, 912.
“Office spirometry”, R.E. Hyatt - P.L. Enright, Lea & Febiger
82 - microQuark User Manual
 Loading...
Loading...