Page 1

T34™
EC REP
0344
Syringe Pump
rd
(3
Directions For Use
Edition)
Caesarea Medical Electronics Ltd.
16 Shacham Street
Industrial Park Caesarea North
P.O.Box 3009
Caesarea 3088900, Israel
MedNet GmbH
Borkstrasse 10
48163 Muenster
Germany
Tel.: +49 251 32266 0
DFU999-103EN Rev. 04
Page 2

T34™ Syringe Pump (3
rd
Edition)
The information in this document is subject to change and does not represent a commitment on the part of Caesarea
Medical Electronics Ltd. to provide additional services or enhancements. The screens illustrated in the document are for
reference purposes only and might be different than the screens displayed on your pump. Documentation provided with
this product might reference product not present in your facility or not yet available for sale in your area.
BD, CME, CME logo, Plastipak, and T34 are trademarks of Becton, Dickinson and Company or its affiliates. All other
trademarks are the property of their respective owners.
©2019 BD. All rights reserved.
DFU999-103EN Rev. 04
2/60
Page 3

Contents
Section 1: General Information
1.1 Preface
1.2 About this Directions For Use
1.3 Advisory Terms and Warnings
1.4 Intended Use
1.5 Contraindications
1.6 System Symbols
1.7 Syringe Pump Inspection and Unpacking
1.8 Syringe Pump Specifications
Section 2: Disposables and Accessories
2.1 Syringe Brands and Sizes
2.2 Syringe Extension Sets
2.3 Battery Power Supply
2.4 Lockbox (Optional)
2.5 Carry Pouches (Optional)
Section 3: Pump Features and Description
3.1 Overview
3.2 Pump Description
3.3 Event Log
3.4 Post Occlusion Bolus Reduction System (POBRS)
Section 4: Modes of Operation
4.1 Modes of Operation
Section 5: Pump Configuration
5.1 Pump Configuration
5.2 Pump Access Codes
5.3 Pump Info and Configuration Menus
5.4 Pump Configurable Settings for Modes of Operation
5.5 Optional Configurable Settings
5.6 Practice Scenarios for Changing Pump Configuration
Section 6: Starting A New Infusion
6.1 Sequence for Starting an Infusion
6.2 Releasing a Trapped Foreign Object from the Actuator
Section 7: Monitoring and Managing Infusions
7.1 Pump and Infusion Safety Checks
7.2 Keypad Lock
7.3 Program Protection and Resume
7.4 Stopping/Pausing the Infusion and Powering Off
7.5 Alerts, Alarms and Troubleshooting
7.6 Bolus
7.7 Changing Syringes/Syringe Extension Sets
Section 8: Servicing and Maintenance
8.1 Servicing, Maintenance and Periodic Checks
8.2 Cleaning
8.3 Pump Storage
8.4 Disposal/Decommissioning
.............................................................................................4
.......................................................................................7
....................................................................................7
.........................................................................................19
.........................................................................................22
......................................................................................46
.............................................................................................51
..........................................................................................54
.....................................................................................55
............................................................................4
..................................................................................7
.............................................................................14
..............................................................................16
................................................................................18
.................................................................................19
............................................................................24
...............................................................................24
...........................................................................27
...............................................................................27
...............................................................................27
T34™ Syringe Pump (3
.......................................................................4
.......................................................................5
...........................................................9
.......................................................................10
...................................................................13
..........................................................................13
..........................................................................18
................................................................19
...................................................23
...............................................................28
...............................................30
....................................................................31
...............................................33
........................................................................37
..................................................................37
..............................................43
............................................................44
..................................................................44
...................................................................47
...................................................48
................................................................49
.........................................................52
.....................................................................53
.......................................................53
........................................................................55
rd
Edition)
Page
DFU999-103EN Rev. 04
3/60
Page 4
T34™ Syringe Pump (3
Section 1: General Information
rd
Edition)
Section 1: General Information
1.1 Preface
Duplication or distribution of this
written permission of CME Ltd. This
transmitted in any form, or by any means, for any purpose, without the express written permission of CME Ltd. To order additional copies
of this
Directions For Use
The information in this
is assumed for inaccuracies. Furthermore, CME Ltd. reserves the right to make changes to any products herein to improve readability,
function, or design. CME Ltd. does not assume any liability arising out of the application or use of any product described herein; neither
does it cover any license under its patent rights nor the rights of others.
, or other related manuals, contact your BD representative.
Directions For Use
1.2 About this Directions For Use
The operator must be thoroughly familiar with the T34™ Syringe Pump described in this
must read and understand any warnings and precautions stated herein.
All illustrations used in this
syringe pump. These settings and values are for illustrative use only. The complete range of settings and values are listed in the
specifications section of this
Directions For Use
This
the technical specifications reflect specific test conditions defined in this standard. Other external factors such as varying back pressure,
temperature, head height, syringe extension set usage, fluid restrictions, solution viscosity or combinations of these factors, may result in
deviations from the performance data enclosed.
Keep this
Note:
has been developed with consideration to the requirements in relevant Harmonised Standards. Data presented in
Directions For Use
Directions For Use
Directions For Use
has been carefully compiled, and is believed to be entirely reliable. However, no responsibility
Directions For Use
Directions For Use
for future reference during the syringe pump's operational life.
and any information contained within is strictly prohibited without the express
and any information contained within, may not be reproduced, distributed, or
Directions For Use
show typical settings and values that may be used in setting up the functions of the
.
prior to use, and in particular
DFU999-103EN Rev. 04
4/60
Page 5
1.3 Advisory Terms and Warnings
WARNINGS, CAUTIONS AND NOTES
Warnings, cautions and notes will be seen throughout this
Warnings advise of circumstances that could result in injury or death to the patient or operator.
Read and understand this
Cautions advise of circumstances that could result in damage to the T34™ Syringe Pump.
Read and understand this
A Note indicates that the information that follows is additional important information, a tip that will help you recover from an
Note:
error or refer you to related information within the
Directions For Use
Directions For Use
OPERATING PRECAUTIONS AND WARNINGS
T34™ Syringe Pump (3
Section 1: General Information
Directions For Use
and all warnings completely before operating the T34™ Syringe Pump.
and all cautions completely before operating the T34™ Syringe Pump.
Directions For Use
. These are defined as follows:
.
rd
Edition)
Read the entire
Only trained service personnel should open the syringe pump cover.
A kinked or occluded syringe extension set may impair the operation of the syringe pump and the accuracy of the
infusion. Before operation, verify that the syringe extension set is not kinked or occluded.
Unsafe operation may result from using improper accessories. Use only accessories and options designed for this
system and supplied or recommended by the T34™ Syringe Pump distributor.
Do not use this equipment with other infusion systems or accessories that are not designed to be used with this
pump system.
Do not let the syringe pump operate when battery is fully depleted. Pump may turn off during operation on fully
depleted battery. Before beginning infusion, ensure the battery is fully charged.
Use of this equipment adjacent to or stacked with other equipment should be avoided because it could result in
improper operation. If such use is necessary, this equipment and the other equipment should be observed to verify
that they are operating normally.
Directions For Use
before using the syringe pump, since the text includes important precautions.
Use of accessories, transducers and cables other than those specified or provided by the manufacturer of this
equipment could result in increased electromagnetic emissions or decreased electromagnetic immunity of this
equipment and result in improper operation.
Portable RF communications equipment (including peripherals such as antenna cables and external antennas)
should be used no closer than 30 cm (12 inches) to any part of the T34TM Syringe Pump, including cables specified
by the manufacturer. Otherwise, degradation of the performance of this equipment could result.
Although the T34™ Syringe Pump has been designed and manufactured to exact specifications, it is not intended to
replace trained personnel in the supervision of infusions.
The specified accuracy of the syringe pump can only be maintained if the syringe pump is used in accordance with
the
Directions For Use
Adjustments, maintenance, or repair made by un-certified service personnel may impair the operation of the
syringe pump and/or the accuracy of the infusion. Make sure any adjustments, maintenance, or repair of the syringe
pump are carried out only by authorised and skilled technicians.
Refer all service, repair and adjustments only to qualified and certified technical personnel. Unauthorised
modifications or the use of any spare parts, other than those supplied by the manufacturer or their distributor, will
void any warranty.
DFU999-103EN Rev. 04
and is maintained and serviced by a certified CME technician.
5/60
Page 6

T34™ Syringe Pump (3
Section 1: General Information
If the syringe pump is subjected to excessive moisture, humidity or high temperature, or otherwise suspected to
have been damaged, remove it from service for inspection by qualified personnel.
The syringe pump has been designed to be as safe as possible to handle; however, care should be exercised to avoid
trapping of fingers or other body parts in the mechanism.
The T34™ Syringe Pump should be operated within the recommended environmental operating range. Operation at
temperatures and/or humidity outside this range may adversely affect accuracy.
This syringe pump is designed for use and should withstand everyday handling. If the syringe pump is dropped
onto a hard surface, or is suspected of being dropped, the operation and calibration should be checked by a
qualified technician.
Pump should be stored with the battery removed to prevent battery corrosion and decay.
CME Ltd. will assume no responsibility for incidents which may occur if the product is not used, stored or
transported in accordance with the environmental conditions stipulated in this document or on the package
labelling.
rd
Edition)
INFUSION PRECAUTIONS AND WARNINGS
Carefully read and follow accompanying syringe extension set instructions for priming the set and the
recommended set change interval.
The syringe and syringe extension set should be disposed of in an appropriate manner, considering the nature of
the residual fluid that may be contained within and in accordance with the hospital/homecare provider's disposal
practices.
Drugs for infusion to be used with the syringe pump may only be prescribed by a qualified medical practitioner.
Caution must be exercised in the selection of drugs and the amount and rate intended to be delivered via syringe
pump.
If the drug contained in the syringe will be exposed to extreme environmental conditions for prolonged time
periods, it is important to select drugs that will not change pharmacologically upon such exposure.
As with all automatic syringe pumps, whenever a toxic or dangerous level of drug is stored in the reservoir,
constant/frequent monitoring of the infusion is required.
In all applications, time to alarm under occlusion or other fault conditions will depend on the infusion rate and
levels of alarm settings. It is recommended to consider these parameters when using drugs requiring infusion
stability or low flow rates and therefore a quick time to alarm.
Do not use Slip-tip syringes. Luer Lock syringes must always be used to ensure secure connection of the syringe
extension set and the syringe pump.
GENERAL PRECAUTIONS AND WARNINGS
The maximum volume that may be infused under single fault condition is 0.1 ml.
Potential strangulation may occur if the cables/tubing are of excessive length.
Potential choking may occur if small parts are inhaled or swallowed.
DFU999-103EN Rev. 04
6/60
Page 7
T34™ Syringe Pump (3
Section 1: General Information
Potential allergic reactions may occur due to materials used in the syringe pump.
The T34™ Syringe Pump is not certified for use in oxygen-enriched environments.
Do not operate the syringe pump near high-energy radio-frequency emitting equipment, (e.g. Imaging equipment
(i.e., X-Ray, MRI, CT Scan, etc.), High Frequency (RF) Surgical Equipment, Defibrillator, etc.) as this may cause
degradation in performance of the syringe pump, which may affect proper infusate delivery.
Do not use hard or sharp objects on the keypad.
Do not bathe or shower whilst using the syringe pump. The syringe pump is resistant to a limited amount of
splashing, but its construction does not make it resistant to large amounts of spraying or immersion in liquids.
Damage to the internal components may result.
rd
Edition)
1.4 Intended Use
The T34™ Syringe Pump is designed for infusion of medications or fluids requiring continuous or intermittent delivery at precisely
controlled infusion rates through all clinically acceptable routes of administration including intravenous, subcutaneous, percutaneous, in
close proximity to nerves, and into an intraoperative site (soft tissue/body cavity/surgical wound site). The system is intended for patients
who require maintenance medications, analgesics, Immunoglobulins, biosimilar, chemotherapeutic agents and general fluids therapy in
hospital and homecare environments.
1.5 Contraindications
• Infusion of blood and blood products
• Infusion of insulin
• Infusion of critical medications whose stoppage or interruption could cause serious injury or death
• Use in ambulatory regimens by patients who do not possess the mental, physical or emotional capability to self-administer their
therapy, or who are not under the care of a responsible individual
1.6 System Symbols
The following symbols are used on the T34™ Syringe Pump and components. Labels on the syringe pump or statements in this
Directions For Use
operate the syringe pump in a safe and successful manner.
preceded by any of the following words and/or symbols are of special significance and/or are intended to help you to
System Symbol Identification and Description
Symbol Description
Syringe Pump
Warnings
Read and understand this
Pump.
Cautions
Read and understand this
Pump.
Refer to
Read the entire
advise of circumstances that could result in injury or death to the patient or operator.
Directions For Use
advise of circumstances that could result in damage to the T34™ Syringe Pump.
Directions For Use
Directions For Use
Directions For Use
:
before using the syringe pump.
and all warnings completely before operating the T34™ Syringe
and all cautions completely before operating the T34™ Syringe
DFU999-103EN Rev. 04
CE mark indicates conformance to Medical Device Directive 93/42/EEC.
Do not dispose of in municipal waste. Symbol indicates separate collection for electrical and electronic
equipment. (WEEE Directive 2012/19/EU). NOTE: Does not apply to the battery.
7/60
Page 8

T34™ Syringe Pump (3
Section 1: General Information
Type CF applied part.
Class II Medical Electrical Equipment providing double insulation for operator and patient safety.
rd
Edition)
IP22
Symbol for degree of protection against ingress of water and solid objects.
Battery.
Direct current.
Manufactured by.
Date of manufacture.
Serial number.
Reference number. Indicates the manufacturer's catalogue number so that the medical device can be
identified.
Indicates the authorised representative in the European Community.
Indicates the acceptable upper and lower limits of atmospheric pressure (altitude).
Indicates the acceptable upper and lower limits of relative humidity.
Indicates the range of temperatures to which the medical device can safely be exposed.
Indicates a medical device that should not be used if its packaging has been damaged or opened.
Indicates the number of drops per millilitre.
Disposables
The use of single-use disposable components on more than one patient is a biological hazard.
Do not reuse single-use disposable components.
DFU999-103EN Rev. 04
8/60
Page 9
T34™ Syringe Pump (3
Expiry date (consumables).
Lot number (consumables).
Sterilized with Ethylene Oxide (applies to syringe extension sets).
1.7 Syringe Pump Inspection and Unpacking
INSPECTING THE SYRINGE PUMP BEFORE USE
Remove the T34™ Syringe Pump from the packaging and inspect for damage during shipment or storage.
Make sure you have the following items:
• T34™ Syringe Pump
•
Directions For Use
• Quick Reference Guide for homecare
If any items are missing or damaged, contact your supplies department.
rd
Edition)
Section 1: General Information
Visually inspect packaging and contents before each use.
Do not use the T34™ Syringe Pump and accessories if there are any obvious signs of damage.
Return for inspection by authorised service personnel.
ACCESSORIES (IF PURCHASED)
• Lockbox (supplied with two keys)
• Carry Pouch (re-usable or disposable)
Refer to the product catalogue for more details.
If any items are missing or damaged, contact your supplies department.
Unsafe operation may result from using improper accessories. Use only accessories and options designed for this
system and supplied or recommended by the T34™ Syringe Pump distributor.
DFU999-103EN Rev. 04
9/60
Page 10
T34™ Syringe Pump (3
Section 1: General Information
rd
Edition)
1.8 Syringe Pump Specifications
T34™ SYRINGE PUMP SPECIFICATIONS
Type: Linear syringe driver mechanism, pulsed motion (540 pulses per mm).
0.1–10 ml/h in 0.01 ml/h increments;
Flow Rate:
Bolus Parameters:
Actuator Travel: 67 mm available.
Syringe Sizes: 2 ml to 50 ml (most commonly used manufacturers).
Accuracy:
Occlusion Pressure:
Battery: 9 V alkaline, IEC 6LR61 type.
Operating Time: Rate Approximate battery life
Indicators: 4 line LCD display (122 × 32 pixels), dual color operation LED.
Alarms: When a problem is detected, the T34™ displays the following alarm messages, sounds an audible
Dimensions:
Classification:
Housing: ABS (fire retardant). Complies with standard UL94V-1.
Weight:
Electrical Safety:
Standards:
Flow rate is adjustable between 0.1 ml/h and
650 ml/h
Bolus flow rate 1–650 ml/h:
1–10 ml/h in 0.01 ml/h increments;
10–29.9 ml/h in 0.1 ml/h increments;
30–49.5 ml/h in 0.5 ml/h increments;
50–299 ml/h in 1 ml/h increments;
300–650 ml/h in 5 ml/h increments.
± 5% system accuracy (syringe pump and set combined) by volume under nominal conditions,
defined as follows:
Flow rates: 1 ml/h and 5 ml/h;
Tested with syringe extension set model M100-172SB;
Needle: 18 gauge;
Solution Type: Distilled water;
Temperature: 22°C ± 3°C;
Back Pressure: 0 ± 10 mmHg;
Syringe size and brand: BD Plastipak 20 ml.
Accuracy measured using the trumpet curve test method defined in EN/IEC60601-2-24.
200–1500 mmHg configurable (10 mmHg increments).
1 ml/h 25 hours
5 ml/h 20 hours
alarm and the LED lights red:
Occlusion or Syringe Empty Syringe Displaced during infusion
End Program System Error
End Battery
167 × 68 × 39 mm (L x W x H)
Type CF Equipment, degree of protection against electrical shock;
IP22 protection against ingress of water and solid objects.
Definition of code:
I = Ingress
P = Protection
2 = Protection from solid objects ≥12.5 mm
2 = Protection from dripping water (15° tilted)
230 g without battery.
Complies with: IEC 60601-1, IEC 60601-1-2, IEC 60601-1-6, IEC 60601-1-8, IEC 60601-1-11,
IEC60601-2-24.
Manufactured in accordance with ISO 13485, IEC 62304 and IEC 62366.
CE marked in accordance with the Medical Devices Directive 93/42/EEC.
10–29.9 ml/h in 0.1 ml/h increments;
30–49.5 ml/h in 0.5 ml/h increments;
50–299 ml/h in 1 ml/h increments;
300–650 ml/h in 5 ml/h increments.
Bolus volume:
0–20 ml in 0.1 ml increments.
Maximum bolus volume is 20 ml.
DFU999-103EN Rev. 04
10/60
Page 11
T34™ Syringe Pump (3
Section 1: General Information
Environmental Specifications: Operating Environment Range:
Ambient Temperature: 5°C to 40°C
Relative Humidity: 15% to 90%, non-condensing
Ambient pressure: 70 kPa to 106 kPa.
Transport and Storage Conditions:
Ambient Temperature: −25°C to 70°C
Relative Humidity: 0% to 90%, non-condensing
Ambient pressure: 48 kPa to 110 kPa.
The T34™ Syringe Pump is designed to be in compliance with IEC 60601-1 (safety), IEC 60601-1-2
(EMC), and IEC 60601-2-24 (infusion pump).
The T34™ Syringe Pumps have been tested and found to comply with the limits for a Class B
digital device. These limits are designed to provide reasonable protection against harmful
interference in a residential installation. This equipment generates, uses and can radiate radio
frequency energy and, if not installed and used in accordance with the instructions, may cause
harmful interference to radio communications. However, there is no guarantee that interference
EMC Specifications:
EMC – Emissions Compliance EMC Standard Range Compliance
Radiated emissions CISPR 11:2015
EMC – Immunity Compliance EMC Standard Test level Compliance
Electrostatic Discharge (ESD)
Immunity
Radiated RF Immunity
Proximity fields from RF wireless
communications equipment
Conducted RF Immunity IEC 61000-4-6:2013
Power Frequency Magnetic Field
Immunity
will not occur in a particular installation. If this equipment does cause harmful interference to
radio or television reception, which can be determined by turning the equipment off and on, the
user is encouraged to try to correct the interference by one or more of the following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Connect the equipment into an outlet on a circuit different from that to which the
receiver is connected.
• Consult the dealer or an experienced radio/TV technician for help.
The T34™ Syringe Pump has been tested to comply with the requirements of IEC 60601-1-2:2014.
30 MHz – 1 GHz Class B, Group 1
IEC 61000-4-2
IEC 60601-2-24
IEC 61000-4-3:2006 +A1:2007
+A2:2010
IEC 61000-4-3:2006 +A1:2007
+A2:2010
IEC 61000-4-8:2009
Contact discharge
Air discharge
Contact discharge ± 8 kV Operator intervention may
Air discharge
10 V/m
80 MHz – 2.7 GHz
80% AM at 1 kHz
380 – 390 MHz 27 V/m
430 – 470 MHz 28 V/m
704 – 787 MHz 9 V/m
800 – 960 MHz 28 V/m
1.7 – 1.99 GHz 28 V/m
2.4 – 2.57 GHz 28 V/m
5.1 – 5.80 GHz 9 V/m
3 V/m 0.15 MHz – 80 MHz
6 V/m in ISM and amateur radio
bands between 0.15 MHz and
80 MHz
80% AM at 1 kHz
30 A/m
50 Hz
± 2 kV, ± 4 kV, ± 6 kV
± 2 kV, ± 4 kV, ± 8 kV
± 15 kV
No degradation of
performance
be required as the syringe
pump may intermittently
reset, requiring user to
restart the infusion.
Yes
Yes
Yes
Yes
rd
Edition)
Use of this equipment adjacent to or stacked with other equipment should be avoided because it could result in
improper operation. If such use is necessary, this equipment and the other equipment should be observed to verify
that they are operating normally.
DFU999-103EN Rev. 04
11/60
Page 12
T34™ Syringe Pump (3
10.00
Long Term Rate Accuracy
0.00
0.20
0.40
0.60
0.80
1.00
1.20
1.40
1.60
1.80
2.00
Long Term Rate Accuracy
-4
-3
-2
-1
0 5 10 15 20 25 30 35
T34 Trumpet Curve at 5ml/h
0 5 10 15 20 25 30 35
T34 Trumpet Curve at 1ml/h
rd
Edition)
Section 1: General Information
Use of accessories other than those specified or provided by the manufacturer of this equipment could result in
increased electromagnetic emissions or decreased electromagnetic immunity of this equipment and result in
improper operation.
Do not operate the syringe pump near high-energy radio-frequency emitting equipment, (e.g. Imaging equipment
(i.e., X-Ray, MRI, CT Scan, etc.), High Frequency (RF) Surgical Equipment, Defibrillator, etc.) as this may cause
degradation in performance of the syringe pump, which may affect proper infusate delivery.
Portable RF communications equipment (including peripherals such as antenna cables and external antennas)
should be used no closer than 30 cm (12 inches) to any part of the T34TM syringe pump, including cables specified by
the manufacturer. Otherwise, degradation of the performance of this equipment could result.
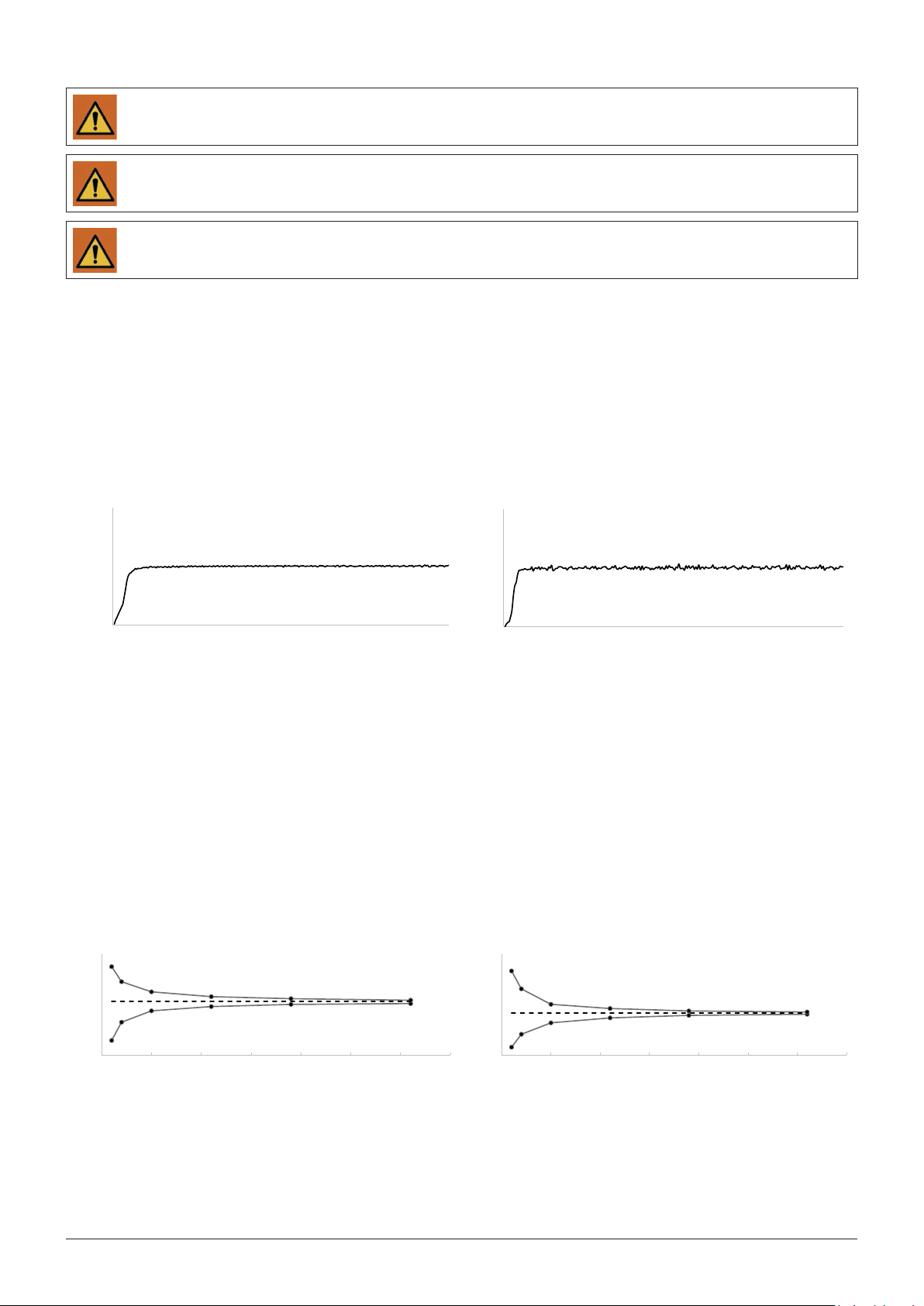
PUMP ACCURACY
The following graphs and curves were derived from testing described in IEC60601-2-24. Testing was performed under normal conditions
at room temperature (72°F or 22°C). Any deviations from normal conditions and room temperature may cause changes in the accuracy of
the syringe pump.
Start-up Curves
The Start-up curves represent continuous flow versus operating time for two hours from the start of the infusion, measured for infusion
rates of 5 ml/h and 1 ml/h. They exhibit the delay in onset of delivery due to mechanical compliance and provide a visual representation
of uniformity. Trumpet curves are derived from the second hour of this data. Tests performed according to IEC 60601-2-24 standard.
T34™LongTermRateAccuracyat5 ml/h T34™LongTermRateAccuracyat1 ml/h
9.00
8.00
7.00
6.00
5.00
4.00
3.00
2.00
Rate(ml/h)
1.00
0.00
0 20 40 60 80 100 120
Rate(ml/h)
0 20 40 60 80 100 120
Time(min) Time(min)
Trumpet Curves
With the T34™, as with all infusion systems, variations cause short term fluctuations in rate accuracy.
The following curves show typical performance of the system in two ways:
1. The accuracy of fluid delivery over various time periods is measured (trumpet curves).
2. The delay in onset of fluid flow when infusion commences (start up curves).
Trumpet curves are named for their characteristic shape. They display discrete data averaged over particular time periods or 'Observation
windows', not continuous data versus operating time. Over long observation windows, short-term fluctuation has little effect on
accuracy as represented by the flat part of the curve. As the observation window is reduced, short-term fluctuations have greater effects
as represented by the 'mouth' of the trumpet. Knowledge of system accuracy over various observation windows may be of interest when
certain drugs are being administered. Short-term fluctuations in rate accuracy may have clinical impact depending on the shelf life of the
drug being infused and the degree of inter-vascular integration. The clinical effect cannot be determined from the trumpet curves alone.
T34™TrumpetCurveat5 ml/h T34™TrumpetCurveat1 ml/h
4
3
3.03
2
1
0
-2.84
1.83
-1.39
1.02
-0.49
0.64
-0.15
0.48
0.02
0.36
0.09
0.257
Flowrateerror(%)
Observationinterval(min) Observationinterval(min)
15
10
-10
Flowrateerror(%)
10.89
6.44
-8.05
-4.84
2.59
-1.99
1.56
-0.77
0.95
-0.15
0.69
0.13
0.397
5
0
-5
DFU999-103EN Rev. 04
12/60
Page 13
T34™ Syringe Pump (3
Section 2: Disposables and Accessories
rd
Edition)
Section 2: Disposables and Accessories
2.1 Syringe Brands and Sizes
The T34™ Syringe Pump is programmed to recognize most commonly used syringes from 2 ml to 50 ml. Luer Lock syringes should always
be used to ensure secure connection of the syringe extension set and syringe.
To avoid accidental selection of an incorrect brand of syringe during setup, it is recommended to disable all syringe types not in regular
use. Unused syringe types can be disabled by an authorised technician.
Do not use Slip-tip syringes. Luer Lock syringes must always be used to ensure secure connection of the syringe
extension set and the syringe pump.
Should you need to operate the T34™ Syringe Pump with a syringe manufacturer and/or brand other than those listed here, please
consult either your local medical engineering department or CME Ltd. Technical Services.
Default Syringe Brands Configured for Use with T34™
Manufacturer Syringe Sizes (ml)
Braun Omnifix 2 5 10 20 30 50
BD Plastipak 3 5 10 20 30 50
Monoject 3 6 12 20 35
Codan/Once 10 20 30 50
Terumo 5 10 20 30 50
Nipro 5 10 20 30 50
Syringe Volumes
Due to the physical length of the screw that drives the syringe plunger forward there are limits to the maximum amount of infusate that
can be delivered from larger syringes and on some smaller syringes there is an undeliverable volume of infusate that will remain in the
syringe once the actuator has driven to the zero position.
Braun 2 ml, BD 10 ml, Codan 10 ml, Monoject 3 ml, Monoject 12 ml, Monoject 35 ml,
Nipro 10 ml and Nipro 20 ml syringes cannot empty completely, potentially a small
volume of infusate will be left in the syringe due to limitations of the syringe pump and
the syringes design. The T34™ Syringe Pump will display the volume as, for example,
Vol 5 (of 5.2)ml. In this example the syringe pump can only deliver 5 ml of the 5.2 ml in
the syringe and when the syringe pump has driven the syringe plunger as far forward
as possible, 0.2 ml will remain in the syringe. The potential volumes that might remain
in the syringes are listed in the table below:
Syringe size and brand Undeliverable infusate volume
Braun Omnifix 2 ml 0.000 ml to 0.114 ml
BD Plastipak 10 ml 0.000 ml to 0.177 ml
Codan/Once 10 ml 0.000 ml to 0.043 ml
Monoject 3 ml 0.000 ml to 0.007 ml
Monoject 12 ml 0.000 ml to 0.388 ml
Monoject 35 ml 0.000 ml to 0.348 ml
Nipro 10 ml 0.000 ml to 0.094 ml
Nipro 20 ml 0.000 ml to 0.006 ml
12 ml Monoject
Vol 5(of 5.2)ml
Change /, Press
DFU999-103EN Rev. 04
13/60
Page 14

Some manufacturers have several brand names within their ranges (e.g. Braun Omnifix and Braun Perfusor). Only
use the brands named above with the T34™, as failing to do so could result in an under- or over-infusion.
Maximum Fill Volume for Syringes 20 ml to 50 ml
Syringe brand Syringe size
20 ml 30 ml 50 ml
Monoject 18.7 ml — —
Braun Omnifix 20 ml 24.4 ml 37.7 ml
BD Plastipak 18 ml 23.5 ml 34.9 ml
Terumo 18.6 ml 24.5 ml 38.0 ml
Codan 20 ml 22.5 ml 35.9 ml
Time to Alarm from Occlusion
T34™ Syringe Pump (3
Section 2: Disposables and Accessories
rd
Edition)
Flow Rate Pressure Threshold
5 ml/h 200 mmHg TTA < 00:06:00
1 ml/h 200 mmHg TTA < 00:35:00
0.1 ml/h 200 mmHg TTA < 06:00:00
5 ml/h 1500 mmHg TTA < 00:25:00
1 ml/h 1500 mmHg TTA < 03:45:00
0.1 ml/h 1500 mmHg TTA < 24:00:00
Note:
Tested at flow rates and occlusion thresholds as described in the table above, using a BD Plastipak 20 ml syringe.
Time to Alarm (TTA)
(hh:mm:ss)
2.2 Syringe Extension Sets
INTRODUCTION
The T34™ Syringe Pump can be operated with any syringe extension set with a Luer connection. However, it is recommended, to
optimise system accuracy and performance, that proprietary syringe extension sets from CME Ltd. are used. All CME Ltd. syringe
extension sets have siphon/free flow protection.
FEATURES AND CHARACTERISTICS
Feature Description
Materials
Tubing
Slide clamp Clamps: to prevent fluid flow to patient (optional on some syringe extension sets).
Pressure activated antisiphon/anti-reflux valve
The syringe extension sets are manufactured using PVC materials that do not contain latex or di-2ethylhexyl phthalate (DEHP).
Micro-bore: require small priming volumes.
Anti-kink: to prevent kinking or occlusion particularly in configuration.
Various lengths available.
All CME Ltd. syringe extension sets contain an anti-syphon valve to prevent uncontrolled flow of fluid
either into or from the patient.
The syringe extension set with pressure activated anti-siphon/anti-reflux valve reduces the potential for
uncontrolled flow and backflow (backtracking).
The pressure-activated anti-siphon valve requires pressure to open. The syringe pump occlusion
pressure setting may require adjustment to prevent occlusion alarms.
The anti-syphon valve enhances pump functioning by:
• Preventing siphoning (uncontrolled flow) in the event the syringe extension set is detached from
the syringe pump or mechanical malfunction, and
• Preventing reflux (back-flow) in the event several infusion pumps are connected simultaneously
to the same patient.
DFU999-103EN Rev. 04
14/60
Page 15
T34™ Syringe Pump (3
Section 2: Disposables and Accessories
rd
Edition)
Luer Lock end connector
Ensure the syringe extension set is NOT connected to the patient during priming.
A kinked or occluded syringe extension set may impair the operation and accuracy of the syringe pump. Before
operation, verify that the syringe extension set is not kinked or occluded.
Note: The recommended syringe extension set change interval is maximum 72 hours.
The syringe extension set is designed to be connected to Luer Lock syringes.
Luer Lock syringes allow a connection between male and female Luer. This provides a secure connection
and prevents accidental removal.
ADVISORY WARNINGS AND NOTES FOR SYRINGES AND SYRINGE EXTENSION SETS
Component damage may occur if the syringe extension set is not correctly attached to the syringe. Assure
all connections are secure: Do not over-tighten connection. This will help minimise leaks, disconnection and
component damage.
Disposables (as with any infusion) used with the syringe pump must be compatible with the drug/fluid being
delivered. Check with the manufacturer of the disposables before use. Consult the fluid or drug manufacturer's
information for precautions, guidelines, and instructions for preparation and use of disposables.
Replace the syringe and/or syringe extension set in accordance with local guidelines.
Use aseptic technique when filling the syringe and priming the syringe extension set. Patient infection may result
from the use of non-sterile components. Maintain sterility of all syringe extension set components and do not
re-use single-use syringe extension sets.
Syringes and syringe extension sets should be disposed of in an appropriate manner, considering the nature of the
residual fluid that may be contained within, in accordance with the hospital/homecare provider's disposal practices.
DFU999-103EN Rev. 04
15/60
Page 16
2.3 Battery Power Supply
Refer to section 7.1 for checking battery levels
BATTERY TYPES AND USE
Always use a 9 volt alkaline disposable battery with the following attributes:
Designation: IEC: 6LR61
Body Size: 46.4 mm × 26.5 mm × 17.5 mm
Impedance: 1700 milli-ohms @ 1 kHz
Do not use batteries marked 6LP3146 or 6LF22 with the T34™ Syringe Pump. 6LP3146 and 6LF22 batteries can cause
issues with the operation of the syringe pump, as the physical construction and internal resistance of this type
of battery are different to the 6LR61 battery. Issues arising from use of 6LP3146 and 6LF22 batteries can include
End Battery messages during Pre-Load, volume test fails, pressure test/calibration issues and reduced amount of
infusions from a battery.
Nominal voltage 9 V
Impedance 1700 milli-ohms @ 1 kHz
Typical weight 45 g (1.6 oz)
Typical volume 22.8 cm3 (1.4 in3)
Terminals Miniature snap
Storage temp. range 5°C to 30°C (41°F to 86°F)
Operating temp. range −20°C to 54°C (−4°F to 130°F)
Designation
ANSI: 1604A IEC: 6LR61
T34™ Syringe Pump (3
Section 2: Disposables and Accessories
rd
Edition)
Note: Tested at flow rates and occlusion thresholds as described in the table above, using a BD Plastipak 20 ml syringe.
If a battery is too tight, do not try to force it into the battery compartment as this may damage the battery contacts.
Only use accessories designed for the system. Unsafe operation may result from using improper accessories. Use
only accessories and options designed for this system and supplied or recommended by the T34™ Syringe Pump
distributor.
BATTERY LIFE
Factors that affect battery life include:
• Pump settings,
• Infusion rate,
• The number of interventions that occur (e.g. stopping/starting infusions, manual movement of actuator and backlight activation),
• The number of key presses that occur, and
• Frequency of LED green light flashing.
The syringe pump battery meter displays battery life remaining as a percentage (%).
• When the battery power is low a low battery alert will activate.
• When the battery power is almost depleted, an end of battery alarm will activate.
• The end of battery alarm will continue until the user presses key to confirm or the battery power is fully depleted.
Refer to alerts, alarms and troubleshooting section for further information on alerts, alarms and troubleshooting.
INDICATIONS TO CHANGE A BATTERY
Guidance for battery changing may vary for different areas according to local policy and where the syringe pump is to be used and who
is managing the syringe pump (healthcare professional or patient).
If the syringe pump is being managed in an environment where designated personnel are available at all times to change a battery if
necessary, the low battery alert can be used as an indication to change a battery.
If the syringe pump is being managed in an environment where designated personnel are unavailable to change a battery
Note:
if necessary, the following rule applies: To ensure delivery of 24 hours at flow rate of up to 1ml/h, always change to a new
battery.
BATTERY FITTING AND REMOVAL
Inserting and removing a battery
The battery should fit securely to ensure good electrical contact.
DFU999-103EN Rev. 04
16/60
Page 17

DO NOT use scissors or metal objects to remove a battery.
To insert the battery into the syringe pump:
1. Slide the compartment cover off at the back of the syringe pump.
2. This reveals the empty battery compartment, with insertion
instructions.
T34™ Syringe Pump (3
Section 2: Disposables and Accessories
rd
Edition)
3. Push the battery into the compartment taking care to ensure
that the battery + / − contacts are aligned on the label inside the
compartment.
4. Slide the cover back on.
To remove the battery from the syringe pump:
1. Slide the compartment cover off at the back of the syringe pump.
2. Remove the battery
3. Slide the cover back on.
DFU999-103EN Rev. 04
17/60
Page 18

T34™ Syringe Pump (3
Section 2: Disposables and Accessories
rd
Edition)
2.4 Lockbox (Optional)
USES AND FEATURES
Lockboxes are designed to help protect the syringe from displacement and/or tampering.
Lock boxes are made from polycarbonate due to its high impact, temperature resistance and optical properties.
TYPES AND SIZES
Lockboxes are available in clear plastic.
• The clear lockbox can be used with any drug delivery route.
• The lockbox will fit most commonly used syringe brands and sizes up to 30 ml.
Refer to your local sales representative for any product-related information.
After the syringe is loaded on the T34™ Syringe Pump and the syringe extension set is connected, place the syringe pump into the
lockbox and carry pouch:
1. Open the lockbox using the standard key that operates all T34™ Syringe Pump lockboxes.
2. Place the syringe pump into the lockbox so that the LCD display and keypad line up with the cut out opening.
3. Close the lockbox, guiding the syringe extension set out of the slot at the side of the top section of the lockbox.
4. Place the lockbox (or the syringe pump without the lockbox) into the carry pouch and secure to the patient.
Note:
You can use the lockbox only with syringes which size is 30 ml or smaller.
Note: Lockboxes are designed for the use with CME Ltd. syringe extension sets. If using an alternate brand of syringe extension set
with a commonly used 30 ml syringe, the syringe extension set design may prevent the lockbox from fully closing and locking.
2.5 Carry Pouches (Optional)
CARRY POUCH USE AND TYPES
Disposable and reusable (washable) pouches are available.
Refer to your local sales representative for any product-related information.
Refer to Section 7.7 for re-usable pouch cleaning/washing instructions.
Using the syringe pump with a CME Ltd. carry pouch or similar receptacle during transportation or patient ambulation whilst the syringe
pump is infusing protects the syringe pump functionality and the medication in the syringe. The carry pouch will also protect the syringe
pump from damage or syringe displacement.
When using the CME Ltd reusable (washable) carry pouch, it is possible to access the screen and keypad of the syringe pump during
infusion by lifting the Velcro flap of the carry pouch whilst the syringe pump remains in the carry pouch.
When using either a CME Ltd reusable (washable) or disposable (single patient use) carry pouch it is possible to remove the forward part
of the syringe pump during infusion from the carry pouch to inspect the syringe without removing the rear section of the syringe pump.
CME Ltd carry pouches can be carried on the shoulder or around the waist for convenience.
DFU999-103EN Rev. 04
18/60
Page 19

Section 3: Pump Features and Description
3.1 Overview
The T34™ Syringe Pump provides the following features:
• Accommodates a range of commonly used syringe brand and sizes
• Three-point syringe detection system
• Capable of small ml/h rate delivery
• Configurable occlusion pressure setting
• LCD display screen with backlight
• Green LED indicator light to indicate if infusion is in progress
• Event log
The following safety features are available:
• Access code protected pump configuration
• Lockable infusion program
• Post Occlusion Bolus Reduction System
• Comprehensive range of alerts and alarms
• Keypad lock
• Lockable lockbox (optional)
T34™ Syringe Pump (3
Section 3: Pump Features and Description
rd
Edition)
3.2 Pump Description
1. Barrel clamp arm sensor 2. Collar sensor 3. Plunger sensor
6. Guide rails
4. Lead screw
5. Actuator
TOP OF PUMP: SYRINGE FITTING
No. Area Function
1. Barrel clamp arm sensor Detects syringe barrel loading and secures syringe in place.
2. Collar sensor Detects correct loading of the syringe collar.
3. Plunger sensor Detects correct loading of the syringe plunger.
4. Lead screw Moves actuator.
5. Actuator Drives the syringe plunger to deliver syringe contents.
6. Guide rails The two guide rails support the actuator position.
DFU999-103EN Rev. 04
19/60
Page 20

FRONT OF PUMP: KEYS AND DISPLAY SCREEN
10. LCD
display screen
1. i+ key
(Info menu)
2. Up
3. Down
4. Start / OK
No. Area Function
• Pressing once during infusion displays an infusion summary.
1.
2.
3.
4.
5.
6.
7.
Info Menu key
Up key
Down key
Start / OK key
Stop / No key
Move Actuator Forward key
Move Actuator Back key
• Pressing a second time during infusion displays the current battery level.
• When pump is in standby mode, accesses the main (Info) menu.
• Activates/deactivates keypad lock.
• Scrolls between options.
• Increases infusion parameters during programming/titration.
• Scrolls between options.
• Decreases infusion parameters during programming/titration.
• Confirms selection.
• Starts infusion.
• Takes user back one step during programming.
• Stops infusion.
• Moves actuator forward when no syringe in place and the barrel clamp
• Accesses purge function (if enabled).
• Accesses bolus function (if enabled).
• Moves actuator backward when no syringe is in place and barrel clamp
5. Stop / No
arm is down.
arm is down.
T34™ Syringe Pump (3
Section 3: Pump Features and Description
Syringe collarBarrel clamp arm sensor Syringe plunger
Plunger sensor
9. LED indicator
8. On / Off key
7. Move Actuator Back
6. Move Actuator Forward,
Purge/Bolus feature
rd
Edition)
Actuator
8.
9. LED indicator
10. LCD display screen
Note:
DFU999-103EN Rev. 04
On / Off key
Instructions on the pump label are for reference only and do not reflect all possible settings. Please, refer to this
Use
for full instructions.
• Powers the syringe pump on and off.
• The LED indicator light is a steady green during system self-tests.
• The LED indicator flashes green to indicate infusion delivery.
• The LED indicator is a steady yellow when the syringe pump is in standby
mode or indicates low priority alarm.
• The LED indicator flashes yellow to indicate medium priority alarm.
• The LED indicator flashes red to indicate a high priority alarm.
• Displays pump and infusion status, programming choices and
instructions.
Directions For
20/60
Page 21

BACK OF PUMP: BATTERY AREA AND PUMP MARKINGS
T34™ Syringe Pump (3
Section 3: Pump Features and Description
rd
Edition)
1. Pump information
2. Battery compartment
No. Area Function
1. Pump information and symbols
2. Battery compartment Includes instructions for inserting the battery correctly.
Labelling (including universal symbols) identify a pump, its manufacturer,
and communicates information on safety, use and performance.
DFU999-103EN Rev. 04
21/60
Page 22

T34™ Syringe Pump (3
Section 3: Pump Features and Description
rd
Edition)
3.3 Event Log
The event log shows a complete time and date stamped record of the last 512 pump events along with a record of pump status (volume
infused, rate, etc.) at the time of the event. Event log data cannot be deleted or altered and it is not patient-specific. i.e. the 512 events
are likely to span multiple patients treated with that particular pump.
Each event is assigned a new number and the syringe pump stores the most recent 512 events in memory. When more than 512 events
have occurred, the oldest event will be deleted each time a new event occurs.
For example, after some time, the first event to appear when you enter the events history might be number 754. This means there have
been 754 events in this pump's life and events 242–754 are currently stored. When event 755 occurs, the oldest event, number 242, will
be deleted and the syringe pump will store events 243–755, then 244–756, and so on.
Events recorded include hourly self-testing when an infusion is running and certain key presses.
When the syringe pump is infusing, the syringe pump will record pump status every hour regardless of any key presses.
The event log can be viewed via the syringe pump menu:
Event log access and navigation
The event log cannot be accessed whilst an infusion is running. If necessary, stop the infusion and remove keypad lock.
1. Press the key:
2. Use / keys to scroll to Event Log
3. Press key.
The most recent event displays:
Event No.: 854
27.07.201 16:01
Start Infusion
Press i+ for Details
When
key is pressed:
VI 1.03ml
VTBI 14.35ml
Rate 0.64ml/h
30ml BD Plastipak
Line 1: Event Number
Line 2: Date and time of event
Line 3: Event description/operating state
Line 4: View details on this event
VI = Volume infused
VTBI = Volume to be infused
Rate = ml/h rate
30 ml BD Plastipak = Syringe brand and size confirmed
Info Menu
Battery Level
Select /, Press
Info Menu
Event Log
Select /, Press
Either use
events or,
Press
/ keys to scroll up/down through
key to display detailed data for the event.
Travel = Actuator travel distance (mm) to deliver 1 ml (for syringe size/brand confirmed)
Travel 2.73mm/ml
1N (0mmHg) = 18mA
10N (540mmHg) = 83mA
Motor Current 0mA
Occlusion 720mmHg
Battery 7.8V
Either use
The event information that displays when key is pressed will vary depending on the operational status of the syringe pump for that
event.
Some events may only record one or two parameters, while other events record numerous parameters.
Note:
DFU999-103EN Rev. 04
/ keys to display further information or to return to the previous screen, press key.
The syringe pump does not automatically change for daylight saving. The date and time can be updated manually via the
syringe pump Change Set up menu.
1 N = Minimum actuator travel force (related to start up motor movement)
10 N = Maximum actuator travel force (related to start up motor movement)
Motor current = Motor current level in mA
Occlusion = Pump occlusion alarm setting
Battery = Battery voltage
22/60
Page 23

T34™ Syringe Pump (3
Section 3: Pump Features and Description
Event Log examples
Switched ON Syringe Removed Anti-bolus Reverse Keypad Lock ON
Switched OFF Occlusion / End Stop Infusion Keypad Lock OFF
Volume Change Purge Pump Operating System Error
rd
Edition)
Other events may be recorded relating to technical information. Refer to the
Note:
Technical Service Manual
for details.
3.4 Post Occlusion Bolus Reduction System (POBRS)
POST OCCLUSION BOLUS REDUCTION SYSTEM
During an occlusion, the pressure in the downstream section of the syringe extension set and/or inside the syringe can increase above
the occlusion pressure defined in pump settings. When the syringe pump alarms the user must check the syringe extension set and
attempt to clear the occlusion. During an occlusion the syringe pump's Post Occlusion Bolus Reduction System feature will reverse the
operation of the motor and drive the actuator to avoid an unintended bolus on release of the occlusion.
When the infusion is resumed, the volume to be infused (VTBI) increases and the volume infused (VI) decreases to indicate the syringe
pump back off feature and the infusion time remaining increases; this protects the ml/h infusion rate.
Following activation of the POBRS and if the user presses
syringe pump back off feature.
Intermediate Rate Occlusion Pressure Unintended Bolus Volume
5 ml/h 200 mmHg (minimum)
1500 mmHg (maximum)
An occlusion may pressurize the syringe extension set and syringe, which can result in an unintended bolus of infusate when the
occlusion is resolved. In order to prevent unintended bolus, disconnect the syringe extension set or relieve the excess pressure through a
stopcock, if present.
The clinician should weigh the relative risks of syringe extension set disconnection with the risks of an unintended bolus.
to resume the infusion VTBI increases and the VI decreases to indicate the
<= 0.5 ml
<= 0.5 ml
OCCLUSION PRESSURE
The occlusion pressure of a pump is the pressure in the system, registered at the syringe pump, when the syringe pump is still operating
but cannot sustain the flow rate. The resultant build-up of pressure sets off the occlusion alarm.
OCCLUSION AND RESPONSE
An occlusion alarm can be activated by:
• A blockage in the syringe extension set – often inadvertently caused by kinking or leaving a roller clamp or a tap closed.
• A clotted-off cannula.
• A partially occluded cannula – if it causes the required driving pressure to rise above the occlusion alarm level.
• A very long or narrow bore cannula or/with syringe extension set.
Occlusion response is characterised in terms of three measurable parameters:
1. Pressure to alarm
2. Time to alarm
3. Bolus release when occlusion is resolved
1. Pressure to alarm
If an occlusion occurs the syringe pump attempts to maintain sufficient pressure on the infusate to cause it to flow through all
restrictions and overcome any additional resistance. Although infusate is incompressible, the syringe extension set and other
components of the system have some give (compliance) and the syringe extension set can expand under the increasing pressure. Other
components of the system, such as the syringe stopper, become compressed. This expansion and compression takes some time to occur.
2. Time to alarm
If the occlusion is present from the beginning of the infusion, the time to alarm will increase. The pressure in the syringe and syringe
extension set increases from zero at the start of the infusion up to the alarm level. Leaving a clamp closed is the most likely cause of
occlusions. Generally, shorter time to occlusion alarm occur with high flow rates and small syringes. Refer to section Time to Alarm from
Occlusion on page 14 for time-to-alarm specifications.
3. Unintended bolus release
In the case of a complete occlusion, there is no flow to the patient whilst pressure in the system is increasing. When the occlusion is
released, the build-up of infusate in the syringe extension set can result in an unintended bolus.
DFU999-103EN Rev. 04
23/60
Page 24

T34™ Syringe Pump (3
Section 4: Modes of Operation
rd
Edition)
Section 4: Modes of Operation
4.1 Modes of Operation
DURATION INFUSION
The primary setting is duration (volume over time infusion) which can be configured with a locked or changeable duration time.
Once the duration time is confirmed, the syringe pump will calculate the ml/h rate.
RATE INFUSION (ML/HOUR)
The primary setting is ml/h rate (rate over time infusion) which can be configured with a locked or changeable ml/h infusion rate.
Once the ml/h rate is confirmed, the syringe pump will calculate the duration time.
COMMON PUMP CONFIGURATIONS
Four common pump configurations are:
• Lock On mode – fixed duration
• Lock Off mode – adjustable duration
• Rate mode (Lock On) – fixed ml/h rate
• Rate mode (Lock Off) – adjustable ml/h rate
Each of these modes can have additional functions enabled or disabled, to suit local requirements.
The syringe pump default mode of operation is Lock On 24 hour duration.
For the correct pump configuration, mode of operation and start-up sequence, you must refer to your local policies.
RESUME
The T34™ Syringe Pump can be configured for a continuous infusion with Rate as the primary setting. The primary setting (e.g. Rate)
is then used to calculate duration. If the Rate is the primary setting it is saved for the current program when Resume is selected after
therapy interruption (e.g. syringe displacement or occlusion). See KEY PRESS OPTIONS OF RESUME AND NEW SYRINGE section for further
instructions.
LOCK ON MODE (FIXED DURATION)
The syringe pump will deliver the syringe volume over the fixed (locked) duration. Once a syringe is detected and confirmed, the syringe
pump calculates the ml/h infusion rate:
syringe volume
fixed duration
• With the Program Lock On, rate change (titration) during infusion cannot be enabled.
• KVO and purge can be enabled if required.
Note:
During programming, the user must check and confirm the infusion summary screen. This includes checking the syringe
volume, duration, and the calculated rate matches the prescription and what is required for that infusion before it
iscommenced.
= ml/h infusion rate
LOCK OFF MODE (ADJUSTABLE DURATION)
The syringe pump will deliver the syringe volume over the confirmed default duration or the duration inputted and confirmed by the
user during programming. The syringe pump then calculates the ml/h infusion rate:
syringe volume
default or duration entered
= ml/h infusion rate
• With Program Lock Off, rate change (titration) during infusion can be enabled if required. There is no option to re-program the
infusion duration during delivery.
• KVO, purge and rate titration can be enabled if required.
Note:
During programming, the user must check, change and/or confirm infusion information and programming screens. This
includes checking that the program summary screen (syringe volume, duration, and the calculated rate) matches the
prescription and what is required for that infusion before it is commenced.
DFU999-103EN Rev. 04
24/60
Page 25

T34™ Syringe Pump (3
COMPARISON OF LOCK ON AND LOCK OFF (DURATION) MODES PROGRAMMING SCREENS
rd
Edition)
Section 4: Modes of Operation
Lock On
(Fixed Duration)
20ml BD Plastipak
Select /, Press
Volume 12.0ml
Duration 24:00
Rate 0.50ml/h
Confirm, Press
Start Infusion?
Lock Off
(Adjustable Duration)
20ml BD Plastipak
Select /, Press
Occlusion 720mmHg
Max. Rate 5ml/h
Program Lock OFF
Battery Status 90%
20ml BD Plastipak
Volume 12ml
Change /, Press
20ml BD Plastipak
Duration 24:00
Change /, Press
20ml BD Plastipak
Rate 0.5ml/h
Confirm, Press
Volume 12.0ml
Duration 24:00
Rate 0.50ml/h
Confirm, Press
Start Infusion?
If the default duration is changed and/or titration is enabled additional screen prompts will display.
There are two alternatives methods for starting an infusion when using a duration (volume over time) mode of operation:
Note:
prime and load or load and prime methods. The method chosen relates to the priming volume of the syringe extension set.
Your local policy will state which method to use.
In Duration mode we recommend using "Prime and Load" and not "Load and Prime". Please be aware if using Load and Prime
Note:
instead of Prime and Load sequence, the rate of delivery will be automatically adjusted to compensate for the lost priming
volume while maintaining the preset duration. If you wish to maintain the rate, please work in Rate mode.
RATE MODE (LOCK ON) – FIXED ML/HOUR RATE
The syringe pump will deliver the syringe volume over the fixed (locked) ml/h rate. Once a syringe is detected and confirmed, the syringe
pump calculates infusion delivery duration:
syringe volume
fixed ml/h rate
= duration
• With the Program Lock ON, rate change (titration) during infusion cannot be enabled.
• KVO and purge can be enabled if required.
Note:
During programming, the user must check and confirm the infusion summary screen. This includes checking the syringe
volume, duration, and the rate matches the prescription and what is required for that infusion before it is commenced.
DFU999-103EN Rev. 04
25/60
Page 26

T34™ Syringe Pump (3
Section 4: Modes of Operation
rd
Edition)
RATE MODE (LOCK OFF) – ADJUSTABLE ML/HOUR RATE
The syringe pump will deliver the syringe volume over the ml/h rate inputted and confirmed by the user during programming the
syringe pump calculates the infusion delivery duration:
syringe volume
confirmed ml/h rate
• With Program Lock OFF, rate change (titration) during infusion can be enabled if required.
• KVO, purge and rate titration can be enabled if required.
= duration
RATE MODE PROGRAMMING SCREENS
a) If the syringe pump is configured with Rate Setting disabled, the default is 0 ml/h.
20ml BD Plastipak
Rate 0ml/h
Change /, Press
b) If the syringe pump is configured with Rate Setting enabled, the programmed rate becomes the default.
20ml BD Plastipak
Rate 2ml/h
Change /, Press
This example shows the default rate is 0 ml/h. The user enters the rate required.
This example shows the default rate is 2 ml/h. The user can either confirm or change the rate.
Note: During programming, the user must check, change and/or confirm infusion information and programming screens.
This includes checking that the program summary screen (syringe volume, duration, and the calculated rate) matches the
prescription and what is required for that infusion before it is commenced.
DFU999-103EN Rev. 04
26/60
Page 27

T34™ Syringe Pump (3
Section 5: Pump Configuration
rd
Edition)
Section 5: Pump Configuration
5.1 Pump Configuration
CONFIGURATION AUTHORISATION
Pump configuration must only be carried out by designated and authorised personnel, you must check with your technical department
and/or line manager if you are designated and have the authority to change the syringe pump configuration.
When configuring a pump, the following must be taken into account, to ensure that:
• The pumps are configured for the required application, e.g. occlusion pressure settings correct for drug delivery route.
• The mode of operation (lock on, lock off and rate modes) configured is correct for the drug prescription. e.g. duration (volumeover-time) or a ml/h infusion
• Optional features and functions that may be required are configured (e.g. Purge, KVO, titration, pump maximum ml/h rate)
Any program/pump changes that are made must be fully documented checked with a second person and against a pump setting
authorisation form which is available from local or CME Ltd. technical service staff.
5.2 Pump Access Codes
ACCESS CODES FOR PUMP CONFIGURATION
The T34™ Syringe Pump has three areas of access code protection to prevent unauthorised changes to set up, configuration or
programming. Certain settings and features may be configured and locked based on patient or clinical need or to configure the syringe
pump for a specific clinical application.
No access code is required to turn the syringe pump on and run an infusion. In normal clinical use the syringe pump user will not see
these fields or be prompted for access codes.
The Change Set Up and Rate Settings menus are available via the syringe pump
The Technician menu code is only provided to fully trained, (by CME Ltd.) and authorised electrical biomedical engineering personnel.
menu.
Do not attempt to access code protected areas if you are not trained or authorised to do so. Authorised personnel
should not share codes with un-authorised personnel and should only give code access to designated personnel.
Codes will only be provided by CME Ltd. to designated and authorised clinical or technical staff when they have been trained
Note:
and certified in their use. No access codes are contained in this
Technical staff must refer to CME Ltd. Technical Services department for access codes and information on pump configuration
Note:
and training.
Directions For Use
.
DFU999-103EN Rev. 04
27/60
Page 28

T34™ Syringe Pump (3
Section 5: Pump Configuration
rd
Edition)
5.3 Pump Info and Configuration Menus
PUMP INFO MENU
The syringe pump Info menu enables the user to navigate to various functions. This includes accessing the syringe pump configuration
settings (Change Set Up and Rate Setting areas).
ACCESSING THE INFO MENU
The menu cannot be accessed during an infusion or with the keypad lock activated.
• With no infusion running, press the key.
• Use / keys to scroll up/down the menu to select the option required, then press key to view the contents.
The tables describes the functions in the menu.
Option Description
Info Menu
Battery Level
Select /, Press
Info Menu
Exit
Select /, Press
View battery life percentage (graph)
Exits Info menu
Info Menu
Rate Setting
Select /, Press
Info Menu
Event Log
Select /, Press
Info Menu
Change Set up
Select /, Press
Change and configure Rate Setting function (access code protected)
View pump event log
Change and configure programming functions (access code protected)
RATE SETTING MENU
The tables shows the configurable parameters in the Rate Setting area.
Parameter Default Range Description
Sets the default rate on Rate Setting Mode.
Rate Setting 0 ml/h
0.1 ml/h – (maximum rate)
Note: Maximum rate can be changed from the technician menu
(Maximum Rate parameter). Default: 5 ml/h. Range: 0.1 ml/h to
650 ml/h.
CHANGE SET UP MENU
The tables shows the configurable parameters in the Change Set Up menu
Parameter Default Range Description
Exit — — Exit the Change Set Up menu.
Language English
Set Time and Date
Key Operation
DFU999-103EN Rev. 04
Current
date/time
5 mm 0.1–100 mm
English,
local language
Month/year
Hour/minute
The syringe pump can be set to English or one local language which
varies according to where the syringe pump is sold.
Sets a date and time stamp for the event log.
This does not automatically change for daylight saving.
Limits the forward movement of the actuator when the key is
pressed with no syringe in place and barrel clamp arm down.
28/60
Page 29

T34™ Syringe Pump (3
Section 5: Pump Configuration
Backlight Duration 5 seconds 0 (OFF)–60 seconds Limits the screen backlight duration following key presses.
rd
Edition)
Info Duration 5 seconds 1–20 seconds
Bolus Dose Rate
Bolus Maximum Volume
Titration Option Disabled Enabled/Disabled
Default Duration 24:00 hours
Occlusion Pressure 720 mmHg
KVO Operation Rate
Program Lock ON OFF/ON With lock on, prevents alteration of default duration or ml/h rate.
300 ml/h 1–650 ml/h
0 ml
(Disabled)
0 ml/h 0 (OFF)–5.0 ml/h
0 (OFF)–20 ml 0–20 ml in 0.1 ml increments.
0:00 hours
(ml/h)
0:01–99:00 hours
(volume over time)
200–1500 mmHg Sets the pressure level at which the occlusion alarm will activate.
Limits the screen information duration which displays when the
key is pressed during an infusion.
1–10 ml/h in 0.01 ml/h increments;
10–29.9 ml/h in 0.1 ml/h increments;
30–49.5 ml/h in 0.5 ml/h increments;
50–299 ml/h in 1 ml/h increments;
300–650 ml/h in 5 ml/h increments.
Enables rate change during infusion. Maximum rate is the syringe
pump max. ml/h rate. Minimum is 0.1 ml/h. Can only be enabled if
program lock is OFF.
With default duration set to 0:00 hours, the syringe pump runs as an
ml/h infusion.
With a nonzero default duration set, the syringe pump runs as a
volume over time infusion.
0–5.0 ml/h in 0.1 ml/h increments.
Activates Keep Vein Open infusion at end program.
DFU999-103EN Rev. 04
29/60
Page 30

T34™ Syringe Pump (3
Section 5: Pump Configuration
rd
Edition)
5.4 Pump Configurable Settings for Modes of Operation
CONFIGURATION SETTINGS FOR LOCK ON AND LOCK OFF (DURATION) MODES
Lock On (Fixed duration) Lock Off (Adjustable duration)
Titration Option Disabled Enable if rate change during infusion is required
Default Duration e.g. 24:00 hours e.g. 24:00 hours
Program Lock On Off
Rate Setting
Occlusion Pressure Set the pressure for the infusate delivery route e.g. subcutaneous, IV.
KVO Set KVO ml/h if required.
Max. Rate
Purge Set purge volume if required. Pump default 0 ml.
0 ml 0 ml
Change if required. Pump default 5 ml/h.
CONFIGURATION SETTINGS FOR RATE (ML/HOUR) MODES
Rate Mode (Lock ON)
(Fixed ml/h rate)
Titration Option Disabled Enable if rate change during infusion required
Default Duration 0:00 hours 0:00 hours
Program Lock On Off
Rate Setting
Occlusion Pressure Set the pressure for the drug delivery route e.g. subcutaneous, IV.
KVO
Max. Rate Change if required. Pump default 5 ml/h.
Purge Set purge volume if required. Pump default 0 ml.
Note: A Pump Configuration Authorisation form is available from CME Ltd. to record authorisation and document pump settings.
Contact your local Sales or Clinical representative.
Pump maximum ml/h rate and purge volume are configured via the syringe pump Technician Menu. If these settings need to
Note:
be changed, you must consult technical staff.
The syringe pump uses an indirect method of pressure detection.
Note:
e.g. 2 ml/h e.g. 2 ml/h
Set KVO ml/h if required. Pump default 0 ml.
Rate Mode (Lock OFF)
(Adjustable ml/h rate)
DFU999-103EN Rev. 04
30/60
Page 31

T34™ Syringe Pump (3
Section 5: Pump Configuration
rd
Edition)
5.5 Optional Configurable Settings
KVO (KEEP VEIN OPEN) OPERATION
The T34™ Syringe Pump can be configured to deliver a KVO infusion to commence at the end of the infusion to keep the patients access
device patent. With KVO enabled the syringe pump applies the KVO rate set until the syringe is empty or to a maximum of 5 ml.
KVO rate can be configured in the syringe pump Change Set Up Menu.
KVO
0.2ml/h
When the KVO volume is delivered the end program alarm activates.
When configuring KVO infusion rate, Medium priority alarm will be heard to confirm the syringe pump
is still infusing in KVO mode.
PURGE
In order to eliminate/reduce mechanical slack (visible spaces at the syringe collar and plunger loading points) and ensure a faster start
up time (time to start delivering the fluid to the patient/reach the programmed infusion rate) the user can purge the system.
• The purge function is available (if enabled) once only, after pre-loading prior to commencing an infusion.
• The purge function is disabled by default (0 ml) and the maximum deliverable is 2.0 ml.
• The purge rate is 650 ml/h.
• The purge function can be configured via the syringe pump Technician menu.
• The purge function can be used with any mode of operation.
Purge sequence (all modes of operation)
1. Turn the syringe pump on without syringe and wait until the preloading process is complete.
2. Load the syringe.
3. Press the key after confirming the syringe size/brand.
20ml BD Plastipak
Select /, Press
Purge
4. Ensure the syringe extension set is disconnected to the patient, confirm by pressing key.
5. Press and hold the key until the slack is removed and purge volume is delivered (a purge volume
will be configured, e.g. 0.2 ml).
Disconnect patient
Press
to Confirm
Purge, hold ! key
Purge 0.00ml
Purge
6. Wait for the next screen to display.
Completed
7. If the syringe size/brand displayed matches the one used, confirm by pressing key. (Use /
keys to select the matching syringe if necessary).
Rate Titration
If enabled, you can titrate (change) continuous infusion flow rates during infusion, it is recommended that the keypad lock is used as an
additional protection against accidental rate change during infusion.
The maximum ml/h rate limit will be the syringe pump maximum rate which is configured via the syringe pump Technician menu. The
minimum rate is 0.1 ml/h. If this setting needs to be changed, you must consult technical staff.
Rate change can be enabled in the following modes of operation:
20ml BD Plastipak
Select /, Press
DFU999-103EN Rev. 04
31/60
Page 32

• Lock Off Mode (duration)
• Rate Mode (ml/h)
• Rate Setting Mode (ml/h, Lock Off )
Rate change cannot be enabled in Lock On mode.
To titrate the ml/h rate during infusion
1. Deactivate keypad lock.
T34™ Syringe Pump (3
Section 5: Pump Configuration
rd
Edition)
2. With infusion running, press / keys to change the rate.
Time Remaining 12:00
Rate 1.0ml/h
<<<< Pump Delivering
3. Enter infusion rate required using / keys, confirm by pressing .
20ml BD Plastipak
Rate 2ml/h
Change /, Press
4. Check that the rate change completed and is correct. (Note change to time remaining). Time Remaining 06:00
Rate 2.0ml/h
<<<< Pump Delivering
5. Activate keypad lock.
DFU999-103EN Rev. 04
32/60
Page 33

T34™ Syringe Pump (3
Section 5: Pump Configuration
rd
Edition)
5.6 Practice Scenarios for Changing Pump Configuration
Changing configuration will affect the operation/functionality of the T34™ Syringe Pump. Set up parameters should only be changed
by clinical or technical staff with user code access rights only and the authority to change pump settings. It is advisable that any
configuration changes are carried out with no syringe in place and barrel clamp arm down.
SCENARIO 1: CHANGE DATE AND TIME
Scenario: Change date and time from 4th February 2009, 16:15 to current date/time.
(10th December 2011, 12:30 is demonstrated below)
1. To power on, press key. Wait until pre-loading completes and screen prompt displays:
2. Press the key.
Load Syringe
Info Menu
Battery Level
Select /, Press
3. Scroll to Change Set Up.
Info Menu
Change Set up
Select /, Press
4. Press key.
Enter Set up Code
00
Change /, Press
5. Enter access code using / keys, confirm by pressing . To obtain a suitable access code, please
contact your local servicecentre.
When the
in thousands thereafter. Scroll to the nearest point in tens and then release the key. Press either the or arrow individually, until the
correct code displays then press to confirm.
arrow is pressed and held down the syringe pump will count up in single digits to ten, then in tens to one hundred and then
6. Scroll to Set Time and Date, press key.
7. Change year using / keys, confirm by pressing key.
8. Change month using / keys, confirm by pressing key.
9. Change date using / keys, confirm by pressing key.
Change Set up
Time & Date
Select /, Press
10.12.2009 16:15:00
Year 11
Change /, Press
10.02.2009 16:15:00
Month 12
Change /, Press
04.02.2009 16:15:00
Date 10
Change /, Press
DFU999-103EN Rev. 04
33/60
Page 34

T34™ Syringe Pump (3
10. Change hour using / keys, confirm by pressing key.
11. Change minutes using / keys, confirm by pressing key.
12. Press to exit Set Up.
13. Check changes by powering off and on, with barrel clamp arm down and observing start up screen
information.
SCENARIO 2: CHANGE PROGRAM LOCK ON TO LOCK OFF
Scenario: Current pump set up: pump default, Lock On 24:00
rd
Edition)
Section 5: Pump Configuration
10.12.2011 16:15:00
Hours 12
Change /, Press
10.12.2011 12:15:00
Minutes 30
Change /, Press
1. To power on, press key. Wait until pre-loading completes and screen prompt displays:
2. Press the key.
Load Syringe
Info Menu
Battery Level
Select /, Press
3. Scroll to Change Set Up.
Info Menu
Change Set up
Select /, Press
4. Press key. Contact your local service centre to obtain a suitable access code.
Enter Set up Code
00
Change /, Press
5. Enter access code using / keys, confirm by pressing key.
When the arrow is pressed and held down the syringe pump will count up in single digits to ten, then in tens to one hundred and then
in thousands thereafter. Scroll to the nearest point in tens and then release the key. Press either the or arrow individually, until the
correct code displays then press key to confirm.
6. Scroll to Program Lock
7. Press key. Change to OFF using / keys, confirm by pressing key.
8. Scroll until Exit displays, confirm by pressing key.
DFU999-103EN Rev. 04
34/60
Change Set Up
Program Lock
Select /, Press
Program Lock
OFF
Change/, Press
Change Set up
Exit
Select /, Press
Page 35

T34™ Syringe Pump (3
Section 5: Pump Configuration
9. Check changes by powering off and on, with barrel clamp arm down and observing start up screen
information (Program Lock status).
rd
Edition)
SCENARIO 3: CHANGE FROM LOCK ON MODE TO RATE SETTING (LOCK ON) MODE
Scenario: Change from Lock On Mode with default duration of 24:00 to Rate Setting (Lock On) Mode with 2 ml/h rate. Change Default
Duration to 0 hours in Change Set-Up Menu and enter 2 ml/h in Rate Setting Menu.
Load Syringe
1. To power on, press key. Wait until pre-loading completes and screen prompt displays:
Info Menu
2. Press the key.
3. Scroll to Change Set Up, press key.
Battery Level
Select /, Press
Info Menu
Change Set up
Select /, Press
4. Enter access code using / keys, confirm by pressing key. Contact your local service centre to
obtain a suitable access code.
5. Scroll to Default Duration.
6. Change to 00:00 using / keys, confirm by pressing key.
7. Scroll until Exit displays.
8. Press the key.
Enter Set up Code
00
Change /, Press
Change Set up
Default Duration
Select /, Press
Default Duration
00:00
Change/, Press
Change Set up
Exit
Select /, Press
Info Menu
Battery Level
Select /, Press
9. Scroll to Rate Setting, press key.
10. Enter access code using / keys, confirm by pressing key. Contact your local service centre to
obtain a suitable access code.
DFU999-103EN Rev. 04
35/60
Info Menu
Rate Setting
Select /, Press
Enter Rate Code
0
Change /, Press
Page 36

11. Change to 2 ml/h using / keys, confirm by pressing key.
12. The Load Syringe screen prompt displays.
13. Check if all setting changes completed by simulating starting an infusion.
T34™ Syringe Pump (3
Section 5: Pump Configuration
Rate setting
2ml/h
Change /, Press
Load Syringe
rd
Edition)
DFU999-103EN Rev. 04
36/60
Page 37

T34™ Syringe Pump (3
Section 6: Starting A New Infusion
rd
Edition)
Section 6: Starting A New Infusion
6.1 Sequence for Starting an Infusion
This section explains the user actions needed for starting a new infusion. The 'Prime and Load' method of syringe extension set priming
is described. This is suitable for the low priming volumes of all CME syringe extension sets.
A. PREPARE SYRINGE AND MANUALLY PRIME THE SET
Prepare syringe with drug(s) as per prescription and local policy, attach drug label, ensuring the label lies flat.
Do not over-label the syringe or apply anything that changes its external diameter at the point where the barrel
clamp is applied as incorrect syringe detection may result.
Manually prime the syringe extension set
Preparing the syringe and the syringe extension set:
1. Select the correct syringe extension set, and remove from packaging.
2. Attach the syringe extension set to prepared Luer Lock syringe, maintaining sterility.
3. Remove cap from end of syringe extension set.
4. Gently push syringe plunger forward until air is expressed from the syringe extension set.
5. Cap the end of the syringe extension set.
B. CHECK THE SYRINGE PUMP
Ensure that the syringe pump is clean, visually intact, appropriate for intended use, and within service date. It is good practice to inspect
medical equipment and accessories between patients and certainly immediately before use (e.g. whilst setting it up on the patient).
Inspection of the syringe pump and/or accessories should include checking that the:
• Pump is undamaged
• Lockbox is locked and intact (if in use)
C. POWERING ON AND PRE-LOADING
Do not insert foreign objects around or near the actuator during automatic actuator movement (pre-loading)
or when manually adjusting the actuator. The procedure for releasing a foreign object from the actuator is in
section6.2.
Powering On
With no syringe in place and the barrel clamp arm down, press the key until the screen illuminates
and the first screen displays.
When the syringe pump is powered on, pre-loading commences.
Pre-loading
Pre-loading is a simultaneous sequence of screen information displaying and automatic actuator movement. During pre-loading:
• The syringe pump performs an internal self-test
• The screens display important pump information
• The actuator moves forwards/backwards automatically
Pre-loading deletes any program in the syringe pump memory and at the end of the pre-loading sequence the actuator returns to the
start position of the last infusion. If the user regularly uses the same syringe brand, size and fills to the same volume, powering off and on
allows automatic actuator movement which returns the actuator to the correct position each time.
Pre-loading only takes place when no syringe is in place and the barrel clamp arm is in the down position.
Note:
If the syringe pump is powered on with no syringe in place but with the barrel clamp arm raised, pre-loading does not take
place.
Version T34xxx.xx
ID: Syringe Pump
T34
The actuator will only move automatically or manually by using the and keys (with no syringe in place and
barrel clamp arm down). Do not use force to try to move the actuator manually as this could cause damage to the
syringe pump and/or affect calibration.
If the keypad lock is on, when a pump is powered on (with no syringe in place and barrel clamp arm down), pre-loading will not take
place and the actuator cannot be moved manually using the
During pre-loading the following screens display in sequence:
DFU999-103EN Rev. 04
/ keys.
37/60
Page 38

T34™ Syringe Pump (3
Section 6: Starting A New Infusion
rd
Edition)
T34
Version T34xxx.xx
ID: Syringe Pump
Pre-Loading
Use to Interrupt
Occlusion 720mmHg
Max. Rate 5ml/h
Program Lock ON
Battery Status 99%
Load Syringe
Pump identification
The syringe pump displays the model name, software version number and pump identification.
The default identification name (ID) is Syringe Pump. This name can be changed by an authorised
technician, e.g. to an asset number or site name. Up to 17 characters are permitted.
Advisory notice
It is advisable not to interrupt the automatic actuator movement to ensure that a previous program
is deleted.
If the user pressed
Pump default settings
Line 1 – Occlusion pressure setting
Line 2 – Pump maximum ml/h Rate that can be set
Line 3 – Program lock status
Line 4 – Battery status*
*During actuator movement this value may fluctuate. Do not rely on this figure as the true battery
percentage.
On completion of pre-loading the screen displays.
This screen displays and flashes until a syringe is detected in all three syringe sensors.
key, the actuator stops moving and the Load Syringe screen will display.
D. CHECK THE BATTERY LEVEL
Check the battery level via the menu
The LED light will display red (no infusion running)
1. Press key.
2. Press key.
3. Wait a few seconds for this screen to display:
Instructions for changing the battery when necessary are given in section 2.3.
Info Menu
Battery Level
Select /, Press
Battery Level
36%
Empty Full
Load Syringe
DFU999-103EN Rev. 04
38/60
Page 39

T34™ Syringe Pump (3
Section 6: Starting A New Infusion
rd
Edition)
E. SYRINGE LOADING, DETECTION AND CONFIRMATION
Manual adjustment of the actuator
1. Ensure the barrel clamp arm is down.
2. Place the prepared syringe above the syringe pump to visually align the syringe collar to the collar sensor.
3. Use the / keys (if required), to move the actuator to the correct position for placing the syringe collar and plunger into the
matching pump sensor areas.
Syringe loading
1. Lift the barrel clamp arm fully and turn the arm 90° (either way).
2. Place the syringe collar vertically (long side) into the syringe pump collar slot and the syringe plunger into the syringe pump plunger
slot,
the syringe should click into position.
3. Turn and lower the barrel clamp arm onto the syringe.
As each point of the syringe is correctly seated, the flashing indicator becomes solid on the screen display. When the collar and plunger
sensors detect a syringe, its size and brand will display. If the syringe is not detected, the display will show 'Check Plunger Sensor' or
'Check Collar Sensor'.
Rear View
Check Plunger Sensor
Check Collar Sensor
Syringe detection and confirmation
The syringe pump identifies the syringe brand, size and volume by measuring the syringe dimensions from the three sensors.
Check that the syringe brand and size inserted into the syringe pump matches the syringe brand and size displayed. If they match,
confirm by pressing
Incorrect syringe size/brand detected
The syringe pump may sometimes misidentify a syringe as a different type or brand from the one being inserted. Common causes are:
• The syringe is not correctly fitted into the 3 sensor areas and the syringe pump has wrongly detected another syringe with very
similar dimensions. (Within ±1 mm of another syringe brand in the syringe library.)
• Over-labelling of the syringe or applying anything that changes its external diameter at the point where the barrel clamp
is applied.
To rectify:
• Scroll between syringe brands of similar dimensions using the / keys.
• When the correct syringe displays, press key to confirm and continue programming.
Failure to detect a syringe
Failure to detect any brand/size of syringe can be caused by:
• The syringe is positioned incorrectly or not fully engaged with any or all of the sensors.
• The syringe brand or size being fitted is not configured into the syringe pump.
To rectify:
• Reposition or refit the syringe ensuring the syringe is firmly placed into the 3 sensor areas. The screen prompt will indicate which
sensor or sensors are affected. For the collar sensor, ensure that the collar of the syringe is facing downwards into the slot because
the sensor is positioned at the bottom of the slot.
key.
DFU999-103EN Rev. 04
39/60
Page 40

T34™ Syringe Pump (3
Section 6: Starting A New Infusion
• Either use a compatible syringe brand or arrange for the new size or brand of syringe to be configured into the syringe pump. This
change can only be performed by authorised personnel.
Never take a syringe that is not empty off the syringe pump if it is still connected to the patient. The syringe
extension set must be disconnected or clamped before removing the syringe to prevent uncontrolled flow and the
risk of serious injury or death to the patient.
If the Volume to be Infused displayed on the syringe pump LCD after confirming the syringe varies by more than 5%
of the actual syringe volume visually confirmed on the syringe scale, remove the syringe, turn off the syringe pump
and, with the barrel clamp arm down, turn the syringe pump on to allow pre-loading to occur. Repeat the syringe
placement and detection steps and ensure the correct syringe size and brand are confirmed. If the calculated
volume reading is still significantly different from the visually confirmed contents, remove the syringe pump from
use and return to an authorized service center for inspection, testing and calibration.
Using a syringe not approved by the syringe pump manufacturer or a syringe type which is not compatible with
syringe pumps, could affect pump performance, resulting in over-delivery or under-delivery of medication to the
patient.
rd
Edition)
F. ENTERING/CONFIRMING THE PROGRAM
When programming the syringe pump for a mode of operation, specific screens and user interactions will depend on the syringe pump
configuration for local use.
This section demonstrates typical programming using the prime and load method for the following modes:
• Lock On mode – fixed duration
• Lock Off mode – adjustable duration
• Rate mode (Lock ON) – fixed ml/h rate
• Rate mode (Lock OFF) – adjustable ml/h rate
Lock On mode – fixed duration
Following syringe confirmation, the program summary displays:
Volume 12.0ml
Duration 24:00
Rate 0.5ml/h
Confirm, Press
The ml/h rate displayed has been calculated from the syringe volume and fixed duration.
Lock Off mode – adjustable duration
Following syringe confirmation, the program sequence commences:
Review pump configuration settings and wait for next screen prompt.
Visually check if the volume in the syringe matches the volume displayed.
Change if necessary, confirm by pressing
Change duration if necessary, by using / arrow keys, confirm by pressing key.
Review the infusion/program summary to check that the parameters displayed match the prescription:
Visually check the volume/duration/rate. To confirm infusion, press the key.
key.
Occlusion 720mmHg
Max. Rate 5ml/h
Program Lock OFF
Battery Status 90%
20ml BD Plastipak
Volume 12ml
Change/, Press
20ml BD Plastipak
Duration 24:00
Change/, Press
If the default duration is changed this screen displays for a few seconds.
DFU999-103EN Rev. 04
Duration Changed,
Please Check Rate
40/60
Page 41

T34™ Syringe Pump (3
The rate displays (calculated from the syringe volume divided by the duration confirmed), check and
confirm by pressing key.
Review the infusion/program summary to check that the parameters displayed match the prescription:
Visually check the volume/duration/rate. To confirm infusion, press the
The ml/h rate is calculated from the syringe volume and confirmed duration.
Rate mode (Lock ON) – fixed ml/h rate
Following syringe confirmation, the program sequence commences:
Review pump configuration settings and wait for next screen prompt.
Review the infusion/program summary to check that the parameters displayed match the prescription.
Visually check the volume/duration/rate, to confirm infusion, press key.
key.
rd
Edition)
Section 6: Starting A New Infusion
20ml BD Plastipak
Rate 1.0ml/h
Confirm, Press
Volume 12.0ml
Duration 12:00
Rate 1.0ml/h
Confirm, press
Occlusion 720mmHg
Max. Rate 5ml/h
Program Lock ON
Battery Status 90%
Volume 12.0ml
Duration 24:00
Rate 0.5ml/h
Confirm, Press
(The duration has been calculated from the syringe volume and fixed ml/h rate).
Rate mode (Lock OFF) – adjustable ml/h rate
Following syringe confirmation, the program sequence commences:
Review pump configuration settings and wait for next screen prompt.
Visually check if the volume in the syringe matches the volume displayed. Change if necessary, confirm
by pressing key.
If adjustable rate setting is enabled, but no value has yet been entered, then an initial default value of
0 ml/h will display.
Enter the infusion rate required and confirm by pressing key.
If a rate setting has been entered, then the ml/h rate will display.
Adjust the infusion rate if necessary, and confirm by pressing the
key.
Occlusion 720mmHg
Max. Rate 5ml/h
Program Lock OFF
Battery Status 90%
20ml BD Plastipak
Volume 12ml
Change/, Press
20ml BD Plastipak
Rate 0.0ml/h
Change/, Press
20ml BD Plastipak
Rate 2.0ml/h
Change/, Press
Review the infusion/program summary to check that the parameters displayed match the prescription.
Visually check the volume/duration/rate. To confirm infusion, press key.
Duration is calculated from the syringe volume and confirmed ml/h rate.
DFU999-103EN Rev. 04
41/60
Volume 12.0ml
Duration 06:00
Rate 2.0ml/h
Confirm, Press
Page 42

T34™ Syringe Pump (3
Section 6: Starting A New Infusion
rd
Edition)
G. START INFUSION (ALL MODES)
Connect the syringe extension set to patient
At this point, site/connect the cannula/syringe extension set to the patient. Follow local policy for the recommended cannula and set
to use.
To commence the infusion, press key.
Check and confirm infusion is running
Visually check that the infusion running screen is visible and the green LED light flashes intermittently.
When the syringe pump is operating, note that the bottom line alternates between the syringe brand and size confirmed and
<<<<PumpDelivering (with moving chevrons):
Time Remaining 24:00
Rate 0.50ml/h
<<<< Pump Delivering
Note:
An alternative sequence which may be used is the 'Load and Prime' method, which involves priming the set after loading the
syringe pump. This method is more suitable for pumps which require a large priming volume. Which method to use should be
decided based on which syringe extension sets are available, local circumstances and hospital policy. Consideration must be
given to clinical risk, ease of use for the syringe pump user and consistency in start-up procedure for all wards/departments
and clinical areas.
In Duration mode we recommend using "Prime and Load" and not "Load and Prime". Please be aware if using Load and Prime
Note:
instead of Prime and Load sequence, the rate of delivery will be automatically adjusted to compensate for the lost priming
volume while maintaining the preset duration. If you wish to maintain the rate, please work in Rate mode.
Time Remaining 24:00
Rate 0.50ml/h
20ml BD Plastipak
Start Infusion?
DFU999-103EN Rev. 04
42/60
Page 43

T34™ Syringe Pump (3
Section 6: Starting A New Infusion
rd
Edition)
6.2 Releasing a Trapped Foreign Object from the Actuator
If an object /finger is trapped either during pre-loading or when manually adjusting the actuator, the alarm and screen prompt that
displays will depend on the battery % level and the force/resistance moving against the actuator. Alerts or alarms that may display
include low battery alert, end battery alarm, system failure alarm or a high motor current alarm.
If a foreign object /finger is trapped in front of, or behind the actuator during pre-loading (automatic actuator movement) or when
manually adjusting the actuator, the user should:
Option 1 (manual adjustment of actuator)
1. Power the syringe pump off
2. With the syringe pump positioned with front of pump facing.
• To move the actuator towards the barrel clamp arm, place finger on guide screw and roll finger towards the syringe pump screen/
keypad.
• To move the actuator towards the front of the syringe pump, place finger on guide screw and roll finger towards the battery
compartment.
Back of pump
(battery compartment area)
Front of pump
(screen and keypad area)
Option 2 (adjustment of actuator using / keys)
1. Power the syringe pump off.
2. Power on.
3. Use the or key to release the object.
Event log interpretation of alarms
Interpretation of the syringe pump event log can assist in identifying the effects on the syringe pump with an object being trapped in
front of, or behind the actuator. Recorded events will reflect the alarm that was activated at the time. If a low or end battery alarm was
activated you may see that the battery voltage has dropped substantially and the activation of a low or end battery alarm is dependent
on the battery % level at the time of the alarm.
DFU999-103EN Rev. 04
43/60
Page 44

T34™ Syringe Pump (3
Section 7: Monitoring and Managing Infusions
rd
Edition)
Section 7: Monitoring and Managing Infusions
7.1 Pump and Infusion Safety Checks
Ensure that the syringe pump is clean, visually intact, appropriate for the intended use, and within service date. It is good practice to
inspect medical equipment and accessories between patients and certainly immediately before use (e.g. whilst setting it up on the
patient).
It is recommended that procedures are established for regular checks on the syringe pump, accessories and the progress of the infusion.
Inspect the lead screw prior to use (refer to section 3.2 Pump Description on page 19, item 4). If there is white plastic debris on the lead
screw, this is an indication of wear on the syringe mechanism. Therefore, discontinue use and send the syringe pump for service.
Inspect for signs of physical damage to the syringe pump, and to lockbox and carry pouch (optional).
PUMP POSITIONING
It is good practice to minimise disturbance to the pumps and to maintain the syringe pump at the same height level throughout an
infusion as far as possible. Optimal operation occurs with positive pressure infusion devices are positioned at the same height level as
the infusion site.
If a pump has been accidentally damaged, dropped or subject to fluid ingress/spillage it should be withdrawn from
service immediately and a suitable replacement pump located. Contact your local service centre.
TO CONFIRM THE INFUSION IS IN PROGRESS
LED indicator
• The indicator LED will flash green.
• The LCD screen will display the following information:
– Line 1 – infusion time remaining
– Line 2 – ml/h infusion rate
– Line 3 – alternates between the syringe size and brand confirmed by the user during set up and Pump Delivering
Regular monitoring should include checking:
• All connections between the syringe and the syringe extension set are secure.
• There are no kinks in the syringe extension set.
• There are no signs of physical damage to the syringe pump, lockbox or carry pouch.
• The keypad lock is on.
• Infusion is in progress.
• Volume history and battery status are as expected.
Note:
Follow local guidance for a full list of infusion monitoring checks.
DFU999-103EN Rev. 04
44/60
Page 45

CHECKING THE BATTERY LEVEL DURING INFUSION (LED LIGHT IS GREEN)
36%
36%
T34™ Syringe Pump (3
Section 7: Monitoring and Managing Infusions
rd
Edition)
Press key twice.
Time Remaining 24:00
Rate 0.50ml/h
<<< Pump Delivering
The battery level displays as a percentage (%).
Wait a few seconds for the screen to default back to infusion running screen again or press the
again, to display infusion volume history screen.
Note: With the infusion running, repeated key presses on the key cycles through volume history, battery level and infusion
running screen. Excessive key presses or usage of the feature will reduce battery life. Use only as required to optimize
battery performance.
key
CHECKING THE BATTERY LEVEL WITH INFUSION PAUSED (LED LIGHT IS RED)
1. Stop infusion by pressing key.
2. Press key once.
3. Press key.
4. The battery level displays as a percentage (%).
Wait a few seconds for the default display to reappear, or press the
immediately.
key to exit the battery screen
Battery Level
Empty Full
Info Menu
Battery Level
Select /, Press
Battery Level
Empty Full
TO CHECK THE VOLUME HISTORY DURING INFUSION
Time Remaining 24:00
Press key once.
The syringe volume to be infused (VTBI) and volume infused (VI) are displayed.
The total of VTBI + VI equals the starting volume.
Note: After pressing the key either a third press or waiting a few seconds returns the display to the base display screen.
Note: Excessive key presses or usage of the feature will reduce battery life. Use only as required to optimize battery performance.
Rate 0.50ml/h
<<< Pump Delivering
Infusion Summary
VTBI 11.00 VI 0.8
DFU999-103EN Rev. 04
45/60
Page 46

T34™ Syringe Pump (3
Section 7: Monitoring and Managing Infusions
rd
Edition)
7.2 Keypad Lock
FEATURES AND USES
The keypad has a locking feature that prevents unintentional powering off of the unit, as well as the ability to lock or limit certain
infusion parameters or pump settings. The T34™ Syringe Pump allows users to lock the operation of the keypad if concerned about
patients, relatives or untrained personnel tampering with the syringe pump.
key and key are active as there may be a need to stop/pause the infusion short-term (e.g. in an emergency situation or for other
The
clinical interventions).
If the syringe pump is stopped/paused for longer than 2 minutes, the Pump Paused Too Long alarm will activate to alert the user to the
syringe pump status. In this instance, the Event Log records these events.
When the syringe pump is used with the keypad lock activated:
The user can stop
stopped/paused, the only option available is to restart the infusion.
• The syringe pump cannot be powered off using the Power key.
• The user cannot scroll through the menu to access the available options
• The user cannot change rate during continuous infusion (if enabled in Lock Off or Rate Modes).
Note the following principles with keypad lock:
• If the power supply is interrupted during an infusion and the user powers the syringe pump on again (with syringe in place): if the
Press to Resume, for New Syringe screen displays, the infusion can be resumed.
• The use of the / keys for manual actuator adjustment is not accessible (when no syringe in place and barrel clamp arm
is down).
• The syringe brand and size displayed cannot be changed using the and arrow keys as this would delete the current program.
• The purpose of pre-loading, (automatic actuator movement) is to delete the current program in the syringe pump. Pre-loading
will not take place even when there is no syringe is in place and the barrel clamp arm is down.
• If the power supply is interrupted during an infusion and the user powers the syringe pump on again (with no syringe in place
and the barrel clamp arm down) Pre-loading does not take place. Because pre-loading (automatic actuator movement) has not
occurred, the program is still available to be resumed. Use of the keypad lock prevents automatic actuator movement.
• If the user then loads and confirms a syringe, the Press to Resume, for New Syringe screen displays, and the infusion can be
resumed.
Note:
It is recommended that the keypad lock is used if rate change (titration) is enabled. This gives additional protection against
unintentional rate change during infusion.
and start an infusion, and with the infusion running use the key to review the infusion status. If the infusion is
APPLYING AND REMOVING THE KEYPAD LOCK
The keypad lock can be activated and deactivated at any time by pressing and holding the key.
To activate the keypad lock
With the syringe pump infusing, press and hold the
left to right. When the progress bar is completely black the syringe pump will beep to confirm that the lock has been activated.
Keypad Lock
OFF ON
To deactivate the keypad lock
Press and hold the
bar is completely white the syringe pump will beep to confirm that the lock has been deactivated.
key down for approximately 5 seconds. The lock indicator graphic will empty from right to left. When the progress
key down for approximately 5 seconds. The lock indicator graphic will fill up from
DFU999-103EN Rev. 04
46/60
Page 47

T34™ Syringe Pump (3
Section 7: Monitoring and Managing Infusions
rd
Edition)
7.3 Program Protection and Resume
Program Protection
The current program (infusion) is the only one available in the syringe pump memory for use. In certain situations, it is possible to
continue use of the current program using Resume after therapy interruption. Program protection and the ability to Resume applies
specifically to the programmed ml/h rate. Pre-loading (automatic actuator movement) will clear a program from the syringe pump
memory. For detailed information on pre-loading, refer to Section 6, Stage C. Powering on and Pre-Loading.
RESUME AND NEW SYRINGE OPTIONS
An infusion can be interrupted, for example by alarm activation (e.g., occlusion), syringe change, or
power interruption. Depending on the cause of interruption, 'Resume' and 'New Syringe' are the two
options that may be presented.
Press to Resume
for New Syringe
RESUME
The Resume option saves the current infusion rate and eliminates the need to reprogram/confirm settings (volume, duration, and rate)
after therapy interruption. The Resume option is available to continue an infusion in the following situations:
• Syringe displacement or occlusion alarm
• Power supply interruption or failure
Resume is not available in the following situations:
• A different syringe brand and/or size is placed in the syringe pump
• The volume in the syringe is changed during therapy interruption
To Resume therapy press
summary and press to start infusion.
Note: Pressing for New Syringe immediately deletes the current program. A new program is then calculated or entered (depending
on mode of operation).
to confirm syringe brand and size then press to Resume. Follow the prompts to confirm the settings
NEW SYRINGE
A program (infusion) may be interrupted to change the syringe (i.e. brand, type, or increase/decrease syringe volume) which requires
programming infusion settings (i.e. volume, duration, and rate). This type of interruption deletes the previous program, rate is not saved,
and the options to Resume, for New Syringe will not be provided.
Following an interruption, verify the syringe brand and size displayed matches the syringe placed into the syringe pump. If they match,
press to confirm. Follow the prompts to change and/or confirm each setting for the current infusion, review the setting summary, and
if correct press to start infusion. If incorrect, press to go back.
Note: Follow local policy/procedure for the appropriate option to press when the to Resume, for New Syringe screen displays
following purge.
Syringe fitting and confirmation:
1. The syringe pump will automatically attempt to detect the syringe brand and size, but if it identifies a syringe incorrectly, lift the
barrel clamp arm and re-seat the syringe.
2. If the incorrect syringe brand and size continue to be displayed, use the and arrows to scroll and select the correct syringe.
When the correct syringe is displayed, to confirm press . The options to Resume, for New Syringe will be provided.
3. If the options for to Resume, for New Syringe are not provided, then there is no current program available and a new program
must be entered.
DFU999-103EN Rev. 04
47/60
Page 48

7.4 Stopping/Pausing the Infusion and Powering Off
TO STOP THE INFUSION (PUMP PAUSED)
T34™ Syringe Pump (3
Section 7: Monitoring and Managing Infusions
rd
Edition)
If the user presses key during an infusion the syringe pump is paused (stopped) for two minutes, the
LED light changes from green to yellow and a screen message displays:
Either press
If the paused state continues with no key presses, after two minutes the syringe pump will alarm and a
'Pump Paused Too Long' screen message displays.
Either press key to restart the infusion or key to pause for another two minutes.
key to restart the infusion or key to pause for another two minutes.
Pump Stopped
Press to Resume
Pump Paused Too Long
Confirm, press
POWERING OFF
1. Remove keypad lock and stop/pause the infusion if running.
2. Press and hold down the key until the progress graphic (moving from left to right) fills completely black, a beep is heard and the
display screen power is removed.
3. Disconnect the syringe extension set from patient access device.
4. Remove syringe from the syringe pump and place the barrel clamp arm down.
5. Remove battery if the syringe pump is no longer required.
DFU999-103EN Rev. 04
48/60
Page 49

7.5 Alerts, Alarms and Troubleshooting
Alarm Condition
When the syringe pump detects a problem, four things may occur:
• If a High priority alarm occurs, infusion will stop. For lower priority alarms, infusion continues.
• An audible alarm is activated,
• A message appears on the display screen indicating the cause of the alarm, and
• The LED indicator will change to red/yellow.
Note:
See the
During the power on sequence the audible speaker is activated and LEDs will illuminate to verify alarm functionality. No action
Note:
Alarm tone settings are normally preserved in the case of power loss, however some system faults will result in loss of alarm
Note:
When you turn off the syringe pump, an event is recorded by the event log, while unexpected power loss events (for example,
Note:
In the event of complete loss of power supply, the backup audio alarm will sound, but there will be no visual indicators.
Note:
The operator should ensure that the current alarm presets are appropriate to use on each patient.
Avoid setting pump limits to extreme values. This may render the alarm system ineffective.
Technical Service Manual
is required during this self-test.
settings.
if you change the battery while the pump is on) are not recorded.
for test procedures to check Alarms functionality.
T34™ Syringe Pump (3
Section 7: Monitoring and Managing Infusions
rd
Edition)
Infusion settings and other setup parameters should only be changed by clinical or technical staff with user code
access rights and the authority to change pump settings.
ALARMS
The syringe pump will activate an alarm when:
Description Alarm Type
Down Occlusion
End Battery
End Of Infusion
Syringe displaced during
infusion
Restart Pump
Switch off & On
ERROR XX
End Of Infusion
(Keep Vein Open)
High priority alarm
Requires immediate user
response
Medium priority alarm
Requires prompt user response
Audio Signal as per
60601-1-8
High priority
5 tones
Volume min. 45 dBA
Medium priority
3 tones
Volume min. 45 dBA
Visual Signal as per
60601-1-8
Operational LED
Red flashing visual
Operation LED flashes red
Yellow flashing signal
Operation LED flashes yellow
Pump Paused Too Long
Low Battery
Near End
Bolus Started / Completed
Keypad Lock / Unlock
Syringe plunger at the limit
of travel
DFU999-103EN Rev. 04
Low priority alarm
Requires user awareness
Informational signal
Provides information that may,
or may not require action from
user
Low priority
3 tones
Volume min. 45 dBA
1 or 2 pulses No visual
49/60
Yellow solid visual
Operation LED is solid yellow
(Not flashing)
Page 50

T34™ Syringe Pump (3
Section 7: Monitoring and Managing Infusions
Syringe loaded
Purge Start / End
Power On / Off
Infusion started / resumed /
stopped by user
KVO stopped by user
Service interval alert
WHEN THE ALARM ACTIVATES:
• If a High priority alarm occurs, infusion will stop. For lower priority alarms, infusion continues.
• The alarm sounds until either the syringe pump is paused or the problem is rectified.
• A screen message indicates the cause of the alarm.
SCREEN PROMPTS
In certain situations screen prompts display to prompt the user and provide information:
Screen prompt Result/cause Possible actions
Keypad Locked
Press
to Resume,
for New Syringe
Pump Stopped
Press
to Resume
Informational signal
Provides information that may,
or may not require action from
user
Only the
The current program has been interrupted
and two options are available for
programming.
The infusion has been stopped.
, and keys are accessible.
1 or 2 pulses No visual
Disengage keypad lock if further access
required.
Press
key resumes the current program.
Press key to delete the current program
(to allow a new program to be set up).
Press
key to resume the infusion or press
key to continue stopped state.
rd
Edition)
TROUBLESHOOTING ALERTS AND ALARMS
Screen information Result/cause Possible actions
Program Nearly
Complete
Low Battery Alert: Battery is almost depleted. Prepare to change battery.
Pump Paused
Too Long
Syringe Empty,
Remove Syringe
End Battery Alarm: Battery will fail imminently. Change battery.
Syringe Displaced,
Check Syringe
Occlusion/Empty
Syringe, Check Line
System Error (High Priority alarm)
Press and hold
If problem persists send pump
for service.
ERROR. Startup MotMov Fail, If problem
persists send pump for service.
key for details.
Alert: Program is about to end/syringe is
almost empty.
Alarm: The syringe pump has been
stopped/paused for more than 2 minutes
without any key presses.
Alarm: Current infusion program has
completed/syringe is empty.
Alarm: One or more of the syringe
detection sensors is not detecting.
Alarm: Clamped set, occluded or kinked.
Actuator has reached the minimum travel
position.
Alarm: An internal system error has
occurred. (Two examples of system failure
screen messages are shown here).
Prepare to change syringe or discontinue
pump use.
Either press
press key to continue pause for another
two minutes or turn the power off.
Prepare to change syringe or discontinue
pump use.
Check the syringe and re-seat as necessary
Check screen messages for assistance.
Release the clamp, flush/replace the access
device or clear the occlusion.
If error recurs: Take pump out of use.
Press
Record error code and summary of fault
and return pump to designated
service centre.
key to resume the infusion,
key to obtain error message.
Technical problem/error and failure identification.
• The syringe pump alarms if an internal system fault has been detected and the unit will be inoperative.
• The user may be prompted to power off and restart, which may rectify the error.
• If the problem cannot be rectified: power off and remove from patient use.
• Refer to the
• Follow local policy and/or contact your authorised Medical Engineering Department for advice.
The Event Log will record the error/alarm event.
DFU999-103EN Rev. 04
Technical Service Manual
for full details of all technical alarms.
50/60
Page 51

T34™ Syringe Pump (3
Section 7: Monitoring and Managing Infusions
rd
Edition)
7.6 Bolus
INTRODUCTION
Bolus Administering a controlled volume of fluid or drug at an increased rate for diagnostic or therapeutic purposes. The syringe
pump should always be infusing and always attached to the patient. (Drugs given by an IV bolus could achieve immediate and
high drug concentration levels.)
Bolus delivery can only be used during an infusion.
Note:
When the infusion is in progress, this screen displays.
To deliver a bolus, press
key and follow the screen prompts.
Time Remaining 24:00
Rate 0.50ml/h
<<< Pump Delivering
BOLUS DELIVERY SETUP
Bolus delivery cannot be performed unless the bolus delivery feature has been configured. Bolus delivery setup is configured via the
Change Set up menu. Note that a suitable access code will be needed.
Press the info button and select Change Set up from the menu.
Enter the access code and press .
Use the / arrow keys to select Bolus Dose Rate and press when selected.
The default Bolus Dose Rate is 300 ml/h. Bolus Dose Rate can adjusted from 1 ml/h to 650 ml/h. Use the
/ arrow keys to enter the desired rate and press to confirm.
Use the / arrow keys to select Bolus Maximum Volume and press when selected.
The Bolus Maximum Volume is preset to 0 ml, and can be configured from 0 to 20 ml in 0.1 ml intervals.
Use the / arrow keys to enter the desired volume and press to confirm.
Info Menu
Change Set up
Select /, Press
Change Set up
Bolus Dose Rate
Select /, Press
Change Set up
Bolus Maximum Volume
Select /, Press
Change Set up
Once the bolus dose rate has been adjusted, use the / arrow keys to select the Exit option and the
key to exit the setup menu.
Exit
Select /, Press
DELIVERING A BOLUS
Once the bolus delivery settings have been configured, boluses may be delivered during infusion. In order to deliver a bolus during an
infusion, press the key. This brings up a confirmation screen:
Press key to activate the bolus menu. To confirm delivery of the bolus, press the key. Once delivery
has been confirmed, the key must be held down continually to deliver the bolus.
Note:
After confirming the bolus, the key must be held continuously. If the key is released, bolus delivery will stop and the
syringe pump will change back to normal infusion.
Press to confirm
Bolus
DFU999-103EN Rev. 04
51/60
Page 52

T34™ Syringe Pump (3
Section 7: Monitoring and Managing Infusions
rd
Edition)
7.7 Changing Syringes/Syringe Extension Sets
INTRODUCTION
This section provides options for managing an infusion in the form of checklists. They are intended as guidance only, not user
instructions.
The way the infusion is managed in your own clinical area will vary, depending on, for example, local policy, types of syringe extension
sets and drugs being infused. You must refer to your local policy for specific infusion management requirements and instructions for
managing infusions.
CHECKLIST FOR CHANGING A SYRINGE (NEW PROGRAM, SAME SET)
Remember to de-activate and activate the keypad lock as necessary.
1. Stop infusion .
a) If the infusion complete alarm has activated, press key to confirm the end of the infusion.
b) If the Program Nearly Completed alert has activated, press the key to access volume history and record the VI (Volume Infused)
then press key to stop the infusion.
2. Power off.
3. Clamp and disconnect the set from the empty syringe.
4. Raise the barrel clamp arm, remove the empty syringe and lower barrel clamp arm.
5. Prepare a new syringe and attach the syringe extension set.
6. Power on, observe pre-loading and wait for the screen to display Load Syringe.
7. Check battery level.
8. Load the new syringe into the syringe pump, check syringe brand/size is correct and to confirm press key.
9. Enter/check new program, if correct, press key.
10. The screen will display Start infusion? Connect set to syringe and when ready to do so, press key.
11. Check that the infusion is running.
CHECKLIST FOR PRIMING A NEW SYRINGE EXTENSION SET FROM THE SAME SYRINGE
Remember to de-activate and activate the keypad lock as necessary.
1. Stop the infusion.
2. Do not power the syringe pump off.
3. Disconnect set from cannula.
4. Remove syringe and set.
5. Attach new set to existing syringe and prime.
6. Align the syringe above the syringe pump and use the key to resize the syringe to the actuator.
7. Raise the barrel clamp arm.
8. Load the new syringe into the syringe pump, check syringe brand/size is correct and to confirm press key.
9. When the screen prompt Press to Resume, for New Syringe displays, press key to resume.
10. Check the program summary screen. If correct, press key.
11. The screen will display Start infusion? Connect set to cannula and when ready to do so, press key.
12. Check that the infusion is running.
CHECKLIST FOR DISCONTINUING THE INFUSION AND PUMP
Remember to de-activate the keypad lock
1. Stop infusion.
a) If the infusion complete alarm has activated, press key to confirm the end of the infusion.
b) If the Program Nearly Complete alert has activated, press the key to access volume history and record the VI then press key
to stop the infusion.
2. Power off.
3. Disconnect the set/cannula from patient.
4. Raise the barrel clamp arm, remove the syringe and lower barrel clamp arm.
5. Remove the battery from the syringe pump.
6. Dispose of the syringe and set according to local policy.
7. Clean and store the syringe pump as per local policy.
DFU999-103EN Rev. 04
52/60
Page 53

T34™ Syringe Pump (3
Section 8: Servicing and Maintenance
rd
Edition)
Section 8: Servicing and Maintenance
8.1 Servicing, Maintenance and Periodic Checks
Periodic maintenance (PM) is recommended every 1 year. In between maintenance the syringe pump requires only cleaning between
patients (or as necessary; refer to section 8.2 Cleaning on page 54 for complete cleaning instructions). The syringe pump will display
a maintenance reminder alert for the user to send the syringe pump for service yearly. The PM is designed to assure the syringe pump's
accuracy and detect and repair any potential pump inconsistencies prior to their occurrence in the field. During the PM, a biomedical
engineer or trained technician should perform the following procedures:
• Clean the syringe pump thoroughly.
• Visually inspect the syringe pump to verify its structural integrity.
• Perform all the manual tests in the Change Set Up menu.
• Perform calibration procedures as per the
• Run the syringe pump for several hours to make sure no abnormalities occur during infusion such as alarms, inaccurate infusion,
and battery inconsistencies.
Note:
Service and maintenance should be performed by a CME technician. The only maintenance a patient can perform is cleaning
the device.
It is the CME technician's responsibility to repair any faults found during the Periodic Maintenance.
Note:
Note: Do not service the syringe pump while it is in use and/or attached to a patient.
Technical Service Manual
.
DFU999-103EN Rev. 04
53/60
Page 54

8.2 Cleaning
PUMP CLEANING - MRC Protocol
IMPORTANT!
• The Manufacturer Recommended Cleaning (MRC) protocol is NOT intended to replace local Infection Prevention and
Control Policy. The decision about the level of decontamination required depends not only on how the device is used,
but also on the risk of the device transmitting infection or acting as a source of infection.
• Best prevention practices against HAI (Hospital Acquired Infections) recommend a 2 steps process: Step 1. Removing
unwanted soils from all surfaces with a cleaning agent (pathogens can use soils for harborage limiting accessibility to
disinfectant agents). Step 2. Disinfecting the freshly cleaned surfaces.
MRC Protocol
INTENT:
• To preserve pump performance.
• To remove soil, particles and chemical residue that could accumulate over time on pump surface. Soil, particles and
chemical residue result from normal use and from the "disinfection protocol" developed by users at point of use.
INSTRUCTIONS
• To clean the pump, wipe the external pump surface using a disposable alcohol wipe impregnated with isopropyl alcohol
(IPA) 70%, to minimize pump exposure to excessive quantities of liquids.
• Isopropyl alcohol (IPA) is volatile and leaves no residue upon evaporation, therefore surfaces are left dry quickly after
wiping.
T34™ Syringe Pump (3
Section 8: Servicing and Maintenance
rd
Edition)
FREQUENCY:
• It is recommended to apply the MRC protocol to the pump after each disinfection sequence as a preventive measure to
maintain pump performance and longevity (removal of chemical residue).
• Note: Preventive maintenance also helps to maintain pump performance over time. This should be performed as
recommended in the Periodic Maintenance section.
Turn off the syringe pump before cleaning.
When fluid ingress is suspected, stop using the syringe pump and request pump verification through maintenance
to identify potential need of corrections.
Immersing the syringe pump into liquid could cause damage to components. Do not soak or immerse any part of
the syringe pump or the syringe pump charger in any type of liquid.
If other chemical cleaning agents are used for "disinfection protocol / regime", ensure to follow the manufacturer
recommended cleaning to preserve pump performance, after completing the "disinfection protocol /regime".
Do not spray or rinse cleaning solutions directly on pump surfaces or in potential liquid retention areas or open
ports such as electrical connections.
Avoid using chemicals that can damage the surfaces of the instrument (for example chlorinated solvents).
When using cleaning solutions containing chemicals (such as corrosive agents), do not use concentrated solutions
and do not expose surfaces above the recommended dwell time. After application, rinse surfaces with IPA
disposable wipes to eliminate chemical residue.
Do not steam, autoclave, EO (ethylene oxide) sterilize, immerse the syringe pump or pump charger in any type of
fluid, or allow fluids to enter the syringe pump case.
LOCKBOX CLEANING
CME Ltd. recommends the use of alcohol sprays and wipes to decontaminate the lockbox. Other products may be used but users should
be aware that extended usage could result in the lock box becoming brittle and susceptible to damage. The substances listed below may
adversely impact products constructed from polycarbonate:
• Alkali bleaches such as sodium hypochlorite
• Butyl acetate
DFU999-103EN Rev. 04
54/60
Page 55

T34™ Syringe Pump (3
Section 8: Servicing and Maintenance
• Methanol
• Acetone
• Sodium hydroxide
• Methyl ethyl ketone
• Acrylonitrile
• Chloroform
• Styrene
• Ammonia
• Dimethylformamide
• Tetrachloroethylene
• Amyl acetate
• Concentrated hydrochloric acid
• Toluene
• Benzene
• Concentrated hydrofluoric acid
• Concentrated sulphuric acid
• Bromine
• Iodine
• Xylene
rd
Edition)
RE-USABLE POUCH CLEANING
Clean fabric made products according to need with wet wipes containing water or alcohol. When thorough cleaning is required, use
machine laundry at 60°C.
• Do not spin wash.
• Do not bleach.
• Do not heat dry.
• Do not iron.
8.3 Pump Storage
If the syringe pump is to be stored for an extended period it should be cleaned and the battery removed. Store in a clean, dry
atmosphere at room temperature and, if available, use the original packaging or a suitable alternative, for protection.
8.4 Disposal/Decommissioning
When the time comes to dispose of the pump, accessories or packaging do so in the best way to minimise any negative impact on
the environment. You may be able to use special recycling or disposal schemes. To find out about these contact your technical service
department or local waste disposal service. Existing national or local regulations concerning waste disposal must take precedence over
the above advice.
Used syringe extension sets should be considered bio-hazardous and treated (handled, disposed or processed) as potentially posing
significant risks of infection transmission to humans or harming the environment. Please follow any applicable national and institutional
guidelines for bio-hazardous materials treatment.
DFU999-103EN Rev. 04
55/60
Page 56

T34™ Syringe Pump (3
rd
Edition)
DFU999-103EN Rev. 04
56/60
Page 57

T34™ Syringe Pump (3
rd
Edition)
DFU999-103EN Rev. 04
57/60
Page 58

T34™ Syringe Pump (3
rd
Edition)
DFU999-103EN Rev. 04
58/60
Page 59

T34™ Syringe Pump (3
rd
Edition)
DFU999-103EN Rev. 04
59/60
Page 60

T34™ Syringe Pump (3
rd
Edition)
DFU999-103EN Rev. 04
60/60
 Loading...
Loading...