CHATTANOOGA Intelect Legend User manual

Intelect® Legend Ultrasound
USER MANUAL
ISO 13485 Certified

Table of Contents
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
Welcome to the Intelect® Legend Ultrasound . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
Features of the Intelect Legend Ultrasound . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
Foreword . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
Liability Disclaimer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2
Precautionary Instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2
Principles of Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
Initial Setup Instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
System Components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
Optional Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
Operator Interface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
Operating Controls . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4
Indications/Contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5 Indications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 Contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5 Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5 Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5 Potential for Burns . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6 To Prevent Overheating of Sound Head . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6 Preventing Adverse Effects . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6 Handle Ultrasound Applicator(s) with Care . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7
US - Ultrasound . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8 Introduction to Ultrasound Therapy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8 Intelect Legend Ultrasound Clinical Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8 General Setup Steps . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8 Power-Up Preset Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9 Quick Start . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9 Detailed Setup Steps . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9 Modifying Treatment Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10 Changing Power-Up Preset Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10 Head Warm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10 Basic Guidelines for Ultrasound Utilization . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10
Appendix . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11
Intelect Legend Ultrasound Two Year Limited Warranty . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11
System Utilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12
Maintenance Instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
System Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14
Description of Ultrasonic Field . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .16
Glossary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .19

Introduction
•Welcome to the Intelect® Legend Ultrasound
•Features
•Foreword
•Before Using the Intelect Legend Ultrasound
•Precautionary Instructions
Welcome to the Intelect Legend Ultrasound
The Intelect Legend US, designed and manufactured by Chattanooga Group offers a new dimension in ultrasound therapy made possible by advanced software design and digital signal
processing. The result is a unit with extraordinary versatility based on simplicity of operation.
The Intelect Legend US allows you to select a frequency of 1 or 3.3 MHz without changing sound heads. Sound heads are available in 2 cm2 , 5 cm2 and 10 cm2 and include the patent pending Electronic Signature™ feature. Duty cycle may be set at 10%, 20%, 50% or Continuous.
Features of the Intelect Legend Ultrasound
• Electronic Signature™
Automatically calibrate the system to any size Intelect Legend sound head.
• Ergonomic applicators
A new ergonomic design that offers a 20 degree contour in the
applicator hand grip. This ergonomic extra will help deliver uniform ultrasound with greater clinician comfort.
• Head Warming
A feature traditionally available in more expensive brands of ultrasound. This will help curb the anxiety of patients during the first moments of treatment.
• Easy as One-Two-Go
In just two steps you are ready to start treatment, just set “Intensity” and press “Start.”
• Tactile Touch Control
Digital electronics and new user interface design give you simple tactile touch control of all system parameters.
• Programmable Start-Up Presets
All power-up presets can be individually customized to meet the clinician’s needs.
Foreword
This manual has been written for the owners and operators of the Intelect Legend Ultrasound. It contains general instructions for operation, precautionary instructions and maintenance recommendations. In order to obtain maximum life and efficiency from your Intelect Legend Ultrasound and to assist in the proper operation of the unit, read and understand this manual thoroughly and become familiar with the controls on the panel as well as the various accessories that come with the unit before operation of the unit.
The specifications put forth in this manual were in effect at the time of publication. However, owing to Chattanooga Group’s policy of continuous improvement, changes to these specifications may be made at any time without obligation on the part of Chattanooga Group.
1
Liability Disclaimer
Before administering any treatment to a patient you should become acquainted with the operating procedures, as well as the indications, contraindications, warnings and precautions. Consult other resources for additional information regarding the application of therapeutic ultrasound.
Precautionary Instructions
1. CAUTION: Read, understand and practice the precautionary and operating instructions. Know the limitations and hazards associated with using any ultrasound device. Observe the precautionary and operational decals placed on the unit.
2. CAUTION: DO NOT operate the Intelect® Legend Ultrasound when connected to any unit other than Chattanooga Group, Inc. devices. DO NOT operate the unit in an environment of shortwave diathermy use.
3. WARNING: Federal law restricts this device to sale by, or on the order of, a physician or licensed practitioner. This device should be used only under the continued supervision of a physician or licensed practitioner.
4. CAUTION: The Ultrasound generator should be routinely checked before each use to determine that all controls function normally; especially that the intensity control does properly adjust the intensity of the ultrasonic power output in a stable manner. Also, determine that the treatment time control does actually terminate ultrasonic power output when the timer reaches zero.
5. CAUTION: Use of controls, adjustments or performance of procedures other than those specified herein may result in hazardous exposure to ultrasonic energy.
6. CAUTION: DO NOT use sharp objects such as a pencil point or ballpoint pen to operate the buttons on the control panel as damage may result.
7. WARNING: Explosion hazard if used in the presence of flammable anesthetics.
The warning symbol for this hazard is prominently displayed on the cabinet.
8. WARNING: For continued protection against fire hazard, replace fuses only with ones of the same type and rating.
9. WARNING: Make certain that the unit is electrically grounded by connecting only to a grounded electrical service receptacle conforming to the applicable national and local electrical codes.
10.WARNING: This device should be kept out of the reach of children.
11.WARNING: This device should be used only under the continued supervision of a
licensed practioner.
12. CAUTION: Meets IEC/EN 60601-1-2 Electromagnetic Compatibility/Interference safety standard. (Care must be taken when operating this equipment around other equipment. Potential electromagnetic or other interference could occur to this or to the other equipment. Try
minimize this interference by not using other equipment in conjunction with it.)
13.WARNING: Type B Equipment
14.CAUTION: This unit should be operated, transported and stored in temperatures between
 - 40° C, with relative humidity ranging from 30% - 60%.
- 40° C, with relative humidity ranging from 30% - 60%.
15.ATTENTION: Consult accompanying documents.
16.DANGER: Patients with an implanted neurostimulation device must not be treated with or be in
close proximity to any shortwave diathermy, microwave diathermy, therapeutic ultrasound diathermy or laser diathermy anywhere on their body. Energy from diathermy (shortwave, microwave, ultrasound and laser) can be transferred through the implanted neurostimulation system, can cause tissue damage and can result in severe injury or death. Injury, damage or death can occur during diathermy therapy even if the implanted neurostimulation system is turned "off."
2

Principles of Operation
•Initial Setup Instructions
•System Components
-Standard and Optional Accessories
•Operator Interface
-Operating Controls
Initial Setup Instructions
Remove the Intelect® Legend US unit and any additional items ordered from the carton and inspect for damage that may have occurred during shipment. Check the voltage rating on the serial decal located on the bottom of the unit. Plug the system power supply in to a 120 Volt to 220/240 Volt AC outlet, as required. DO NOT attempt to use Direct Current (DC). DO NOT attempt to use the unit if it is not properly grounded. DO NOT place unit in a location where the power cord could be tripped over or pulled out during treatment. Follow the procedures listed in the precautionary instructions located later in this section.
System Components
The following accessories are included (standard) with your Intelect Legend Combo.
Item |
Part# |
Description |
|
|
|
1 |
78047 |
Applicator, Ultrasound 5 cm2 |
|
|
|
2 |
4248 |
Conductor™ Gel |
|
|
|
3 |
78201 |
Operator’s Manual |
|
|
|
Optional Accessories
The following is a list of optional accessories available for the Intelect Legend Combo.
Item |
Part# |
Description |
178046 Applicator, Ultrasound 10 cm2
278048 Applicator, Ultrasound 2 cm2
Operator Interface
The operator interface consists of an illustrated control panel with light emitting diodes (LED) and a liquid crystal display (LCD). The operator is able to view parameter options on the LED and LCD readouts and make selections by touching the designated area of the control panel. The displays will provide continual information during the treatments concerning amplitude and elapsed time. Ultrasound and stimulation intensities are adjusted with control panel buttons adjacent to the corresponding LED display. The stimulation / ultrasound output can be stopped by pressing the “PAUSE” or “STOP” buttons located at the bottom of the control panel.
3
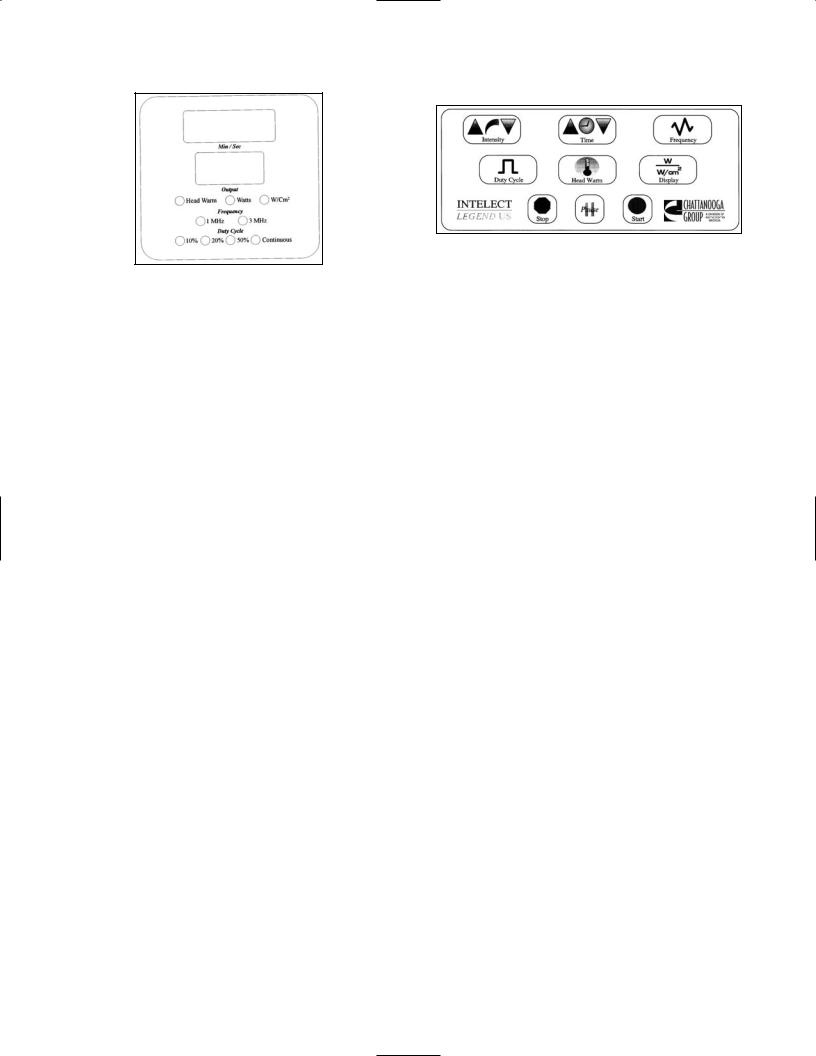
LED Display |
Illustrated Control Panel |
®
Operating Controls
1.LED Screen Display - LED Parameter Display Field.
•Displays Treatment Time, Output Intensity, Frequency, Duty Cycle, Display and Head Warm.
2.Illustrated Control Panel
•Membrane switches control LED screen display and treatment parameters.
3.Intensity (Power)
•Select this prompt to set or modify output power intensity.
4.Time
•Select this prompt to set or modify treatment time in minutes.
5.Frequency
•Select this prompt to change to a frequency of 1 MHz or 3.3 MHz.
6.Duty Cycle
•Select this prompt to change to a duty cycle of 10%, 20%, 50% or Continuous.
7.Head Warm
•Select this button to warm the head of the ultrasound applicator prior to treatment.
Note: Turning Head Warm on will be accompanied by a fan turning on and the lighting of the Head Warm LED on the LED display. The fan and the LED will turn off when Head Warm is turned off. A low level of output will be displayed in the output LED display when Head Warm is active.
8.Display
•Select this button to change output display from W/cm2 (Intensity) to Watts (Power).
9.Stop
•Select this prompt to stop a treatment session.
10.Pause
•Select this prompt to pause a treatment session.
11.Start
•Select this prompt to begin a treatment session.
4

Indications/Contraindications
Indications
Ultrasound for use in applying deep heat can be used for treatment of selected medical conditions such as the relief of pain, muscle spasms and joint contractures. Those conditions may be associated with adhesive capsulitis, bursitis with slight calcification, myositis and soft tissue injuries. The Intelect® Legend Ultrasound can provide therapeutic deep heating between 40° and 45° C in all of its operating modes, while using any of the applicators available for this device.
Contraindications
Ultrasound should not be used over:
•An area of the body where a malignancy is known to be present.
•The eyes
•The reproductive organs
•An acute infection or sepsis
•A pregnant uterus
•A deep vein thrombosis
•An arterial disease
•An anesthetized area or condition that causes impairment of sensation, such as chemotherapy.
•The epiphyses of skeletally immature children.
•The thoracic area if the patient is using a cardiac pacemaker.
•A healing fracture
•Ischemic tissues in individuals with vascular disease where the blood supply would be unable to follow the increase, in metabolic demand and tissue necrosis might result.
•Patients with an implanted neurostimulation device must not be treated with or be in close proximity to any shortwave diathermy, microwave diathermy, therapeutic ultrasound diathermy or laser diathermy anywhere on their body. Energy from diathermy (shortwave, microwave, ultrasound and laser) can be transferred through the implanted neurostimulation system, can cause tissue damage and can result in severe injury or death. Injury, damage or death can occur during diathermy therapy even if the implanted neurostimulation system is turned "off."
Precautions
Precautions should be taken when used:
•For acute conditions of bursitis and tendonitis that can be exacerbated by the use of ultrasound.
•Over an area of the spinal cord following a laminectomy (i.e., when major covering tissues have been removed).
•On patients with a tendency toward hemorrhaging.
Warnings
•Always keep the applicator sound head in constant motion.
•Always keep the sound head in full contact with the patient’s skin or submerged under water when setting intensity.
•Use ample conductive gel to ensure good coupling throughout the treatment. If needed, apply when setting intensity.
•Be sure to read all instructions for operation before treating a patient.
•DO NOT drop the sound head on hard surfaces. DO NOT cool the sound head with ice water or ice packs. DO NOT allow the sound head to overheat repeatedly. All of these conditions are
likely to damage the sound head crystal.
•CAUTION: Use of controls or adjustments or performance of procedures other than those specified herein may result in hazardous exposure to ultrasonic energy.
5
 Loading...
Loading...