Cardioline ECG100L, ECG200L User Manual

ECG100L - ECG200L
User manual
 Rev.04 – 13.09.2018 1936
Rev.04 – 13.09.2018 1936

ECG100L - ECG200L
All rights reserved © Cardioline SpA.
CARDIOLINE® is a registered trademark of Cardioline SpA.
This publication may not be reproduced, in whole or in part, in any form or manner, without prior written authorisation by:
Cardioline Spa
Via Linz, 151
38121 Trento
Italy

ECG100L - ECG200L
Table of Contents |
|
|
1. |
GENERAL INFORMATION ......................................................................................................................... |
1 |
|
1.1. Other important information........................................................................................................... |
1 |
2. |
SAFETY INFORMATION............................................................................................................................. |
2 |
3. |
ELECTROMAGNETIC COMPATIBILITY (EMC)............................................................................................. |
7 |
|
3.1. Guidance and Manufacturer's declaration - Electromagnetic emissions......................................... |
8 |
|
3.2. Guidance and Manufacturer's declaration - Electromagnetic immunity ......................................... |
8 |
|
3.3. Guidance and Manufacturer's declaration - Electromagnetic immunity ......................................... |
9 |
3.4.Recommended separation distances between portable and mobile RF communications
|
equipment and the ECG100L/ECG200L........................................................................................................ |
10 |
||
4. |
SYMBOLS AND LABEL ............................................................................................................................... |
12 |
||
|
4.1. |
Explanation of the symbols.............................................................................................................. |
12 |
|
|
4.2. |
Device label...................................................................................................................................... |
13 |
|
5. |
INTRODUCTION........................................................................................................................................ |
14 |
||
|
5.1. |
Purpose of the manual..................................................................................................................... |
14 |
|
|
5.2. |
Recipients......................................................................................................................................... |
14 |
|
|
5.3. |
Intended use .................................................................................................................................... |
14 |
|
|
5.4. |
Description of the device ................................................................................................................. |
15 |
|
|
5.4.1. |
General overview ..................................................................................................................... |
16 |
|
|
5.4.2. |
Keyboard.................................................................................................................................. |
19 |
|
|
5.4.3. |
Display...................................................................................................................................... |
20 |
|
|
5.4.4. |
Data entry ................................................................................................................................ |
20 |
|
6. |
PREPARATION FOR USE............................................................................................................................ |
21 |
||
|
6.1. |
Initial startup.................................................................................................................................... |
21 |
|
|
6.2. |
Patient cable connection ................................................................................................................. |
21 |
|
|
6.3. |
Loading the paper............................................................................................................................ |
22 |
|
|
6.4. |
Switching the device ON/OFF .......................................................................................................... |
23 |
|
|
6.5. |
Power connection............................................................................................................................ |
23 |
|
|
6.6. |
Battery operation............................................................................................................................. |
24 |
|
7. EXECUTION OF AN EXAM......................................................................................................................... |
26 |
|||
|
7.1. |
General procedure........................................................................................................................... |
26 |
|
|
7.2. |
Before acquisition ............................................................................................................................ |
26 |
|
|
7.2.1. |
Preparing the patient............................................................................................................... |
26 |
|

ECG100L - ECG200L
|
7.2.2. |
Connecting the patient ............................................................................................................ |
27 |
|
7.3. |
Viewing the ECG............................................................................................................................... |
29 |
||
|
7.3.1. |
Disconnection of the leads....................................................................................................... |
30 |
|
7.4. |
Acquiring an ECG.............................................................................................................................. |
30 |
||
|
7.4.1. |
Automatic acquisition of an ECG (AUTO) ................................................................................. |
30 |
|
|
7.4.2. |
Manual acquisition of an ECG (MANUAL) ................................................................................ |
32 |
|
|
7.4.3. |
Manual acquisition of an ECG with Rhythm Printing (MANUAL).............................................. |
33 |
|
|
7.4.4. |
Acquisition of an urgent ECG ................................................................................................... |
33 |
|
7.5. |
Printing an ECG ................................................................................................................................ |
33 |
||
|
7.5.1. |
Automatic Printing formats...................................................................................................... |
34 |
|
7.6. |
Storing an ECG ................................................................................................................................. |
34 |
||
7.7. |
Exporting an ECG ............................................................................................................................. |
34 |
||
|
7.7.1. |
Exporting to a USB flash drive .................................................................................................. |
35 |
|
|
7.7.2. |
Transferring ECG to the PC ...................................................................................................... |
35 |
|
8. |
DEVICE SETTINGS ..................................................................................................................................... |
36 |
||
8.1. |
Settings ............................................................................................................................................ |
36 |
||
8.2. |
Setting date and time....................................................................................................................... |
36 |
||
8.3. |
System settings ................................................................................................................................ |
37 |
||
8.4. |
Rhythm Leads setting....................................................................................................................... |
38 |
||
8.5. |
Service settings ................................................................................................................................ |
38 |
||
|
8.5.1. |
Memory wipe........................................................................................................................... |
38 |
|
|
8.5.2. |
Display calibration.................................................................................................................... |
38 |
|
8.6. |
Memory management ..................................................................................................................... |
38 |
||
9. |
UPGRADING YOUR DEVICE OPTIONS ....................................................................................................... |
40 |
||
10. |
MAINTENANCE AND TROUBLESHOOTING ............................................................................................... |
41 |
||
10.1. |
Precautions ...................................................................................................................................... |
41 |
||
10.2. |
Switching off the device................................................................................................................... |
41 |
||
10.3. |
Regular maintenance ....................................................................................................................... |
41 |
||
|
10.3.1. |
Functional check ...................................................................................................................... |
41 |
|
|
10.3.2. |
Patient cable cleaning .............................................................................................................. |
42 |
|
|
10.3.3. |
Device cleaning ........................................................................................................................ |
42 |
|
|
10.3.4. |
Operation check....................................................................................................................... |
43 |
|
10.4. |
Recommendations ........................................................................................................................... |
43 |
||
10.5. |
Battery maintenance ....................................................................................................................... |
43 |
||
10.6. |
Cleaning the thermal printer............................................................................................................ |
45 |
||

ECG100L - ECG200L
|
10.6.1. Cleaning the printer ................................................................................................................. |
45 |
|
|
10.6.2. Cleaning the thermal head of the printer ................................................................................ |
45 |
|
10.7. |
Troubleshooting table...................................................................................................................... |
45 |
|
10.8. |
Error notifications ............................................................................................................................ |
46 |
|
11. |
TECHNICAL SPECIFICATIONS .................................................................................................................... |
48 |
|
11.1. |
Harmonised standards applied ........................................................................................................ |
49 |
|
11.2. |
Accessories....................................................................................................................................... |
50 |
|
12. |
WARRANTY .............................................................................................................................................. |
51 |
|
13. |
DISPOSAL ................................................................................................................................................. |
52 |
|

ECG100L - ECG200L

ECG100L - ECG200L
1. GENERAL INFORMATION
1.GENERAL INFORMATION
This manual is an integral part of the device and should always be available as support material to the clinical practitioner or the operator. Strict compliance with the information contained in this manual is an essential prerequisite for a proper and reliable use of the device.
Have the operator read the manual thoroughly as the information related to the different chapters is only described once.
1.1.Other important information
This manual was written with the utmost care. Should you find any details which do not correspond to those contained in this manual, please inform Cardioline SpA who will correct such inconsistencies as soon as possible.
The information contained in this manual is subject to change without notice.
All changes will be in compliance with the regulations governing the manufacturing of medical equipment.
All trademarks mentioned in this document are property of their respective owners. Their protection is guaranteed.
No part of this manual may be reprinted, translated or reproduced without the manufacturer's written authorisation.
The code relating to this manual is listed below.
Language |
Code |
|
|
ENGLISH |
36510212_ENG |
|
|
1

ECG100L - ECG200L
2. SAFETY INFORMATION
2.SAFETY INFORMATION
Cardioline SpA will be held responsible for the safety, reliability and functionality of the devices only if:
1.the assembly operations, modifications or repairs are carried out by Cardioline SpA or by its Authorised Service Centre;
2.The device is used in compliance with the instructions provided in the use manual.
Always contact Cardioline SpA should you wish to connect any devices not mentioned in this manual.
 Warnings
Warnings
This manual provides important information on proper use and safety of the device. Failure to comply with the described operating procedure, improper use of the device, ignoring the specifications and recommendations supplied, may cause severe physical injuries to the operators, patients and bystanders, or may damage the device.
No modification of this equipment is allowed.
The device captures and presents the data that reflects the physiological condition of the patient; this information can be examined by specialist medical staff and will be useful in providing an accurate diagnosis. In any event, the data cannot be used as the only means to make an accurate diagnosis of the patient.
The operators for whom this device is intended must have the required competence regarding medical procedures and the treatment of patients. They must also be sufficiently trained in using the device. Have the operator carefully read and understand the contents of the operator manual and the other annexed documents before using the device for clinical applications. Inadequate knowledge or training could be at a greater risk for the physical safety of operators, patients and bystanders, or could damage the device. In case the operators are not trained in using the device, we recommend to contact Cardioline or its Authorised Distributors to plan adequate training courses.
The device ECG100L and its power supply are classified as ME Equipment, since the power supply is considered an integral part of the device.
The ECG200L device is classified as Electromedical equipment.
To avoid the risk of electric shock, this equipment must only be connected to a supply main with protective earth.
If in doubt regarding the integrity of the external earth conductor, use the device with its inner battery.
The positioning of the device must be such as to don't make difficult the operation of disconnection from main supply when an external power supply is used. The plug of the main supply is the main
2

ECG100L - ECG200L
2. SAFETY INFORMATION
switch used to disconnect the device from the main supply. Please, be sure to keep it near the device.
All input and output signal connectors (I/O) are intended to be used only for connection to appropriate devices which comply with IEC 60601-1 standards or further IEC standards (e.g. IEC 60950). Connecting additional equipment to the device could increase leakage current to the chassis and/or patient. To avoid endangering the safety of the operator and patient, keep in mind the requirements of IEC 60601-1:2005+A1 clause 16 and measure the leakage current to confirm that there is no risk of electric shock.
When performing ECG acquisition, ensure the USB port is completely covered by the plastic lid.
For the correct operation of the device and for the safety of the operators, patients and bystanders, the device and the accessories must be exclusively connected as outlined in this manual.
To maintain immunity from potential interference of electromagnetic signals, a system must be used with shielded cables when connecting the device to the mains.
To guarantee the safety of the operator and of the patient, the equipment connected to the same line as the device must comply with IEC 60950 or IEC 60601-1 standards.
The power cable shielding (when present) must be connected to an earthing system appropriate for the area where the device is used. This will avoid electric shocks caused by different earth potentials which could exist between the various points of an electricity distribution system, or else by failures of the external equipment connected to the mains.
The safety of the patient and the operator is guaranteed if the peripheral units and the accessories that can come into direct contact with the patient comply with the UL 60601-1, IEC 60601-1 and IEC 60601-2-25 standards. Only use spare parts and accessories supplied with the device and available from Cardioline SpA. Refer to section 11.2 for the list approved accessories.
The patient cables to be used with the device are defibrillation-proof. Check the patient cables for ruptures or cracks before use.
Conductive parts of the patient cable, electrodes and associated connections of type CF applied parts, including the neutral conductor of the patient cable and electrode, should not come into contact with other conductive parts, including earth ground.
Defibrillation protection of the ECG relies on the use of the provided ECG cable and the use of any other ECG cable may impair the safe use of the equipment leading to electric shock for the patient and operator. Refer to section 11.2 for the list approved accessories.
To prevent death or any serious personal injuries during defibrillation, avoid contact with the device or patient cables. It is furthermore necessary to properly position the defibrillation pads with respect to the electrodes in order to minimize patient skin burns.
This device is designed to be used only with the electrodes specified in this manual. Strictly follow the correct clinical procedures to prepare the skin before the application of the electrodes and monitor the patient in order to avoid any irritation, inflammation or other skin reactions. The electrodes are designed for short-term applications and must be promptly removed once the examination is complete. Refer to section 11.2 for the list approved accessories.
The ECG electrodes may cause skin irritation; check the skin for any irritations or inflammations.
3

ECG100L - ECG200L
2. SAFETY INFORMATION
To prevent any infections, use the disposable components (e.g. the electrodes) only once. To ensure safety and use efficiency, do not use electrodes after their expiration date.
The quality of the signal produced by the electrocardiograph may be adversely affected by the use of other medical equipment such as defibrillators and ultrasound machines.
The device is intended for external use and is not intended for direct cardiac application.
There is a risk of explosion. Do not use the device in the presence of flammable anaesthetics.
There is no safety hazard if other equipment, such as pacemakers or other stimulators, is used simultaneously with the device; however, disturbance to the signal may occur.
The device is not designed for use with high-frequency (HF) surgical equipment, and does not provide any protective means against hazards to the patient.
The operation may be adversely affected by the presence of strong magnetic fields such as those produced by electro surgery equipment.
The use of the device is not recommended in the presence of medical diagnostic imaging equipment such as the Magnetic Resonance Imaging (MRI) or Computerised Axial Tomography (CAT) in the same environment.
Only use the recommended batteries. Using other types of batteries may cause danger of fire or explosion.
The internal rechargeable battery is hermetically sealed NiMH and requires no maintenance. Should the battery be faulty, contact Cardioline technical assistance service.
The low battery warning is designed for the recommended batteries only. Using other types of batteries may lead to a lack of indication resulting in device failure. If the battery is low, connect the device to the electrical mains.
The device is not intended as a general purpose storage device, thus no files should be stored except from those automatically created by the device itself. Using the electrocardiograph as a general purpose storage device may results in unwanted radio frequency emissions.
Do not clean the device or the patient cables by submersing them in liquid, autoclaving, or steam cleaning. This may cause serious damage to equipment or reduce its lifespan. Using non-specific detergents/disinfectants, failure to comply with the recommended procedures or contact with nonspecific materials may cause additional risks to operators, patients or bystanders or may damage the device. Do not sterilise the device or the patient cable with ethylene oxide gas (EO). Refer to Section 10 for instructions on proper cleaning and disinfection.
Do not leave the patient cable unattended in the presence of children as they could be accidentally strangled.
Do not leave the electrodes unattended in the presence of children as they could cause suffocation if accidentally swallowed.
4

ECG100L - ECG200L
2. SAFETY INFORMATION
 Attention
Attention
To prevent any damage to the keyboard do not use sharp or heavy objects to press the keys, only use your fingertips.
The device and the patient cable should be cleaned before use. Check the connections for any damage or excessive wear before each use. Replace the patient cable should it present any damage or be excessively worn.
Do not pull or stretch patient cables as this could result in mechanical and/or electrical failures. Patient cables should be stored after forming them into a loose loop.
There are no user-serviceable parts inside the device. The device can only be dismantled by qualified service personnel. Any malfunctioning or defective device must be excluded from use and be checked/repaired by qualified service personnel before being reused.
The device does not require any calibration or special instrumentation for correct use and maintenance.
When it is necessary to dispose of the device, its components and accessories (e.g.: batteries, cables, electrodes) and/or packaging material, comply with local standards for waste disposal.
Notes
The movements of the patient may generate excessive noise and affect the quality of the ECG tracing or the correct analysis of the device.
An appropriate preparation of the patient is important in order to guarantee a proper application of the ECG electrodes and the correct operation of the device.
The incorrect positioning of the electrodes for the detection of the algorithm depends on the normal physiology and on the order of the ECG leads and tries to identify the most likely exchange. However, it is recommended to check the positioning of the electrodes of the same group (limbs or chest).
If the electrodes are not properly connected to the patient, or one or more patient leads are damaged, the display will show a message “Lead fail”. When the ECG is printed the device will add the indication of inoperable device on the printout.
As defined by the IEC 60601-1 and IEC 60601-2-25 safety standards, the device is classified as follows:
ECG100L: Internal Power equipment - class I on external AC/DC power supply.
ECG200L: Internal Power equipment - class I.
Defibrillation-proof Type CF applied parts.
Ordinary equipment.
Not suitable for use in the presence of flammable anaesthetics.
Continuous operation
5

ECG100L - ECG200L
2. SAFETY INFORMATION
NOTE: From a safety view point, the power supply is declared “Class I” based on IEC 60601-1 standard. A three-pole plug is used to guarantee earthing together with the power lines. The earth terminal of the power cable is the only point where the unit is earthed. Exposed metal parts which are accessible during standard operation have a double insulation from the power lines. The internal earth connections are a functional earthing.
Accuracy of measurements taken with the device is compliant with IEC 60601-2-25.
ECG100L has the following power supply features:
Model: AFM60US18
Manufacturer: XP Power Limited
Rated Input: 100-240 VAC, 50-60 Hz, 1.5-0.9 A
Rated Output: 60 W, 18 V, 3.34 A
Protection Class: I
Degree of Protection: IP20
The device is a Class IIa in compliance with Directive 93/42/EEC.
The device is a prescription device according to FDA regulation
In order to prevent damage to the device during transportation and storage (when still in its original packaging), comply with the following environmental conditions:
Ambient temperature ..................... |
+5°C to +40°C |
Relative humidity ............................ |
20% to 90% |
Atmospheric pressure ..................... |
700 hPa to 1060 hPa |
The device is intended for use in hospitals or doctor's offices and should comply with the following environmental requirements:
Ambient temperature ..................... |
+10°C to +40°C |
Relative humidity ............................ |
50% to 90% |
Atmospheric pressure ..................... |
700 hPa to 1060 hPa |
After using the device on battery power, always reconnect the power cable. This will guarantee that the batteries are recharged automatically the next time the device is used.
6

ECG100L - ECG200L
3. ELECTROMAGNETIC COMPABILITY (EMC)
3.ELECTROMAGNETIC COMPATIBILITY (EMC)
This device requires particular precautions regarding Electromagnetic Compatibility. It must therefore be installed and commissioned in compliance with the information on Electromagnetic Compatibility contained in this manual.
Portable and mobile radio communication equipment can affect operation of the device.
Using accessories, transducers or cables different than those specified in par. 11.2 can increase the emissions or decrease the immunity of the appliance.
 Warnings
Warnings
This device is only intended to be used by professional healthcare personnel. This device could generate radio interference or disturb operation of the equipment in the vicinity. Therefore it could be necessary to take measures to mitigate these effects, such as re-directing or repositioning the device or shielding the room.
The use of accessories and cables other than those recommended by Cardioline may cause an increase in emissions or a lowering in the protection of the system.
The device must not be used near or superimposed to other equipment. If necessary, check that the device works according to its standard operation.
Use of this equipment adjacent to or stacked with other equipment should be avoided because it could result in improper operation. If such use is necessary, this equipment and the other equipment should be observed to verify that they are operating normally.
Use of accessories, transducers and cables other than those specified or provided by the manufacturer of this equipment could result in increased electromagnetic emissions or decreased electromagnetic immunity of this equipment and result in improper operation.
Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 inches) to any part of the device, including cables specified by the manufacturer. Otherwise, degradation of the performance of this equipment could result.
Notes
The EMISSIONS characteristics of this equipment make it suitable for use in industrial areas and hospitals (CISPR 11 class A). If it is used in a residential environment (for which CISPR 11 class B is normally required) this equipment might not offer adequate protection to radio-frequency communication services. The user might need to take mitigation measures, such as relocating or re-orienting the equipment.
Electromagnetic compatibility during the use of the device is required with the surrounding devices.
An electronic device can generate or receive electromagnetic interference. The electromagnetic compatibility test (EMC) has been carried out on the electrocardiograph in compliance with the international EMC directive for medical equipment (IEC 60601-1-2). This IEC standard has been adopted as a European standard (EN 60601-1-2).
7

ECG100L - ECG200L
3. ELECTROMAGNETIC COMPABILITY (EMC)
Fixed, portable and mobile equipment for RF communication may affect the protection of the medical equipment. See par. 3.4 for the recommended separation distance between the radio equipment and the device.
The purpose of the device is the acquisition of ECG signals and the presentation of ECG reports for diagnostic purposes, as defined in IEC 60601-2-25.
Electromagnetic disturbances can cause disturb or degradation of the acquired ECG signal, resulting in misdiagnosis or delayed treatment.
3.1.Guidance and Manufacturer's declaration - Electromagnetic emissions
The ECG100L/ECG200L is intended to operate in the electromagnetic environment specified below. The customer or the user of the ECG100L/ECG200L must guarantee that it is used in this environment.
Emission test |
|
Compliance |
|
Electromagnetic environment – guidance |
|
|
|
|
|
Radio frequency (RF) emissions |
|
|
|
The ECG100L/ECG200L uses RF energy only for its internal function. |
|
Group 1 |
|
Therefore, its RF emissions are very low and are not likely to cause any |
|
CISPR 11 |
|
|
||
|
|
|
interference in nearby electronic equipment. |
|
|
|
|
|
|
Radio frequency (RF) emissions |
|
Class B |
|
|
CISPR 11 |
|
|
|
|
|
|
|
The device is suitable for use in all establishments other than domestic |
|
Harmonics emissions |
|
|
|
|
|
Class B |
|
environments and those directly connected to the public low-voltage |
|
IEC 61000-3-2 |
|
|
||
|
|
|
power supply network that supplies buildings used for domestic |
|
Voltage fluctuations/ |
|
|
|
|
|
|
|
purposes. |
|
flicker emissions |
|
Complies |
|
|
|
|
|
||
IEC 61000-3-3 |
|
|
|
|
3.2.Guidance and Manufacturer's declaration - Electromagnetic immunity
The ECG100L/ECG200L is intended for use in the electromagnetic environment specified below. The customer or the user of the ECG100L/ECG200L assure that it is used in such an environment.
Immunity test |
|
Conformity |
|
Compliance level |
|
Electromagnetic Environmental Information |
|
|
|
|
|
|
|
Electrostatic discharge |
|
+/- 6 kV contact |
|
+/- 6 kV contact |
|
Floors should be wood, concrete or ceramic tile. If |
(ESD) |
|
|
|
floors are covered with synthetic material, the |
||
|
+/- 8 kV air |
|
+/- 8 kV air |
|
||
IEC 61000-4-2 |
|
|
|
relative humidity should be at least 30 %. |
||
|
|
|
|
|
||
Electrical Fast Transient/ |
|
+/- 2 kV for power |
|
+/- 2 kV for power |
|
|
|
supply lines |
|
supply lines |
|
Mains power quality should be that of a typical |
|
Burst |
|
|
|
|||
|
+/- 1 kV for |
|
+/- 1 kV for |
|
commercial or hospital environment. |
|
IEC 61000-4-4 |
|
|
|
|||
|
input/output lines |
|
input/output lines |
|
|
|
|
|
|
|
|
||
|
|
+/- 1 kV line(s) to |
|
+/- 1 kV line(s) to |
|
|
Surge |
|
line(s) |
|
line(s) |
|
Mains power quality should be that of a typical |
IEC 61000-4-5 |
|
+/- 2 kV line(s) to |
|
+/- 2 kV line(s) to |
|
commercial or hospital environment. |
|
|
earth |
|
earth |
|
|
8

|
|
|
ECG100L - ECG200L |
|
|
|
|
3. ELECTROMAGNETIC COMPABILITY (EMC) |
|
|
|
|
|
|
|
|
|
|
|
|
<5% UT |
<5% UT |
|
|
|
(>95% dip in UT) |
(>95% dip in UT) |
|
|
|
for 0.5 cycle |
for 0.5 cycle |
|
|
Voltage dips, |
40% UT |
40% UT |
Mains power quality should be that of a typical |
|
short interruption and |
||||
(60% dip in UT) |
(60% dip in UT) |
commercial or hospital environment. If the user of |
||
voltage variations |
||||
for 5 cycles |
for 5 cycles |
the ECG100L/ECG200L requires continued operation |
||
on power supply |
||||
|
|
|||
70% UT |
70% UT |
during power mains interruptions, it is recommended |
||
input |
||||
that ECG100L/ECG200L be powered from an |
||||
lines |
(60% dip in UT) |
(60% dip in UT) |
||
uninterruptible power supply or a battery. |
||||
IEC 61000-4-11 |
for 25 cycles |
for 25 cycles |
||
|
||||
|
|
|
||
|
<5% UT |
<5% UT |
|
|
|
(>95% dip in UT) |
(>95% dip in UT) |
|
|
|
for 5 s |
for 5 s |
|
|
Power frequency and |
|
|
Power frequency magnetic fields should be at levels |
|
magnetic field |
3 A/m |
3 A/m |
characteristic of a typical location in a typical |
|
(50/60 Hz) |
|
|
commercial or hospital environment. |
NOTE: UT is the AC mains voltage prior to the application of the test level.
3.3.Guidance and Manufacturer's declaration - Electromagnetic immunity
The ECG100L/ECG200L is intended for use in the electromagnetic environment specified below. The customer or the user of the ECG100L/ECG200L should assure that it is used in such an environment.
Emission test |
|
IEC 60601 test level |
|
Compliance level |
|
Electromagnetic Environmental Information |
|
|
|
|
|
|
|
Conducted RF |
|
3 V rms |
|
|
|
Portable and mobile RF communications equipment |
|
From 150 kHz to 80 |
|
3 V |
|
should be used no closer to any part of the |
|
IEC 61000-4-6 |
|
|
|
|||
|
MHz |
|
|
|
ECG100L/ECG200L, including cables, than the |
|
|
|
|
|
|
||
|
|
|
|
|
|
recommended separation distance calculated from |
|
|
|
|
|
|
the equation applicable to the frequency of the |
|
|
|
|
|
|
transmitter. |
|
|
|
|
|
|
Recommended separation distance: |
Radiated RF |
|
3 V/m |
|
|
|
|
|
From 80 MHz to 2.5 |
|
3 V/m |
|
|
|
IEC 61000-4-3 |
|
|
|
|
||
|
GHz |
|
|
|
From 80 MHz to 800 MHz |
|
|
|
|
|
|
||
|
|
|
|
|
|
From 800 MHz to 2.5 GHz |
|
|
|
|
|
|
where P is the maximum output power rating of the |
|
|
|
|
|
|
transmitter in watts (W) according to the transmitter |
|
|
|
|
|
|
manufacturer and d is the recommended separation |
9

ECG100L - ECG200L
3. ELECTROMAGNETIC COMPABILITY (EMC)
distance in metres (m).
Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey (a), should be less than the compliance level in each frequency range (b).
Interference may occur in the vicinity of equipment marked with the following symbol:
NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
a)Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the ECG100L/ECG200L is used exceeds the applicable RF compliance level above, the ECG100L/ECG200L should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the ECG100L/ECG200L.
b)Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
3.4.Recommended separation distances between portable and mobile RF communications equipment and the ECG100L/ECG200L
The ECG100L/ECG200L is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the ECG100L/ECG200L can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the ECG100L/ECG200L as recommended below, according to the maximum output power of the communications equipment.
|
Maximum rated power output of the |
|
|
Separation distance according to frequency of transmitter (m) |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
transmitter (W) |
|
|
150 KHz to 800 MHz |
|
|
800 MHz to 2.5 GHz |
|
|
800 MHz to 2.5 GHz |
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
0.01 |
|
0.12 |
|
0.12 |
|
0.23 |
|
||||
|
|
|
|
|
|
|
|
||||
0.1 |
|
0.37 |
|
0.37 |
|
0.74 |
|
||||
|
|
|
|
|
|
|
|
||||
1 |
|
1.17 |
|
1.17 |
|
2.33 |
|
||||
|
|
|
|
|
|
|
|
||||
10 |
|
3.69 |
|
3.69 |
|
7.38 |
|
||||
|
|
|
|
|
|
|
|
||||
100 |
|
11.67 |
|
11.67 |
|
23.33 |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
10

ECG100L - ECG200L
3. ELECTROMAGNETIC COMPABILITY (EMC)
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in metres (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
11
ECG100L - ECG200L
4. SYMBOLS AND LABEL
4.SYMBOLS AND LABEL
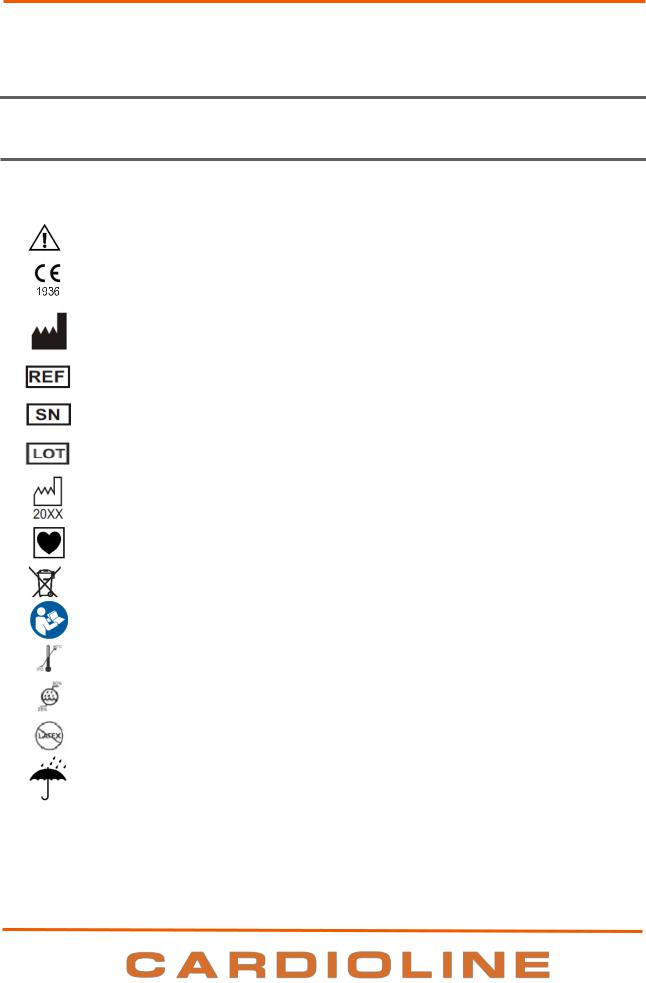
4.1.Explanation of the symbols
Symbol |
Description |
|
|
|
Comply with the instructions in the use manual |
|
|
|
CE marking – compliance with the European Union directives |
|
|
|
Manufacturer |
|
|
|
Reference number (product code) |
|
|
|
Serial Number |
|
|
|
Lot number |
|
|
|
Year of manufacture |
|
|
|
Type CF equipment |
|
|
|
Separate collection of electrical waste and electronic equipment |
|
|
|
Consult instructions for use – placed beside the input connector for the power supply |
|
|
|
Temperature variation |
|
|
|
Humidity variation |
|
|
|
No latex |
|
|
|
Keep dry |
|
|
12
 Loading...
Loading...