Page 1

Biomek is a trademark of Beckman Coulter, Inc. HyperD is a trademark of Pall
Life Science
Group
06-0679 1206 Sig 1106
10008245 US/EG Rev D
Bio-Rad
Laboratories, Inc.
Web site www.bio-rad.com USA 800 4BIORAD
Australia 61 02 9914 2800 Austria 01 877 89 01
Belgium 09 385 55 11 Brazil 55 21 3237 9400
Canada 905 712 2771 China 86 21 6426 0808
Czech Republic 420 241 430 532 Denmark 44 52 10 00
Finland 09 804 22 00 France 01 47 95 69 65
Germany 089 318 84 0 Greece 30 210 777 4396
Hong Kong 852 2789 3300 Hungary 36 1 455 8800
India 91 124 4029300 Israel 03 963 6050 Italy 39 02 216091
Japan 03 5811 6270 Korea 82 2 3473 4460
Mexico 52 555 488 7670 The Netherlands 0318 540666
New Zealand 0508 805 500 Norway 23 38 41 30
Poland 48 22 331 99 99 Portugal 351 21 472 7700
Russia 7 495 721 14 04 Singapore 65 6415 3188
South Africa 27 861 246 723 Spain 34 91 590 5200
Sweden 08 555 12700 Switzerland 061 717 95 55
Taiwan 886 2 2578 7189 United Kingdom 020 8328 2000
Corporation. MicroMix is a trademark of Diagnostic Products Corporation.
he SELDI process is covered by US patents 5,719,060, 6,225,047, 6,579,719, and
T
,818,411 and other issued patents and pending applications in the US and other
6
jurisdictions.
ProteinChip®Serum
Fractionation Kit
Instruction Manual
Catalog #K10-00007
For technical support,
call your local Bio-Rad office, or
in the US, call 1-800-4BIORAD
(1-800-424-6723).
Page 2

Introduction
The ProteinChip serum fractionation kit is designed to facilitate the
analysis of crude serum samples by fractionating proteins based on
their biophysical properties.
The kit allows high-throughput fractionation by using anion exchange
media in a 96-well microplate format. The anion exchange support is
supplied in a 96-well ProteinChip Q filtration plate and requires
rehydration before use. The samples are added to the plate and then
eluted in a stepwise manner by altering the pH of the wash buffer
until six fractions are collected. The fractions can then be analyzed
on a ProteinChip array using a profiling protocol. Each of the six
fractions is collected twice, and the two collections are pooled. This
helps to ensure that the pH changes appropriately, and also results in
greater reproducibility in the fractionation.
The fractions can be analyzed in your particular profiling application.
If you are using multiple array types or conditions, we recommend
that the same fraction is profiled at one time to avoid multiple
freeze-thaw cycles.
Materials
Materials Included
n
ProteinChip U9 buffer (9 M urea, 2% CHAPS, 50 mM Tris-HCl,
pH 9), 20 ml
n
Rehydration buffer (50 mM Tris-HCl, pH 9), 250 ml
n
Wash buffer 1 (50 mM Tris-HCl, 0.1% OGP, pH 9), 20 ml
n
Wash buffer 2 (50 mM HEPES, 0.1% OGP, pH 7), 30 ml
n
Wash buffer 3 (100 mM Na acetate, 0.1% OGP, pH 5), 30 ml
n
Wash buffer 4 (100 mM Na acetate, 0.1% OGP, pH 4), 30 ml
n
Wash buffer 5 (50 mM Na citrate, 0.1% OGP, pH 3), 30 ml
n
Wash buffer 6 (33.3% isopropanol, 16.7% acetonitrile,
0.1% trifluoroacetic acid), 30 ml
n
ProteinChip Q filtration plate, filled with dehydrated anion
exchange Q ceramic HyperD F sorbent, 1
© 2006 Bio-Rad Laboratories, Inc.© 2006 Bio-Rad Laboratories, Inc.
Page 3
n
Microplate sealing strips, 10
n
Instruction manual
Note: The volume of supplied reagents allow for processing of the 96-well filtration
plate in a maximum of two runs (2 x 48 samples). ProteinChip serum fractionation kit
eplacement buffers (catalog #K10-00008) are available to order.
r
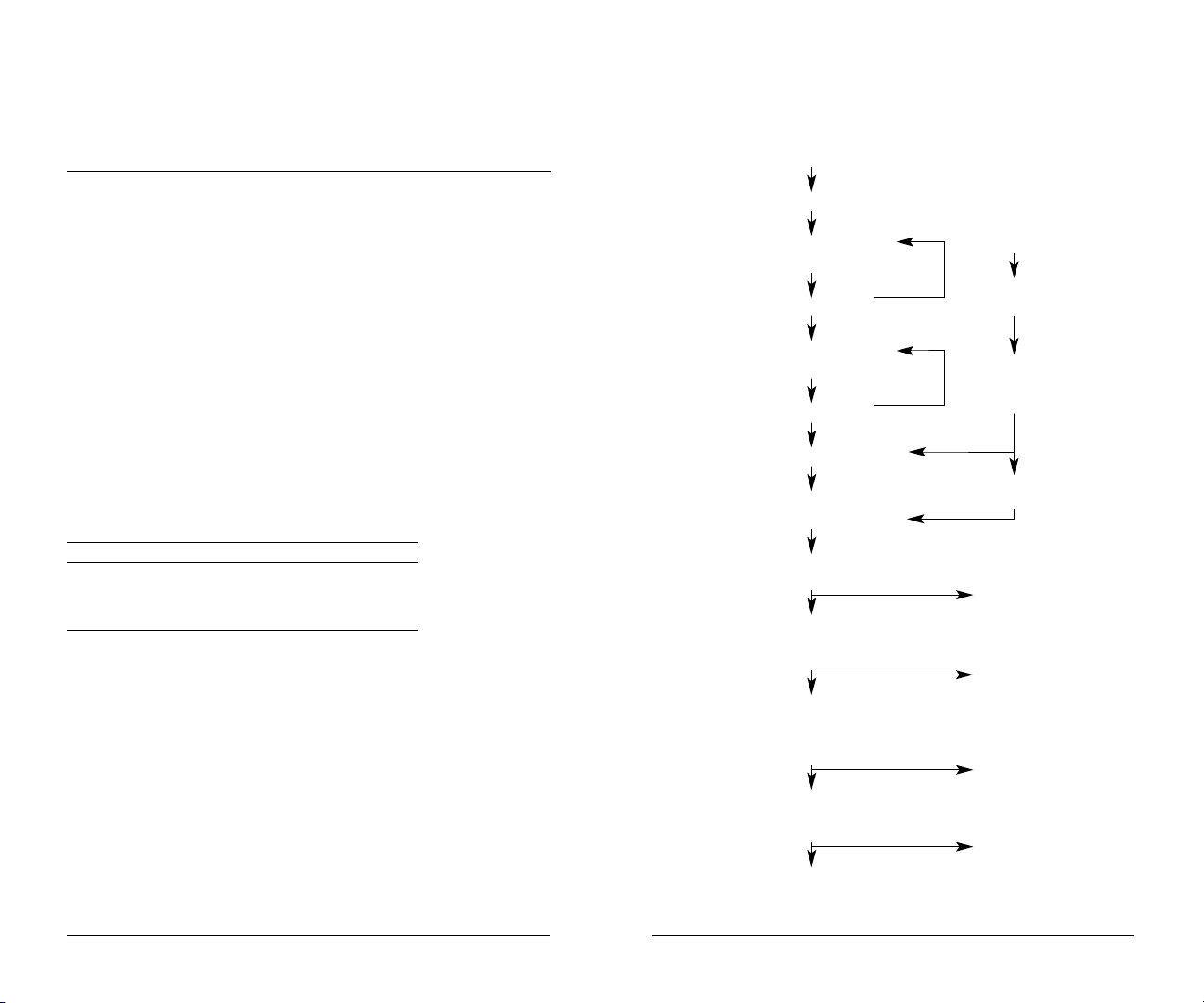
Protocol Flow Chart
ehydrationAdd 200 µl rehydration
R
buffer to each well of filtration
plate; mix 60 min at room
temperature (RT)
Materials Needed but Not Included
n
V-bottom 96-well microplate labeled samples
n
V-bottom 96-well microplates labeled F1–F6
n
96-well microplate for collection of waste
n
Adhesive sealing film for microplates
n
12-column partitioned buffer reservoir
n
Pipet tips
n
MicroMix 5 plate and tube shaker (Diagnostic Products Corp.)
n
Biomek 3000 workstation integration package
(catalog #Z33-00030)
n
Vacuum manifold (for manual use)
Storage
Table 1. Storage conditions for kit components.
Item Storage
ProteinChip U9 buffer –20 to –50°C
Rehydration and wash buffers 2–8°C
ProteinChip Q filtration plate 2–8°C
Remove buffer
Add 200 µl rehydration
buffer to each well
Remove buffer
Equilibration Add 200 µl U1 buffer
to each well
Remove buffer
Sample Add 50 µl sample to each well
binding
Add 50 µl sample and rinse to
each well; mix 30 min at 4°C
Fractionation Apply vacuum
and collect eluent
Add 100 µl wash buffer 1 to each well;
mix 10 min at room temperature; apply
vacuum and collect eluent
Add 100 µl wash buffer 2 to each well;
mix 10 min at room temperature;
apply vacuum and collect eluent;
repeat and pool eluents
Repeat double washes with
wash buffers 3–6, pooling
eluents for each wash buffer
Washes
Washes
Bring sample
to RT
Spin
Add 20 µl sample
to each well
Sample
Preparation
Add 30 µl
ProteinChip U9
buffer to each well
Mix 20 min
at 4°C
Add 50 µl U1 buffer
to each well; mix
Flow-through fraction
Fraction 1 (pH 9)
Fraction 2 (pH 8)
Fraction 3–6
Analyze fractions on
ProteinChip arrays
© 2006 Bio-Rad Laboratories, Inc.© 2006 Bio-Rad Laboratories, Inc.
Page 4

Detailed Use Protocol
The following protocol can be used with the Biomek 2000 or 3000
laboratory automation workstation or can be performed manually
using a vacuum manifold. We recommend a vacuum setting of
15 in Hg.
Notes:
1. When using the filtration plate in the MicroMix 5, make sure that the plate is securely
held in the manifold and that the bottom of the plate is not touching the surface of
the mixer.
2. The settings recommended for the MicroMix 5 may vary from mixer to mixer. If you
experience problems with mixing, you may need to adjust the settings. See
Appendix B for instructions.
3. When removing the foil seal from the ProteinChip Q filtration plate, you may notice
some of the sorbent adhered to the foil seal. The amount should be relatively
uniform across all wells. This is normal and has not been found to adversely affect
the performance of the kit.
Step 1: Q Ceramic HyperD F Sorbent Rehydration
The filtration plate should be used directly after rehydration.
1. Tap the filtration plate on the workbench several times to make
sure that all of the dry Q ceramic HyperD F sorbent is settled to
the bottom of the plate.
2. Take the filtration plate out of the pouch and carefully remove the
top seal on the filtration plate.
Note: If using only part of the filtration plate, with a sharp blade remove only enough of
the foil seal to reveal the columns needed. Alternatively, remove the whole seal, and
reseal the unneeded wells with the microplate strips provided. While processing
samples, only the wells in use should be uncovered. After using part of the plate,
reseal the used wells with microplate strips and mark those columns as used.
3. With an 8-channel pipet, add 200 µl of rehydration buffer to
each well.
4. Carefully seal the plates with microplate sealing strips and
shake by hand for a few minutes (some liquid may come
through the membrane) until the sorbent appears to be
resuspended in solution.
5. Mix the filtration plate on the MicroMix 5 (form 48, amp 7) for
60 minutes at RT. Carefully remove the sealing strips. Place
plate on vacuum collar and then place the vacuum collar and
plate on vacuum manifold.
Note: It is extremely important to mix the sorbent and rehydration buffer well to avoid
plugging wells during fractionation. Check visually that mixing is adequate. If necessary,
adjust the MicroMix form and amp settings. Mixing should be vigorous enough to
ensure that all sorbent is in contact with buffer without the mixture reaching the top of
the well. If mixing is still not adequate after changing the MicroMix settings, it may be
necessary to adjust settings in the MicroMix 5 software (see Appendix B).
Step 2: Sample Preparation
1. Bring serum samples to ambient temperature. Spin at
20,000 g for 10 minutes at 2–8°C.
2. Aliquot 20 µl of serum sample to each well of a standard
V-bottom 96-well microplate.
3. Add 30 µl of ProteinChip U9 buffer to each well.
4. Cover microplate with adhesive sealing film and mix on the
MicroMix 5 (set at 20, 5, 20) for 20 minutes at 2–8°C.
Step 3: Preparation of U1 Buffer
1. Add 10 ml of ProteinChip U9 buffer solution to 80 ml of
rehydration buffer (50 mM Tris-HCl) to produce U1 buffer
(1 M urea, 0.2% CHAPS, 50 mM Tris-HCl, pH9). The volume is
enough for 1 complete plate. If you are using part of the plate,
adjust volume accordingly.
© 2006 Bio-Rad Laboratories, Inc.
© 2006 Bio-Rad Laboratories, Inc.
Page 5

Step 4: Equilibration of Q Ceramic HyperD F Sorbent With U1 Buffer
1. After step 1.4, place the waste collection plate underneath the
filtration plate and apply a vacuum to remove the buffer from the
filtration plate.
2. Add 200 µl of rehydration buffer to each well.
3. Apply vacuum to remove the buffer in the filtration plate.
4. Repeat steps 4.2 and 4.3 three times with rehydration buffer and
an additional three times with U1 buffer. Empty the waste plate
under the filtration plate as necessary between washes.
5. The sorbent in the filtration plate is now ready to bind sample.
Step 5: Binding Sample With Sorbent
1. Pipet 50 µl of sample from each well of the sample microplate to
the corresponding well in the 96-well filtration plate.
2. Add 50 µl of U1 buffer to each well of the sample microplate.
3. Mix 5 times.
4. Pipet 50 µl from each well of the sample microplate to the
corresponding well in the 96-well filtration plate.
Note: This step is included because there is a dead volume when pipetting with the
robot. When the robot pipets to collect the sample initially, it does not collect all of the
material. The addition of 50 µl of U1 buffer and mixing allows the residual material to
be collected and added to the first 50 µl.
Step 6: Fraction Collection
Note: To avoid cross-contamination between wells, apply adhesive sealing film on the
microplate during the mixing step. Remove the film before applying a vacuum, and
replace with a new piece for each mixing.
Fraction 1
1. Place the 96-well microplate labeled F1 underneath the
filtration plate.
2. Apply the vacuum and collect the flowthrough into the F1 plate.
3. Add 100 µl of wash buffer 1 to each well of the filtration plate.
4. Mix for 10 minutes on the MicroMix 5 (set at 20, 7, 10) at RT.
5. Apply the vacuum and collect the eluent into the F1 plate.
Note: Fraction 1 contains the flowthrough and the pH 9 eluent.
Fraction 2
1. Add 100 µl of wash buffer 2 to each well of the filtration plate.
2. Mix for 10 minutes on the MicroMix 5 (set at 20, 7, 10) at RT.
3. Place the 96-well microplate labeled F2 underneath the
filtration plate.
4. Apply the vacuum and collect the eluent into the F2 plate.
5. Repeat procedures 1–4.
Note: Fraction 2 contains the pH 7 eluent.
5. Cover the filtration plate with adhesive sealing film and mix on
the MicroMix 5 (set at 20, 7, 30) for 30 minutes at 2–8°C.
© 2006 Bio-Rad Laboratories, Inc.
Fraction 3
1. Add 100 µl of wash buffer 3 to each well of the filtration plate.
2. Mix for 10 minutes on the MicroMix 5 (set at 20, 7, 10) at RT.
3. Place the 96-well microplate labeled F3 underneath the
filtration plate.
4. Apply the vacuum and collect the eluent into the F3 plate.
5. Repeat procedures 1–4.
Note: Fraction 3 contains the pH 5 eluent.
© 2006 Bio-Rad Laboratories, Inc.
Page 6

Fraction 4
1. Add 100 µl of wash buffer 4 to each well of the filtration plate.
2. Mix for 10 minutes on the MicroMix 5 (set at 20, 7, 10) at RT.
3. Place the 96-well microplate labeled F4 underneath the
filtration plate.
4. Apply the vacuum and collect the eluent into the F4 plate.
5. Repeat procedures 1–4.
Note: Fraction 4 contains the pH 4 eluent.
Fraction 5
1. Add 100 µl of wash buffer 5 to each well of the filtration plate.
2. Mix for 10 minutes on the MicroMix 5 (set at 20, 7, 10) at RT.
3. Place the 96-well microplate labeled F5 underneath the
filtration plate.
4. Apply the vacuum and collect the eluent into the F5 plate.
5 Repeat procedures 1–4.
Note: Fraction 5 contains the pH 3 eluent.
Fraction 6
1. Aliquot wash buffer 6 into the buffer tray.
2. Mix 100 µl of wash buffer 6 to each well of the filtration plate.
3. Vortex for 10 minutes on the MicroMix 5 (set at 20, 7, 10) at RT.
4. Place the 96-well microplate labeled F6 underneath the
filtration plate.
5. Apply the vacuum and collect the eluent into the F6 plate.
6. Repeat procedures 2–5.
Note: Fraction 6 contains the organic solvent eluent.
Step 7
Seal the six collection microplates and store until proceeding with
the ProteinChip array binding protocol. If the samples are to be
analyzed within 24 hours, store at 4°; longer term storage should
be at –20°C.
Step 8
Dispose of the sample plate as biohazard waste if human serum
sample is used.
Step 9
If you only used part of the filtration plate, put the filtration plate
back in the pouch; seal and store at 4°C.
Ordering Information
Catalog # Description
K10-00007
K10-00010
K10-00008
C54-00018
C57-30075
Z33-00030
ProteinChip Serum Fractionation Kit, includes 1 x 96-well
ProteinChip Q filtration plate packed with Q ceramic HyperD F sorbent,
buffers, sealing strips, instructions
ProteinChip U9 Buffer, for serum fractionation kit, 20 ml
ProteinChip Serum Fractionation Kit Replacement Buffers,
includes wash buffers 1–6
ProteinChip Q Filtration Plate, 1 x 96-well
ProteinChip CM10 Arrays, A-H format, 12
Biomek 3000 Workstation Integration Package, includes Biomek
3000 workstation Windows XP operating system and 17" flat-panel
monitor, integrated custom mixer/shaker, Biomek software with
CFR 21 Part 11 compliance capability, wash station, 8-channel pipet,
wash tools, vacuum system
© 2006 Bio-Rad Laboratories, Inc. © 2006 Bio-Rad Laboratories, Inc.
Page 7

50,000 100,000 150,000 200,000
0
1.0
0
0
.5
1
.0
0
1.0
0
1.0
0
0
.2
0.4
0.6
0
1.0
2.0
3.0
Fraction 6
Fraction 5
F
raction 3
Fraction 2
F
raction 1
Fraction 4
1.5
0.5
0.5
0.5
1.5
4.0
5,000 10,000 15,000 20,000
0
10
20
30
4
0
0
5
.0
1
0
0
1.0
2
.0
3
.0
0
2.5
5.0
7.5
0
1
0
20
30
0
25
50
75
Fraction 6
F
raction 5
Fraction 3
F
raction 2
Fraction 1
Fraction 4
7.5
2
.5
Appendix A: Performance Specification
When the ProteinChip serum fractionation kit is used according to
this protocol, each sample processed will produce six fractions
(see Figure 1).
40
30
20
10
Fraction 1
0
10
7.5
5.0
2.5
3.0
0
Fraction 2
2.0
1.0
Fraction 3
0
7.5
Intensity
5.0
2.5
Fraction 4
0
30
10
0
75
50
25
0
5,000
10,000 15,000 20,000
m/z
Fig. 1. Example fractionation of human serum sample. Profiled on
ProteinChip CM10 arrays (2–20 kD).
© 2006 Bio-Rad Laboratories, Inc. © 2006 Bio-Rad Laboratories, Inc.
20
Fraction 5
Fraction 6
1.0
0.5
0
1.5
1.0
0.5
0
1.0
0.5
0
1.5
Intensity
1.0
0.5
0
0.6
0.4
0.2
0
4.0
3.0
2.0
1.0
0
5,000
10,000 15,000 20,000
m/z
Fig. 2. Example fractionation of human serum sample. Profiled on
ProteinChip CM10 arrays (20–200 kD).
Fraction 1
Fraction 2
Fraction 3
Fraction 4
Fraction 5
Fraction 6
Page 8

Appendix B: Adjusting MicroMix 5 Settings
In some instances when rehydration of the sorbent in the plate has
been problematic, the settings of the MicroMix shaker can be
adjusted to improve the vigorousness of the shaking. Adjustments
are made to the Gain Factor in the advanced hardware settings of
the MicroMix 5 software. Generally, the manufacturer of the shaker
recommends that advanced hardware settings not be changed.
However, some advanced users may find changing these settings
useful during troubleshooting.
CAUTION: Changing the Gain Factor and/or Damping Factor affects the calibration of
the shaker, and recalibration is necessary.
To change the Advanced Hardware settings:
1. Choose
Fig. 3. Advanced Hardware settings warning dialog box.
Settings>Advanced Hardware. Warning appears.
2. Choose Ye s. The Advanced Hardware dialog box appears.
3. Enter the Gain Factor.
The Gain Factor is the scaling of the amplitude of shake. It is
adjusted by changing the multiplication factor used by the
firmware for amplitude control. The nominal value of the Gain
Factor is factory set and should normally be 14. The Gain Factor
should not go below 10 or above 18.
Fig. 4. Advanced Hardware dialog box.
© 2006 Bio-Rad Laboratories, Inc.© 2006 Bio-Rad Laboratories, Inc.
 Loading...
Loading...