Belimed WD-290 User manual

Instruction Manual
WD290
Article number: 10268-0068
Version: 001-03/08

Inhaltsverzeichnis
1 |
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4 |
1.1 |
Before you read on . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4 |
1.2 |
Target group . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4 |
1.3 |
Amendments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4 |
1.4 |
Symbols and references used . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4 |
2 |
For your safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5 |
2.1 |
Intended use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5 |
2.2 |
Duty of care in handling the device . . . . . . . . . . . . . . . . . . . . . . . . . . |
5 |
2.3 |
Non-intended use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5 |
2.4 |
Instruction of personnel. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5 |
2.5 |
Fields of application for the device . . . . . . . . . . . . . . . . . . . . . . . . . . |
6 |
2.6 |
Process validation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
6 |
3 |
Device description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
7 |
3.1 |
Device unclean side (US) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
7 |
3.2 |
Device clean side (CS) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8 |
3.3 |
Controller unclean side (US) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
9 |
3.4 |
Controller clean side (CS) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
9 |
4 |
Pre-treating medical devices . . . . . . . . . . . . . . . |
. . . . . . . . . . . . . 10 |
4.1 |
Responsibility for pre-treatment . . . . . . . . . . . . . . . |
. . . . . . . . . . . . 10 |
4.1.1 |
SOPs (Standard Operating Procedures) . . . . . . . . |
. . . . . . . . . . . . 10 |
4.2 |
Preparing medical devices . . . . . . . . . . . . . . . . . . . |
. . . . . . . . . . . . 10 |
4.3 |
Pre-cleaning pre-treatment . . . . . . . . . . . . . . . . . . . |
. . . . . . . . . . . . 11 |
4.3.1 |
Avoiding subsequent cleaning . . . . . . . . . . . . . . . . |
. . . . . . . . . . . . 11 |
5 |
Preparing the device. . . . . . . . . . . . . . . . . . . . . . . |
. . . . . . . . . . . . 12 |
6 |
Self-disinfection . . . . . . . . . . . . . . . . . . . . . . . . . . |
. . . . . . . . . . . . 13 |
6.1 |
Why self-disinfection? . . . . . . . . . . . . . . . . . . . . . . . |
. . . . . . . . . . . . 13 |
6.2 |
Starting self-disinfection . . . . . . . . . . . . . . . . . . . . . |
. . . . . . . . . . . . 13 |
7 |
User identification. . . . . . . . . . . . . . . . . . . . . . . . . |
. . . . . . . . . . . . 15 |
7.1 |
Identification via operating panel . . . . . . . . . . . . . . |
. . . . . . . . . . . . 15 |
7.2 |
Identification via barcode reader . . . . . . . . . . . . . . . |
. . . . . . . . . . . . 16 |
8 |
Loading and identifying racks . . . . . . . . . . . . . . . |
. . . . . . . . . . . . 17 |
8.1 |
Loading racks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. . . . . . . . . . . . 17 |
8.2 |
Rack identification . . . . . . . . . . . . . . . . . . . . . . . . . . |
. . . . . . . . . . . . 17 |
8.2.1 |
Rack identification via the operating panel . . . . . . . |
. . . . . . . . . . . . 17 |
8.2.2 |
Rack identification via barcode . . . . . . . . . . . . . . . . |
. . . . . . . . . . . . 17 |
9 |
Identification of batch content. . . . . . . . . . . . . . . |
. . . . . . . . . . . . 18 |
9.1 |
Batch content identification via operating panel . . . |
. . . . . . . . . . . . 18 |
9.2 |
Batch content identification via barcode . . . . . . . . . |
. . . . . . . . . . . . 18 |
10 |
Loading from the unclean side . . . . . . . . . . . . . . |
. . . . . . . . . . . . 19 |
10.1 |
Manual loading . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
. . . . . . . . . . . . 19 |
10.2 |
Automatic rack station (optional). . . . . . . . . . . . . . . |
. . . . . . . . . . . . 21 |
11 |
Washing and disinfection . . . . . . . . . . . . . . . . . . |
. . . . . . . . . . . . 22 |
|
|
|
2/39 |
001 |
Instruction Manual WD290 |
|
10268 |
© Belimed |

11.1 General information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22 11.2 Washing, disinfecting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
12 Unloading from the clean side. . . . . . . . . . . . . . . . . . . . . . . . . . . 23 13 Switching off the device . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24 14 Daily servicing and cleaning work . . . . . . . . . . . . . . . . . . . . . . . 25
14.1 Servicing in general . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25 14.2 Servicing the bottom washing arm . . . . . . . . . . . . . . . . . . . . . . . . . 25 14.3 Servicing the top washing arm . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26 14.4 Cleaning the surface sieve and coarse sieve . . . . . . . . . . . . . . . . . 27
15 |
Device fails to clean properly . . . . . . . . . . . . . . . . . . . . . . . . . . . |
28 |
15.1 Checking the device . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28 15.2 Checking dosing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28 15.3 Checking rack loading. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
16 Faults . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
16.1 Fault text without process interruption . . . . . . . . . . . . . . . . . . . . . . 29 16.2 Fault text with process interruption . . . . . . . . . . . . . . . . . . . . . . . . . 30 16.3 Device does not run . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
17 |
Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
31 |
17.1 Modem connection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31 17.1.1 Activating the connection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31 17.2 Independent measurement data acquisition IPD . . . . . . . . . . . . . . 31 17.3 Tank heating manually switchable . . . . . . . . . . . . . . . . . . . . . . . . . 32 17.3.1 Function . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32 17.3.2 Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32 17.4 Built-in printer CS / US . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33 17.4.1 Function . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33 17.4.2 Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34 17.4.3 Changing paper rolls . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34 17.5 Emergency stop CS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34 17.5.1 Function . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34 17.5.2 Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
18 Conformity and certifications . . . . . . . . . . . . . . . . . . . . . . . . . . . 35 19 Glossary. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36 20 Organisation Belimed AG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
20.1 Manufacturer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
20.2 Subsidiaries, Customer Service . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
21 |
Notes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
39 |
Instruction Manual WD290 |
001 |
3/39 |
© Belimed |
10268 |
|

Introduction
1Introduction
1.1 Before you read on
Your product meets high standards and is easy to operate. Nevertheless, please take time to read these instructions carefully. You will become familiarised with your product and be able to use it to its best.
1.2Target group
These instructions are a component of the product and are intended for the owner, users, operators, as well as servicing personnel. They must be accessible for this group of persons.
1.3Amendments
The text, graphics and data correspond to the technical status of the product at the time of going to print. Amendments in the sense of technical development remain reserved.
1.4Symbols and references used
The following symbols and references to occupational safety used throughout the documentation are important to avoid harm to health and life.
DANGER!
There is an imminent risk to the life and health of persons.
WARNING!
There may be a risk to the life and health of persons.
CAUTION!
A situation for which there is a warning of damage to property and equipment.
NOTE
User tips and useful information on the best possible use of the equipment.
4/39 |
001 |
Instruction Manual WD290 |
|
10268 |
© Belimed |

For your safety
2For your safety
With the EC Declaration of Conformity and the CE mark, we affiirm that this product complies with the basic health and safety requirements in accordance with Directive 93/42/EEC Annex II (see Chap. 18 "Conformity and certifications")
Hazards may still arise from the product if it is used incorrectly by inadequately trained personnel or not as intended.
2.1Intended use
This product is exclusively approved for the uses stated in the instructions. Namely for central sterilisation, substerilisation in surgery, in hospitals, clinical laboratories and in industry.
2.2Duty of care in handling the device
•Only use original racks, spare parts and accessories
•Load the racks as intended (see Chap. 8 "Loading and identifying racks")
•Daily servicing work on the device must be carried out regularly and in accordance with regulations (see Chap. 14 "Daily servicing and cleaning work")
•Validation of the programme parameters must be performed regularly (see Chap. 2.6 "Process validation")
•Installation, deinstallation, servicing or modification must only be carried out by persons authorised by Belimed
•In the case ofincorrectly installed, operated or maintained devices all warranty claims are invalidated
2.3Non-intended use
All other applications are considered as non-intended use.
Damages caused by operator error, non-intended use, failure to observe the instructions, operation by untrained personnel, unauthorised modifications and conversions without the written consent of the manufacturer, are not permitted and preclude the manufacturer's liability for the ensuing damage to property and personal injury.
2.4Instruction of personnel
This product must only be used, maintained and repaired by authorised, trained and briefed personnel. This assumes that these instructions are read and understood.
Responsibilities and competencies in operation, servicing and maintenance must be clearly defined and observed.
Instruction Manual WD290 |
001 |
5/39 |
© Belimed |
10268 |
|

For your safety
2.5Fields of application for the device
Cleaning and conditioning of:
•Surgical instruments
•Minimal-invasive instruments
•Instruments for anaesthesia and intensive care
•Baby bottles and teats
•Containers
•OP shoes
•Laboratory instruments from research and production
•Rigid endoscopes
•Eye instruments
•Neurosurgery
2.6Process validation
The aim of process validation is to achieve a high level of safety in the reconditioning of medical devices in order to afford the operators and patients the greatest possible protection.
Process validation consists of:
a)Type testing / factory testing
b)Process validation consisting of:
•IQ - Installation Qualification
•OQ - Operational Qualification
•PQ - Performance Qualification
c)Routine testing / Annual requalification
(refer to appendix EN ISO 15883-1 November 2001, Chap. 6, Pages 35-37)
NOTE
Further information on process validation may be obtained from Belimed
Customer Service.
CAUTION!
Validation must only be carried out by authorised persons!
Devices must only be operated with processes validated in accordance with regulations! Only use components (items to be washed, racks, programmes, chemicals) which have been validated together.
The safety of operators and patients may be compromised if the devices used are not validated in accordance with regulations.
6/39 |
001 |
Instruction Manual WD290 |
|
10268 |
© Belimed |
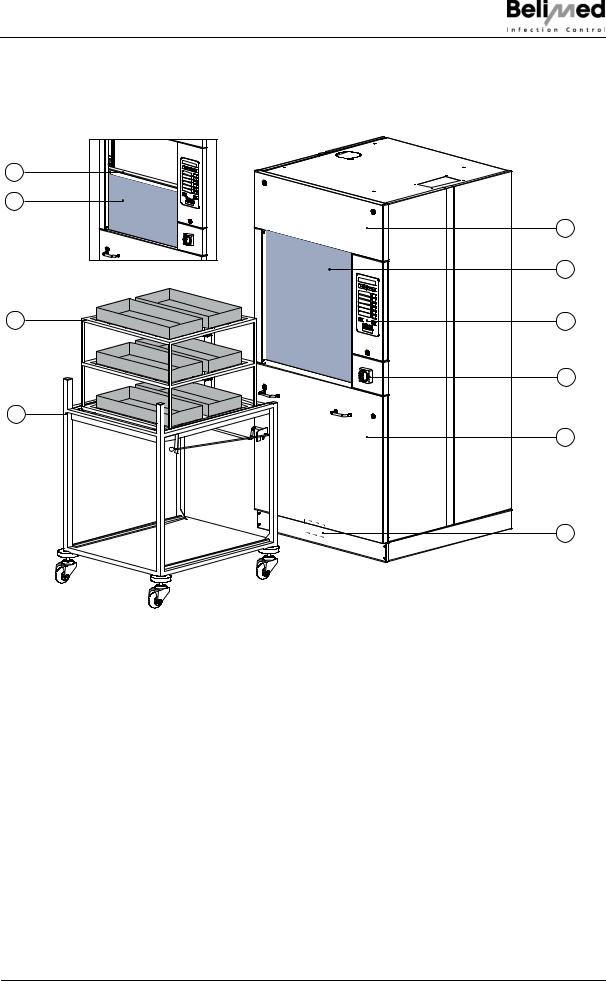
Device description
3Device description
3.1 Device unclean side (US)
10
9
8
7
1 |
2 |
3 |
4 |
5 |
6 |
Abb 201
1Door drive (behind the panel)
2 Wash compartment doors made of double-layer safety glass
3Operating panel
4 Main switch (with EMERGENCY OFF function)
5Service panel
6 Location type plate
7Transfer trolley
8Rack
9 Automatic wash compartment doors
10 Door safety switching strip
Instruction Manual WD290 |
001 |
7/39 |
© Belimed |
10268 |
|

Device description
3.2Device clean side (CS)
1 |
2 |
3 |
4 |
Abb 301
1Door drive (behind the panel)
2Operating panel with door button
3 Wash compartment doors made of double-layer safety glass
4Service panel
8/39 |
001 |
Instruction Manual WD290 |
|
10268 |
© Belimed |

Device description
3.3Controller unclean side (US)
|
|
|
|
|
1) |
Display |
|
|
|
|
|
|
|
– With screensaver "BELIMED INFEC- |
|
|
|
|
|
1 |
|
|
TION CONTROL"; this means that |
2 |
3 |
4 |
5 |
|
|
|
this display automatically appears af- |
|
|
|
|
|
|
|
ter approx. 1 h. Press any button, dis- |
|
|
|
|
|
|
|
play"Program ready" appears again |
Program 1 |
|
|
1 |
|
2) |
Cursor left |
|
|
|
|
|
||||
Program 2 |
|
|
2 |
|
|
– Print operating data such as program |
|
Program 3 |
|
|
3 |
|
|
|
formula and setup parameters |
|
|
|
|
– |
Self disinfection On/Off |
||
|
|
|
|
6 |
|
||
|
|
|
4 |
|
|
|
|
Program 4 |
|
|
|
|
– |
IPD verification On/Off |
|
Program 5 |
|
|
5 |
|
3) |
Cursor right |
|
Program 6 |
|
|
6 |
|
|
– |
Acoustic signal On/Off |
|
|
|
|
|
|
||
|
|
|
|
7 |
4) |
Cursor down |
|
|
|
|
|
|
– |
Printer On/Off |
|
|
|
|
|
8 |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
5) |
Cursor up |
|
|
|
|
|
|
|
– Shift button Programs P7 - P12 |
|
|
|
|
|
|
6) |
Program buttons |
|
|
|
|
|
|
|
– Selection of Programs P1 - P6, with |
|
|
|
|
|
Abb 179 |
|
|
Shift P7 - P12 |
|
|
|
|
|
7) |
Door button |
|
|
|
|
|
|
|
– |
Opens/close door |
|
|
|
|
|
8) |
On/Off button |
|
|
|
|
|
|
|
– Display batch number (press 4 sec- |
|
|
|
|
|
|
|
|
onds) |
3.4Controller clean side (CS)
|
3 |
|
1 |
RUN |
|
ERROR |
2 |
|
|
|
4 |
1)Display
–With screensaverBELIMED INFECTION CONTROL; this means that this display automatically appears after approx. 1 h. Press any button, display"Program ready" appears again
2)Door button
–Door opens/closes
3)RUN
LED lit = In progress
Abb 178
4)ERROR
LED lit = Fault
(See Chap. 16 "Faults")
Instruction Manual WD290 |
001 |
9/39 |
© Belimed |
10268 |
|

Pre-treating medical devices
4Pre-treating medical devices
4.1 Responsibility for pre-treatment
CAUTION!
Always observe the specifications from the medical device manufacturer!
The owner is responsible for pre-treatment of medical devices. The best possible washing results are only to be achieved with correct pre-treatment as intended.
Various treatments fix proteins and may contribute to preserving prion infectivity.
CAUTION!
The air bubbles in the foam prevent pressure building-up in the cleaning system and therefore the best possible contact between the cleaning agent and the items to be washed.
4.1.1SOPs (Standard Operating Procedures)
The contents of the following criteria and specifications must be regulated:
•Product responsibility
•Transport routes and duration of waste disposal (time for soiling to dry in)
•Type of soiling (blood, ointments, bone meal...)
•Material properties and compatibility of the items to be washed (risk groups acc. to RKI Ordinance)
•Consideration of all operating instructions and reconditioning regulations for medical devices
•Necessary knowledge of the medical devices to be reconditioned
•Maintenance plan and regular inspections
Belimed recommends producing work instructions which describe the procedure within a working process.
4.2Preparing medical devices
CAUTION!
Not all medical devices are suitable for mechanical reconditioning (see Chap. 2.5 "Fields of application for the device")
All inner and outer surfaces must be accessible for cleaning (open valves, taps, joint instruments...) Special caution is required for the lumen. Disassemble MIC or other complex instruments according to the manufacturer's specifications.
10/39 |
001 |
Instruction Manual WD290 |
|
10268 |
© Belimed |

Pre-treating medical devices
4.3Pre-cleaning pre-treatment
Remove coarse soiling immediately after use. Dried-on blood or tissue reduces the effectiveness of cleaning.
4.3.1Avoiding subsequent cleaning
Various treatments fix proteins and may contribute to preserving prion infectivity.
The following pre-treatment methods may cause impairments in subsequent washing:
•Pre-treatment with aldehydic disinfectants
•Pre-treatment with alcohol solutions
•Pouring antiseptic solutions on the items to be rinsed
•Aldehyde and alcohol vapours
•Heat pre-treatment
Small quantities of foam are permissible with manual cleaning in the immersion bath or ultrasonic cleaning.
Instruction Manual WD290 |
001 |
11/39 |
© Belimed |
10268 |
|

Preparing the device
5Preparing the device
WARNING!
Only operate the device if it is in a technically faultless condition! Damaged or defected components must be reported to the technical specialist.
After a prolonged period of disuse (approx. 1 week), the daily servicing work must be carried out to prepare the device (see Chap.14 "Daily servicing and cleaning work").
Check the quantity of detergents (see Chap. 16 "Faults")
Switch the device on at the main switch (Fig. 209)  0 I with the button. Four possible display texts may now appear
0 I with the button. Four possible display texts may now appear
Abb 209
Performing self-disinfection
(See Chap. 6 "Self-disinfection")
Display text:
Self-disinfection
Start
Program ready
Continue with program flow (see Chap. 7 "User identification")
Display text:
Program ready
Fault without process interruption
Rectify fault (see Chap. 16 "Faults")
Example display text:
Dosing device
Empty
Fault with process interruption
Report to the technical specialist
Example display text:
No pressure
Fault Code 110
12/39 |
001 |
Instruction Manual WD290 |
|
10268 |
© Belimed |
 Loading...
Loading...