Page 1

s
Alaris® Syringe Pump
Technical Service Manual
Page 2

Alaris® Syringe Pump
Contents
General Information .............................................................5
Introduction ...........................................................................................5
Product Familiarity
Purpose of this manual
Conventions Used in this Manual
General precautions
Front panel and main display
Configuration and Calibration ....................................................8
Access codes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8
Dedication options (301/302)
Data Set Activation (612)
Handsfree Bolus (175)
Power Lock (711)
Configuration options (251)
Teach Learn
Teach Learn Procedure (Software versions V1.4.13 and above)
Data Set Transfer
Data Set Upload and Download (401 and 499)
Download CQI Event Log (402)
Calibration procedures (243)
SYRINGE CLAMP calibration
PLUNGER POS (position) calibration ................................................................17
SYRINGE FORCE calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .18
LINE PRESSURE calibration – Alaris® CC Syringe Pump only ............................................
20
BATTERY calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .21
...........................................................................................14
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6
...........................................................................7
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9
...................................................................................9
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14
......................................................................................15
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15
....................................................................15
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .16
........................................................................16
Preventative Maintenance ..................................................... 22
Preventative Maintenance ............................................................................22
Visual Inspection
Recommended Cleaning
Updates
Battery Test and Replacement
Self-test Procedure (123)
Calibration Verification Mode (240)
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .24
Upgrading software
Pole Clamp Arm Update
Motor Plate Strain Beam Update
Transmission Buffer Pad Update
Linear (PL3) Update. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .28
Replace the Main Battery
Self-tests included in full test
Self-tests not included in full test
Comms Test (123). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .31
......................................................................................22
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .23
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .24
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .27
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .27
....................................................................28
.........................................................................29
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .29
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .30
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .30
...................................................................31
....................................................................32
1000SM00001 Iss. 18
2/86
Page 3

Alaris® Syringe Pump
Performance Verification Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .33
Troubleshooting .............................................................. 34
Review logs ...........................................................................................34
Event Log download
Information Logs (376)
Software fault codes
PL3 Error
Exception error handling
General fault diagnosis
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .39
Introduction
Failure causes
Diagnosis
Actions
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .39
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .39
............................................................................................39
...............................................................................34
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .34
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .35
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .39
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .42
................................................................................42
Circuit Descriptions ............................................................ 43
Functional module block diagram .....................................................................43
Module overview functional description
Control PCB
Pressure Transducer (Model CC)
Power Supply Unit PCB
Display PCB
Battery
Transmission
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .44
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .44
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .44
............................................................................................45
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .45
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .44
....................................................................44
Corrective Maintenance. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 46
Corrective Maintenance ...............................................................................46
Access to pump
Rear case and subassemblies
Power Supply Unit and Speaker
Mains inlet, PE stud and magnet
Pole clamp and RS232
Rail cam
Front case and subassemblies
Control PCB and RS232 (if option fitted)
Display PCB
Chassis PCB and Plunger assembly
Chassis assembly and Pressure Transducer (Model CC only)
Syringe Sizing assembly
Chassis assembly breakdown
Plunger assembly breakdown
Pressure Transducer Assembly (Model CC only)
Keypads and labels
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .47
..........................................................................49
....................................................................49
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .50
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .51
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .52
.........................................................................53
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .53
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .55
.................................................................56
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .57
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .58
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .60
......................................................................62
........................................................64
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .65
Appendix . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 70
Electromagnetic Compatibility . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .70
Guidance and Manufacturer’s Declaration – Electromagnetic Emissions
Guidance and Manufacturer’s Declaration - Electromagnetic Immunity
1000SM00001 Iss. 18
. . . . . . . . . . . . . . . . . . . . . . . . . . . . .70
. . . . . . . . . . . . . . . . . . . . . . . . . . . . .71
3/86
Page 4

Alaris® Syringe Pump
Guidance and Manufacturer’s Declaration—Electromagnetic Immunity . . . . . . . . . . . . . . . . . . . . . . . . . . . .72
Recommended Separation Distances for LIFE SUPPORT Equipment between portable and mobile RF
communications equipment and the Alaris
Disposal
..............................................................................................74
Information on Disposal for Users of Waste Electrical and Electronic Equipment
Information on Disposal in Countries outside the European Union
Battery Removal
Spare Parts Listing
Electrical Parts Listing
Front Case Parts Listing
Rear Case Parts Listing
Keypads and Labels
Transmission Parts Listings
Software
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .79
...................................................................................74
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .75
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .75
............................................................................76
.............................................................................77
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .78
.........................................................................79
Test Equipment. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .80
Fitting and Replacement Guidelines
General assembly information
Torque guide
Service Contacts
Document History
Software Upgrade Record
......................................................................................81
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .83
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .84
.............................................................................85
...................................................................81
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .81
® Syringe Pump . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .73
.....................74
..................................74
1000SM00001 Iss. 18
4/86
Page 5
Alaris® Syringe Pump
General Information
General Information1
Introduction
The Alaris® Syringe Pumps are designed to deliver a continuous and accurate infusion whenever small fluid volumes need to be
administered with great precision. High performance, comprehensive alarm protection and sophisticated monitoring systems
combined with simple operation make these syringe pumps suitable for both general and critical infusions in a variety of areas within a
hospital.
The Asena® brand name has been changed to the Alaris® brand name. This change in brand name has no effect on the intended use or
functionality of the product. Recommended disposable products for use with this product may refer to either the Asena® brand name or
Alaris® brand name and both types are suitable for use with this infusion pump.
Product Familiarity
Ensure that you are fully familiar with this syringe pump by carefully studying the Directions for Use (DFU) prior to attempting any
repairs or servicing.
As part of a policy of continuous improvement, product enhancements and changes are introduced from time to time.
Purpose of this manual
This Technical Service Manual shows how to set up, test and maintain the following Alaris® Syringe Pump models:
Alaris® CC Syringe Pump• Alaris® GH Syringe Pump (with Plus software)•
Alaris® GH Syringe Pump• Alaris® GH Guardrails® Syringe Pump (with Plus software)•
Alaris® TIVA Syringe Pump• Alaris® CC Syringe Pump (with Plus software)•
Alaris® GS Syringe Pump• Alaris® CC Guardrails® Syringe Pump (with Plus software)•
Alaris® PK Syringe Pump• Alaris® CC Guardrails® Syringe Pump (with Compatible Pre-filled syringe)•
Alaris® GH Guardrails® Syringe Pump• Alaris® GH Guardrails® Syringe Pump (with Compatible Pre-filled syringe)•
Alaris® CC Guardrails® Syringe Pump•
It is intended for use by personnel experienced in medical equipment testing and maintenance procedures .
Conventions Used in this Manual
BOLD Used for Display names, self-test codes, controls and indicators referenced in this manual, for example,
Battery Indicator, access code 212, ON/OFF button.
'Single quotes' Used to indicate cross-references made to another section of this manual. For example, see Chapter 2,
'Configuration and Calibration'.
underline Used to indicate a link to another section within this manual.
Italics Used to refer to other documents or manuals. For example, refer to the relevant Directions for Use (DFU) for
further information. Also used for emphasis, for example, ...if the gap still measures less than...
A
Wherever this symbol is shown a Hints and Tips note is found. These notes provide useful advice or
information that may help to perform the task more effectively.
Wherever this symbol is shown an Update note is found. A typical example is drawing attention to a software
upgrade that should be confirmed has been installed.
Wherever this symbol is shown an Important note is found. These notes highlight an aspect of test or
maintenance that is important to know about.
1000SM00001 Iss. 18
5/86
Page 6
General precautions
Please read the general Operating Precautions described in the Directions for Use carefully prior to using the
pump.
w
This pump contains static-sensitive components. Observe strict precautions for the protection of static
sensitive components when attempting to repair and service the pump.
An explosion hazard exists if the pump is used in the presence of flammable anaesthetics. Exercise care to
locate the pump away from any such hazardous sources.
B
An electrical shock hazard exists if the pumps casing is opened or removed. Refer all servicing to qualified
service personnel.
A
This pump is protected against the effects of high energy radio frequency emissions and is designed to fail safe
if extremely high levels of interference are encountered. Should false alarm conditions be encountered, either
M
remove the source of the interference or regulate the infusion by another appropriate means.
If the pump is dropped, subjected to excessive moisture, humidity or high temperature, or otherwise
suspected to have been damaged, remove it from service for inspection by qualified service personnel.
L
When connected to an external power source, a three-wire (Live, Neutral, Earth) supply must be used. If the
integrity of the external protective conductor in the installation or its arrangement is in doubt, the pump
should be operated from the battery.
Alaris® Syringe Pump
General Information
1000SM00001 Iss. 18
6/86
Page 7
Alaris® Syringe Pump
General Information
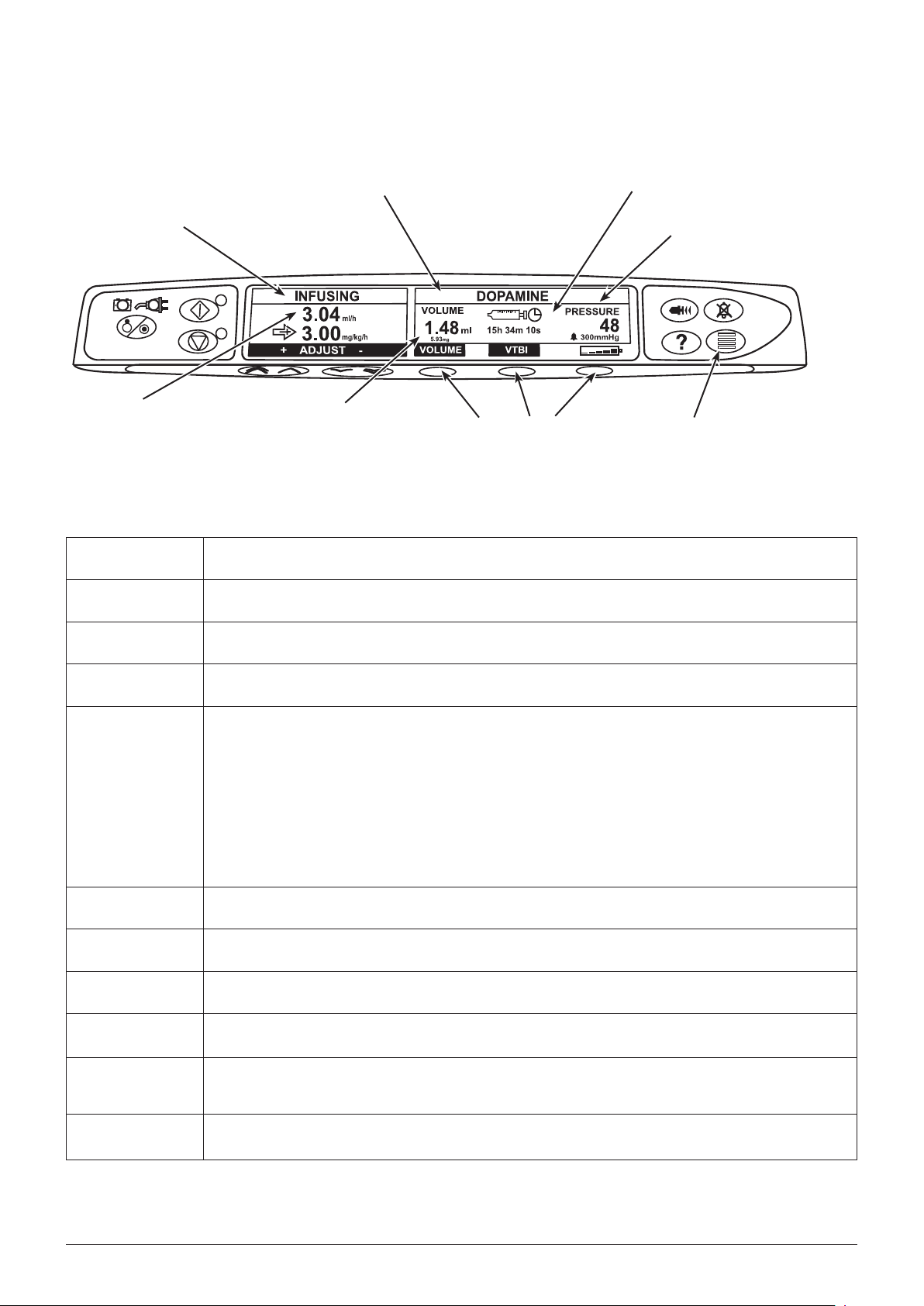
Front panel and main display
The display shown is for general guidance only. For pump specific front panel and main display information refer to relevant Directions
For Use.
Syringe Type Fitted / Drug Name / Profile*
Time Remaining Icon
Infusion Status
Infusion Rate Volume Infused
Blank Softkeys
* “Profile” is only available on an Alaris® Syringe Pumps with a Data Set loaded.
** Pressure Information is only displayed on the Alaris® CC Syringe Pumps.
Controls and indicators
a
b
h
ON/OFF button - Press once to switch the pump ON. Press and hold down for 3 seconds to switch the pump
OFF.
RUN button - Press to start the infusion. The Green LED will flash during infusion.
HOLD button - Press to put the infusion on hold. The amber LED will be lit while on hold.
Pressure Information** /
Pressure Icon (if enabled)
Pressure button (not available on the
Alaris® GS Syringe Pump)
c
i
d
e
g
f
j
k
MUTE button - Press to silence alarms.
PURGE/BOLUS button - Press to access PURGE or BOLUS soft keys. Press and hold down soft key to operate.
PURGE the extension set during set up.
Pump is on hold •
Extension set is not connected to the patient•
Volume Infused (VI) is not added •
BOLUS fluid or drug delivered at an accelerated rate.
Pump is infusing •
Extension set is connected to the patient •
VI is added•
OPTION button - Press to access optional features.
PRESSURE button - Press to display the pumping pressure and alarm level.
BLANK SOFTKEYS - Use in conjunction with the prompts shown on the display.
CHEVRON keys - Double or single for faster/slower, increase or decrease of values shown on main display.
BATTERY indicator - When illuminated, indicates that the pump is running on the internal battery. When
flashing, indicates that the battery power is low, with less than 30 minutes of use remaining.
AC POWER indicator - When illuminated, indicates that the pump is connected to an AC power supply and the
battery is being charged.
1000SM00001 Iss. 18
7/86
Page 8
Alaris® Syringe Pump
Configuration and Calibration
Configuration and Calibration2
Access codes
The syringe pump software contains a number of configuration and test routines that can be accessed by the user. The majority of tests
are ‘MENU’ driven from a technical access code (see below).
Note: The pump
should be power
cycled after entering
any new calibration or
configuration information
prior to performing any
validation tests.
Code Description
123 Self Test Procedure
166 External Reprogramming
167 Teach Learn Procedure
175 Handsfree Bolus
243 Calibration Selection Menu
251 User Configuration Menu
301 Fully Dedicated
302 Semi-dedicated
376 Service Access Menu
401 Upload Data Set to Pump (Guardrails® Software enabled Pumps and the Alaris® PK Syringe Pump)
402 Download CQI Event Log from Pump (Guardrails® Software enabled Pumps only)
418 Alternative Alarm Tone. (Not available for Guardrails® Software enabled Pumps and the Alaris® PK Syringe Pump)
499 Download Data Set from Pump (Guardrails® Software enabled Pumps and the Alaris® PK Syringe Pump)
611 Cold Start (RAM Clear)
612 Data Set activation (Alaris® PK Syringe Pump)
711 Power Lock (Alaris® PK Syringe Pump)
Codes available on Alaris® Syringe Pumps (with Plus software):
Code Description
123 Self Test Procedure
166 External Reprogramming
167 Teach Learn Procedure
240 Calibration Verification Mode
243 Calibration Selection Menu
251 User Configuration Menu
301 Fully Dedicated
302 Semi-dedicated
376 Service Access Menu
401 Upload Data Set to Pump
402 Download CQI Event Log from Pump (Guardrails® Software enabled Pumps)
611 Cold Start (RAM Clear)
Each MENU (and some unique items) has its own three-digit access code that can be entered using the following procedure:
1. b and turn the pump ON.
Hold down
Enter the required access code using the
2. f keys and the NEXT softkey.
When the required code shows on screen, press
3. OK to confirm.
1000SM00001 Iss. 18
8/86
Page 9

Alaris® Syringe Pump
Configuration and Calibration
Dedication options (301/302)
Fully Dedicated (set using access code 301) will remind a user that a pressure disc must be fitted to start any infusion. In this mode
occlusion pressures are always displayed in mmHg.
Semi-Dedicated (set using access code 302) will remind a user that a pressure disc must be fitted when drugs and dosing features are
used. When a pressure disc is not in use, pressure levels L-0 to L-10 will be displayed.
Data Set Activation (612)
This code is used to load the predefined pump configuration and drug setup into the non-volatile storage. It is necessary to enter the
code 612 after a cold start (code 611); the configuration and drug setup will then be available in normal operation.
Alternatively a data set may be uploaded as appropriate. See directions for use contained within the Alaris® PK Editor Software package.
Handsfree Bolus (175)
Enable or disable the Handsfree Bolus. If enabled pressing bolus button displays screen prompting for hands free or hands on. Default
volume after clear setup is 0.0. Upper amount restricted to bolus volume limit in general options or drug protocol bolus volume limit.
Power Lock (711)
Available on the Alaris® PK Syringe Pumps with software V2.3.11 and above.
Disabled The new alternative Power Down sequence now allows the user to Power Down the pump whilst the infusion is
suspended (on hold) in TCI mode and predictive TIVA mode.
Enabled The Power Down sequence (Power Lock) remains the same where the user may only Power Down the pump by stopping
the infusion, selecting ‘new operation’ from the options menu, confirming the selection, then Powering Down the pump.
1000SM00001 Iss. 18
9/86
Page 10
Configuration options (251)
Enter access code 251 to display the User Configuration menu:
Alaris® Syringe Pump
Configuration and Calibration
Drug Library*
Set drug names list on a Model GH - Select Character Group f(double chevrons) Select Character
f(single chevrons). To go to next Character use NEXT.
Set drug names and protocols for Models CC and TIVA (see drug protocol setup instructions on following
pages).
General Options* See general options table later in this chapter.
Clock Set
Hospital Name* Enables establishment name (max 20 characters) to be displayed during the power-up sequence.
Enable Syringes* Configure the type and size of syringes permitted for use.
Language Configure the language used for messages shown on display.
Contrast
Enable Units*
Warning -
A
When entering a drug name the character "%" should not be used as it may cause the pump to lock up and the safety
processor alarm to sound. The word "percent" or an abbreviation is recommended for use. Only the Models GH and
CC are affected by this anomaly.
Set the current date and time. To set the clock, use
To set the hospital name, use
To enable syringes, use
Select language required using
Set the display panel contrast. Use
Select the type of units permitted for use on the pump. To enable units, use
enable/disable and OK to store.
f and NEXT to adjust and OK to store.
f and SELECT, to enable/disable and OK to store.
f and SELECT to store.
f to adjust contrast and OK to store.
f and NEXT to adjust and OK to store.
f and MODIFY, to
*Note: For Guardrails® Software
enabled pumps, pumps with Plus
software and the Alaris® PK Syringe
Pump these options may vary or
will not be available. Please refer to
the relevant pump or PC software
Directions For Use for comprehensive
information.
1000SM00001 Iss. 18
10/86
Page 11
Alaris® Syringe Pump
Configuration and Calibration
Alaris® TIVA Syringe Pump drug protocol setup
Select Drug Library from Configuration Options (1. 251).
Use
2. f to select drug and press MODIFY to modify selected drug or NEW to create new drug name.
QUIT
3. will return to 251 main menu.
When modifying a drug protocol, pressing
4. BACK at any time will take you to the previous step.
Modify - Existing drug
5.
ENABLE/DISABLEa) - Enables or disables the drug being available.
DELETE
b) - Select Yes to delete from drug library.
EDIT
c) - See table below.
Edit Drug Protocol - New or existing drug
6.
Press a) OK softkey to confirm each step.
Drug Option
Drug Name
Concentration Units
Minimum Concentration
Default Concentration
Maximum Concentration
Dose Rate Units
Induction Dose
Induction Time
Pause After Induction MODIFY
Maintenance Rate
Bolus Dose
Bolus Rate RATE
To Adjust
(Softkeys are shown in Bold)
Select Character Group
Select Character
To go to next Character NEXT
f
f or OFF
f or OFF
f or OFF
f
f or OFF
f
f
f
f (double chevrons)
f (single chevrons)
Hands Free Bolus MODIFY
1000SM00001 Iss. 18
11/86
Page 12
Alaris® Syringe Pump
Configuration and Calibration
Alaris® CC Syringe Pump* drug protocol setup
Select Drug Library from Configuration Options (1. 251).
Use
2. f to select drug and press MODIFY to modify selected drug or NEW to create new drug name.
QUIT
3. will return to 251 main menu.
When modifying a drug protocol, pressing
4. BACK at any time will take you to the previous step.
Modify - Existing drug
5.
ENABLE/DISABLEa) - Enables or disables the drug being available.
DELETE
b) - Select Yes to delete from drug library.
EDIT
c) - See table below.
Edit Drug Protocol - New or existing drug
6.
Press a) OK softkey to confirm each step.
*Note:
For Guardrails® Software enabled pumps this option will not be available. Please refer to the relevant Alaris® Syringe Pump
Directions For Use or Guardrails® Editor Directions For Use for comprehensive information.
Drug Option
Drug Name
Dose Rate Units
Maximum Dose
Default Dose
Minimum Dose
Concentration Units
Minimum Concentration
Default Concentration
Maximum Concentration
Maximum Bolus
Bolus Rate
Pressure Alarm
To Adjust
(Softkeys are shown in Bold)
Select Character Group
Select Character
To go to next Character NEXT
f (double chevrons)
f (single chevrons)
f
f or OFF
f or OFF
f or OFF
f
f or OFF
f or OFF
f or OFF
f or OFF
f
f or OFF
A
Warning -
When entering a drug name the character "%" should not be used as it may cause the pump to lock up and the safety
processor alarm to sound. The word "percent" or an abbreviation is recommended for use. Only the Models GH and
CC are affected by this anomaly.
1000SM00001 Iss. 18
12/86
Page 13
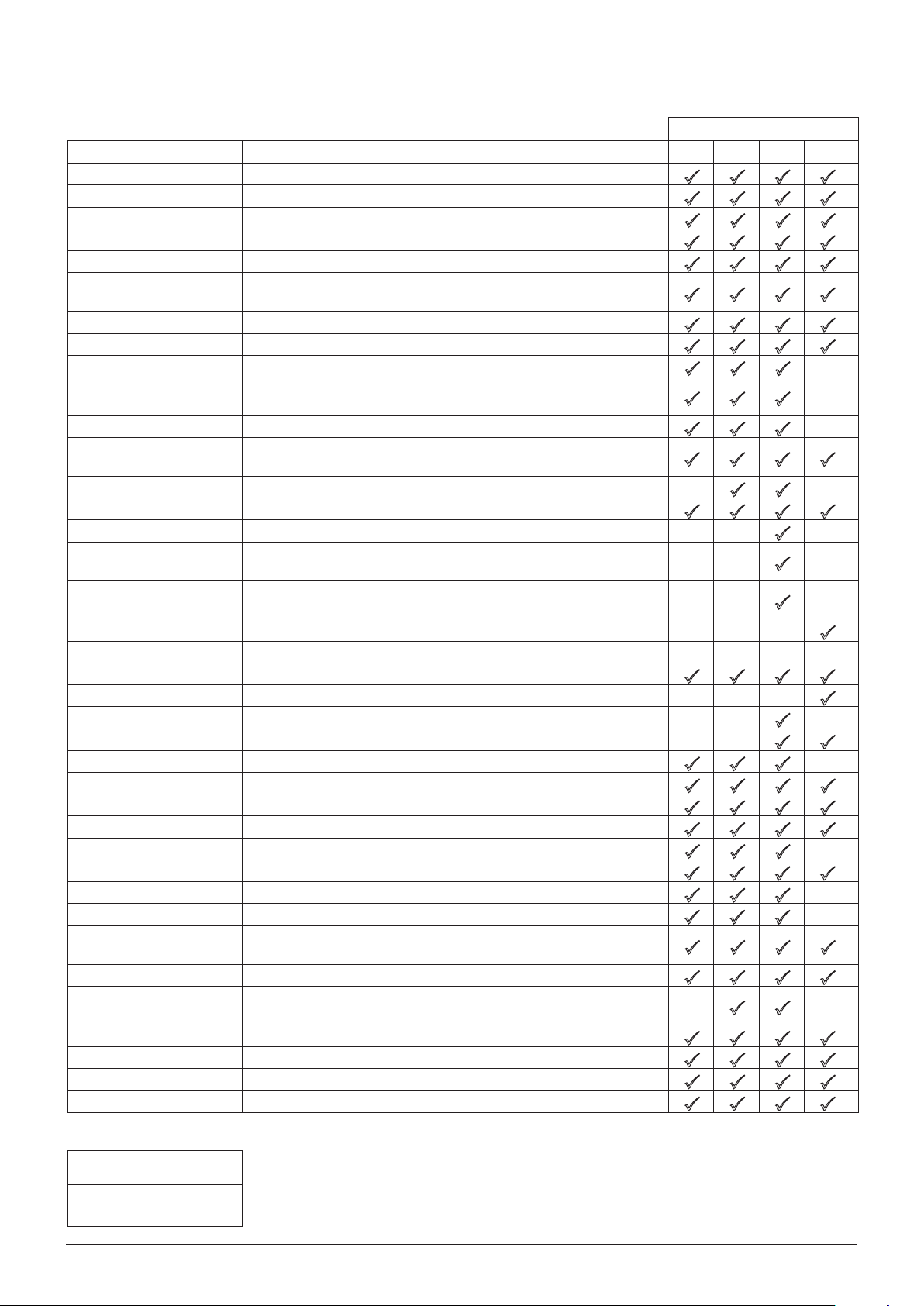
General options
Option Description
NURSE CALL FITTED Enables Nurse Call (hardware option).
NURSE CALL INVERT When enabled, the nurse call output is inverted.
RS232 SELECTED Sets the pump’s communications to use RS232 (hardware option).
NEOI WARNING Sets the Near End Of Infusion (NEOI) warning time.
EOI POINT Sets the End Of Infusion volume.
KVO AT EOI Enables pump to run at the Keep Vein Open (KVO) rate when End Of
Infusion (EOI) is reached.
KVO RATE Sets the Keep Vein Open (KVO) rate.
BACK OFF Motor will reverse to relieve line pressure when an occlusion occurs.
AUTO SAVE When disabled, the patient information is cleared on power up.
RATE LOCK When enabled, the rate can be locked to prevent unwanted changes of
the set infusion rate.
QUIET MODE When enabled, the button beeps are muted.
AC FAIL When enabled, the AC Power Failure Alarm will sound if AC power is
disconnected.
RATE TITRATION When enabled, the rate can be changed whilst the pump is infusing.
PRESSURE DISPLAY Enables / disables the Pressure Icon on the main display.
AUTO PRESSURE Enables / disables the automatic pressure alarm level option.
AUTO SET PRESSURE Automatically sets the line occlusion pressure alarm level to a specified
amount above the current pressure.
AUTO OFFSET Adjusts the automatic offset value used by auto pressure and auto set
pressure.
HANDS FREE BOLUS Enables / disables hands-free bolus.
CAP PRESSURE Sets the maximum pressure limit.
PRESSURE DEFAULT Sets the default occlusion alarm level.
DEFAULT BOLUS VOLUME Sets the default hands-free bolus volume for No Drug mode only.
MAX PRESSURE Sets the maximum pressure limit.
WEIGHT Sets the default patient weight in kg.
CAP RATE Sets the maximum value for infusion rate.
PURGE RATE Sets the purge rate.
PURGE VOLUME LIMIT Sets the maximum permissible purge volume.
PURGE SYRINGE Prompt to purge syringe after confirmation.
BOLUS Enables / disables the bolus feature.
DEFAULT BOLUS Sets the default bolus rate.
CAP BOLUS RATE Sets the maximum value for bolus rate.
BOLUS VOL LIMIT Sets the maximum permissible bolus volume.
MANUAL BOLUS Volume infused will be increased if plunger is manually moved in and
syringe remains confirmed.
CALL BACK TIME Adjusts the time for the pump to sound the call back alarm.
VTBI CLEAR RATE Rate will be set to zero when VTBI has been set-up with stop as the end
rate.
EVENT LOG DISPLAY Enables / disables the event log display.
BATTERY ICON Enables / Disable the Battery Icon on the main display.**
AUDIO VOLUME Sets the alarm volume of the pump at high, medium or low.
AUTO NIGHT MODE Sets Backlight to dim between 21:00 and 06:00hrs.
* For Guardrails® Software enabled pumps, pumps with Plus software and the Alaris® PK Syringe Pump
Key:
= available option
= unavailable option
these options may vary or will not be available, with only the first three options listed in table above
adjustable in the General Options on the pump. Please refer to the relevant Pump or PC Software
Directions For Use for comprehensive information.
** For Alaris® GS Syringe Pump the battery icon can be seen via the Options menu by pressing the
key.
Alaris® Syringe Pump
Configuration and Calibration
Models
GS GH* CC* TIVA
d
1000SM00001 Iss. 18
13/86
Page 14
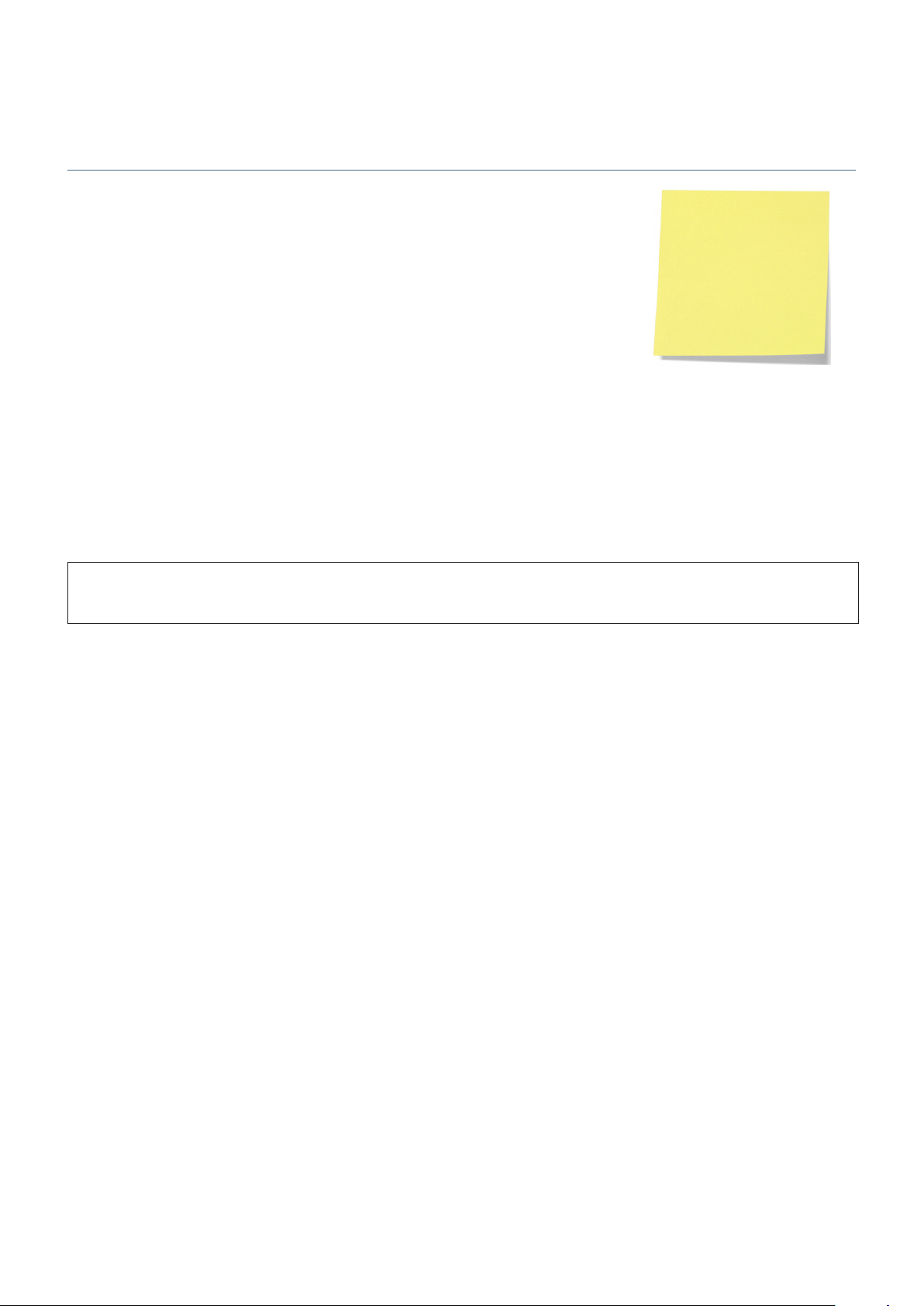
Teach Learn
Teach Learn Procedure (Software versions V1.4.13 and above)
Alaris® Syringe Pump
Configuration and Calibration
For the teacher pump only (not required for learn pumps), in General Options 251, switch off 1.
RS232 before commencing Teach Learn procedure.
Turn the teacher pump on in normal operation.2.
Enter the access code 167 on learn pump.3.
Align the two IrDA ports on the pumps (optimum distance is 5cm).4.
Press OK and then Yes to confirm.5.
A progress bar will travel across the learn pump.6.
When completed, select No to cancel retry.7.
Note: For multiple
Teach Learn procedures,
to avoid call-back alarm
every 2 minutes, turn
teacher pump on in
access code mode.
Possible reasons for failure:
RS232 is not switched off.•
If software versions are different, confirm Teach Learn procedure on learner pump to continue. Verify settings after completion of •
learn.
The pump models are different. For example, an Alaris® GS Syringe Pump can only teach an Alaris® GS Syringe Pump.•
The line of sight between the IrDA windows was obstructed during data transfer.•
Important: During the Teach Learn procedure a note should be taken of any parameters that fail. These should then be adjusted •
manually in the relevant option setting. The final screen will show “Incomplete Data Transfer” if any commands have failed. Verify
learn pump configuration prior to returning the pump to clinical use.
Check protocols are correct on learn pump after Teach Learn procedure, before returning pump to use.
A
After a Teach Learn procedure it is necessary to clear the previous patient setup in order to update the syringe
information. On power-up after Teach Learn procedure and when prompted with CLEAR SETUP, select YES.
1000SM00001 Iss. 18
14/86
Page 15

Alaris® Syringe Pump
Configuration and Calibration
Data Set Transfer
Data Set Upload and Download (401 and 499)
Upload Data Set to an Alaris® Syringe Pump with Guardrails® Safety Software or an Alaris® PK Syringe Pump (401)
Using the Guardrails® Editor Transfer Tool or Alaris® PK Editor Software Transfer Tool allows a released Data Set to be uploaded to an
Alaris® Syringe Pump.
Download Data Set from an Alaris® Syringe Pump with Guardrails® Safety Software or an Alaris® PK Syringe Pump (499)
Using the Verification Tool allows an uploaded Data Set in an Alaris® Syringe Pump to be downloaded to a PC for comparison and
verification.
Note: After data
set upload the new
parameters will not take
effect until the pump
has been powered up in
normal operation mode
and a new profile has
been selected.
Download CQI Event Log (402)
Download CQI Event Log from an Alaris® Syringe Pump with Guardrails® Safety Software (402)
Using the CQI Event Log Downloader allows the CQI Event Log to be downloaded from an Alaris® Syringe Pump to a PC for use with the
Guardrails® CQI Reporter. The Guardrails® CQI Reporter is a program for querying and reporting on the collective event data allowing the
user to analyse trends in medication administration and track medication errors.
Warning -
A
Note: For more
information relating to
the Guardrails® Editor, the
Alaris® PK Editor Software
and the Guardrails® CQI
Reporter refer to the
relevant Directions For
Use supplied with the
software.
At no time should the Guardrails® Safety Software or the Alaris® PK Editor Software be used to upload to or download
from an Alaris®
Syringe Pump while the pump is connected to a patient.
1000SM00001 Iss. 18
15/86
Page 16

Calibration procedures (243)
Enter access code 243 to display the Calibration Selection menu (see Access Codes).
SYRINGE CLAMP calibration
Fit calibration tool into position on pump as shown in Steps 1-2 and close the •
clamp.
At each step, • CAL is displayed if value is within tolerances.
Press • CAL button to store calibration point.
Note: If CAL is not displayed,
check for correct positioning of
calibration tool.
If calibration cannot be
performed, repairs to pump may
be necessary.
Note: The calibration values
shown on the displays are for
illustrative use only and may
vary.
Note: The pump should be
power cycled after entering any
new calibration or configuration
information prior to performing
any validation tests.
Alaris® Syringe Pump
Configuration and Calibration
Calibration tool required: 1000TG00095
Step 1
Step 2
Step 3
1000SM00001 Iss. 18
16/86
Page 17
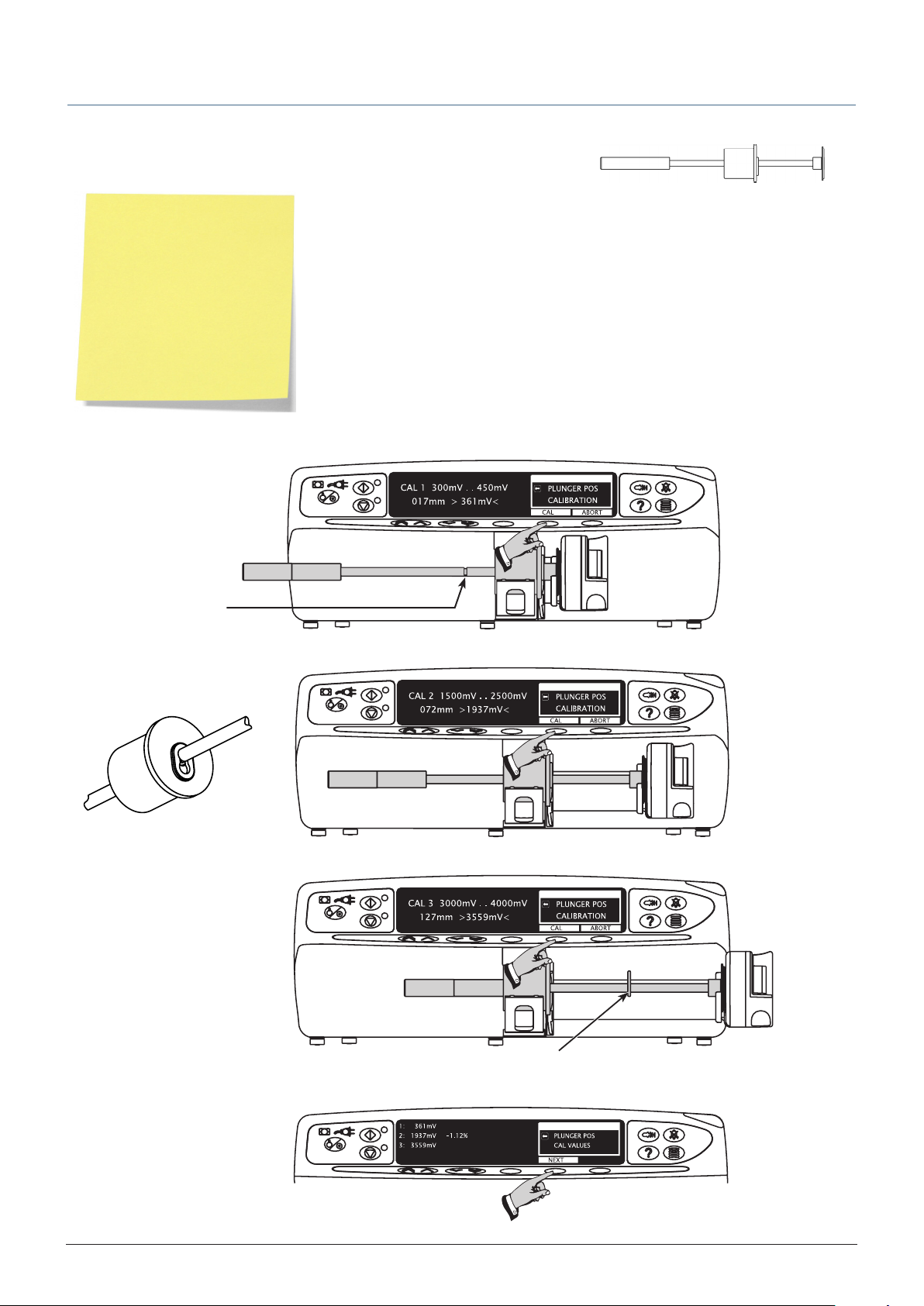
PLUNGER POS (position) calibration
Fit calibration tool into position on pump as shown in Steps 1-3. •
At each step, • CAL is displayed if value is within tolerances.
Press • CAL button to store calibration point.
Alaris® Syringe Pump
Configuration and Calibration
Note: If CAL is not displayed,
check for correct positioning of
calibration tool.
If calibration cannot be
performed, repairs to pump may
be necessary.
Note: The calibration values
shown on the displays are for
illustrative use only and may
vary.
Step 1
Channel for locking clip
Step 2
Close-up of calibration tool,
showing locking clip in position.
Calibration tool required: 1000TG00095
Step 3
Step 4
1000SM00001 Iss. 18
Locking clip
17/86
Page 18
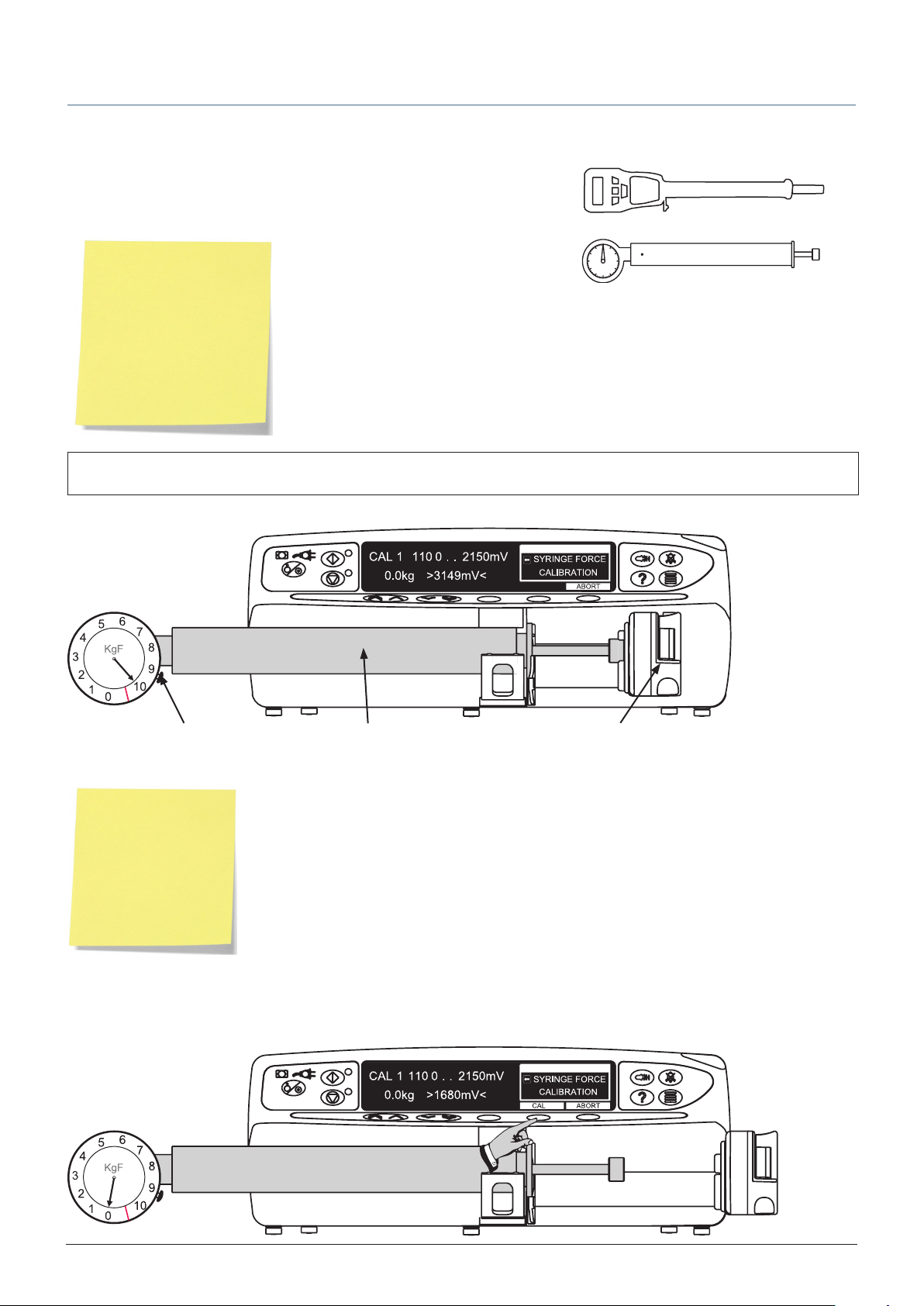
SYRINGE FORCE calibration
Precondition:
This preconditions the mechanism and should only be done if motorplate or chassis
has been replaced. Fit Calibration tool as shown, zero the gauge, run until gauge
registers 10kgf and then carefully declutch mechanism and withdraw plunger. Do
not press any button during this procedure.
Note: To convert Kilograms
of Force (kgf) to Newtons
(N) multiply by 9.806650. For
example 10kgf = 98.07N.
Note: The calibration values
shown on the displays are for
illustrative use only and may
vary.
Excessive force will damage the plunger mechanism. Do not apply more than 10 kgf ±0.05kgf to the plunger
A
mechanism at any time.
Alaris® Syringe Pump
Configuration and Calibration
Calibration tool required:
0000TG00200 (top) or
0000TG00020 (bottom)
10kgf ±0.05kgf
Zero Gauge Syringe Force Calibration Tool Plunger
Fit Calibration tool and position plunger as shown in Steps 1 to 3, zero the gauge. At each step press CAL when required calibration
force is reached.
Note: If CAL is not
displayed, check for
correct positioning of
tool.
If calibration cannot
be performed, repairs
to pump may be
necessary.
Allow 30 seconds for pressure to stabilise following any preconditioning calibration.
Step 1
0kgf ±0.05kgf
1000SM00001 Iss. 18
18/86
Page 19
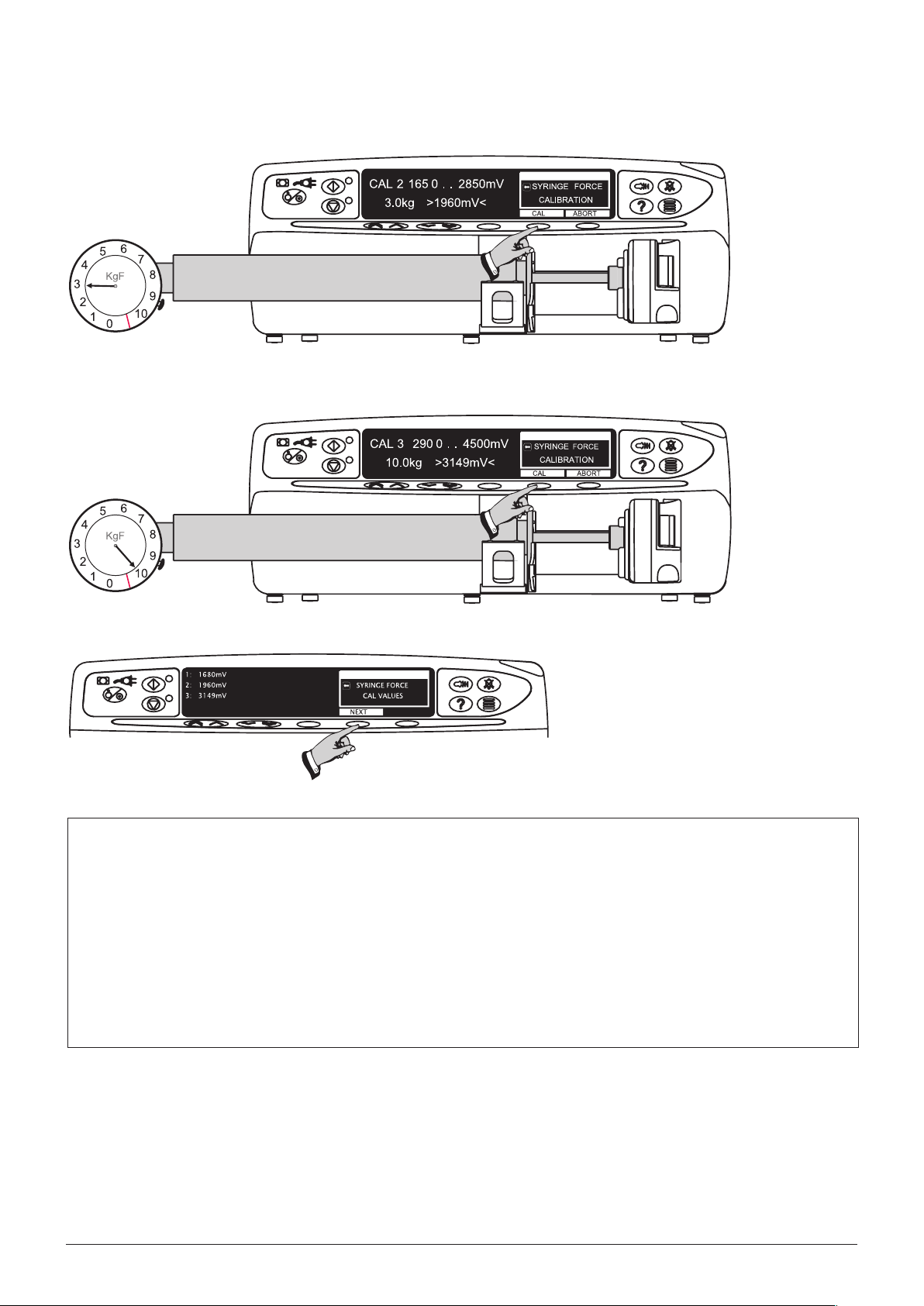
Step 2
3kgf ±0.05kgf
Step 3
10kgf ±0.05kgf
Alaris® Syringe Pump
Configuration and Calibration
Step 4
A
Use of the 0000TG00200 Digital Occlusion Testgear.
The 0000TG00200 Occlusion testgear uses a digital force gauge to register applied forces.
Please refer to the MecMesin Compact Gauge Operation Instructions supplied for detailed operational information
and power options and requirements.
To prepare the testgear for use, load into the syringe pump.
Ensure there is nothing touching the testgear plunger (such as the syringe plunger drive).•
Turn on the Compact Gauge using the ‘On/Zero’ key.•
Select ‘kg’ force units, and ‘MAX’ reading option.•
If the display indicates other than 0.00kg, zero the system using the ‘On/Zero’ key.•
Operate the system as required for performing the calibration activity.
Before the next use, ensure the ‘MAX’ reading is cleared using the ‘On/Zero’ key.
1000SM00001 Iss. 18
19/86
Page 20
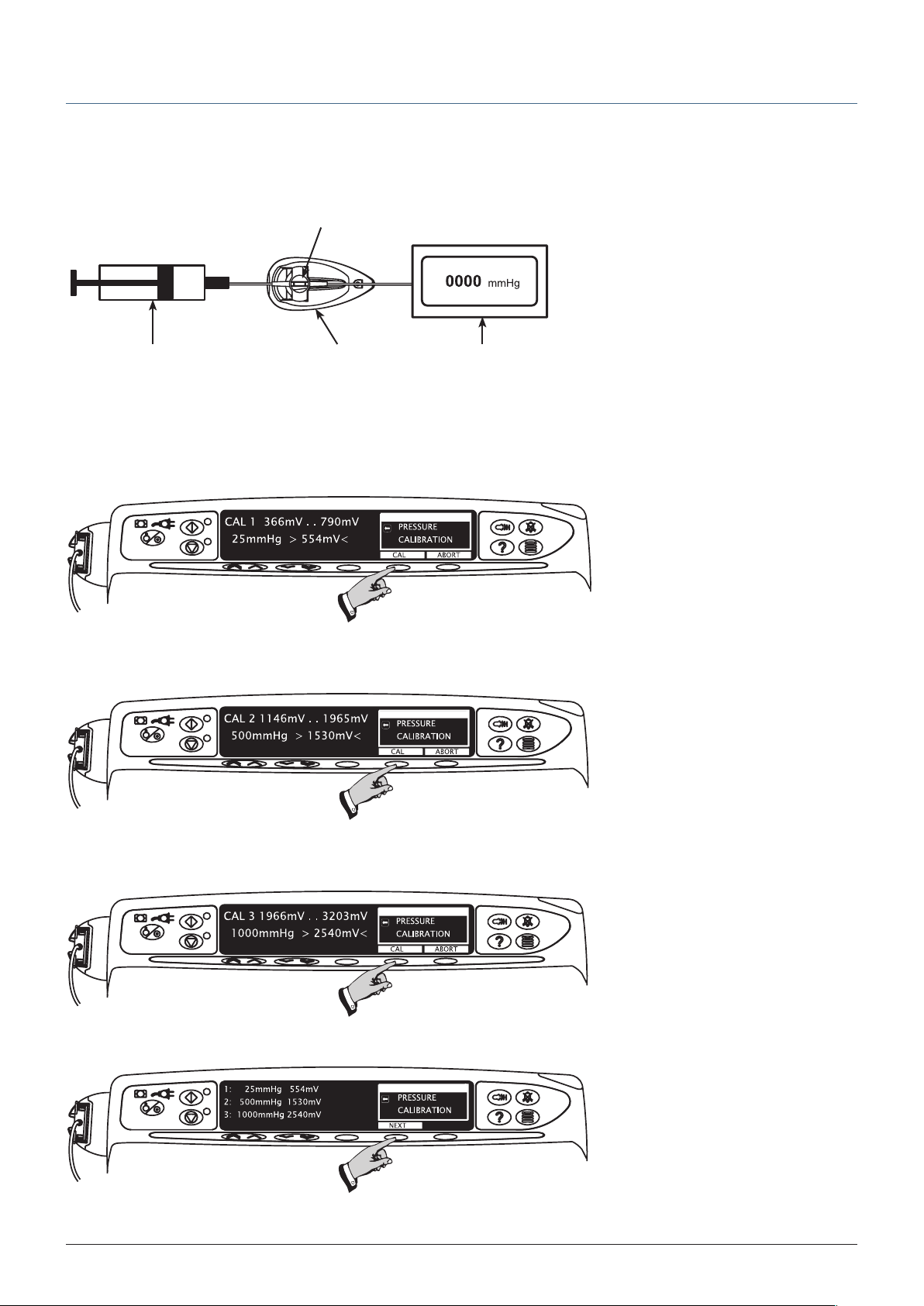
LINE PRESSURE calibration – Alaris® CC Syringe Pump only
Tools required:
Pressure gauge (range 0-1400 mmHg) (Tolerance +/- 0.1% Full Scale Accuracy)•
Dedicated pressure sensing disc extension set (i.e. G30402M)•
50ml Luer-lock syringe•
Dedicated pressure sensing disc extension set
Alaris® Syringe Pump
Configuration and Calibration
Pressure Transducer
Load pressure disc infusion set into transducer. Connect infusion set to syringe and gauge. Using syringe, apply pressure required as
shown at steps 1-3. At each step press CAL when required calibration pressure is displayed on pressure gauge.
The calibration values shown on the displays are for illustrative use only and may vary.
Note:
Pressure gauge50ml Luer-lock syringe
Step 1
25mmHg ± 1mmHg
Step 2
500mmHg ± 1mmHg
Step 3
1000mmHg ± 1mmHg
Step 4
1000SM00001 Iss. 18
20/86
Page 21

Alaris® Syringe Pump
BATTERY STATE
FINISH
OK
CAP 2488 mAh 00:09
CHR 2362 mAh 05:28
VOL 8.21 V 02:05
Configuration and Calibration
BATTERY calibration
Connect the Pump to AC mains.1.
Select BATTERY CALIBRATION from menu and press OK.2.
The pump will automatically run the battery calibration. Battery calibration cycles the battery through a charge, discharge and re-3.
charge sequence during which the gas gauge within the battery pack will be updated with a measurement of the current capacity
of the cells.
Battery compartment should be ventilated during calibration (open battery cover). Pump may fail calibration if too
A
hot, so care should be taken not to calibrate too many pumps in close proximity (in a docking station, for example).
Ensure that the battery is supported as you open the battery compartment.
Disconnecting the AC mains at any time during calibration will cause battery calibration to fail.
A
When calibration is complete, the following is shown on the display:4.
2
1
3
Value Description Pass Criteria
1 Battery Capacity Pack capacity value updated after measured
2 Current Battery Charge Level Current charge in pack. n/a
3 Battery Voltage Current pack voltage. n/a
4 Initial Charge Time Time taken during initial charge phase. Initial charge
5 Discharge Time Time taken during measured discharge phase. Pack
6 Final Charge Time Time taken during final charge phase. Pack is fully
4
6
5
discharge phase (if changed).
phase checks pack is fully charged and if not it is
charged.
is discharged to determine how much charge is
available from the pack.
recharged ready for use.
Greater than 2100mAh
Lower than 2 hours 59 minutes
Between 4 hours 15 minutes and 10
hours
Lower than 2 hours 59 minutes
All pass criteria (see table above) should be met and the pump should display FINISH at the end of the calibration otherwise 5.
calibration has failed. If calibration has failed retry calibration or replace battery.
Press 6. OK to exit.
Note: The plunger drive
will move automatically
during the discharge
phase, so ensure that
the plunger drive is
not obstructed during
calibration (remove
syringes etc).
1000SM00001 Iss. 18
21/86
Page 22
Alaris® Syringe Pump
Preventative Maintenance
Preventative Maintenance 3
Preventative Maintenance
To ensure the pump remains in good operating condition, routine and preventative maintenance inspections are required. Routine
maintenance inspections should be performed by hospital/facility before each use, see Directions For Use for details.
Preventative maintenance inspections should be performed at least every three years.
For the preventative maintenance inspection the following should be performed:
Full visual inspection of the pump, internal and external•
Fitting of all updates required•
Battery test and/or replacement•
Clean the pump•
Performance Verification Procedure•
Following all spare part replacement and repair activities, testing must be performed in accordance with the
A
Performance Verification Procedure (PVP). Additional testing and calibration may be required after certain repairs
are completed, see table in Chapter 6 Corrective Maintenance for more information.
Visual Inspection
Open the pump, as per Chapter 6 Corrective Maintenance and visually inspect the interior of the pump.
Visually inspect the exterior of the pump checking the following:
Labels should be replaced as required if not flat, legible or fully adhered.•
Check Keypad for any sign of wear and replace as required. •
Case components must be checked for damage and replaced if necessary. •
Check the pole clamp is not damaged and that it functions correctly.•
Inspect the AC power supply plug and cable for damage.•
The case should be clean and free from IV solution residue, especially near moving parts. •
Check for dried solution deposits on accessible areas of pressure transducer and plunger mechanism.•
1000SM00001 Iss. 18
22/86
Page 23
Alaris® Syringe Pump
Preventative Maintenance
Recommended Cleaning
To ensure this pump remains in good operating condition, it is important to keep it clean and carry out the routine procedures
described below. All servicing should only be performed by a qualified service engineer.
Thoroughly clean external surfaces of the pump, by wiping over with a lint-free cloth, lightly dampened with warm water and a
standard disinfectant/detergent solution.
Do not use the following disinfectant types:
NaDcc (such as PRESEPT)•
Hypochlorites (such as CHLORASOL)•
Aldehydes (such as CIDEX)•
Cationic Surfactants (such as Benzalkonium Chloride)•
Iodine (such as Betadine)•
Recommended cleaners are:
Brand Concentration
Hibiscrub 20% (v/v)
Virkon 1% (w/v)
The following products were tested and are acceptable for use on the Alaris® Syringe Pump range if used in accordance with the
specified manufacturer’s guidelines.
Warm soapy water•
Mild detergent in water (e.g. Young’s Hospec)•
70% Isopropyl Alcohol in water•
Chlor-Clean•
Clinell Sporicidal wipes•
Hibiscrub•
TriGene Advance•
Tristel Fuse sachets•
Tristel Trio wipes system•
Tuffie 5 wipe•
Virkon Disinfectant•
Virusolve+ (Ready To Use)•
Virusolve+ (Wipes) •
Before cleaning always switch OFF and disconnect from the AC power supply. Never allow fluid to enter the casing
A
and avoid excess fluid build up on the pump.
Do not use aggressive cleaning agents as these may damage the exterior surface of the pump.
Do not steam autoclave, ethylene oxide sterilise or immerse this pump in any fluid.
Use an appropriate cleaning method that does not allow an excess of fluid to accumulate around the keypads.
Aggressive cleaning can potentially create a fluid ingress path into the shelf keypad which can result in keypad
failure.
In case of failure, usually resulting in a KY1 error code, the shelf keypad must be replaced. As a preventive
measure, shelf keypads manufactured after week number 15, 2003 should be used since they offer more
protection to excessive cleaning. The week number may be found on the keypad connection tail.
We recommend that all pumps within the following serial numbers -
Alaris® GS Syringe Pump 08510 - 09976
Alaris® GH Syringe Pump 16437 - 22286
Alaris® CC Syringe Pump 03471 - 06632
Alaris® TIVA Syringe Pump 01310 - 02369
(or pumps outside of this range which had their shelf keypad replaced between 2nd July 2002 and 30th April
2003) have their shelf keypad replaced at the next routine service. All other pumps have a shelf keypad that does
not exhibit this potential risk.
1000SM00001 Iss. 18
23/86
Page 24
Updates
Upgrading software
Recommended at the next service: If the pump has software versions V1.9.3 (MK1/2) or V2.3.5 (MK3) and below,
then upgrade to software version V1.9.4 (MK1/2) or V2.3.6 (MK3) or greater.
Strongly recommended: If the pump has software version V1.5.9 and below then upgrade the Alaris® Syringe
Pump (except the Alaris® TIVA Syringe Pump) software to the latest software versions, as this will address a
potential issue that may result in a condition where the running LED is flashing, the infusion status shows
"INFUSING" but the volume infused display will not increment and no drug will be infused into the patient.
This potential issue may occur under the following remote circumstances :-
If the pump is subsequently stopped and restarted, the infusion will start normally.
Mandatory: If the Alaris® PK Syringe Pump software is below V3.2.16 then upgrade to software version V3.2.16
or greater. This will provide an additional advice screen for the Propofol Schnider model usage, alerting the user
that a Tpeak of 1.6 minutes is used.
When upgrading a pump from one software version to another where the first or middle digit changes, cold start
will be required before and after software upgrade, unless otherwise stated in a Technical Information Notice.
Calibration will also be required after software upgrade and cold start.
Alaris® Syringe Pump
Preventative Maintenance
A new syringe was recently fitted into the drive mechanism and•
An infusion is started, very quickly stopped and then restarted. (The pump must be stopped between •
0.375 secs and 0.435 secs after starting - a window of 0.06 secs.)
Complete and return the 'Software Upgrade Record' in the 'Appendix' section after performing any software
A
Tools required
upgrade.
The Software Distribution Disk (See table below) •
IrDA port on PC or Comms Port•
Programming kit 1000SP00172 (Includes Programme Header and IrDA cable)•
RS232 cable 1000SP00336•
Ver. 3 Software Maintenance Utility (SMU) 1000CD00028•
IrDA power-down test
To check PC is set up correctly for communication with Alaris® Syringe Pumps the Power Down Test needs to be performed on one
Alaris® Syringe Pump only as follows:
Load the IrDA Power Down Test program on your PC.1.
Select GO on the PC software program.2.
Align the IrDA converter with the pump IrDA window (optimum distance is 5cm). 3.
Connect to serial port.4.
Enter access code 5. 166.
Press 6. Yes to continue Bootstrap.
Select IrDA interface.7.
Select a Baud rate of 115200.8.
The pump will then display Bootstrap in progress.9.
Press the 10. c button to silence the alarm.
Select Transmit on PC. Check progress bar moves on PC and pump powers down.11.
1000SM00001 Iss. 18
24/86
Page 25
Software Versions available
Alaris® Syringe Pump
Preventative Maintenance
Syringe Pump Model
Alaris® GS Syringe Pump
Alaris® GH Syringe Pump
Alaris® CC Syringe Pump
Alaris® TIVA Syringe Pump
Alaris® PK Syringe Pump
Key: MP = Main Processor. Mk1/Mk2/Mk3 are the released versions of the Control PCB.
Syringe Pump Model with Plus Software
Alaris® GH Syringe Pump
Alaris® GH Guardrails® Syringe Pump
Mk1/Mk2 Mk3 Mk1/Mk2 Mk3 Mk3
1000SP01221
(MP v1 5.10)
1000SP01221
(MP v1 5.10)
1000SP01221
(MP v1 5.10)
1000SP01221
(MP v1.6.2)
Software Enhanced Software Guardrails® Safety Software
1000SP01225
(MP v2.0.0)
1000SP01226
(MP v2.0.0)
1000SP01227
(MP v2.0.0)
1000SP01228
(MP v2.1.0)
1000SP01469
(MP v4.1.4)
1000SP01469
(MP v4.1.4)
1000SP01270
(MP v1.9.4)
1000SP01270
(MP v1.9.4)
1000SP01270
(MP v1.9.4)
1000SP01270
(MP v1.9.4)
1000SP01276
(MP v2.3.6)
1000SP01268
(MP v2.3.6)
1000SP01267
(MP v2.3.6)
1000SP01269
(MP v2.3.6)
1000SP01454
(MP v3.2.16)
MP v3.1.4
(Installed by CareFusion
Personnel)
MP v3.1.4
(Installed by CareFusion
Personnel)
Alaris® CC Syringe Pump
Alaris® CC Guardrails® Syringe Pump
Soft bootstrap
Load the software program onto your PC. Start the ‘MP Only’ version of relevant pump software. Check the correct pump type is 1.
displayed.
Select GO.2.
Align the IrDA converter pump with the IrDA window (optimum distance is 5cm), or connect RS232 cable. 3.
Connect to serial port.4.
Enter access code 166.5.
Press Yes to continue Bootstrap.6.
Select IrDA interface or RS232 interface.7.
Select a Baud rate of 115200.8.
The pump will then display Bootstrap in progress.9.
Press the c button to silence the alarm.10.
Select Start on PC. Monitor progress of all selected channels11.
Power down pump.12.
1000SP01476
(MP v4.1.4)
1000SP01476
(MP v4.1.4)
1000SM00001 Iss. 18
25/86
Page 26
Alaris® Syringe Pump
Preventative Maintenance
Hard bootstrap
Load the software program onto your PC. Start the relevant pump software (not the ‘MP Only’ version).1.
Disconnect the battery and separate the pump.2.
Fit the Programme header onto the control board.3.
Reconnect the battery. The pump will alarm, press the c button to silence.4.
Align the IrDA converter pump with the IrDA window (optimum distance is 5cm), or connect RS232 cable. 5.
Connect to serial port.6.
Switch the Programme header to the correct position either RS232 or IrDA.7.
Switch on the Programme header.8.
Select GO on the PC software program.9.
Select Start on PC. Monitor progress of all selected channels10.
Power down pump.11.
Cold start
It may be necessary to carry out a cold start if the pump has changed between certain software. Refer to documentation supplied with
the software disk to see if cold start is required.
Enter access code 611, then power down when prompted.1.
Perform a full calibration.2.
Caution - Potential Erasure of Data:
A
Cold Start erases ALL information from the pump. This feature should only be used when changing between
incompatible software versions. Full recalibration and reconfiguration will be required. CareFusion technicians
should not re-instate drug information (this MUST be left to the customer).
Power Failure
Failures may occur when using laptops when communicating with Alaris® Syringe Pumps, due to power
requirements.
External power supply may be used in conjunction with IrDA or RS232 cable to compensate for lack of power
from laptop.
Please Note IrDA data transfer can be affected by bright sunlight or fluorescent lighting.
1000SM00001 Iss. 18
26/86
Page 27

Alaris® Syringe Pump
Preventative Maintenance
Pole Clamp Arm Update
The Pole Clamp Arm material has been changed to a stronger material to prevent the arm from bending when tightened.
The Pole Clamp Arm spares kit (part number 1000SP00589) replaces parts of the Pole Clamp assembly to address bent or slipping Pole
Clamps. Note: There is no requirement to remove the V Clamp. (see Figure 1)
V Clamp (remains fixed to
the pump case).
Arm clamp
Modified Knob Pole
Clamp Screw.
Apply Loctite 7471 here.
Figure 1 - Pole Clamp Arm replacement
Apply small amount of Castrol
LMX grease to this surface.
Apply Loctite 243 and Loctite 7471 here.
Spring
Ball Bearing
Pivot Screw (Torque to 2Nm
using Driver)
Motor Plate Strain Beam Update
Check motor plate serial number, if code is numeric only, numeric barcode or is alphanumeric beginning with prefix "PH", then this is
the current version of motor plate. The current version of motor plate does not require the motor plate beam support (see Figure 2). All
other versions of motor plate require the motor plate beam support (see Figure 3).
Motor Plate
Motor Plate Strain Beam Support
Figure 2 - Current Motor Plate Figure 3 - Motor Plate Strain Beam Support
1000SM00001 Iss. 18
27/86
Page 28

Alaris® Syringe Pump
Preventative Maintenance
Transmission Buffer Pad Update
Check Buffer Pad fitted if manufactured prior to March 2001 and serial numbers are within either of the ranges 8001-03468 and below
or 8002-06788 and below . If not fitted, clean the surface of the carriage face nearest the plunger drive tube and fit Buffer Pad in the
position shown (sloping edge to match carriage profile, see Figure 4). (see Chapter 6 Corrective Maintenance for instructions on how to
fit part)
Buffer Pad
Figure 4 - Buffer Pad location
Linear (PL3) Update
Fit the Linear upgrade kit (1000SP01488) to any pump that exhibits error PL3 and was manufactured prior to September 2008 with
serial number within the ranges:
8001-20585 and below •
8002-20783 and below•
8003-55260 and below•
8004-09725 and below•
8005-08623 and below•
This update includes a chassis with linear potentiometer fitted and Chassis PCB. See Chapter 6 Corrective Maintenance for instructions
on how to fit part.
Parts in the kit have the following enhancements:
Linear potentiometer - new gold plated and high insertion force crimp contacts. •
Chassis PCB - new gold plated contacts for the connector to the linear potentiometer and change to track layout.•
The enhancements will improve the contact quality between the Chassis PCB pins and the linear potentiometer crimp terminals. Also
the change to track layout on the Chassis PCB have been made to eliminate the exposed via hole contacts.
Follow additional instructions on how to deal with PL3 errors in Chapter 4 Troubleshooting.
1000SM00001 Iss. 18
28/86
Page 29

Alaris® Syringe Pump
Preventative Maintenance
Battery Test and Replacement
To test the battery perform the battery calibration, as outlined in the procedure in Chapter 2 Configuration and Calibration, and verify
that all pass criteria are met. If pass criteria are not met then replace the battery.
Battery charge retention will eventually degrade. So where retention is critical the internal battery should be replaced every three
years.
Replace the Main Battery
Remove the two case screws in battery cover, remove cover and battery.1.
Fit new battery.2.
Replace battery cover and secure with 2 screws.3.
A
A
Battery Cover
Battery
It is essential that the battery pack is calibrated after fitting as failure to do so will degrade the quoted auxiliary
battery power on this product.
The battery pack used in this Alaris® Syringe Pump is manufactured by CareFusion and includes a proprietary PCB
(printed circuit board) designed specifically for the Alaris® Syringe Pump, and in conjunction with Alaris® Syringe
Pump software, controls battery use, charge and temperature. Any use of battery packs that are not manufactured
by CareFusion in the Alaris® Syringe Pump is at your sole risk, and CareFusion does not provide any warranty for or
endorsement on any battery packs that are not manufactured by CareFusion. CareFusion’s product warranty shall not
apply in the event the Alaris® Syringe Pump has suffered damage or premature wear, or malfunctions or otherwise
operates incorrectly, as a result of use with a battery pack that is not manufactured by CareFusion.
1000SM00001 Iss. 18
29/86
Page 30

Alaris® Syringe Pump
Preventative Maintenance
Self-test Procedure (123)
Self-tests included in full test
Enter access code 123 to view the Test Selection menu (see Access Codes in chapter 2). Refer to table below for the tests in each menu
item.
Test Section Test Action
Software
Safety Processor
Full only
Sensor
Software info Displays the software version.
Data Set Info Displays the Data Set information. (pumps with Guardrails® Safety Software only)
Safety ID Check displays the version of the safety ID.
Safety LED Check red LED illuminated.
Safety Alarm Check Backup alarm sounds.
Serial Number Check displays serial number of unit.
Language Check displays correct language.
Real-time Clock Check displays correct date and time.
Service Date Check displays date when service is next required.
Disc Detect Check the display changes correctly to indicate if a disc is Out or In (Model CC only).
Line Pressure Check pressure is 000mmHg +/-20mmHg with no pressure applied (Model CC only).
Motor Encoder Check motor runs and Passed is displayed.
Drive Engage Check display indicates Drive Engaged or Disengaged when clutched/declutched.
Plunger Fit Check display indicates if the Plunger button is Out or In.
Plunger Position Check display smoothly and continuously changes during full plunger travel.
Insert the syringe size calibration tool (1000TG00095) and check the following values are
Syringe Clamp
displayed for diameters inserted:
12mm diameter = 11.5 to 12.5mm
32mm diameter = 31.5 to 32.5mm
Syringe Force Check motor runs and syringe force is displayed.
Battery Battery Check displays values in CAP, CHR and VOL; no dashes should be seen.
Audio Audio Speaker Check the main audible alarm sounds.
Display Check that all of the display pixels are illuminated.
Backlight Check that the backlight switches from LOW to HIGH when indicated.
Battery LED Check the Battery LED (Amber) flashes.
Visual Indicator
Key Keypad Press the key indicated and check changes to next key.
Comms Comms RS232 only. Check Nurse call and RS232 operation.
Start LED Check the Start LED (Green) flashes.
Stop LED Check the Stop LED (Amber) flashes.
Warning LED Check the Warning LED (Amber) flashes.
Alarm LED Check the Alarm LED (Red) flashes.
1000SM00001 Iss. 18
30/86
Page 31

Alaris® Syringe Pump
GND DSR RXD TXD NC C
NC
COM
5
4
32
1
NC O CTS
9876
Preventative Maintenance
Self-tests not included in full test
Test Section Test Action
Remote Remote Check the function of the IrDA output for remote access
Syringe clamp Displays calibration values for Closed and Open positions.
Calibration records
Linearity
Comms Test (123)
Select COMMS TEST from the displayed menu.
Section only applicable if RS232 Hardware option is fitted.
Note:
No specific customer test equipment is available to conduct the RS232 on nurse call alarm tests. It is assumed that the customer will have
associated systems that make use of the RS232 and nurse call options, hence:
The nurse call system can be tested, once connected to the customer facility, by running the pump and simulating an alarm condition
(e.g. Disengaging the drive while running).
The RS232 system can be tested by communicating with the pump using a customer application.
If no customer systems are available for in-use testing, the following connections to the 9 pin D type output socket will permit testing. It
is recommended that all test connections are made via a 9 way D type plug that can be fitted into the pump socket.
Plunger position Displays calibration values for Left, Middle and Right positions.
Syringe force Displays calibration values for 0, 3 and 10 kgf.
Line pressure Displays calibration values for 25, 500 and 1000mmHg (Model CC only)
Linearity Check the mechanism runs full travel and graph displays smooth linear travel.
Occlusion base Check the occlusion base level is within tolerance shown on graph.
Test Description
RS232 Test Only available when Nurse Call option is fitted.
NURSE CALL FITTED and RS232 SELECTED must be enabled () in access code 251 General Options
Note:
for this test to work. Connect the 9-pin D type connector to the 9 pin D type output socket at the rear
of the pump. The display ‘_ _ _ _’ will change to PASS if the communications test is successful.
Nurse Call Only available when Nurse Call option is fitted.
NURSE CALL FITTED and RS232 SELECTED must be enabled () in access code 251 General Options
Note:
for this test to work. Locate the 9-pin D type connector at the rear of the pump. Check that the Nurse
Call relay switches from NC to NO connections during test.
RS232 pinout
Pin Number
Required Action Comments
(Pump Socket Id)
1 Nurse call relay - normally closed connection With nurse call test in progress - Confirm continuity with pin 5 -
Alternately switches with pin 8.
2 Link pin 2 to pin 3 RS232 Tx and RX test link. With RS232 test in progress - Confirm
PASS is displayed on test screen.
3 See pin 2 -----
4 Not used -----
5 0 volt line With respect to pin 7.
6 Not used -----
7 Apply 9 to12 volts DC RS232 Power supply - with respect to pin 9.
8 Nurse call relay - normally open connection With nurse call test in progress - Check continuity to pin 5 -
9 Nurse call relay - common connection ----
1000SM00001 Iss. 18
Alternately switches with pin 1.
31/86
Page 32

Alaris® Syringe Pump
Preventative Maintenance
Calibration Verification Mode (240)
The Calibration Verification Mode allows a qualified service engineer to verify the required calibration in normal operation mode by
selecting to use the Calibration Verification profile. This tech mode screen allows activation of this profile with the required settings only
for the next time the pump is powered up in normal operation mode. Confirmation of this profile on power up is required. This profile
and the appropriate dedication mode set to allow verification will be discarded when the pump is powered down.
1. 240.
Enter the access code
Pressing the
2. OK key will activate the Calibration Verification profile as defined below as the currently selected profile, clear the
current drug setup, and return to the access code screen.
Pressing the
3. QUIT key will return to the access code screen
Note:
The Calibration Verification profile Name will be CALIBRATION VERIF.
CALIBRATION VERIF. settings:
Profile name is displayed on the main screen during normal operation mode. This provides a clear indication of the pump being •
set in this profile. Likewise all logging will be against this profile and can be filtered out with the CQI PC package,
Infusion Rate Maximum will be 200ml/h•
Pressure Maximum will be 1000mmHg / L10•
Number of Syringe Brands will be 1 i.e. BD Plastipak•
Syringe enabled will be the BD Plastipak 50ml syringe model•
Note:
If BD Plastipak 50ml physical characteristics are not available in the current data set then these settings will be extracted from
the default data set.
Profile will not have any drug setups•
The Calibration Verification profile will take the default values for all remaining parameters, except ml/h will be the only enabled •
dosing only unit and Auto Save will be disabled.
The Calibration Verification profile will count as an additional profile in the data set only for the next time the pump is powered •
up in normal operation mode, but will not be selectable from the list of data set profiles.
1000SM00001 Iss. 18
32/86
Page 33

Performance Verification Procedure
Model / Serial Number: Service Order / Inventory Number:
Hospital Name / Reference: Software Version:
Inspection Physical inspection and clean
Error Log
376
Self Test
123
Infusing
ch4
ch3
Check/set serial number, set service date (optional)
Check all functions in self-test
Check date and time is correct (set as required (251) ch2
Syringe size detection test
12 mm spacer (11.5 to 12.5)•
32 mm spacer (31.5 to 32.5)•
Alarms functionality check
Drive Disengaged, Check Syringe, AC power fail, Pressure Disc out (CC), Near End of Infusion, End of Infusion
Ensure pump works on battery and AC mains
Alaris® Syringe Pump
Preventative Maintenance
Linear speed test*
Pump set to 200 ml/h, syringe type BD Plastipak 50, for a distance of 15 mm.
2 min 27.01 secs to 2 mins 30.59 secs
Occlusion test
Pump set to 100 ml/h, syringe type BD Plastipak 50, alarm level L-3,
2.4 kgf to 3.8 kgf
Verification Tests
Setup Set rate to zero (or lowest value possible), Clear Volume Infused and VTBI
Electrical Safety Tests
Verification
Performed By _______________________________
chX
indicates the chapter number in the Technical Service Manual (TSM) - 1000SM00001.
CH3
= Refer to TSM Chapter 3
E.G.
CH3
OR Dedicated (CC), alarm level 200 mmHg, drive occlusion at
2.4 kgf to 3.8 kgf
Line pressure readings (CC)
Alarm set to 50 mmHg – pump alarms 40 mmHg to 60 mmHg
Alarm set to 750 mmHg – pump alarms 710 mmHg to 790 mmHg
Clear Error / Alarm/Battery logs (as required)
Class I Type CF
Test in accordance with the standard
EN 60601-1 and test equipment operation
manual.
Sign
Test results are stored:
Electronically ¨
Print-out ¨
Other ¨
____________________
___________________
Print
______ mins ______ secs
_____________ kgf
_____________ mmHg
_____________ mmHg
PASS / FAIL
_____________________
Date
A
* Latest issue of the plunger protector jig (0000JG00014 Issue 7) has been improved so that the needle of the dial
gauge rests upon the plunger head (avoid resting the needle on the moulding flash line) of the pump. This improves
the linear speed accuracy test results as any variation caused by the jig movement during the test are eliminated.
1000SM00001 Iss. 18
33/86
Page 34

Alaris® Syringe Pump
Troubleshooting
Troubleshooting4
Review logs
Event Log download
A PC application known as the Event Log Download Utility (ELDU) (part number 1000SP00209) is available to download logs from Alaris®
Syringe Pumps.
The Event Log holds up to 1500 individual events. Pumps with Guardrails® Software enabled retain one year of events.
For Alaris® Syringe Pumps (with Plus software) the event log is downloaded via the Alaris® Transfer Tool (1000SP01463), refer to the
relevant Directions For Use for further details.
ELDU operation
Click on ELDU icon on PC.1.
Click Accept to agree with Restrictions of Use and continue.2.
Select Configure from drop-down menu.3.
Select Setup Pump and choose Alaris® as pump type.4.
Select Settings to select log to be downloaded.5.
Check communications are set as follows:6.
Required PC com port selected.•
Set baud rate to 38400.•
Click OK to confirm.
7.
Align the IrDA converter pump with the IrDA window (optimum distance is 5cm), or connect RS232 cable.8.
Power up pump.9.
Click Download Log from main screen.10.
Press Close, when finished.11.
Select File from drop-down menu and save file. Log may be printed here as required.12.
Information Logs (376)
Use access code 376 to view the information logs (see Access Codes in chapter 2).
Log View Notes
Service Displays the last 10 fault codes.
Clear Service Clears any information stored in the service log.
Displays the complete event log (maximum 1500 events except
Event
Key Displays the last 200 key presses and the time they occurred. Does not record while in Tech mode.
Use Displays the hours of use since reset and since last cold start. Press OK to clear hours since reset.
Access code 376 provides the following additional service options:
Service Date Set the date when pump will display ‘Service due’ and any service message entered.
Service Message Enter message to be displayed on service date.
Serial Number Record the serial number of the pump.
Unit Reference Free-form text field for user reference only.
Event Log Access provided when standard power-up mode leads to errors such that the Event Log access from the
PCB Identification
Pumps with Guardrails® Software enabled which have one year of
events).
Options
Allows Control PCB ID to be reviewed. (Pumps with Guardrails® Safety Software only) Number
d button cannot be accessed.
Option to view the time and date at which
they occur.
Will not be available if there is no data in the
service log.
Option to view the time and date at which
they occur.
1000SM00001 Iss. 18
34/86
Page 35

Software fault codes
The following errors, MT1, DE1, PF1, PP1 and SC1 may be experienced if the self test operation or calibration
A
Code Module Failure Action/Replace
AC1
AC2 AC VCO failure
AD1 ADC Converter Voltage reference/Power regulation Control PCB
AM1
AM2 VCO failure
AS1
AS3 Speaker current test at power up
BT1
BT2 Battery cell voltage is low
BT3 Battery cell voltage is high
BT4 Battery discharging when connected to mains.
CK1 Clock Excessive timing drift Control PCB
DE1
DE2 Drive engagement opto self test
DE3 Emitter in wrong state
DB1
DB2 Drug database file retrieval
DB3 Drug database file storage
DB4 CRC Error
DS1
DS2 Dosing storage failure
DS3 Dosing data failure
DS4 Dosing drug library failure
DS5 Dosing patient data failure
DS6 Dosing IDFS failure
DS7 Dosing Data Set manager failure
DS8 Dosing Profile manager failure
DSM1
DSM2 Data Set integrity failure
DSM3 Data Set incompatible format
DSM4 Data Set CRC failure
DSM5 Data Set read failure
DSM6 Data Set element CRC failure
DSM7 Data Set storage failure
DSM9 Data Set data corruption
operation has been accessed by quitting from the configuration menu. If these are displayed the pump should be
power cycled and these operations entered directly.
AC Alarm manager
Audio manager
Audio Status
Battery
Drive Engage Detect
Drugs Manager
Dosing Manager
Data Set Manager
AC alarm manager failure
Audio status output driver
Software execution
Battery gas gauge
Drive engagement software module Control PCB
Drug database file system
Dosing retrieval failure
Data Set loading failure
Control PCB
Control PCB
Speaker, wiring or Control PCBAS2 Audio status monitoring input ADC
Battery or Control PCB
Plunger drive flexi, Control PCB or
Transmission PCB flexi
Control PCB
Control PCB
Upload or check Data Set
Upload or check Data Set Control
PCB
Control PCBDSM8 Data Set storage retrieval failure
Alaris® Syringe Pump
Troubleshooting
1000SM00001 Iss. 18
35/86
Page 36

Alaris® Syringe Pump
Code Module Failure Action/Replace
EV1
EV2 File storage software module
EV3 Log read index
EV4 Log write index
EV5 Log data read
EV6 Log data write
EV7 Log data seek
EV8 Log repair failure
EV9 Log format failure
EV10 Log reporting failure
EV11 Log extracting failure
EV12 Log pack failure
EV13 Log unpack failure
FD1
FD2 Alarm manager software module
FD3 Plunger drive software module
FD4 Pressure monitor software module
FD5 Syringe software module
FD6 User configuration software module
FD7 Fluid delivery setup data retrieval
FD8 Fluid delivery setup data storage
FD9 Fluid delivery critical data
FD11 VI cross check error
FL1
FL2 File storage software module
FL3 Log read index
FL4 Log write index
FL5 Log data read
FL6 Log data write
FL7 Log data seek
FL8 Log repair failure
FL9 Log format failure
FL10 Log reporting failure
FL11 Log extracting failure
FL12 Log pack failure
FL13 Log unpack failure
GG1 Gas Gauge Communications with gas gauge Battery or Control PCB
GR1 Guardrails® Limit Guardrails® Limit failure Control PCB
IM1
IM2 Serial number to set
IM3 RTC to set
IM4 Storage failure Control PCB
Event Log
Fluid Delivery
Fluid Log
Identification Manager
Open log file at power up
Control PCB
Fluid delivery software module
Control PCB
Open fluid log file at power up
Control PCB
Data corrupt Control PCB
Set Serial Number in access code
376
Set RTC in access code 251.
Control PCB
Troubleshooting
1000SM00001 Iss. 18
36/86
Page 37

Alaris® Syringe Pump
Code Module Failure Action/Replace
KL1
KL2 File storage software module
KL3 Log read index
KL4 Log write index
KL5 Log data read
KL6 Log data write
KL7 Log data seek
KL8 Log repair failure
KL9 Log format failure
KL10 Log reporting failure
KL11 Log extracting failure
KL12 Log pack failure
KL13 Log unpack failure
KY1
KY2 Line failure
LC2
LC3 LCD display memory test
ME1
ME2 Overrun detected
ME3
MT1
MT2
MT3
MT4 Motor rotation in wrong direction
MT5 Motor rotation speed has drifted
MT6 Running at wrong rate
MT7 Motor rotation detected when it should be stopped
MT8 Motor not rotating when it should be
MT9 Motor rotation inhibit control of Safety Processor
MT10
MT11
MT12 Motor critical data
NC1
NC2 Monitor signal is out of range
NC3 Relay current monitor drive
NC4 Safety processor failure
PA1
PA2 Patient Data storage failure
PA3 Patient Data data error
PA4 Patient Data IDFS error
PB1
PB2 Plunger button opto self test
PB3 Emitter in wrong state
Key Log
Keypad
LCD
Motor Encoder
Stepper Motor
Nurse Call
Patient Data
Plunger Button
Open key log file at power up
Keypad key stuck down for 10mins
LCD control parameter storage
Motor encoder module software
Motor encoder interrupt service software run when
encoder is disabled
Motor module software
Encoder has failed, preventing motor software
continuing to run
Safety processor has failed, preventing motor software
continuing to run
Motor rotation travel data has reached maximum value
ADC preventing nurse call operation
Patient Data retrieval failure
Plunger button module software
Control PCB
Keypad or Control PCB
Control PCB or Display PCB
Motor encoder or Control PCB
Check motor wire connections, opto
flag not slipping and encoder gear
is not in line with opto. Chassis PCB,
Control PCB.
Control PCB or RS232/Nurse Call
PCB
Control PCB
Plunger drive flexi, Control PCB or
Transmission PCB flexi
Troubleshooting
1000SM00001 Iss. 18
37/86
Page 38

Alaris® Syringe Pump
Code Module Failure Action/Replace
PC1
PC2 PIP Controller critical data
PC3 Phase module critical data
PC4 Fluid delivery software module
PC5 PIP Controller setup data retrieval
PC6 PIP Controller setup data storage
PD1
PD3 Emitter in wrong state
PF1
PF2 Gripper opto module software
PF3 Plunger button opto module software
PFM1
PFM2 Profile retrieval failure
PFM3 Profile startup failure
PFM4 Profile data corruption
PG1
PG2 Plunger gripper opto self test
PG3 Emitter in wrong state
PL1
PL2 Alarm manager software module
PL3 Plunger drive travel deviation See next page.
PL4 Plunger position monitor software
PL5 Motor software module
PL6 Syringe software module
PL7 User configuration option software
PL8
PL9 Excessive plunger drive travel deviation when in motion
PIP Controller
Pressure Disc
Plunger Fitment
Profile Manager
Plunger Grippers
Plunger Drive
PIP Controller software module
Pressure disc module software
Plunger fitment software module
Profile storage failure
Plunger gripper module software
Plunger drive software module
Excessive plunger drive travel deviation when
stationary
Control PCB
Pressure disc opto or Control PCBPD2 Pressure disc opto self test
Optos,Cables, Control PCB or
Plunger assy.
Control PCB
Plunger gripper opto, Transmission
PCB, or Control PCB
Control PCB or Chassis PCB
Control PCB or Chassis PCB
Perform Plunger Postion and
Syringe Force calibrations.
Carriage assembly, Motor plate,
Linear Potientiometer or Chassis
PCB
Troubleshooting
1000SM00001 Iss. 18
38/86
Page 39

Alaris® Syringe Pump
Troubleshooting
PL3 Error
Introduction
A PL3 is an alarm code that is triggered when the pump observes deviation within the linear measurement system. Deviation is
calculated between the measured value (linear potentiometer) and the calculated values derived from the motor travel. The alarm is
designed to detect failures within the transmission.
This alarm code is not available in the Alaris® Syringe Pumps (with Plus software).
Note:
Failure causes
Worn half nut•
Fluid ingress on or around the linear potentiometer•
Loose motor plate bearing•
Contact resistance linear potentiometer/Chassis PCB•
Diagnosis
To establish where the fault lies perform the following test and checks:
Perform precondition test as detailed in the Syringe Force Calibration procedure see Chapter 2, Configuration and Calibration. If •
the pump does not reach 10kgf this would indicate a worn half nut.
Open the case and internally inspect the pump for any signs of fluid ingress.•
With the case open and the mechanism NOT declutched, try to push the plunger in the direction shown in figure 1. Check that •
there is no movement of the motor plate bearing (figure 2). If there is movement this would indicate a loose motor plate bearing.
Actions
Check the pump was manufactured prior to September 2008 and serial number is as listed or below (Serial numbers 8001-20585, •
8002-20783, 8003-55260, 8004-09725 or 8005-08623), if so then fit the Linear upgrade kit (1000SP01488) that contains chassis
with linear potentiometer and Chassis PCB.
Motor Plate
bearing
Replace the carriage assembly (1000SP01107) if the half nut is worn, as indicated by precondition test.•
Replace the motor plate (1000SP01110) if the motor plate has a loose motor plate bearing.•
Replace any parts damaged by fluid ingress.•
Perform Plunger Position and Syringe Force calibrations, see Chapter 2, Configuration and Calibration.•
Testing must be performed in accordance with the Performance Verification Procedure (PVP), see Chapter 3, Preventative •
Maintenance.
A
Figure 1 Figure 2
Linear potentiometer and Chassis PCB included in the Linear upgrade kit have had the following enhancements:
Linear Potentiometer - Crimp contacts changed to gold plated and high insertion force to improve contact
Chassis PCB - Connector changed to gold plated to improve contact with linear potentiometer and track layout
change to eliminate bridging connections on the linear signal tracks
1000SM00001 Iss. 18
39/86
Page 40

Alaris® Syringe Pump
Troubleshooting
Code Module Failure Action/Replace
PM1
PM2 Pressure sensor
PM3 Syringe force
PM4 User configuration options
PM5 Syringe
PM6 Setup retrieval failure
PM7 Setup storage failure
PP1
PP2 ADC
PP3 Plunger position sensor
PP4 Plunger position sensor calibration Calibrate linear travel
PP5 Plunger position cal data retrieval
PP7 Drive engagement software
PS1
PS2 ADC
PS3 Current reading invalid
PS5 Voltage (Test) reading invalid
PS6 Pressure sensor calibration Calibrate pressure sensor
PS7 Pressure sensor cal data retrieval
PS8 Pressure sensor cal data storage
PS9 Pressure sensor amplifier gain
PS10 Pressure sensor shift failure
SC1
SC2 ADC
SC3 Syringe clamp sensor readings
SC4 Syringe clamp calibration Calibrate syringe clamp
SC5 Syringe clamp cal data retrieval
SC6 Syringe clamp cal data storage
SD1
SD3 Service data file contents
SF1
SF2 ADC
SF3 Syringe force sensor current reading
SF5 Voltage (test) reading
SF6 Syringe force sensor calibration Calibrate syringe force
SF7 Syringe force cal data retrieval
SF8 Syringe force cal data storage
SF9 Syringe force sensor amplifier Motor plate or Control PCB
SF10 Syringe force output Calibrate syringe force. Motor plate.
SP1
SP3 Safety processor
Pressure Measurement
Plunger Position
Monitor
Pressure Sensor
Syringe Clamp
SD Data
Syringe Force
Safety Processor
Pressure measurement software
Plunger position monitor software
Pressure sensor software module
Syringe clamp software module
Service data file retrieval
Syringe force sensor software
Safety processor program memory
Control PCB
Control PCB
Plunger position linear
potentiometer.
Control PCBPP6 Plunger position cal data storage
Control PCB
Pressure sensor or Control PCBPS4 Voltage (Normal) reading invalid
Control PCB
Pressure sensor or Control PCB
Control PCB or syringe clamp
potentiometer
Control PCB
Perform Cold start. Control PCBSD2 Service data file storage
Control PCB
Motor plate or Control PCBSF4 Syringe force sensor normal reading
Control PCB
Control PCBSP2 Safety processor ID version
1000SM00001 Iss. 18
40/86
Page 41

Alaris® Syringe Pump
Troubleshooting
Code Module Failure Action/Replace
SV1
SV2 File storage software module
SV3 Log read index
SV4 Log write index
SV5 Log data read
SV6 Log data write
SV7 Log data seek
SV8 Log repair failure
SV9 Log format failure
SV10 Log reporting failure
SV11 Log extracting failure
SV12 Log pack failure
SV13 Log unpack failure
SY1
SY4 Plunger fitment
SY5 Plunger button stuck in Check plunger button. Control PCB.
SY6 Syringe data table retrieval
SY7 Syringe data table storage
TC0
TC1 TCI data corruption
TC2 TCI not configured
TC3 TCI incorrect state
TC4-12 PK Model error 4-12
TC13 PK Model parameters error
TC14 PK Model init error
UC1
UC3 User config. option data retrieval
UC4 User configuration model Perform Cold start
UT1
UT2 User timer software request
Service Log
Syringe Manager
PK / TCI
User Config. options
User Timer
Service Log software module
Syringe manager software module
No fault found
User config. option file retrieval
User timer master clock
Control PCB
Control PCBSY2 Syringe clamp
Control PCB
Contact your local CareFusion,
Alaris® Products service
representative
Control PCBUC2 User config. option file storage
Control PCB
1000SM00001 Iss. 18
41/86
Page 42

Alaris® Syringe Pump
Troubleshooting
Exception error handling
Exception errors include Assertion Errors and Enum Failure Errors and are used to trap logical errors in the software execution.
The pump will display the error type, the title of the software module in which the error occurred and the line number. The user should
make a note of these for use in diagnosis. This information is stored in the service log (access code 376).
After an error, the pump will not store information when powered down. When the pump is switched on again, the user should always
confirm clear setup if this is not done automatically.
General fault diagnosis
Parts to Check/Test
Dropped or damaged
Exposed to fluids
No battery power
General Fault
No AC mains power
Delivery rates out of tolerance
Front Case
Rear Case
Labels and Keypads
Mechanism
Control PCB
Power PCB
Display PCB
Battery
Mains Lead
Fuses
1000SM00001 Iss. 18
42/86
Page 43

Circuit Descriptions5
Functional module block diagram
Alaris® Syringe Pump
Circuit Descriptions
Start/Stop
Keypad
Rate/Soft
Keypad
Function
Keypad
Display
PCB and
Backlight
Syringe sizing
potentiometer
PL5
PL7
PL9 PL6
PL3
PL15
Keypad
and LED
Indicators
Display
CCFL
Backlight
Syringe
Clamp
Control PCB
Transmission
Interface
Real-time
Clock
Backup
Battery
Safety
Processor
PL8
Transmission
Linear Travel
Potentiometer
Motor
Plate
PL4
Chassis PCB
IrDA
PL3
Carriage PCB
PL5
PL2
Main Audio
Speaker
Pressure
Transducer
(Model CC)
Options
RS232/Nurse
Call
Programme
Header
PL18
PL1
PL2
Audio Alarm
Pressure
Transducer
Serial
Comms
Main Processor
Backup
Speaker
Motor Drive
Power
Distribution
(Battery
Charger)
PL10
PL13
PL12
PL1
Plunger
PCB
Stepper
Motor
Battery with
Gas Gauge
Mains
Inlet
Power PCB
1000SM00001 Iss. 18
43/86
Page 44

Alaris® Syringe Pump
Circuit Descriptions
Module overview functional description
The Alaris® Syringe Pumps are designed to be serviced generally to major assembly level. The PCBs are designed as non-serviceable
items and as such, can only be replaced as complete parts.
The major assemblies are:
Control PCB •
Power Supply Unit PCB•
Display PCB •
Battery•
Transmission •
Transducer (Model CC)•
CareFusion will make available, on request, circuit diagrams which will assist appropriately qualified technical personnel to repair those
parts of the device which are designated by the manufacturer as repairable.
Control PCB
Contains the main processor module, which provides the control functions for almost all aspects of the pump. It drives and monitors
all other modules using the program code stored in the flash eprom. The main processor runs the main application program. The main
processor directly interfaces to:
Safety Processor•
Keypads•
Display•
Real Time Clock•
Communications Switch to IrDA and RS232 (optional) interfaces•
Nurse Call Output•
Indicator LEDs•
Audible Alarm•
Motor – controller and dual channel coil driver DAC•
System Sensors (including: syringe clamp, plunger position, drive engagement opto, plunger button opto, syringe force sensor, •
line pressure sensor, pressure disc opto, motor encoder, AC power).
Backlight•
Power supply•
Battery gas gauge•
The function of the Safety Processor Module is to ensure the correct operation of the Main Processor by constantly exchanging data. If
an error is detected, the module can independently disable the stepper motor that drives the transmission. Additionally, it can create
both audible and visible alarms using its dedicated piezoelectric buzzer, alarm LED and, if fitted, the Nurse Call Interface.
The Safety Processor controls (independent of main processor):
Audio sounder•
Visual indicator LED•
Control signal to inhibit motor drive•
Power supply hold up control•
Pressure Transducer (Model CC)
Monitors the line pressure when the pressure disc is inserted and flags the presence of a pressure disc. The Control PCB checks the
transducer for presence of a pressure disc and the line pressure when a disc is present.
Power Supply Unit PCB
The Power Supply Unit (PSU) is a single output switched-mode power supply rated at 15 VDC/1.6 A continuous duty. The PSU has a wide
input voltage range of 85 to 264VAC, 47-63 Hz single phase.
Display PCB
The Pump uses a Cold Cathode Fluorescent Lamp (CCFL) as a backlight for the negative mode LCD display. The CCFL Backlight Supply
Module generates the high voltages required to drive the lamp and facilitates software based brightness control.
1000SM00001 Iss. 18
44/86
Page 45

Alaris® Syringe Pump
Circuit Descriptions
Battery
The Battery Pack Module provides system power in the absence of a mains supply.
The Battery Pack Module consists of six 1 2V 2.7Ah NiMH battery cells connected in series, a thermal fuse, thermal circuit breaker and
Gas Gauge Module sealed in a heat shrink sleeving.
The Gas Gauge Module is permanently connected across the battery terminals so that it can monitor terminal voltage, charge /
discharge current and the battery pack temperature.
Through charge monitoring information, from the Gas Gauge Module, the Control PCB Main Processor Module determines the battery
charge level and hence ‘Battery Low’ and ‘Battery Empty’ conditions.
Battery capacity will reduce over time.
Battery calibration will update the Gas Gauge Module with ‘up to date’ battery capacity information.
If the battery pack fails to achieve the calibration limits, it is recommended that the battery pack is replaced and calibration performed.
Transmission
The Transmission Interface Module provides the Pump with the capability of monitoring a number of critical parameters associated with
the transmission operation. The device can detect failures or incorrect operation of the transmission and prevent incorrect drug dosage
administration.
The electronics for this module occupy three separate PCBs that are located in various areas of the transmission as follows:
Plunger PCB
Carriage PCB
Chassis PCB
Located inside the Plunger Holder Assembly. Contains the electronics that detect correct syringe plunger
location and gripper motion.
Located on the Transmission Carriage. Contains electronics that detect correct engagement of the Half Nut
with the Leadscrew.
Located on the Chassis Extrusion. This facilitates measurement of motor speed, syringe drive force and linear
plunger position. Additionally, the IrDA compliant infrared transceiver is positioned on the reverse side of
this PCB. The stepper motor which drives the mechanical transmission of the pump is controlled by the Motor
Drive module on the Control PCB.
1000SM00001 Iss. 18
45/86
Page 46

Alaris® Syringe Pump
Corrective Maintenance
Corrective Maintenance6
Corrective Maintenance
This chapter contains procedures required to properly disassemble, repair and replace parts and then to reassemble the pump.
Following all spare part replacement and repair activities, testing must be performed in accordance with the Performance Verification
Procedure (PVP), see Chapter 3, Preventative Maintenance. Additional testing and calibration may be required after certain repairs are
completed, see table below for more information.
Ensure the pump is disconnected from the AC power supply and switched off before attempting to service.
A
The pump contains static-sensitive components and therefore strict ESD precautions should be observed at all
times.
Always protect the plunger holder and syringe clamp when the pump is upside down. For regular servicing, the use
of the case support cradle Part No. 0000JG00047 is recommended.
Batteries should be disposed of as outlined by the local country regulations. Do not send batteries back to the
manufacturer.
For fastener torque settings, please refer to Appendix D Fitting and Replacement guidelines.
Only use CareFusion recommended spare parts.
Performance Verification Procedure
Battery Calibration
Syringe Clamp Calibration
Plunger Position Calibration
Test/calibration to perform
Line Pressure Calibration
Syringe Force Calibration
Repair/Replacement of
Front Case
Rear Case
Labels and Keypads
Mechanism
Control PCB
Power PCB
Display PCB
Chassis PCB
Battery
Syringe Size clamp /
potentiometer
Pressure Transducer
= Required
Blank = Optional
1000SM00001 Iss. 18
46/86
Page 47

Access to pump
Replacement Procedure
Remove the two case screws in battery cover, remove cover and battery.1.
Remove the six case screws.2.
Model CC only: Insert a flat-blade screwdriver into the blanking plug of transducer, prise plug away from transducer and remove 3.
securing screw.
Carefully separate case halves and disconnect cables.4.
Where necessary, remove the foot rivets with a flat-blade screwdriver and remove the feet from the case. Refer to additional 5.
information on the following page concerning rivet orientation.
Reassemble in reverse order.6.
(H/J) Case Screws
Alaris® Syringe Pump
Corrective Maintenance
C Blanking Plug
F Pressure Transducer
I Feet
Note: Feet shown are the rivet
B Battery Cover
E Rear Case
D Front Case
A Battery
Note: It is essential that the battery pack is calibrated
after fitting as failure to do so will degrade
the quoted auxiliary battery power on this
product.)
Item Description Part Number
a
b
c
d
d
d
d
d ASENA PK, Kit, Front Case 1000SP01204
e
e
f
g
h
i ASENA SP, Kit, Spare adhesive foot rivet replacement 1000SP00593
i ASENA SP, Kit, Spare adhesive foot 1000SP00595
j Alaris SP main case screws 80 off 1000SP01325
ASENA SP, Assy, Battery 1000SP01122
ASENA SP, Battery Cover/Handle 1000SP01121
ASENA CC, Assy, Plug Blanking Transducer 1000ME01317
ASENA GS, Kit, Front Case 1000SP00478
ASENA GH, Kit, Front Case 1000SP00479
ASENA TIVA, Kit, Front Case 1000SP00480
ASENA CC, Kit, Front Case 1000SP01153
Alaris GS/GH/TIVA/PK, Kit, Rear Case 1000SP01115
ASENA CC, Kit, Rear case 1000SP01154
ASENA CC, Kit, Pressure Transducer 1000SP01155
ASENA SP, Case Sealing Cord (1m) (internal, not shown) 1000ME00311
ASENA SP, Kit, Fixings (screws, washers etc) 1000SP00466
style. Adhesive backed
feet are now available.
1000SM00001 Iss. 18
47/86
Page 48

Alaris® Syringe Pump
Corrective Maintenance
The Pump has two types of rivet feet, one type is white and the other is black. The rivet feet can be removed and
replaced without opening the pump, and may be replaced with adhesive backed feet as described below.
There are also two types of cases, one that had rivet feet and therefore holes in the cases for them and the other
is without the holes but only a recess for the adhesive backed foot to fit into.
For cases that had rivet feet and therefore holes, kit 1000SP00593 is required. For cases without the holes, kit
1000SP00595 is required.
Kit 1000SP00593 contains a fitting instruction, 242 adhesive backed feet, 242 foot bonding pads and one 20g
tube of Loctite 454 gel adhesive. This kit is intended as a replacement for the rivet style foot and has enough
feet for 48 pumps.
Kit 1000SP00595 contains 11 adhesive backed feet only. This kit is intended for cases that do not have the rivet
feet and therefore no holes in the cases. Only a recess is present for the adhesive backed foot to be placed into.
1000SM00001 Iss. 18
48/86
Page 49

Rear case and subassemblies
Power Supply Unit and Speaker
Replacement Procedure
Disconnect the PSU cable.1.
Remove the three PSU screws.2.
Remove earth wire screw and washer.3.
Remove PSU and insulator.4.
With a pair of soft-faced pliers, carefully compress the catch holding the internal speaker and pull the speaker up and out.5.
Reassemble in reverse order.6.
Alaris® Syringe Pump
Corrective Maintenance
C PSU Screws
A Speaker
B PSU
D PSU Cable
E PSU Insulator
C Earth Screw
Earth Wire
Item Description Part Number
a
b PSU / Insulator Assy SP E / DHR 1000SP01448
c
d Assy Cable PSU P8000 1000SP00094
e Insulator PSU Asena SP 1000ME01306
ASENA SP, Kit, Speaker 1000SP01130
ASENA SP, Kit, Fixings (screws, washers etc) 1000SP00466
1000SM00001 Iss. 18
49/86
Page 50

Mains inlet, PE stud and magnet
Replacement Procedure
Remove two nuts to remove PE stud.1.
Remove the two screws on Mains inlet.2.
Remove mains inlet and retainer.3.
Remove magnet by lifting one end.4.
Reassemble in reverse order.5.
Alaris® Syringe Pump
Corrective Maintenance
Retainer
B Mains Inlet
A PE Stud
C Magnet
E Mains Inlet Retainer Screws
Item Description Part Number
a ASENA SP/GW, Kit, PE Stud 1000SP00467
b ASENA SP, Kit, Mains Inlet 1000SP01124
c Magnet IR Detect 1000ME01303
d ASENA SP, Fuse, T-1.25A Slow Blow, Mains (not shown) 1000EL00222
e
ASENA SP, Kit, Fixings (screws, washers etc) 1000SP00466
1000SM00001 Iss. 18
50/86
Page 51

Pole clamp and RS232
Replacement Procedure
Remove three pole clamp screws.1.
Remove two tube restraint screws.2.
Remove two nuts and washers from RS232 socket screws.3.
Reassemble in reverse order.4.
Alaris® Syringe Pump
Corrective Maintenance
E RS232 Socket Connector
C Tube Restraint RS232
B Tube Restraint
A Pole clamp
F
Restraint
screw
F Pole Clamp screw
The Pole Clamp Arm material has been changed to a stronger material to prevent the arm from bending when
tightened.
The Pole Clamp Arm spares kit replaces parts of the Pole Clamp assembly to address bent or slipping Pole
Clamps. Note: There is no requirement to remove the V Clamp.
V Clamp (remains fixed to
the pump case)
D Arm clamp
Apply small amount of Castrol
LMX grease to this surface
Apply Loctite 243 and Loctite 7471 here
Spring
Ball Bearing
D Modified Knob
Pole Clamp Screw
Apply Loctite 7471 here
Item Description Part Number
a ASENA SP, Assy, Pole clamp 1000SP00115
b ASENA SP, Tube restraint blank 1000ME01213
c ASENA SP, Tube restraint RS232 1000ME01214
d SPARE KIT POLE CLAMP ARM 1000SP00589
e ASENA SP/GW, Kit, RS232 connector 1000SP00468
f
g Pole Clamp Snake Eye Driver (not shown) 1000ME01466
ASENA SP, Kit, Fixings (screws, washers etc) 1000SP00466
Pivot Screw (Torque to 2Nm
using Driver)
1000SM00001 Iss. 18
51/86
Page 52

Rail cam
Replacement Procedure
Remove screw from lever release.1.
Remove screw from lever rail cam.2.
Remove locking washer from spring.3.
Reassemble in reverse order.4.
A Lever Release
A Rail Cam Kit
Alaris® Syringe Pump
Corrective Maintenance
C Lever Release
B Lever Rail Cam
A Lever Rail Cam
Item Description Part Number
a ASENA SP, Kit, Rail Cam 1000SP01114
b Alaris SP Cam rail clamp only kit 1000SP01323
c Alaris SP Cam rail relase level only kit 1000SP01324
1000SM00001 Iss. 18
52/86
Page 53

Front case and subassemblies
Control PCB and RS232 (if option fitted)
Replacement Procedure
Disconnect cable from RS232 PCB.1.
Remove the four retaining screws and washers from RS232 PCB.2.
Remove the three retaining screws and disconnect all flexi and cable connections. Push the motor towards the transmission to ease 3.
removal of Control PCB.
When fitting Control PCB ensure all flexi and cables are routed clear of PCB.4.
Connect all flexi and cable connections - secure with the three retaining screws.5.
Reassemble RS232 PCB in reverse order.6.
Alaris® Syringe Pump
Corrective Maintenance
B RS232 PCB and Fixings
Motor
A Control PCB
C Retaining screws
1000SM00001 Iss. 18
53/86
Page 54

Control PCB Reverse side
Alaris® Syringe Pump
Corrective Maintenance
E Piezo sounder
The removal and replacement of soldered components should only be undertaken by engineers trained to IPC
A
Item Description Part Number
a Asena GS, Control PCB, Mk3 1000SP01275
a Asena GH, Control PCB, Mk3 1000SP01272
a Asena CC, Control PCB, Mk3 1000SP01271
a Asena TIVA, Control PCB, Mk3 1000SP01273
a Asena PK, Control PCB, Mk3 1000SP01455
a Alaris GH GR Control Panel Spares Kit 1000SP01359
a Alaris CC GR Control Panel Spares Kit 1000SP01360
a Alaris GH Guardrails Control Board/RS232 1000SP01318
a Alaris CC Guardrails Control Board/RS232 1000SP01317
a GH Cntrl PCB Plus S/W & Plus Dataset 1000SP01472
a GH Cntrl PCB Plus S/W & Plus GR Dataset 1000SP01473
a CC Cntrl PCB Plus S/W & Plus Dataset 1000SP01474
a CC Cntrl PCB Plus S/W & Plus GR Dataset 1000SP01475
b ASENA SP, Kit, RS232 (PCB and Fixings) 1000SP01160
c
d Asena SP, Battery BT1 3.6V 70mAh NiMH 1000EL00719
e Alaris SP, Buzzer LS2 (SMD) (MKIII) 1000EL00718
standards.
The pump contains static sensitive components and therefore strict ESD precautions should be observed at all
times.
Prior to removing any component it should be established if the PCB being reworked is a lead or lead free device. If
in doubt, contact your CareFusion affiliate office or distributor for further information.
ASENA SP, Kit, Fixings (screws, washers etc) 1000SP00466
D Battery
1000SM00001 Iss. 18
54/86
Page 55

Display PCB
Replacement Procedure
Remove the flexible circuit ferrite.1.
Remove the four display fixing screws and two display mounting brackets.2.
Reassemble in reverse order. For the Model GS fit the adaptor bracket to the display.3.
Secure the shelf keypad flexi to the display using double-sided adhesive pad.4.
D Mounting Bracket
K Mounting Bracket
Screws (x4)
A Display PCB
Models GH/CC/TIVA/PK Display
Option Shown Fitted to Display PCB
Alaris® Syringe Pump
Corrective Maintenance
C Window Adaptor
F Display Insulator
H Gasket
Model GS Display Option
J Backlight shield
Item Description Part Number
a ASENA GS, Display PCB 1000SP01118
b ASENA GH/CC/TIVA, Display PCB 1000SP01119
c Adaptor GS Display Mk
d ASENA SP, Assy, Bracket Display Mounting 1000ME00261
e Pad Self-adhesive Double-sided 12x12mm 0000ME00423
f ASENA GS, Assy, Display Insulator 1000SP00187
g ASENA GH/CC/TIVA, Assy, Display Insulator 1000SP00188
h ASENA SP, Assy, Gasket Display 1000ME01301
i Ferrite FP-24.5x5x20(J70) 0000EL00821
j ASENA GS, Kit, Backlight Shield 1000SP00273
k
ASENA SP, Kit, Fixings (screws, washers etc) 1000SP00466
II Asena 1000ME01500
H Gasket
Models GH/CC/TIVA/PK Display Option
(not shown on back of Display PCB)
(not shown on flexible circuit of Display PCB)
B Display PCB
G Display Insulator
1000SM00001 Iss. 18
55/86
Page 56

Chassis PCB and Plunger assembly
Replacement Procedure
Remove the two Chassis PCB screws. Disconnect all cables.1.
Extend the plunger out to its full extent and fully loosen the two plunger retaining screws in the carriage.2.
Carefully remove the plunger flexi from the carriage PCB and straighten. While applying controlled force to the plunger, extract it 3.
from the carriage and withdraw it completely.
Reassemble in reverse order.4.
Alaris® Syringe Pump
Corrective Maintenance
D Plunger Screw x2
Carriage PCB
Check Buffer Pad fitted if manufactured prior to March
2001 and serial numbers are within either of the ranges
8001-03468 and below or 8002-06788 and below. If not
fitted, clean the surface of the carriage face nearest the
plunger drive tube and fit Buffer Pad in the position shown
(sloping edge to match carriage profile, see diagram).
D Chassis PCB Screw x2
B Plunger Flexi
A Chassis PCB
C Buffer Pad
Item Description Part Number
a ASENA SP, Kit, Chassis PCB 1000SP00189
b ASENA SP, Kit, Plunger assembly (not shown) 1000SP01113
c ASENA SP, Kit, Carriage buffer 1000SP00230
d
ASENA SP, Kit, Fixings (screws, washers etc) 1000SP00466
1000SM00001 Iss. 18
56/86
Page 57

Chassis assembly and Pressure Transducer (Model CC only)
Replacement Procedure
Carefully peel away the label on the bottom of the front case to gain access to the chassis screw. Remove this screw.1.
Remove the two screws securing the syringe flange clamp.2.
Carefully withdraw the chassis.3.
Remove the two screws from the pressure transducer assembly and carefully withdraw (Model CC only).4.
Reassemble in reverse order.5.
Alaris® Syringe Pump
Corrective Maintenance
A Chassis
E Pressure Transducer
Screws (x2)
C Pressure
Transducer
B Syringe Flange Clamp (2 piece)
B Flange Clamp
Screws
Syringe Flange Clamp has been enhanced in response to market feedback indicating under certain conditions,
false error alarms may occur on the pump close to the End of Infusion (EOI) particularly when syringes of small
sizes are used (5,10ml etc.) if incorrectly fitted to the pump.
Fit Enhanced 1 piece Syringe Flange Clamp if manufactured prior to March 2001 and serial numbers are within
either of the ranges 8001-02315 and below or 8002-04311 and below.
Alternatively the latest 2 piece Syringe Flange Clamp may be fitted if the pump is required to have an EOI point
below 5%. Pump must have software versions v1.8.1 or higher if fitting the 2 piece Syringe Flange Clamp.
Original syringe
flange clamp
Enhanced 1 piece
syringe flange clamp
E Chassis
Screw
D Label
Enhanced 2 piece syringe
flange clamp
Item Description Part Number
a ASENA SP, Kit, Chassis Assembly 1000SP01112
b ASENA SP, Kit, Syringe Flange Clamps (1 piece) 1000SP00577
b ASENA SP, Kit, Syringe Flange Clamps (2 piece) 1000SP00570
c ASENA CC, Kit, Pressure Transducer 1000SP01155
d ASENA SP, LBL, Label Chassis Screw Cover 1000LB00431
e
ASENA SP, Kit, Fixings (screws, washers etc) 1000SP00466
1000SM00001 Iss. 18
57/86
Page 58

Syringe Sizing assembly
Replacement Procedure
Remove the syringe sizing retainer screws, case brace and retainer.1.
Remove the shim and discard, then lever the syringe sizing mechanism from the housing and withdraw the potentiometer.2.
Pull the syringe clamp back to its full extent. Carefully remove the bung, screw and washer. Pull hard on the syringe clamp to remove.3.
Carefully lever the syringe sizing mechanism from the housing and pull through the case. Remove the shaft bearing and the v-ring 4.
seal.
Secure the assembly loading jig to the syringe sizing mechanism. Fit the shaft bearing and v-ring seal onto the end of the jig.5.
Lay the assembly on one side, potentiometer to the left, wires exiting upwards. The injection 'pip' feature on the 6.
pre-moulded shaft should be visible.
Fit the seal protector into the upper case and load the syringe sizing mechanism. Compress the v-seal against the protector.7.
Shim
(If Required)
Alaris® Syringe Pump
Corrective Maintenance
E Retainer Screw x2
Retainer
B Syringe Sizing Mechanism
C Syringe Sizing Clamp
Potentiometer
Apply Catrol LMX
Grease liberally
Withdraw the protector and push the syringe sizing mechanism through the hole in the front case until the flat sides locate in the 8.
case and the potentiometer aligns with the case recess. Ensure the moulding pip is located on the side.
Slide the shim component, if required, down the side wall of the syringe potentiometer recess. Bend the shim 'outward'.9.
Fit the syringe sizing retainer so that the shim is visible protruding from the retainer. Fit the case brace. Secure with two screws.10.
Remove the assembly loading jig.11.
The syringe shaft flats to be moved into the open position.12.
Fit the syringe clamp over the shaft, fit the screw, washer and bung into the shaft end.13.
Bung
1000SM00001 Iss. 18
58/86
Page 59

A Cam Kit
Alaris® Syringe Pump
Corrective Maintenance
F Syringe Clamp Jig
Check for presence of shim if the potentiometer is the earlier type with blue casing. If shim is not fitted, or the
cam has sharp edges, fit the Cam Kit on reassembly. If the cam has sharp edges or the shim is folded incorrectly,
these may cause excessive wear of areas around the front case. Mechanical movement and small changes in the
syringe diameter can result in a syringe detect failure, which may occur if shim is not present or case is worn.
Potentiometers with black casing do not require a shim to be fitted. When replacing a potentiometer that had a
shim with a new potentiometer with black casing do not re-fit the shim.
If pump was manufactured prior to March 2001 and has serial numbers within either of the ranges 8001-02574
and below or 8002-04778 and below fit enhanced syringe clamp 1000SP01123 on reassembly. Old syringe
clamps can be recognised as clear plastic; new syringe clamps are solid blue plastic. Old syringe clamps may
crack and fail after being subjected to Isopropyl alcohol used in the cleaning process.
D Potentiometer
B Syringe Sizer Kit
Note: Replace the foot
last if replacing the case.
If fitting a new syringe
sizing mechanism, apply
generous amounts
of grease (CASTROL
LMX) to the slot of the
mechanism and lightly
grease the v-seal.
Item Description Part Number
a ASENA SP, Kit, CAM Kit 1000SP00170
b Vishay Syringe Sizing Spare 1000SP01407
c ASENA SP, Kit, Syringe and Flange Clamps (see previous page) 1000SP01123
d Vishay Potentiometer Spare 1000SP01408
e
f ASENA SP, Test, Syringe Clamp Jig 1000SP00481
ASENA SP, Kit, Fixings (screws, washers etc) 1000SP00466
1000SM00001 Iss. 18
59/86
Page 60

Chassis assembly breakdown
Replacement Procedure
Remove the pulley nut, washers and withdraw the pulley and toothed belt. 1.
Remove three screws securing stepper motor.2.
Remove the leadscrew by driving out the roll pin using a suitable punch. 3.
Remove three screws securing motor plate.4.
Remove three screws securing bearing block.5.
Refit plunger into carriage, declutch plunger and withdraw plunger and carriage. Hold linear potentiometer actuator and spring on 6.
the side of the carriage.
Remove one screw holding carriage PCB. 7.
Remove the linear travel potentiometer.8.
Fit new linear potentiometer to centre area of chassis and flush to rear of chassis slot and flush to motor-plate end.9.
Reassemble in reverse order.10.
Alaris® Syringe Pump
Corrective Maintenance
D Chassis
A Chassis Assembly
E Carriage PCB
B Motor
D Linear Potentiometer
I Bearing Block
H Carriage
J Leadscrew
G Bearing Block Screw x3
There are 2 different chassis assemblies available therefore before fitting a replacement chassis assembly check
software version to ensure fitting the correct part, see table below. Control PCB assembly numbers are supplied
for reference when the software is inaccessible.
Chassis Part Number Mark Software version Control PCB assembly number
1000SP01136 MkI and MkII V1.x.x 8000EL00008 and 8000EL00070
1000SP01328 MkIII V2.x.x and above 8000EL00100
1000SM00001 Iss. 18
60/86
Page 61

Alaris® Syringe Pump
Corrective Maintenance
G Motor screw x3
Figure 1 - Current Motor Plate
C Motor Plate
G Motor Plate Screw x3
F Motor Plate Strain Beam Support
Figure 2 - Motor Plate Strain Beam Support
Check motor plate serial number, if code is numeric barcode or is alphanumeric beginning with prefix "PH", then
this is the current version of motor plate. The current version of motor plate does not require the motor plate
beam support (see Figure 1). All other versions of motor plate require the motor plate beam support (see Figure
2).
New bearing block (1000SP01478) is required if replacing the bearing block on a new style chassis. If upgrading
the pump from an old chassis to a new chassis, this bearing block must be fitted and the old one discarded. The
new chassis can be identified by having pre tapped holes instead of self tapping holes.
Item Description Part Number
A ASENA SP, Kit, Chassis Assembly 1000SP01112
B ASENA SP, Kit, Stepper Motor 1000SP01109
C ASENA SP, Kit, Motor Plate 1000SP01110
D ASENA SP, Kit, Chassis Enhancement (MkI and MkII) 1000SP01136
D ASENA SP, Kit, Chassis Enhancement MkIII Kit 1000SP01328
E ASENA SP, Carriage PCB 8000EL00022
F SP Kit Motor Plate Strain Beam Support 1000SP00408
G
H Spare Carriage Asena 1000SP01107
I Spare Bearing Block Asena (P8) 1000SP01111
I Bearing Block New Chassis Kit 1000SP01478
J Alaris SP leadscrew kit 1000SP01327
ASENA SP, Kit, Fixings (screws, washers etc) 1000SP00466
1000SM00001 Iss. 18
61/86
Page 62

Plunger assembly breakdown
Replacement Procedure
Remove three screws holding plunger backplate.1.
Remove components as required. Follow diagrams step ii to step ix.2.
Reassemble in reverse order.3.
i
Alaris® Syringe Pump
Corrective Maintenance
ii
H Backplate
E Backplate screws
G Plunger Fixings kit
I Intermediate tube
iii
F Gear declutch
Item Description Part Number
a ASENA SP, Kit, Plunger Assembly 1000SP01113
(Complete Plunger Assembly, not shown)
b ASENA SP, Plunger Detect PCB 8000EL00019
c ASENA SP, Gripper Bottom 1000ME01218
d ASENA SP, Gripper Top 1000ME01219
e
f GEAR DECLUTCH 1000ME01198
g Alaris SP plunger fixings kit 1000SP01320
h Alaris SP Plunger back plate kit 1000SP01321
i Alaris SP Intermediate tube kit 1000SP01322
ASENA SP, Kit, Fixings (screws, washers etc) 1000SP00466
iv
1000SM00001 Iss. 18
62/86
Page 63

Alaris® Syringe Pump
Corrective Maintenance
vii
v
C Gripper Bottom
G Plunger Fixings kit
vi
D Gripper Top
a
viii
B Plunger Detect PCB
ix
Ensure Keyway locates correctly
between these 2 parts
Ensure flexible circuit exits via this slot
View on arrow a
Feed flexible circuit
through this slot after
routing as shown
1000SM00001 Iss. 18
63/86
Page 64

Pressure Transducer Assembly (Model CC only)
The following instructions detail the fitting of the Pressure Transducer Assembly.
Replacement Procedure
Fit the mylar gasket.1.
Align the hole in the gasket with the hole in the centre disc holder and 2.
ensure label is square to the Centre Disc Holder.
Use a clean wipe and apply pressure to the mylar gasket.3.
Crease the mylar gasket along the ledge of the centre disc holder and 4.
ensure it is well adhered along the front face of the step edge.
Load the spring onto the disc-detect flag shaft.5.
Locate spring arms to spring retainer on the flag and to the recess in 6.
the disc holder top.
Sealing Cord
D Mylar Gasket
Centre Disc Holder
Rotate and install the plastic flag.7.
Fit the sealing cord starting at the break bar.8.
Load the disc holder centre onto the disc holder top.9.
Secure disc holder centre to disc holder top using 10.
screw.
11.
Load pressure transducer into assembled housing.
Once the PCB is located, apply pressure over the 12.
sensor area of the PCB to ensure good location.
Alaris® Syringe Pump
Corrective Maintenance
D Flag
B Top Disc Holder
Slide the flexible circuit ferrite into place and ensure the flexible circuit 13.
is formed at 90° to the PCB.
Lower the Base Disc Holder onto the Centre Disc Holder.14.
Secure the base with the screws.15.
Use a lint-free cloth and approved cleaner to wipe the surface clean.16.
Start the gasket at the pointed end. Work around the perimeter and 17.
minimise the gap at the join (if using cord - N/A if using single-piece
gasket).
C Transducer Screw x4
Ferrite
A Pressure Transducer
Item Description Part Number
a ASENA CC, Kit, Pressure Transducer 1000SP01155
b ASENA CC, Assy, Top Disc Holder 1000ME00450
c
d Alaris SP CC disc detect parts kit 1000SP01326
ASENA SP, Kit, Fixings (screws, washers etc) 1000SP00466
1000SM00001 Iss. 18
64/86
Page 65

Keypads and labels
Replacement Procedure
Discard any keypads removed as they cannot be reused.1.
Ensure all residual adhesive is removed from bonding surfaces.2.
Fit replacement keypads after removing backing paper from underside. Handle replacement keypads carefully to avoid damage.3.
Apply finger pressure to keypads working from one end, to drive the air out of the adhesive/case interface.4.
Remove label(s) from case as required.5.
Clean case where replacement label(s) to be fitted.6.
Fit replacement label(s) taken from label sheet as required.7.
Ensure all keypad membrane flexi tails are routed and secured with double sided adhesive pads (0000ME00423).8.
To ensure an effective case fluid seal, ensure the top 5mm edge of the shelf keypad is given careful attention as
A
described in step 4 above.
Alaris® Syringe Pump
Corrective Maintenance
(A/B) Keypad
(A/D) Keypad
(A/C) Keypad
Top Edge
Item Description Part Number
a ASENA GH/CC, Key, Switch Overlay Keypad 1000SP01126
ASENA GS, Key, Switch Overlay Keypad 1000SP01127
ASENA TIVA, Key, Switch Overlay Keypad 1000SP00403
ASENA PK, Key, Switch Overlay Keypad 1000SP01203
b Keypad Asena SP On/Off 1000LB00625
PK Keypad - On/Off BOM 1000LB01405
Asena
c Keypad Asena
PK Keypad - Shelf- BOM 1000LB01404
Asena
Keypad Asena
d Keypad Asena
PK Keypad - Options - BOM 1000LB01403
Asena
Keypad Asena
Keypad Asena
GH Shelf 1000LB00626
GS Shelf 1000LB00628
GH Options 1000LB00627
GS Options 1000LB00629
TIVA Options 1000LB00630
1000SM00001 Iss. 18
65/86
Page 66

A
Note: Item A additionally
provides a clear window
for the combined serial
number and status label
Part No. 1000LB00590.
Alaris® Syringe Pump
Corrective Maintenance
C
B
Note: Item
Part No. 1000LB00431
B also available separately.
1000SM00001 Iss. 18
66/86
Page 67

115-230V ~50-60Hz 20VA
1000LB01410 Iss 3
Alaris
®
GS Label Set
1000LB01410 Iss 3
1000AW01933 Iss 3
1000LB01 10 ss 3
Cardinal H lth
1180 Roll
witzer d
Patents, Patenter, Pa entes, Brevets, Pae Brevet i, Patenten, 特許
AU 144122; 144123; 144125; 723884; 737149; 906; 91584; DE 29920378.6;
49910883.3; FR 997137; GB 2083560; 20835 83563; IE 003;
D13007; JP 1117996; 1117997; 1117999; US 64 7335; 6428
GS
1000LB01410 Iss 3
Alaris®
A
F
E
Alaris® Syringe Pump
Corrective Maintenance
B (one spare)
C
D
Picture shows Alaris® GS Syringe Pump Label Set. Refer to the following model types for correct label set.
Description Part Number
Label Set GS Syringe Pump 1000LB01410
Label Set CC Syringe Pump 1000LB01536
Label Set GH Syringe Pump 1000LB01537
Label Set TIVA Syringe Pump 1000LB01539
Label Set PK Syringe Pump 1000LB01540
Alaris CC With Guardrails Label Set 1000LB01544
Alaris GH With Guardrails Label Set 1000LB01545
Alaris GH PFS Label Set 1000LB01550
Alaris CC PFS Label Set 1000LB01551
Alaris GH Plus Label Set 1000LB01558
Alaris CC Plus Label Set 1000LB01559
Alaris CC Guardrails Plus Label Set 1000LB01560
Alaris GH Guardrails Plus Label Set 1000LB01561
SAMPLE
1000SM00001 Iss. 18
67/86
Page 68

E
Alaris® Syringe Pump
Corrective Maintenance
F
D
1000SM00001 Iss. 18
68/86
Page 69

A
Note: Label A is a blank
combined serial number
and status label. Transfer
information from old label.
This label should be used in
conjunction with the clear
protective cover from the
universal label set.
Alaris® Syringe Pump
Corrective Maintenance
B/C
The picture above shows the label set that is available as a separate item from the standard Alaris® Syringe Pump label sets.
Item Description Part Number
abc Instrument Label 1”x1 1/2” 1000LB00590
Use in conjunction with universal label set.
1000SM00001 Iss. 18
69/86
Page 70

Alaris® Syringe Pump
Appendix7
Electromagnetic Compatibility
Warning:
The use of any accessory, transducer, or cable with the Alaris® Syringe Pump other than those specified may result in increased •
emissions or decreased immunity of the pump.
The Alaris® Syringe Pump should not be used adjacent to or stacked with other equipment, however if adjacent or stacked use is •
necessary, the Alaris® Syringe Pump should be observed to verify normal operation in the configuration in which it will be used.
Caution:
The Alaris® Syringe Pump is a CISPR 11 Group 1 Class A Medical Equipment System and intended for use by healthcare •
professionals only.
Medical Electrical Equipment needs special precautions regarding EMC and needs to be installed, put into service and used •
according to the EMC information provided in the accompanying documents.
Portable and Mobile RF communications can affect Medical Electrical Equipment.•
Operating the pump near equipment which radiates high energy radio frequencies (electro surgical or cauterizing equipment, •
portable radios, cellular telephones, etc.) may cause false alarm conditions. If this happens, reposition the pump away from the
source of interference or turn off the pump and manually regulate the flow.
Appendix
Guidance and Manufacturer’s Declaration – Electromagnetic Emissions
The Alaris® Syringe Pump is intended for use in the electromagnetic environment specified below.
The customer or the user of the Alaris® Syringe Pump should assure that it is used in such an environment.
Emissions Test Compliance Electromagnetic Environment – Guidance
CISPR 11
RF Emissions
CISPR 11
RF Emissions
EN 61000-3-2
Harmonic Emissions
EN 61000-3-3
Voltage Fluctuations,
Flicker Emissions
Group 1
Class A
Class A
Complies
The pump uses RF energy only for its internal function in the normal product
offering. Therefore, its RF emissions are very low and are not likely to cause
any interface in nearby electronic equipment.
The pump is suitable for use in all establishments, other than domestic, and
those directly connected to the public low-voltage power supply network that
supplies buildings used for domestic purposes.
1000SM00001 Iss. 18
70/86
Page 71

Guidance and Manufacturer’s Declaration - Electromagnetic Immunity
The Alaris® Syringe Pump is intended for use in the electromagnetic environment specified below.
The customer or the user of Alaris® Syringe Pump should assure that it is used in such an environment.
Immunity Test
EN 60601-1-2
Test Level
Compliance Level Electromagnetic Environment – Guidance
Alaris® Syringe Pump
Appendix
EN 61000-4-2 Electro-Static
Discharge (ESD)
EN 61000-4-4
Electrical Fast Transient, Burst
(EFT) (Note 3)
EN 61000-4-5
Power Line Surge
(Note 3)
EN 61000-4-8 Power Frequency
Magnetic Field (50/60 Hz)
EN 61000-4-11
Voltage Dips, Short
Interruptions, and Voltage
Variations
(Note 3)
±6 kV contact
±8 kV air
±2 kV for power
supply lines
±1 kV for input/
output lines
±1 kV Line(s) to
Line(s)
±2 kV Line(s) to
Earth
3 A/m 400 A/m 50 Hz (Note 2)
<5 % UT (Note 1)
(>95 % dip in UT)
for 0.5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
for 25 cycles
±8 kV contact (Note 2)
±15 kV air (Note 2)
±2 kV for power supply
lines
N/A (Note 4)
±1 kV Line(s) to Line(s)
±2 kV Line(s) to Earth
<5 % UT
(>95 % dip in UT)
for 0.5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
for 25 cycles
Floors should be wood, concrete, or ceramic tile.
If floors are covered with synthetic material, the
relative humidity should be at least 30 %.
Mains power quality should be that of a typical
commercial or hospital environment.
Mains power quality should be that of a typical
commercial or hospital environment.
Power frequency magnetic fields should be at
levels characteristic of a typical location in a
typical commercial or hospital environment.
Mains power quality should be that of a typical
commercial or hospital environment.
If the user of the pump requires continued
operation during power mains interruptions, it is
recommended that the pump be powered from
an uninterruptible power supply or a battery.
The pump does employ an internal short
duration battery.
<5 % UT
(>95 % dip in UT)
for 5 sec
Note 1—UT is the AC mains voltage prior to application of the test level.
Note 2—Compliance levels raised by EN 60601-2-24.
Note 3—Performed at the Minimum and Maximum Rated Input Voltage.
Note 4—CareFusion recommends using signal cables of less than 3 metres in length and this requirement is applicable only if signal
cables are 3 metres or more in length. (EN 60601-1-2:2002, Clause 36.202.4)
1000SM00001 Iss. 18
<5 % UT
(>95 % dip in UT)
for 5 sec
71/86
Page 72

Guidance and Manufacturer’s Declaration—Electromagnetic Immunity
LIFE SUPPORT Equipment
The Alaris® Syringe Pump is intended for use in the electromagnetic environment specified below.
The customer or the user of the Alaris® Syringe Pump should ensure that it is used in such an environment.
Immunity Test
EN 61000-4-6
Conducted RF
EN 61000-4-3
Radiated RF
EN 60601-1-2
Test Level
3 V rms
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5 GHz
Compliance
Level
10 V rms
(Note 3)
10 V/m
(Note 3)
Electromagnetic Environment – Guidance
Portable and mobile RF communications equipment should be
used no closer to any part of the pump, including cables, than the
recommended separation distance calculated from the equation
applicable to the frequency of the transmitter.
Recommended Separation Distance
3.5
d = [-----] √P
V1
12
d = [-----] √P 80 MHz to 800 MHz
V2
12
d = [-----] √P 80 MHz to 2 5 GHz
E1
23
d = [-----] √P 800 MHz to 2.5 GHz
E1
Alaris® Syringe Pump
Appendix
where P is the maximum output power rating of the transmitter in
watts (W) according to the transmitter manufacturer and d is the
recommended separation distance in metres (m).
a
Field strengths from fixed RF transmitters, as determined by an
electromagnetic site survey, b should be less than the compliance level
in each frequency range. c
Interference may occur in the vicinity of equipment marked with the
following symbol:
Note 1—At 80 MHz and 800 MHz, the higher frequency range applies.
Note 2—These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from
structures, objects, and people.
Note 3—Compliance levels raised by EN 60601-2-24.
a
The compliance levels in the ISM frequency bands between 150 kHz and 80 MHz and in the frequency range 80 MHz to 2.5 GHz are
intended to decrease the likelihood that mobile/portable communications equipment could cause interference if it is inadvertently
brought into patient areas. For this reason, an additional factor of 10/3 is used in calculating the recommended separation distance
for transmitters in these frequency ranges.
b
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur
radio, AM and FM radio broadcast, and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic
environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the
location in which the pump is used exceeds the applicable RF compliance level above, the pump should be observed to verify normal
operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the pump.
c
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 10 V/m.
1000SM00001 Iss. 18
72/86
Page 73

Alaris® Syringe Pump
Appendix
Recommended Separation Distances for LIFE SUPPORT Equipment between portable and mobile RF communications equipment and the Alaris® Syringe Pump
The Alaris® Syringe Pump is intended for use in an electromagnetic environment in which radiated RF disturbances
are controlled.
The user of the Alaris® Syringe Pump can help prevent electromagnetic interference by maintaining a minimum distance between
portable and mobile RF communications equipment (transmitters) and the Alaris® Syringe Pump as recommended below, according
to the maximum output power of the communications equipment.
Separation Distance According to Frequency of Transmitter
m
Rated Maximum Output
Power of Transmitter
W
0.01 0.03 0.12 0.12 0.23
0.1 0.11 0.38 0.38 0.73
1 0.35 1.20 1.20 2.30
10 1.11 3.80 3.80 7.28
100 3.50 12.00 12.00 23.00
150 kHz to 80 MHz
Outside ISM bands
3.5
d = [------] √P
V1
150 kHz to 80 MHz
In ISM bands
12
d = [------] √P
V2
80 MHz to 800 MHz
12
d = [ ------] √P
E1
800 MHz to 2.5 GHz
23
d = [------] √P
E1
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in metres (m) can be
determined using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter manufacturer.
Note 1—At 80 MHz and 800 MHz, the separation distance for the higher frequency range apply.
Note 2—The ISM (Industrial, Scientific, and Medical) bands between 150 kHz and 80 MHz are 6.765 MHz to 6.795 MHz; 13.553 MHz to
13.567 MHz; 26.957 MHz to 27.283 MHz; and 40.66 MHz to 40.70 MHz.
Note 3—An additional factor of 10/3 is used in calculating the recommended separation distance for transmitters in the ISM
frequency bands between 150 kHz and 80 MHz and in the frequency range 80 MHz to 2.5 GHz to decrease the likelihood that mobile/
portable communications equipment could cause interference if it is inadvertently brought into patient areas.
Note 4—These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from
structures, objects, and people.
1000SM00001 Iss. 18
73/86
Page 74

Alaris® Syringe Pump
Appendix
Disposal
Information on Disposal for Users of Waste Electrical and Electronic Equipment
This U symbol on the product and/or accompanying documents means that used electrical and electronic products should not be
mixed with municipal waste.
If you wish to discard electrical and electronic equipment, please contact your CareFusion affiliate office or distributor for further
information.
Disposing of this product correctly will help to save valuable resources and prevent any potential negative effects on human health and
the environment which could otherwise arise from inappropriate waste handling.
Information on Disposal in Countries outside the European Union
This symbol is only valid in the European Union. The product should be disposed of taking environmental factors into consideration.
To ensure no risk or hazard, remove the internal rechargeable battery and the Nickel Metal Hydride battery from the control board and
dispose of as outlined by the local country regulations. All other components can be safely disposed of as per local regulations.
Battery Removal
Remove the Main Battery
Remove the two case screws in battery cover. 1.
Remove cover and battery.2.
Remove the Battery on Control PCB
Remove the Control PCB from the pump, see 'Spare Parts 1.
Replacement Procedures'.
2.
Remove the protective plastic cover from the Control PCB.
Desolder battery from the Control PCB.3.
Control PCB Reverse side
Battery Cover
Battery
Plastic cover
1000SM00001 Iss. 18
Battery
74/86
Page 75

Spare Parts Listing
Electrical Parts Listing
Part Number Description
1000EL00222 FUSE, T-1.25A SLOW BLOW, MAINS
1000SP00189 ASENA SP, KIT, CHASSIS PCB
1000SP01273 Asena TIVA, Control PCB, Mk3
1000SP01118 ASENA GS, DISPLAY PCB
1000SP01119 ASENA GH/CC/TIVA, DISPLAY PCB
1000SP01272 Asena GH, Control PCB, Mk3
1000SP01122 ASENA SP, ASSY, BATTERY
1000SP01124 ASENA SP, KIT, MAINS INLET
1000SP01408 VISHAY POTENTIOMETER SPARE
1000SP01130 ASENA SP, KIT, SPEAKER
1000SP01275 Asena GS, Control PCB, Mk3
Alaris® Syringe Pump
Appendix
1000SP01155 ASENA CC, KIT, PRESSURE TRANSDUCER
1000SP01160 ASENA SP, KIT, RS232 (PCB and FIXINGS)
1000SP01271 Asena CC, Control PCB, Mk3
8000EL00019 ASENA SP, PLUNGER DETECT PCB
8000EL00022 ASENA SP, CARRIAGE PCB
1000SP01455 Asena PK, Control PCB, Mk3
1000SP01359 Alaris GH GR Control Panel Spares Kit
1000SP01360 Alaris CC GR Control Panel Spares Kit
1000SP01448 PSU / Insulator Assy SP E / DHR
0000EL00821 Ferrite FP-24.5x5x20(J70)
1000SP00094 Assy Cable PSU P8000
1000SP01472 GH Cntrl PCB Plus S/W & Plus Dataset
1000SP01473 GH Cntrl PCB Plus S/W & Plus GR Dataset
1000SP01474 CC Cntrl PCB Plus S/W & Plus Dataset
1000SP01475 CC Cntrl PCB Plus S/W & Plus GR Dataset
1000SP01318 Alaris GH Guardrails Control Board/RS232
1000SP01317 Alaris CC Guardrails Control Board/RS232
1000EL00719 Asena SP, Battery BT1 3.6V 70mAh NiMH
1000EL00718 Alaris SP, Buzzer LS2 (SMD) (MKIII)
1000SM00001 Iss. 18
75/86
Page 76

Front Case Parts Listing
Part Number Description
1000SP01153 ASENA CC, KIT, FRONT CASE
1000SP00478 ASENA GS, KIT, FRONT CASE
1000SP00479 ASENA GH, KIT, FRONT CASE
1000SP00480 ASENA TIVA , KIT, FRONT CASE
1000SP01204 ASENA PK , KIT, FRONT CASE
1000SP00593 ASENA SP, KIT, SPARE ADHESIVE FOOT RIVET REPLACEMENT
1000SP00595 ASENA SP, KIT, SPARE ADHESIVE FOOT
1000SP01123 ASENA SP, KIT, SYRINGE and FLANGE CLAMPS
1000SP01407 Vishay Syringe Sizing Spare
1000ME01500 Adaptor GS Display Mkii Asena
1000SP00187 ASENA GS, ASSY, DISPLAY INSULATOR
1000SP00188 ASENA GH/CC/TIVA, ASSY, DISPLAY INSULATOR
0000ME00423 PAD SELF ADHESIVE DOUBLE SIDED 12X12MM
Alaris® Syringe Pump
Appendix
1000ME00261 ASENA SP, ASSY, BRACKET DISPLAY MOUNTING
1000ME01301 ASENA SP, ASSY, GASKET DISPLAY
1000ME00450 ASENA CC, ASSY, TOP DISC HOLDER
1000ME00311 ASENA SP, CASE SEALING CORD (1M)
1000SP01326 Alaris SP CC Disc Detect parts Kit
1000SP00466 ASENA SP, KIT, FIXINGS (SCREWS,WASHERS,ETC)
1000SP00170 ASENA SP, Kit, CAM Kit
1000SP00570 ASENA SP, Kit, Syringe Flange Clamps (2 piece)
1000SP00577 ASENA SP, Kit, Syringe Flange Clamps (1 piece)
1000SP00273 ASENA GS, Kit, Backlight Shield
1000SM00001 Iss. 18
76/86
Page 77

Rear Case Parts Listing
Part Number Description
1000SP01115 Alaris GS/GH/TIVA/PK, KIT, REAR CASE
1000SP01154 ASENA CC, KIT, REAR CASE
1000SP01121 ASENA SP, BATTERY COVER/HANDLE
1000SP00593 ASENA SP, KIT, SPARE ADHESIVE FOOT RIVET REPLACEMENT
1000SP00595 ASENA SP, KIT, SPARE ADHESIVE FOOT
1000SP01114 ASENA SP, KIT, RAIL CAM
1000SP00115 ASENA SP, ASSY, POLE CLAMP
1000ME01213 ASENA SP, TUBE RESTRAINT BLANK
1000ME01214 ASENA SP, TUBE RESTRAINT RS232
1000ME01303 MAGNET IR DETECT
1000ME01317 ASENA CC, ASSY, PLUG BLANKING TRANSDUCER
1000SP00466 ASENA SP, KIT, FIXINGS (SCREWS,WASHERS,ETC)
1000SP00467 ASENA SP/GW, KIT, PE STUD
Alaris® Syringe Pump
Appendix
1000SP00468 ASENA SP/GW, KIT, RS232 CONNECTOR
1000SP01323 Alaris SP Cam Rail Clamp only Kit
1000SP01324 Alaris SP Cam Rail Release Lever only Kit
1000SP01325 Alaris SP Main Case Screws 80 off
1000SP00589 SPARE KIT POLE CLAMP ARM
1000ME01306 Insulator PSU Asena SP
1000SM00001 Iss. 18
77/86
Page 78

Keypads and Labels
Part Number Description
1000SP01126 ASENA GH/CC, KEY, SWITCH OVERLAY KEYPAD
1000SP01127 ASENA GS, KEY, SWITCH OVERLAY KEYPAD
1000SP00403 ASENA TIVA, KEY, SWITCH OVERLAY KEYPAD
1000SP01203 ASENA PK, KEY, SWITCH OVERLAY KEYPAD
1000LB00625 Keypad Asena SP On/Off
1000LB01405 Asena PK Keypad - On/off Bom
1000LB00626 Keypad Asena GH Shelf
1000LB01404 Asena PK Keypad - Shelf- Bom
1000LB00628 Keypad Asena GS Shelf
1000LB00627 Keypad Asena GH Options
1000LB01403 Asena PK Keypad - Options - Bom
1000LB00629 Keypad Asena GS Options
1000LB00630 Keypad Asena TIVA Options
Alaris® Syringe Pump
Appendix
1000LB00431 ASENA SP, LBL, LABEL CHASSIS SCREW COVER
1000LB00590 Instrument Label 1”x1 1/2”
1000LB01410 Label Set GS Syringe Pump
1000LB01536 Label Set CC Syringe Pump
1000LB01537 Label Set GH Syringe Pump
1000LB01539 Label Set TIVA Syringe Pump
1000LB01540 Label Set PK Syringe Pump
1000LB01544 Alaris CC With Guardrails Label Set
1000LB01545 Alaris GH With Guardrails Label Set
1000LB01550 Alaris GH PFS Label Set
1000LB01551 Alaris CC PFS Label Set
1000LB01558 Alaris GH Plus Label Set
1000LB01559 Alaris CC Plus Label Set
1000LB01560 Alaris CC Guardrails Plus Label Set
1000LB01561 Alaris GH Guardrails Plus Label Set
1000SM00001 Iss. 18
78/86
Page 79

Transmission Parts Listings
Part Number Description
1000ME01218 ASENA SP, GRIPPER BOTTOM
1000ME01219 ASENA SP, GRIPPER TOP
1000SP00230 ASENA SP, KIT, CARRIAGE BUFFER
1000SP01109 ASENA SP, KIT, STEPPER MOTOR
1000SP01111 Spare Bearing Block Asena (P8)
1000SP01112 ASENA SP, KIT, CHASSIS ASSEMBLY
1000SP01113 ASENA SP, KIT, PLUNGER ASSEMBLY
1000SP01136 ASENA SP, KIT, CHASSIS ENHANCEMENT (MkI and MkII)
1000SP01328 ASENA SP, KIT, CHASSIS ENHANCEMENT MkIII Kit
1000SP01110 ASENA SP, KIT, MOTOR PLATE
1000SP01107 SPARE CARRAIGE ASENA
1000ME01198 GEAR DECLUTCH
1000SP00408 SP KIT MOTOR PLATE STRAIN BEAM SUPPORT
Alaris® Syringe Pump
Appendix
1000SP01320 Alaris SP Plunger Fixings Kit
1000SP01321 Alaris SP Plunger Back Plate Kit
1000SP01322 Alaris SP Intermediate Tube Kit
1000SP01327 Alaris SP Leadscrew Kit
1000SP01488 Linear Upgrade Kit
1000SP01478 Bearing Block New Chassis Kit
Software
Part Number Description
1000SP01221 Asena Mk1and2 V1.5.10 and V1.6 2 Sprs Kit
1000SP01270 ASENA SYRINGE PUMP, SOFT, SOFTWARE CD V1.9.4 (MK1 and 2)
1000SP01227 ASENA CC, SOFT, SOFTWARE CD V2.0.0 (MK3)
1000SP01225 ASENA GS, SOFT, SOFTWARE CD V2.0.0 (MK3)
1000SP01226 ASENA GH, SOFT, SOFTWARE CD V2.0.0 (MK3)
1000SP01228 ASENA TIVA, SOFT, SOFTWARE CD V2.1.0 (MK3)
1000SP01267 ASENA CC, SOFT, SOFTWARE CD V2 3.6 (MK3)
1000SP01276 ASENA GS, SOFT, SOFTWARE CD V2 3.6 (MK3)
1000SP01268 ASENA GH, SOFT, SOFTWARE CD V2.3.6 (MK3)
1000SP01269 ASENA TIVA, SOFT, SOFTWARE CD V2.3.6 (MK3)
1000SP01454 Asena PK Spares Kit V3.2.16 (Smuv3)
1000CD00028 Alaris® Software Maintenance Utility CD
1000SP01469 Alaris GH/GH GR Plus S/W Upgrade Kit
1000SP01476 Alaris CC/CC GR Plus S/W Upgrade Kit
1000SM00001 Iss. 18
79/86
Page 80

Test Equipment
Part Number Description
1000SP00373 Alaris Calibration Kit
Includes:
Alaris® Syringe Pump
Appendix
1000TG00080 Linear Speed
0000JG00175 Syringe Sizing Shim
1000TG00010 Syringe Sizing Spacer Bom
1000TG00011 Syringe Sizing Spacer
0000JG00014 Asena SP and P Series,test,plunger Protect
0000TG00200 Digital Occlusion Test Gear (Cal)
0000TG00033 Test Gear Stopwatch
1000TG00095 Linear Sizing Spacer Bom
1000TG00055 Syringe Sizing Spacer Bom
1000TG00059 Linear Sizing Spacer Bom
5000SP00010 Spare Key Elec/mech P5000
0000TG00032 Test Gear Magnet PCAM
0000JG00047 ASENA SP, TEST, CASE CRADLE JIG
0000JG00053 ASENA SP, TEST, PE STUD SOCKET
1000SP00172 ASENA SP, KIT, IRDA PORT CABLE and HEADER PCB
1000SP00209 ASENA SP, KIT, EVENT LOG DOWNLOAD UTILITY
DEC001 RS232 CABLE
Test Gear Bom
1000SP00481 ASENA SP, TEST, SYRINGE CLAMP JIG
1000ME01466 POLE CLAMP SNAKE EYE DRIVER
G30402M EXT SET, 200cm, P-DISC
1000SM00001 Iss. 18
80/86
Page 81

Fitting and Replacement Guidelines
General assembly information
A wide range of self-tapping fasteners are available.1.
PT screws are for plastic, self-tapping applications.2.
TAPTITE screws are for metal self thread-forming applications. These can be recognised by a triangular cross-section on the end.3.
Almost all fasteners on the Alaris® Syringe Pumps are self-tapping and have the potential to be over-tightened (over-torqued).4.
The force required to create a thread for the first time is more than when reassembling a previously made joint.5.
Always use the correct torque level when first making an assembly stage.6.
Take care with the torque applied when re-assembling parts. Less torque is required, so a hand tool may be more appropriate.7.
In many situations a stripped thread will require replacement of the failed component.8.
The head patterns of the fasteners are of the following types:9.
Pozi Number 1 (smaller X head)•
Pozi Number 2 (larger X head)•
Torx Number T8 (Small star profile, used typically on countersunk parts with smaller heads - Backplate / Mains Inlet / Carriage PCB •
screw).
Torx Number T10 (Medium star profile, used on the majority of Alaris® Syringe Pump Torx fasteners)•
Torx Number T20 (Larger star shape, typically for case securing screws)•
M3 (Hex head with 5.5mm across flats (AF) drivers)•
M4 nuts (Hex head with 7mm across flats (AF) drivers)•
Always select the correct tool and bit pattern for the fastener.10.
Alaris® Syringe Pump
Appendix
Torque guide
Note: Where a torque
level is not stated then
fixing should be hand
tight.
When selecting a torque for a servicing activity, be aware that refastening will require less torque than the initial manufacture. 1.
Use this information as a guide to the 'do not exceed' torque levels when servicing the equipment. When servicing it is 2.
recommended that torque is applied gradually until the component is secure. In any process do not exceed the stated levels.
If a torque driver is available for servicing this will help control the applied torque. Otherwise, be aware that excess force may cause 3.
the component to fail.
Plunger Drive Assembly:
Stage Description Component Description Qty Established Process Torque
Intermediate Tube Bearing Plate Screw - PT K30x8 Pan Hd Torx (T10) 2 50 cNm
Fixing Gripper Gears Screw - PT K22x12 Pan Hd (T6) 2 50 cNm
Screw on the Backplate Assembly Screw - PT K30x12 Csk Torx (T8) Rogard 3 40 cNm
Main Chassis Assembly:
Stage Description Component Description Qty Established Process Torque
Mount motor onto motor plate Screw - M3x12 Pan Hd Torx (T10) 3 60 cNm
Mount motor plate to chassis assembly Screw - Taptite M4x10 Csk Pozi 3 1.0 Nm
Attach drive belt and leadscrew pulley Nut - M4 Full 1 40 cNm
Secure Carriage PCB to carriage Screw - PT K30x6 Csk Pozi 1 30 cNm
Secure carriage plate to carriage Screw - PT K30x6 Pan Hd Torx (T10) 1 40 cNm
Secure bearing block to chassis Screw - Taptite M4x10 Csk Pozi 3 1.0 Nm
1000SM00001 Iss. 18
81/86
Page 82

Alaris® Syringe Pump
Appendix
Front Case Assembly:
Stage Description Component Description Qty Established Process Torque
Secure syringe sizing retainer Screw - PT K30x8 Csk Pozi 2 40 cNm
Attach syringe clamp Screw M3 x 8 Pan Hd Torx (T10) 1 50 cNm
Secure display / mounting brackets to front case Screw PT K30x12 Csk Pozi 4 50 cNm
Secure syringe flange clamp to chassis / bearing
block
Secure chassis to front case Screw M3x8 Taptite Csk Pozi 1 50 cNm
Secure plunger drive to carriage Screw - PT K30x6 Pan Hd Torx (T10) 2 30 cNm
Secure RS232 option Spacer 8mm Hex Br/Ni Pl M3x6mm 4 hand tight
Secure Control board Screw - PT K30x6 Pan Hd Torx (T10) 3 30 cNm
Secure Chassis PCB to chassis Screw - M3x8 Taptite Pan Hd 2 50 cNm
Secure Model CC pressure transducer assembly
to front case
Assembly Rear Case:
Stage Description Component Description Qty Established Process Torque
Attach pole clamp to rear case Screw - M3x8 Pan Hd Torx (T10) 3 70 cNm
Attach pole clamp arm to pole v clamp Pivot screw 1 2 Nm
Secure rail clamp to cam Screw - PT K30x10 Csk Torx (T8) Rogard 1 70 cNm
Attach camera lever Screw PT K30x8 Pan Hd Torx (T10) 1 60 cNm
Secure mains inlet assy to retainer Screw - PT K30x12 Csk Torx (T8) Rogard 2 40 cNm
Secure PE stud M6 Nut 2 hand tight
Secure PSU to case Screw - PT K30x8 Pan Hd Torx (T10) 3 40 cNm
Secure earth lead to PSU metal frame Screw M3x6 Pan Hd Pozi 1 hand tight
Fit tube restraint / RS232 cover Screw PT K30x8 Csk 2 40 cNm
Fit RS232 male / female connectors Jack Socket RS232 P8000 1 hand tight
Secure Model CC pressure transducer assembly
to rear case
Final Assembly:
Stage Description Component Description Qty Established Process Torque
Secure front case to rear case Screw PT K40x12 Pan Hd Torx (T20) 6 70 cNm
Secure battery cover / handle Screw PT K40x12 Pan Hd Torx (T20) 2 70 cNm
Pressure Transducer Assembly for Model CC only:
Stage Description Component Description Qty Established Process Torque
Secure disk holder centre to top Screw PT K30x8 Pan Hd Torx (T10) 1 50 cNm
Secure disk holder base to centre Screw PT K30x8 Pan Hd Torx (T10) 4 50 cNm
Screw - PT K30x14 Pan Hd Torx (T10) 2 70 cNm
Nut M3 St. St A2 4 40 cNm
Screw M3x6 Pan Hd Pozi Z+C 4 40 cNm
Screw - PT K30x12 Pan Hd Torx (T10) 2 70 cNm
Screw - 4gx1/2” S/T B Zn Clr Pz1 1 60 cNm
1000SM00001 Iss. 18
82/86
Page 83

Alaris® Syringe Pump
Service Contacts
For service, contact your local CareFusion Affiliate Office or Distributor.
AE CN GB NZ
CareFusion,
PO Box 5527,
Dubai, United Arab Emirates.
Tel: (971) 4 28 22 842 Tel: (86) 21 58368028 Tel: (44) 0800 917 8776 Tel: 09 270 2420
Fax: (971) 4 28 22 914 Fax: (86) 21 58368017 Fax: (44) 1256 330860 Fax: 09 270 6285
AU DE HU SE
CareFusion,
3/167 Prospect Highway,
PO Box 355
Seven Hills, NSW 2147,
Australia.
Tel: (61) 2 9838 0255 Tel: (49) 2401 604 0 Tel: (36) 14 88 0232
Fax: (61) 2 9674 4444 Fax: (49) 2401 604 121 Fax: (36) 12 01 5987 Fax: (46) 8 544 43 225
BE DK IT US
CareFusion,
Leuvensesteenweg 248 D,
1800 Vilvoorde,
Belgium.
Tel: (32) 2 267 38 99 Tlf. (45)70 20 30 74 Tél: (39) 055 30 33 93 00 Tel: (1) 800 854 7128
Fax: (32) 2 267 99 21 Fax. (45)70 20 30 98 Fax: (39) 055 34 00 24 Fax: (1) 858 458 6179
CA ES NL ZA
CareFusion,
235 Shields Court,
Markham,
Ontario L3R 8V2,
Canada.
Tel: (1) 905-752-3333 Tel: (34) 902 555 660 Tel: (31) 30 228 97 11 Tel: (27) (0) 860 597 572
Fax: (1) 905-752-3343 Fax: (34) 902 555 661 Fax: (31) 30 225 86 58 Fax: (27) 21 5107567
CH FR NO
CareFusion Switzerland 221
Sàrl
Critical Care
A-One Business Centre
Zone d’activitiés Vers-la-Pièce
n° 10
1180 Rolle / Switzerland
Ph.: 0848 244 433 Tél: (33) 1 30 05 34 00 Tel: (47) 66 98 76 00
Fax: 0848 244 100 Fax: (33) 1 30 05 34 43 Fax: (47) 66 98 76 01
CareFusion,
Shanghai Representative Office, Suite
A, Floor 24,
Shanghai Times Square Office Building,
No.500 Zhangyang Road,
Shanghai 200122, China.
CareFusion,
Pascalstr. 2,
52499 Baesweiler,
Deutschland.
CareFusion,
Firskovvej 25 B,
2800 Lyngby,
Danmark.
CareFusion,
Edificio Veganova,
Avenida de La Vega, nº1,
Bloque 1 - Planta 1,
28108 Alcobendas, Madrid,
España.
CareFusion,
Parc d’affaire le Val Saint Quentin
2, rue René Caudron
78960 Voisins le Bretonneux
France
CareFusion,
The Crescent, Jays Close,
Basingstoke,
Hampshire, RG22 4BS,
United Kingdom.
CareFusion,
Döbrentei tér 1,
H-1013 Budapest,
Magyarország.
Tel: (36) 14 88 0233
CareFusion,
Via Ticino 4,
50019 Sesto Fiorentino,
Firenze, Italia.
CareFusion,
De Molen 8-10,
3994 DB Houten,
Nederland.
CareFusion,
Solbråveien 10 A,
1383 ASKER,
Norge.
CareFusion,
14B George Bourke Drive,
Mt Wellington 1060,
PO Box 14-518,
Panmure 1741, Auckland,
New Zealand
Freephone: 0508 422734
CareFusion,
Hammarbacken 4B,
191 46 Sollentuna,
Sverige.
Tel: (46) 8 544 43 200
CareFusion,
10020 Pacific Mesa Blvd.,
San Diego, CA 92121,
USA.
CareFusion,
Unit 2 Oude Molen Business Park,
Oude Molen Road, Ndabeni,
Cape Town 7405, South Africa.
Tel: (27) 21 510 7562
Appendix
1000SM00001 Iss. 18
83/86
Page 84

Document History
Issue Date CO No. Author Update Description
1 19/12/02 4091 Ian Tyler Initial release
2 14/01/03 4268 Ian Tyler
3 24/04/03 4432 Ian Tyler
4 10/06/04 4710 Ian Tyler
5 27/09/04 5478 Ian Tyler Administration change.
6 6/04/05 5688 Ian Tyler Add Alaris® PK Syringe Pump
7 20/04/05 5920 Ian Tyler
8 4/11/05 6064 Ian Tyler
9 23/06/06 6932 Ian Tyler
10 01/07 7322 Ian Tyler Update of Spare Parts
11 07/07 7805 Ian Tyler Update of Spare Parts
12 10/2007 7988 Ian Tyler Update of Spare Parts
13 05/2008 8408 Ian Tyler
14 06/2008 8606 Ian Tyler Administration change.
15 07/2008 8616 Ian Tyler Administration change.
16 Jan 2009 8827 Ian Tyler
17
18 April 2010 10534 Ian Tyler
February
2010
9361 Ian Tyler Rebrand to CareFusion
RS232 pin-out table pin descriptions pin5 and 9 switched.
Drug Protocol Setup table - Dose Rate Max and Min headings switched.
Note added on cleaning with reference to shelf keypads.
Added pole clamp pivot screw and changed one screw description.
Added pole clamp arm and updated part numbers.
Drawings of Pole clamp and Transducer to show latest versions.
Information relating to Guardrails® Safety Software.
Pole clamp arm replacement.
Updates for latest Software versions, including - configuration options, part
numbers, error codes and new screen display.
Enhanced 2 piece syringe flange clamp.
Adhesive feet availability information.
Added additional diagrams for Chassis and Plunger assembly.
Add more information regarding battery calibration
Updated TIVA Drug setup and protocol to include more units in column
headings
Add access code 612
Update Speed test values
New PK error codes
Strain plate update
New software settings
Pole clamp part numbers update
Rebranded from ALARIS Medical Systems to CareFusion
Added access codes 175 and 711
Administration rebrand change.
Updated Motor Plate replacement instructions and drawings
Revision of Maintenance procedures
Update of syringe potentiometer part
Update of Spare Parts
Update of PL3 Error diagnosis
Inclusion of several Information Notices
Correct calibration pressure gauge tolerance.
Updated Plunger Assembly breakdown.
Alaris® Syringe Pump
Appendix
1000SM00001 Iss. 18
84/86
Page 85

Alaris® Syringe Pump
Appendix
Software Upgrade Record
Please fill out the table below and return to the local CareFusion representative, see Service Contacts for address details, to ensure the
records are upto date so that any future product actions can be directed to the correct institution(s).
Hospital Name: Country:
Product SKU Serial Number Processor/Software
version after upgrade
Date Updated
(dd/mm/yyyy)
Comments/Reference
(e.g. Field Safety Notice number,
Information Notice number, etc.)
Signature: Name: Position:
1000SM00001 Iss. 18
85/86
Page 86

This manual has been prepared for use by qualified
service personnel only.
CareFusion cannot accept any liability for any
breakdown or deterioration in performance of
parts or equipment resulting from unauthorised
repair or modification.
Alaris, Guardrails and Asena are registered
trademarks of CareFusion Corporation or one of its
subsidiaries. All rights reserved.
All other trademarks are property of their
respective owners.
© 2002-2010 CareFusion Corporation or one of its
subsidiaries. All rights reserved.
CareFusion Switzerland 317 Sàrl,
t
CH-1180, Rolle
EC REP CareFusion U.K. 305 Ltd., RG22 4BS, UK
carefusion.com
 Loading...
Loading...