Abaxis Piccolo xpress User manual

piccolo® xpress™ Chemistry Analyzer
Operator’s Manual
For In-Vitro Diagnostic Use Only
Abaxis, Inc.
3240 Whipple Road Union City, CA, USA 94587
Abaxis Europe
Heidelberger Landstrasse 230
D-64297 Darmstadt
Customer and Technical Support: 1-800-822-2947
PN: 1100-7008 Manual Text, Rev. B
PN: 1100-7009 Manual Assembly, Rev. A
© 2006, Abaxis, Inc.


Table of Contents
|
|
|
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Section 1: General Information |
1-1 |
|
1.1 |
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-1 |
1.2 |
Universal Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-1 |
1.3 |
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-2 |
1.4 |
Technical Support . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-3 |
1.5 |
Symbols Used in Labeling . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1-3 |
Section 2: Setup and Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2-1 |
|
2.1 |
Unpacking . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2-1 |
2.2 |
Physical & Environmental Specifications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2-2 |
2.3 |
Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2-2 |
2.4 |
piccolo xpress™ System Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2-4 |
2.5 |
Touchscreen and Front Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2-5 |
2.6 |
Reagent Discs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2-7 |
2.7 |
Ancillary Products. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2-9 |
Section 3: Testing and Results . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-1
3.1 |
Sample Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
3-1 |
3.2 |
Preparing the Reagent Disc . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
3-2 |
3.3 |
Running a Patient Sample . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
3-4 |
3.4 |
Canceling Analysis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
3-10 |
3.5 |
Results . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
3-11 |
3.6 |
Testing Procedure Summary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
3-13 |
Section 4: Configuring the Analyzer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-1
4.1 |
Using the Settings Screens . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-2 |
4.2 |
Customizing Reference Ranges. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-2 |
4.3 |
Printing and Archiving Reference Ranges . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-12 |
4.4 |
Retrieving Reference Ranges . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-14 |
4.5 |
Transmitting Reference Ranges . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-16 |
4.6 |
Viewing Analyzer Identification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-17 |
4.7 |
Changing Date and Time. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-18 |
4.8 |
Selecting the Language . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-19 |
4.9 |
Selecting Units . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-20 |
4.10 |
Setting Sound Volumes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-24 |
4.11 |
Adjusting the Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-25 |
4.12 |
Printer Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-28 |
4.13 |
Setting Communication Protocol . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-32 |
4.14 |
Using Optional Data Functions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-33 |
4.15 |
Running Controls . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
4-34 |
Table of Contents |
TOC-1 |

Section 5: Recalling Results . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-1 |
|
5.1 |
Recalling the Results of the Last Disc Run. . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-2 |
5.2 |
Searching for Results. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-3 |
5.3 |
Browsing Results. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-8 |
5.4 |
Transmitting All Results . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
5-10 |
Section 6: Calibration & Q. C. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
6-1 |
|
6.1 |
Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
6-1 |
6.2 |
Quality Control Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
6-1 |
6.3 |
Running External Controls . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
6-3 |
Section 7: Troubleshooting. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
7-1 |
|
7.1 |
Error Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
7-1 |
7.2 |
Electrostatic Discharge . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
7-1 |
7.3 |
Technical Support . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
7-1 |
7.4 |
Disc Cancellations. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
7-1 |
7.5 |
Instrument and Result Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
7-2 |
7.6 |
Reinitializing the Analyzer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
7-4 |
Section 8: Operating Principles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-1 |
|
8.1 |
Principles of Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-1 |
8.2 |
Principles of Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
8-2 |
Section 9: Maintenance & Service . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
9-1 |
|
9.1 |
Cleaning the Analyzer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
9-1 |
9.2 |
Cleaning Spills . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
9-2 |
9.3 |
Cleaning the Air Filter. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
9-2 |
9.4 |
Updating the Analyzer Software . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
9-3 |
9.5 |
Returning the Analyzer to the Manufacturer for Service . . . . . . . . . . . . . . . . . . |
9-4 |
Section 10: Connecting a Computer/Printer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-1
10.1 Connecting an External Printer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-1 10.2 Connecting a Computer. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-1 10.3 Transmission Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-2 10.4 Installing the Abaxis® Driver . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-3 10.5 Setting Up HyperTerminal . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-10 10.6 Capturing Results with HyperTerminal . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-13
INDEX . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . I-1
TOC-2 |
Table of Contents |
|
|
|
|
General Information |
||
|
Section 1 |
|
|
|||
|
|
1.1 |
Intended Use |
|||
|
|
|
|
The piccolo xpress™ chemistry analyzer provides quantitative in-vitro |
||
|
|
|
|
determinations of clinical chemistry analytes in lithium-heparinized |
||
|
|
|
|
whole blood, heparinized plasma, or serum. |
||
|
|
|
|
|
CAUTION: |
If the piccolo xpress™ chemistry analyzer is used |
|
|
|
|
|
|
in any way other than described in this manual, |
|
|
|
|
|
|
the analyzer may not operate as intended, may |
|
|
|
|
|
|
produce inaccurate or no results, and may pose a |
|
|
|
|
|
|
safety hazard. |
|
|
|
|
|
Note: |
Use only piccolo® reagent discs with the |
|
|
|
|
|
|
piccolo xpress™ chemistry analyzer. |
|
|
1.2 |
Universal Precautions |
|||
Operator health and safety regulations require that Universal Precautions be observed at all times while handling human blood samples or working with the piccolo xpress™ chemistry analyzer in any way. For details, see OSHA 29 CFR Part 1910 (“Occupational Safety and Health Standards”), Standard Number 1910.1030 (“Toxic and Hazardous Substances: Bloodborne Pathogens”), which can be found on the Internet by going to http://www.osha.gov and searching for “1910.1030”.
For further guidelines on handling and disposing of hazardous laboratory wastes, refer to “Clinical Laboratory Waste Management; Approved Guideline—Second Edition” (GP5-A2), from the Clinical and Laboratory Standards Institute (formerly NCCLS). This can be found on the Internet at http://www.clsi.org.
General Information |
1-1 |

1.3Introduction
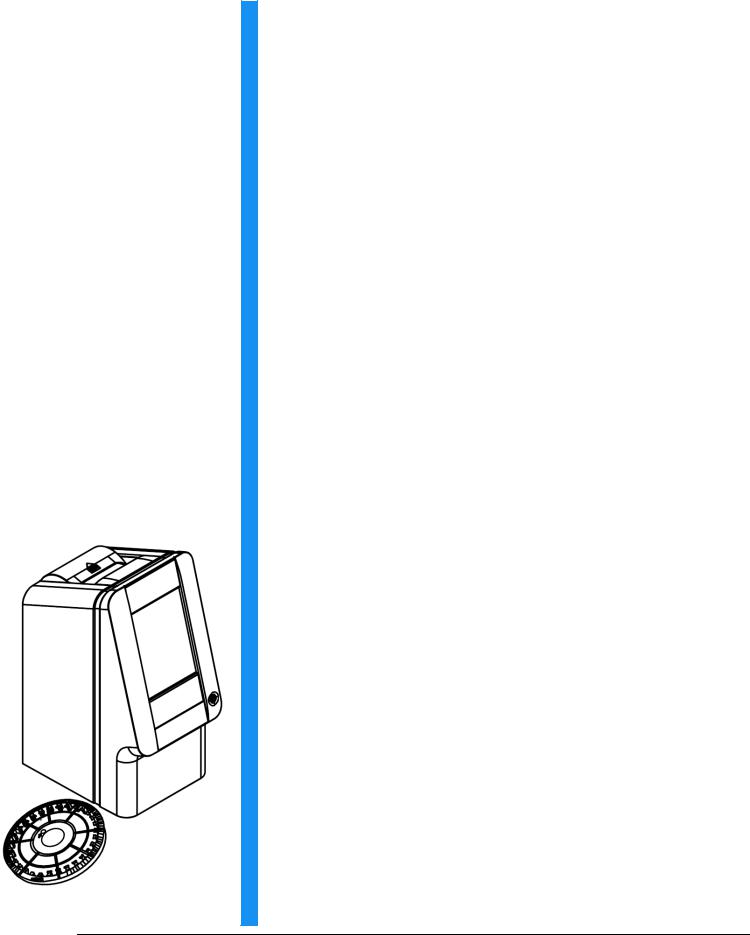
The piccolo xpress™ chemistry system is compact and easy to transport. The system consists of a portable analyzer and single-use disposable reagent discs. The analyzer contains the following features and components:
■a variable-speed motor to spin the disc
■a photometer to measure analyte concentrations
■two microprocessors to control testing and analytical functions
■a thermal line printer to print out results
■a VGA color touchscreen for communicating with the analyzer
■optional data functions for more detailed analysis information
Each reagent disc is self-contained clear plastic, 8 cm in diameter and 2 cm thick, and containing an aqueous diluent in its center and dry reagent beads in cuvettes around its edge. All blood separation and sample diluent mixing is performed within the disc itself.
To perform an analysis, the operator needs only to collect a blood sample (lithium-heparinized whole blood or plasma, serum), place the sample in the reagent disc, put the disc into the analyzer drawer on the front of the analyzer, and input patient information. When analysis is finished, the results print automatically.
Results are printed on thermal paper with adhesive backing for inclusion within the patient's medical record. Five USB ports are provided so data can be sent to an external printer, computer, memory stick, or laboratory information systems/electronic medical record systems (LIS/EMR).
The entire analysis requires ~100 μL of sample and is capable of providing results in about 12 minutes.
Note: This manual includes a number of analyzer screens and computer screen captures. The analyzer screens are illustrations only, not exact duplications. All screens represent typical use and installations, and may differ in some minor details from the screens on your system.
1-2 |
General Information |
1.4Technical Support
Abaxis Technical Support personnel are trained to answer questions regarding the operation of the piccolo xpress™ chemistry analyzer. Call Abaxis Technical Support at 800-822-2947.
1.5Symbols Used in Labeling
The following symbols are found on the back of the analyzer on the piccolo xpress™ label and above the connectors.
Direct current.
!Caution. Refer to any accompanying documents.
Biohazard. In accordance with good laboratory practice, consider all material from human sources to be potentially infectious, and handle them with the same precautions used with patient specimens (see Section 1.2).
USB connection.
General Information |
1-3 |

1-4 |
General Information |
|
|
|
|
Setup and Description |
|
|
Section 2 |
|
|
||
|
|
2.1 |
Unpacking |
||
|
|
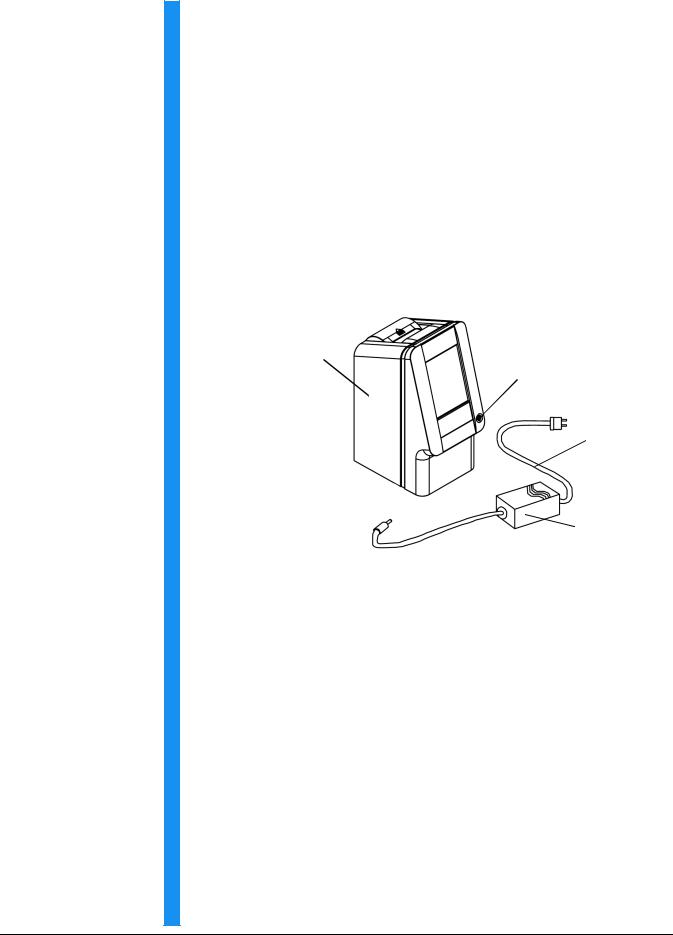
1. |
Remove the piccolo xpress™ chemistry analyzer from the ship- |
||
|
|
|
|
|
ping carton. Place the analyzer on a level surface that is free of |
|
|
|
|
|
hair, dust, and other contaminants. Do not place the analyzer |
|
|
|
|
|
near a sunny window or any other heat source. |
|
|
2. |
Check the components received with the piccolo xpress™ |
||
|
|
|
|
|
against the following figure to make sure everything required to |
|
|
|
|
|
set up the analyzer is included. |
piccolo xpress™ chemistry analyzer 
 Power button
Power button
 Power cord
Power cord
Power adapter with cord
The shipping carton also includes a USB cable, Abaxis Driver CD, piccolo xpress™ Operator's Manual (this manual), Warranty card, and Start-up kit (not shown above).
3.After setup, complete the warranty card and mail it to Abaxis® within 10 days to start the warranty period. Customers are placed on the customer mailing list to receive any information pertaining to the piccolo xpress™ and ancillary products, such as software upgrades.
Setup and Description |
2-1 |

2.2Physical & Environmental Specifications
Analyzer dimensions: |
Height: |
32.4 cm |
(12.75 inches) |
|
Width: |
15.2 cm |
(6 inches) |
|
Depth: |
20.3 cm |
(8 inches) |
Weight: |
Analyzer: |
5.1 kg |
(11.2 pounds) |
|
Power adapter: |
0.7 kg |
(1.6 pounds) |
Mode of operation: |
Continuous |
|
|
Protection against ingress of fluids: |
Ordinary equipment (IPXO) |
|
|
Altitude: |
2000 m (6562 ft) |
|
|
Ambient operating temperature: |
0–40 ºC (32–104 ºF), indoor use |
||
Humidity: |
80% relative humidity for temperatures up to 31 ºC |
||
|
(88 ºF), decreasing linearly to 50% relative humidity |
||
|
at 40 ºC (104 ºF) |
|
|
Reaction temperature: |
37 ºC (98.6 ºF) |
|
|
Thermal protection rating: |
70 ºC (158 ºF) |
|
|
Power requirements: |
100–240 volts AC, 50–60 Hz or 15 Volts DC, 5.0A |
||
|
Main unit: 1.1 to 0.45 amps, 15 volts DC, 5.0 A |
||
Main supply voltage: |
Fluctuations not to exceed ± 10% of the nominal |
||
|
voltage |
|
|
Transient overvoltages: |
Installation Category II in accordance with |
||
|
UL 61010A-1 first edition Annex J |
||
Pollution: |
Degree 2 in accordance with IEC 664 |
||
|
|
|
|
2.3Setup
1.Set up the piccolo xpress™ on a surface as follows:
■Level.
■Free of vibration and sudden jolts.
■Free of hair, dust, and other contaminants.
■Located in an ambient operating temperature of 15–32 ºC (59–90 ºF).
■Away from sunny windows and all other potential heat sources.
■At least six inches from any wall to provide adequate ventilation and access to the power connection and USB ports.
2-2 |
Setup and Description |
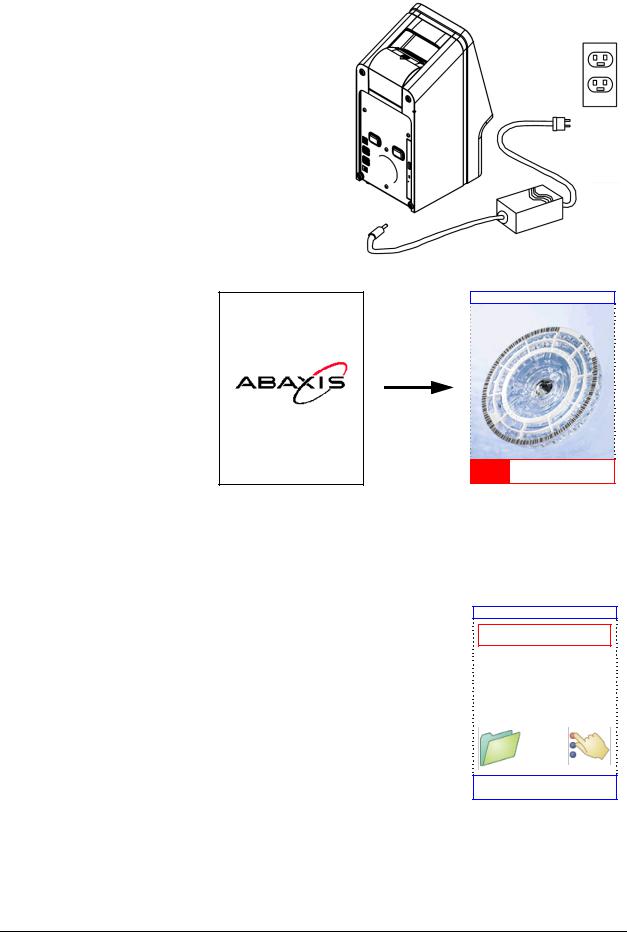
2.Plug the power jack into the analyzer, then plug the detachable power supply cord into the power adapter and into a grounded electrical outlet.
CAUTION: To prevent power surges or drain, DO NOT plug the analyzer into the same circuit as a centrifuge or any other high-current device. Abaxis also recommends using a surge protector of the same type used for computers.
3.Press the Power button to turn on the analyzer.
The display then shows the following:
piccolo xpress V1.2.3.4
Performing iQC...
Note: If the display is blank (shows no message), remove the plug from the power adapter, then re-insert the plug and press firmly to make sure it seats correctly.
During the warming period, the display reads as shown:
Home
Warming Up
Monday 16 Aug 2006 10:30AM
Setup and Description |
2-3 |
4.After passing the self test and reaching operating temperature, the analyzer is ready to run the first reagent disc, and displays “Analyze”:
See Section 6.2, “Quality Control Features” for details about the analyzer self test. The analyzer may require additional time for the heaters to warm the analyzer to operating temperature.
5.Check the analyzer date and time to ensure they are correct. Refer to Section 4.7, “Changing Date and Time” for directions.
Home
Analyze
Monday 16 Aug 2006 10:45AM
6.The analyzer can be connected to an external computer or
printer to transmit or print patient and control results. See Section 10, “Connecting a Computer/Printer” for instructions.
7.Reference ranges are preset when the analyzer is shipped from the factory. The range values can be changed using the Customizing Reference Range feature described in
Section 4.2.
2.4piccolo xpress™ System Description
The piccolo chemistry system consists of a portable analyzer and disposable single-use reagent discs. Each reagent disc contains all the reagents needed to perform a panel of tests on a single sample.
Be sure to become familiar with the system before running samples.
The piccolo xpress™ analyzer uses centrifugal and capillary forces to process heparinized whole blood samples and distribute diluted plasma to the reaction chambers (cuvettes) in the reagent disc. Serum and heparinized plasma samples are processed in a similar manner. The analyzer optically measures the chemical reactions and calculates analyte concentrations from these measurements and from encoded calibration data contained on the bar code ring on the reagent disc.
Results are stored in memory, and can be printed using the built-in thermal printer or downloaded to an external personal computer for data management.
2-4 |
Setup and Description |

The touchscreen display provides easy communication with the analyzer. The touchscreen shows procedural instructions, indicates the status of the analyzer, and presents any error messages. For details, see Section 2.5.
The reagent disc drawer slides out from the front of the analyzer, and transports the reagent disc (see Section 2.6) into the analyzer and automatically positions it for analysis.
The analyzer prints results on adhesive-backed thermal paper tape, through its internal printer.
The external power adapter changes the electrical outlet voltage to DC voltage required by the analyzer. Two power supply cords are provided: one cord is connected to the power adapter and plugs into the back of the analyze, while the other connects the power adapter to a surge protector designed for use with a computer.
The analyzer has several USB connectors. Use the top USB connector for connecting to an external computer (see Section 10, “Connecting a Computer/Printer” for details).
The analyzer is easy to carry using the recessed handle incorporated into the top of the unit.
2.5Touchscreen and Front Panel
You’ll use the analyzer’s touchscreen display to operate the analyzer.
■Use the ten numbers on the touchscreen to input patient information.
■The left and right arrow touchscreen keys move the display cursor forward or
backward to change a number on the display. The right arrow key (  ) functions as a dash (–) when entering a patient identification number. The left arrow key
) functions as a dash (–) when entering a patient identification number. The left arrow key
(  ) functions as a backspace.
) functions as a backspace.
■Use the up (S) and down (T) arrow keys to scroll through a displayed list of items, or to increase or decrease a displayed value.
■Operating functions are available through various icons on the touchscreen, as well as specific keys that appear on the touchscreen according to the actions performed.
■Press Back on the touchscreen to cancel a choice and return to the previous screen, or to move backward through a series of screens.
Setup and Description |
2-5 |
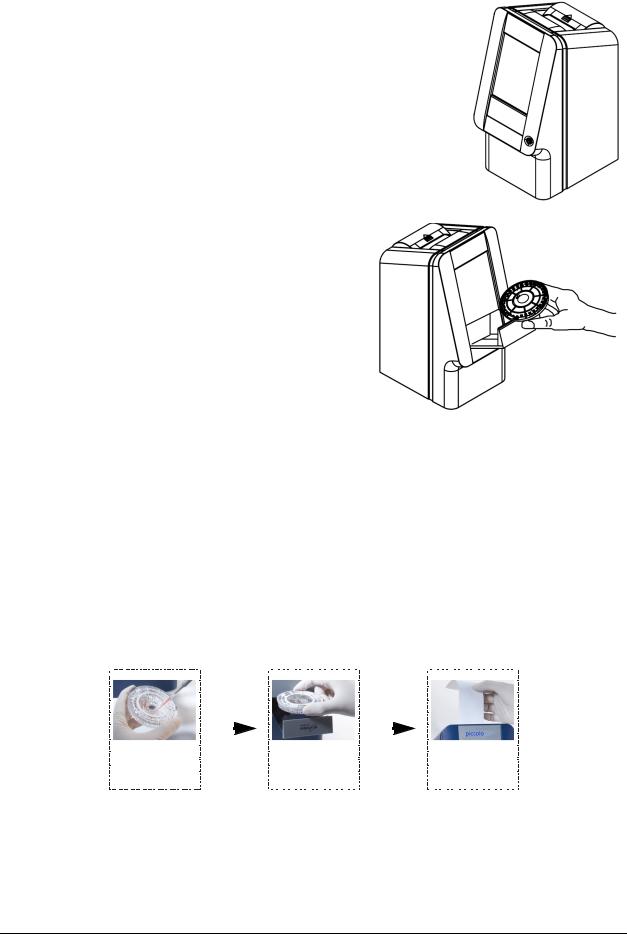
Turn the analyzer’s power on and off by pressing the
Power button on the front of the instrument.
(This button can only turn the power turned off if the drawer is closed and there is no disc in the analyzer.)
Power button 
The drawer opens or closes by pressing Analyze or
CLOSE on the touchscreen. Closing a drawer containing a reagent disc initiates an analysis.
Results for previously analyzed samples can be accessed using the Recall function — see “Recalling Results” on page 5-1 for instructions.
Information Icons
A number of the piccolo xpress™ touchscreen displays include an information icon  that you can press to get additional information about the procedure you’re performing.
that you can press to get additional information about the procedure you’re performing.
For example, in the screen that appears when you load a disc, you can press  to view screens outlining the basic analysis procedure:
to view screens outlining the basic analysis procedure:
|
Insert Sample |
|
|
|
|
Insert Disc |
|
|
|
|
Read Results |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Remove reagent disc from its foil pouch and transfer 100 ul of whole blood, plasma, or serum into the disc.
Insert the reagentdisc into the analyzer, close drawer and follow on screen prompts.
View, read or download results.
Exit |
Next |
|
Back |
Exit |
Next |
|
Back |
Exit |
|
|
|
|
|
|
|
|
|
Press Next and Back to move through the information screens, and Exit to close them.
2-6 |
Setup and Description |

2.6Reagent Discs
Disc structure and function
In the piccolo® system, all chemistry reactions are performed inside clear plastic reagent discs, 8 cm in diameter and 2 cm thick, specially designed to perform all the steps required to convert a few drops of whole blood, plasma, or serum into a panel of test results. Each disc contains all the components and reagents needed to perform one or more tests on a single sample.
A total of 30 cuvettes are located around the periphery:
■four system cuvettes contain QC reagent beads for instrument and chemistry quality control
■a minimum and a maximum absorbance cuvette are used to calibrate the spectrophotometer
■a specially designed cuvette detects whether sample volume was sufficient
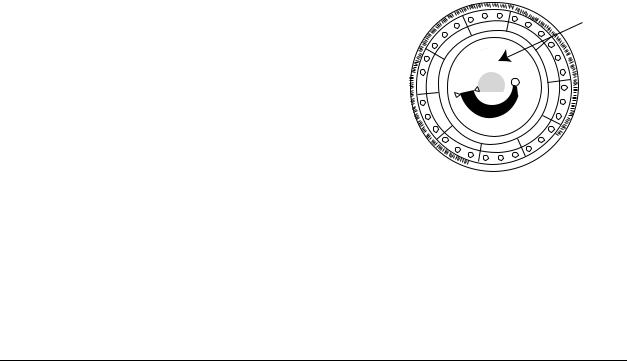
Bar Code Ring
bar code ring
Cuvettes
cuvettes
Sample Port
sample port
Diluent
diluent
Container
container
Sample Fill Line
sample fill line
■a cuvette verifies that sufficient diluted sample was delivered to the reaction cuvettes
■an empty cuvette captures excess fluids
■21 cuvettes contain test-specific lyophilized reagent beads.
The bar code ring attached to the top of the reagent disc contains calibration data for the specific chemistries in the disc. It also contains the disc identification code, lot number, and expiration date. The analyzer automatically checks the code and rejects any expired disc. The bar code ring also protects the optical surfaces of the cuvette from fingerprints and debris, and helps minimize contamination of the analyzer by capturing small drops of blood that may be on the disc surface.
The sample port, marked by an arrow pointing to a molded circle on the disc’s upper surface, provides access to the sample chamber. When sufficient sample has been loaded into the sample chamber, the sample fill line forms between two molded arrows on the disc surface.
A sample diluent is sealed in a container inside the center of the disc. At the beginning of the reaction cycle, the analyzer opens this container and releases the diluent.
The analyzer separates a lithium-heparinized whole blood sample by centrifugation inside the disc. Plasma and serum samples are unaffected. Precisely measured quantities of sample and diluent are delivered to the mixing chamber. Centrifugal and capillary forces then deliver the diluted sample to the cuvettes, where it dissolves the reagent beads and initiates the chemical reactions. Reaction products in the cuvettes are then measured photometrically.
Setup and Description |
2-7 |
Disc Storage and Handling
CAUTION: Discs are fragile — always handle with care. Do not tap discs on the table or work bench to empty the sample port. Do not use a disk that has been dropped.
Inspect every reagent disc for damage before use. Never use a damaged disc.
■Store each reagent disc as described on its label. This keeps the disc’s reagents stable until the expiration date printed on the disc’s foil pouch and encoded in its bar code ring. The analyzer automatically rejects any expired disc.
■Discs can be used directly from the refrigerator (stored at 2–8 °C) without warming.
■A disc can remain in its sealed pouch at room temperature for a cumulative period of 48 hours. Longer time at room temperature can cause suppression of chemistries and disc cancellations.
■Do not expose discs — in or out of their foil pouches — to direct sunlight or to temperatures above 32 °C (90 °F).
■Inspect the unopened foil pouch for tears and punctures. A torn or damaged pouch can allow moisture to reach the disc and reduce reagent performance.
■Open the disc pouch at the notch on the top right edge of the package.
Note: You must use the disc within 20 minutes of opening its pouch.
■Once the pouch is opened, do not place the disc back in the refrigerator for later use.
■Keep discs clean. Handle them only by their edges to avoid smudges on the optical surfaces. Use a lint-free tissue to remove any spilled blood from disc surfaces.
■Optional: Write the patient identification number on the disc surface in the space shown at right. Do not write anywhere else on the disc, or on the bar code ring.
■Hold reagent discs flat after introducing the sample or control to avoid spillage.
Write patient ID here
WARNING: |
BIOHAZARD: Used reagent discs contain body fluids. Follow good labora- |
|
tory working practices. Handle all used discs as if they are contaminated |
|
with hepatitis or other infectious diseases. Check with the appropriate state |
|
agency regarding disposal regulations. For more information, see |
|
Section 1.2, “Universal Precautions,” on page 1-1. |
2-8 |
Setup and Description |

2.7Ancillary Products
To order consumables or supplies such as reagent discs, pipettes, tips, printer paper, or control samples, contact your authorized distributor, or call Abaxis Customer Service at 800-822-2947.
Setup and Description |
2-9 |

2-10 |
Setup and Description |
|
|
|
|
Testing and Results |
|
Section 3 |
|
|
|||
|
|
|
|
3.1 |
Sample Requirements |
|
|
|
|
■ |
The piccolo xpress™ chemistry analyzer accepts lithium- |
|
|
|
|
|
heparinized whole blood, plasma, or serum samples. |
|
|
|
|
■ |
Lithium heparin is the only anticoagulant recommended |
|
|
|
|
|
for use with the piccolo xpress™. |
|
|
|
|
|
Note: When collecting the sample in lithium heparin |
|
|
|
|
|
collection tubes, fill the tube at least half-way |
|
|
|
|
|
so the anticoagulant does not become too |
|
|
|
|
|
concentrated in the sample. |
|
|
|
|
■ |
If using CLIA '88 Waived reagent discs: only lithium- |
|
|
|
|
|
heparinized whole blood can be used as a sample. |
|
|
|
|
■ |
If performing a Basic Metabolic Panel Plus: only lith- |
|
|
|
|
|
ium-heparinized plasma or serum can be used as a sam- |
|
|
|
|
|
ple. Heparinized whole blood cannot be used. |
|
|
|
|
■ |
A sample size of 90–120 µL is required. |
|
|
|
|
■ |
Whole blood must be analyzed within 60 minutes of col- |
|
|
|
|
|
lection, or separated into plasma or serum. |
|
|
|
|
■ |
To prevent hemolysis, do not refrigerate or shake whole |
|
|
|
|
|
blood. |
|
|
|
|
■ |
If not analyzed immediately, plasma or serum can be |
|
|
|
|
|
stored at room temperature for no longer than 5 hours |
|
|
|
|
|
after centrifugation. If storage for more than 5 hours is |
|
|
|
|
|
required, refrigerate the sample in the stoppered tube at |
|
|
|
|
|
2–8 °C (36–46 °F) for no longer than 48 hours, or store it |
|
|
|
|
|
at –10 °C for up to 5 weeks in a freezer with no self- |
|
|
|
|
|
defrost cycle. Under these conditions, there will be no |
|
|
|
|
|
clinically important changes in most analyte concentra- |
|
|
|
|
|
tions. |
|
|
|
|
■ |
For accurate interpretation of glucose results, the patient |
|
|
|
|
|
should fast for at least 12 hours before the sample is col- |
|
|
|
|
|
lected. |
|
|
|
|
|
|
|
|
|
|
|
|
Testing and Results |
3-1 |

WARNING: |
Operator health and safety regulations require that Universal Precautions be |
|
observed at all times while handling human blood samples or working with |
|
the piccolo xpress™ chemistry analyzer in any way. |
|
For details, please refer to OSHA 29 CFR Part 1910 (“Occupational Safety |
|
and Health Standards”), Standard Number 1910.1030 (“Toxic and Hazard- |
|
ous Substances: Bloodborne Pathogens”), which can be found on the Inter- |
|
net by going to this address: |
|
http://www.osha.gov |
|
and searching for “1910.1030”. |
Note: |
Operators must also be aware of state and local OSHA biohazard regu- |
|
lations that may specify requirements in addition to the federal regula- |
|
tions, and must also ensure compliance procedures are in place. |
3.2Preparing the Reagent Disc
■Refer to Section 2.6, “Reagent Discs,” on page 2-7 for complete information about piccolo® reagent discs, including handling instructions. Make sure you are thoroughly familiar with this information before beginning to use the analyzer.
■Refer to Section 3.1, “Sample Requirements,” on page 3-1 for sample handling and storage requirements.
Note: Analysis must begin immediately (no more than 10 minutes) after dispensing the sample into the reagent disc.
CAUTION: Discs are fragile — always handle with care. Do not tap discs on the table or work bench to empty the sample port. Do not use a disk that has been dropped.
Inspect every reagent disc for damage before use. Never use a damaged disc.
3-2 |
Testing and Results |
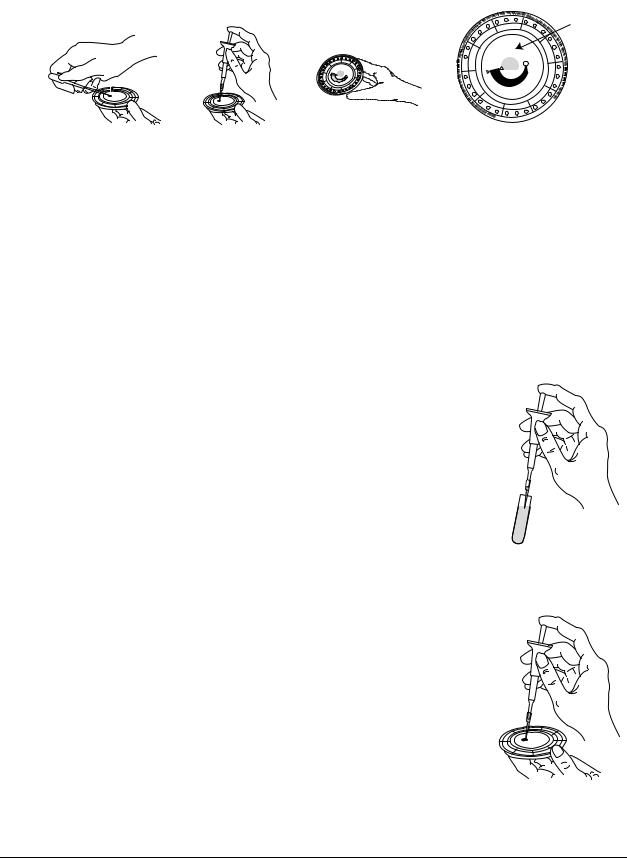
Step 1 — Dispense the Sample
Use a micropipette (one is included with the piccolo xpress™) or other transfer device to dispense
approximately 100 µL of sample into the disc via the sample chamber.
Write patient
ID here
CAUTION: Wear powder-free gloves while handling reagent discs or operating the ana-
lyzer. Powder can disrupt the analyzer’s optical components.
a.Fill the sample chamber.
1.Using the Piccolo 100 µl volume pipette, firmly attach a new tip to the end of the pipette. DO NOT touch the tip (this could cause a false elevation of amylase).
2.With your index finger or thumb, push the pipette button to the stop position and hold it down for sample pickup.
3.Immerse the tip 2–3 mm below the surface of the sample, as shown at right.
4.SLOWLY release the button to pick up the sample. Pause, then remove the pipette from the sample tube.
5.Make sure there are no air bubbles or air gaps in the pipette tip.
6.Place the pipette tip into the disc’s sample chamber, and tilt the disc to 45° with the sample port above the fill line, so that the entire sample flows into the sample chamber. The tip should touch the sample chamber, as shown at right.
Testing and Results |
3-3 |

7.Push the plunger down with a slow, continuous motion. Take care not to overfill the sample chamber. A 90 µL sample will fill the sample chamber and form a line between the two arrows molded on the disc. More than 120 µL of sample will overfill the chamber.
8.Discard the pipette tip into a biohazard container.
90 μL
> 90 μL < 120 μL
<90 μL
> 120 μL
9.Clean the reagent disc. Use a lint-free tissue to remove any sample spilled on the outside of the disc, taking care that the tissue does not withdraw any sample from the sample port. Dispose of the tissue in a biohazard container.
b.Optional: Label the disc.
Write the patient ID on the disc surface in the space shown on page 3-3. Do not write anywhere else on the disc or on the bar code ring.
c.Carry the prepared disc to the analyzer.
Hold the disc by its edges in a flat position.
CAUTION: Do not remove the sample and try to reintroduce it into the disc.
3.3Running a Patient Sample
This section includes detailed, step-by-step instructions for performing analyses using the piccolo xpress™.
Note: The analyzer includes a number of optional data functions that can be used to enter more detailed information about samples.
In this manual, the steps for these optional functions are marked with the Advanced Setting icon: 
The screens for these optional functions appear on the analyzer only if the functions are enabled. If a screen shown in a procedure step in this
manual does not appear on the analyzer screen, skip that step. (See
“Using Optional Data Functions” on page 4-33 for details about these
functions.)
3-4 |
Testing and Results |
1.Turn on the analyzer by pressing the Power button on the front of the analyzer. Do this before removing reagent discs from the refrigerator.
The analyzer starts up,
then performs a self test.
See Section 6.2, “Quality Control Features” for information about the analyzer self test.
If the analyzer needs time to warm the disc chamber to operating temperature, the display shows “Warming Up.”
When the analyzer reaches operating temperature, it displays “Analyze” on the Home screen, as shown at right.
Home
Warming Up
Monday 16 Aug 2006 10:30AM
piccolo xpress V1.2.3.4
Performing iQC...
LoadHomeDisc
Analyze
Close drawer to analyze a sample
Monday 16 AugCLOSE2006 10:45AM
2.Press Analyze on the touchscreen to open the disc drawer.
The following messages are then displayed:
Load Disc
Opening Drawer... |
Close drawer to |
|
|
|
analyze a sample |
|
|
|
|
|
|
CLOSE
Note: You can press the information icon  in the Load Disc screen to see help screens outlining the basic analysis procedure. Press Next and
in the Load Disc screen to see help screens outlining the basic analysis procedure. Press Next and
Back to move through the help screens, and Exit to close them.
Testing and Results |
3-5 |
3.Place the disc in the recessed area in the drawer.
4.Press CLOSE on the touchscreen. The analyzer then closes the drawer.
5.Optional: Enter the operator ID using the touchscreen, then press Done.
The operator ID is a number of up to 14 digits.
The right arrow key ( X) functions as a dash (–), and the left arrow key (W ) functions as a backspace.
Closing Drawer...
Enter Operator ID
12345678901234
1 2 3
4 5 6
7 8 9
0
Cancel Done
6.Select the sample type from those shown in the display.
The figure at left shows the default selections: Patient and Control. Your actual choices will depend on how the analyzer is configured — see “Using Optional Data Functions” on page 4-33.
Select Type
Patient
Control
Back Cancel
Select Type
Patient
Control
Control Level 1
Control Level 2
Special 1
Back Cancel
Note: The correct sample Type is necessary for results to be interpreted correctly.
3-6 |
Testing and Results |
7. Optional: Select the patient’s gender.
Select Gender
Unknown
Male
Female
8.Optional: Enter the patient’s age using the up (S) and down (T) arrow keys, and adjust the units if needed.
Then press Done.
Back Cancel
Enter Age
0 |
|
|
2 |
|
|
Yrs. |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
9.Enter an ID number for the sample (up to 14 characters), then press Done.
The right arrow key ( X) functions as a dash (–), and the left arrow key (W ) functions as a backspace.
Back |
Done |
|
|
Enter Sample ID
12345678901234
1 2 3
4 5 6
7 8 9
0
Back |
Done |
|
|
Testing and Results |
3-7 |
10.Optional: Enter an alternate ID (up to 14 characters), then press Done.
11. The analyzer then
Sample: 12345678901234
checks the disc type,
Analyzing Sample...
and begins processing
the sample. Determining disc
type...
CANCEL
Enter Alternate Sample ID
12345678901234
1 2 3
4 5 6
7 8 9
0
Back |
Done |
|
|
Sample: 12345678901234
Analyzing Sample...
Comprehensive
Metabolic Panel
CANCEL
Note: If the disc is found to be of an incorrect type or expired, an error message appears. Repeat the analysis with another disc of the correct type.
12. |
When the sample is finished processing, the analyzer stores the |
|
|
Sample: 12345678901234 |
|||
|
|
||
|
results and shows that the analysis is complete. |
|
Analysis Complete
OPEN
3-8 |
Testing and Results |
13.By default, the analyzer automatically prints the results of the analysis.
If the results do not print automatically, they can be recalled from memory and printed — see “Searching for Results” on page 5-3.
Note: Be sure to review the results printout for any suppressed results, which are marked with these symbols: ~~~
14.Press OPEN to open the disc drawer.
|
|
|
Remove Disc |
|
Opening Drawer... |
To analyze additional sample, |
|||
load new disc |
||||
|
|
|
||
|
|
|
|
|
|
|
|
|
|
CLOSE
15.Remove the reagent disc from the drawer.
CAUTION: Dispose of the disc according to the lab’s standard procedures for human patient samples. For additional information, see Section 1.2, “Universal Precautions,” on page 1-1.
16.To analyze another sample, insert the new reagent disc and repeat the above procedure.
17.When finished, press CLOSE to close the drawer and return the
Home
analyzer to standby mode.
Analyze
Monday 16 Aug 2006 10:45AM
Testing and Results |
3-9 |
3.4Canceling Analysis
Occasionally you may need to cancel an analysis in progress. Do this as follows.
1.Press CANCEL on the touchscreen.
Confirm
The display asks for confirmation.
Cancel Analysis?
CANCEL NO
2.Press CANCEL again to confirm. The analysis is then canceled.
Canceling Analysis
Please Wait
The disc drawer then opens.
|
|
|
Remove Disc |
|
Opening Drawer... |
To analyze additional sample, |
|||
load new disc |
||||
|
|
|
||
|
|
|
|
|
|
|
|
|
|
CLOSE
3.Remove the disc from the drawer. The analyzer is now ready to perform another analysis. Press CLOSE to close the drawer.
3-10 |
Testing and Results |
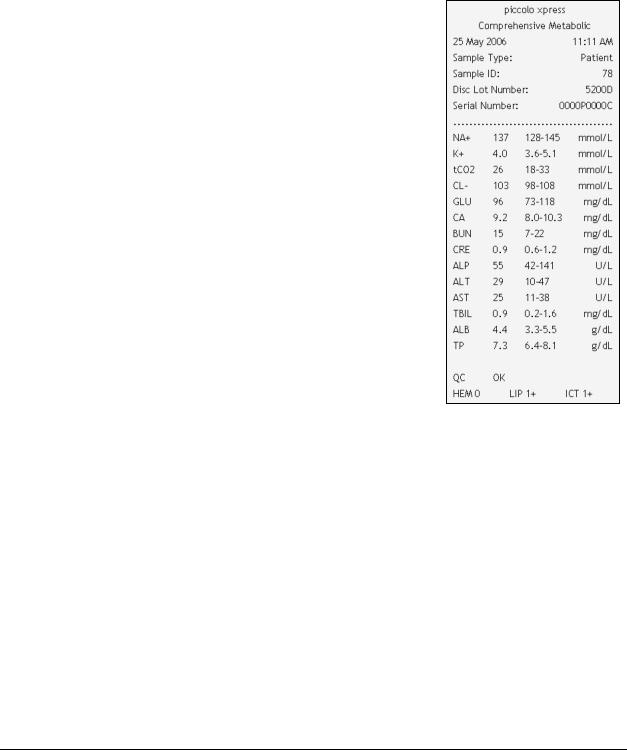
3.5Results
The results calculated by the analyzer are stored in memory and printed automatically, and can also be recalled and printed later as needed. If the analyzer is connected to an external computer, the results are automatically transmitted as soon as they are calculated (see Section 10). See Section 8.2, “Principles of Operation” for the basic procedure and equations used to calculate analyte concentrations.
The figure at the right shows the contents of a typical results printout. The heading of the results printout includes information such as the reagent disc type, test date and time, sample type, sample ID number, alternate ID number, gender, age, operator ID number, disc lot number, and analyzer serial number. The test results section of the card is printed in four columns: chemistry name, analyte concentration, reference range, and specified units, as shown at right. The results printout has an adhesive backing so it can easily be placed into the patient's file.
Each reagent disc contains reagents to detect exposure to extreme conditions such as temperature and humidity. The message “QC OK” is printed on the results when results from these reagents are within the expected ranges. Otherwise, no results are printed, and the analyzer opens the disc drawer.
■Results outside the reference range are indicated in the results by an asterisk (*) printed next to the analyte concentration.
■Results outside the dynamic range are indicated in the results by a “less than” symbol (<) printed next to the lowest value of the dynamic range, or a “greater than” symbol (>) printed next to the highest value of the dynamic range.
For example, the dynamic range of glucose is 10–700 mg/dL. A sample concentration of glucose below this range would be printed as <10 mg/dL, and a concentration above this range would be printed as >700 mg/dL. Results outside the dynamic range should be reported as being below or above the value indicated.
■The symbols “~~~” are printed in place of numbers when a result cannot be determined — that is, when the result is suppressed. A result may be suppressed due to improper mixing of a reagent bead with diluted sample, a nonlinear reaction, an endpoint of a particular reaction not reached, or a concentration outside the analyzer’s capabilities. When a chemistry is suppressed (~~~), the analyzer prompts the operator to print an error report.
Testing and Results |
3-11 |

For the lactate dehydrogenase (LD) assay only:
Blood cells contain significant levels of LD, and therefore all LD assays are sensitive to hemolysis caused by release of LD from red blood cells. In the following circumstances, the results are annotated to help interpret LD activity in the presence of small amounts of hemolysis.
If HEM is greater than 50 and less than or equal to 100 mg/dL, the printed LD value is followed by an “H” indicating additional influence from hemolysis.
If HEM is greater than 100 and less than or equal to 150 mg/dL, the LD value is preceded by “<” and followed by “H”, indicating that the true LD recovery is less than reported.
If HEM is greater than 150, no result will be indicated and only “HEM” will be printed.
HEM, LIP, or ICT is printed in place of the analyte concentration if hemolysis, lipemia, or icterus, respectively, has adversely affected the results. LIP is also printed if both lipemia and icterus have been affected. HEM is also printed if hemolysis and icterus, hemolysis and lipemia, or hemolysis, lipemia, and icterus have affected a particular analyte. Examine the sample indices to determine if more than one interferent is affecting a particular result.
The sample indices are included at the bottom of the results printout. These indices indicate the degree of hemolysis, icterus, and lipemia found in the sample. Hemolysis, icterus, and lipemia are measured on a scale of 0 (clear), 1+ (slight), 2+ (moderate), and 3+ (gross).
If the sample is identified as hemolytic, collect a new sample and run another reagent disc. Abaxis recommends that the new sample be separated into serum or plasma so that the degree of hemolysis can be verified. If the new sample is hemolytic, use an alternative testing method or send the sample to a reference laboratory. Samples with hematocrit in excess of 60% packed red cell volume may appear on the result card as HEM. Follow the instructions above for retesting hemolyzed samples.
High lipemia may be due to diet. Ensure the patient has fasted for at least 12 hours before collecting another sample. For suggestions on testing grossly lipemic or icteric samples, see “Troubleshooting” on page 7-1. For grossly lipemic samples from fasting patients or for icteric samples, use an alternative testing method or send the sample to a reference laboratory.
3-12 |
Testing and Results |
 Loading...
Loading...