Page 1
3B SCIENTIFIC
Instruction sheet
11/07 SP/ALF
®
PHYSICS
Cloud chamber U8483220
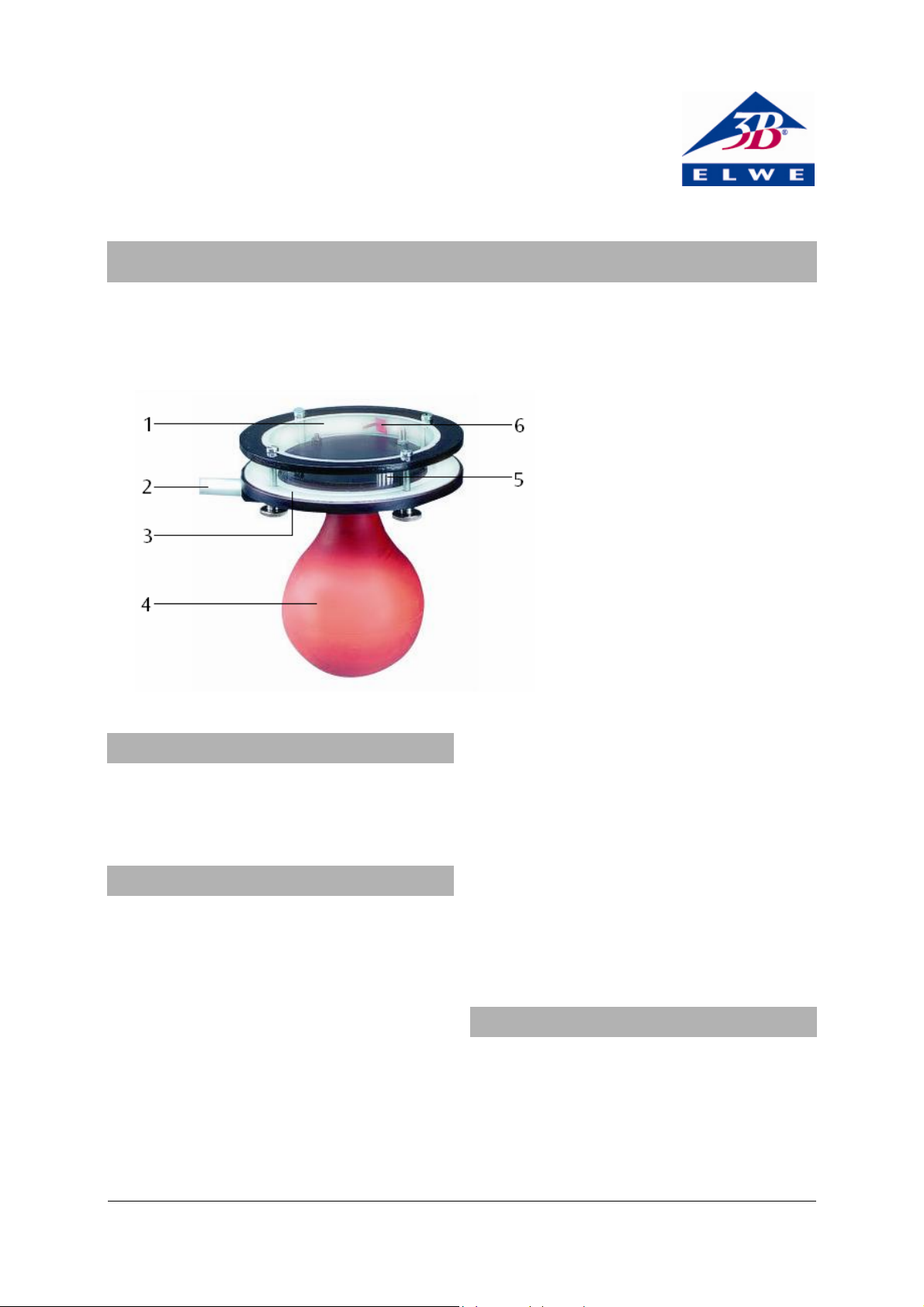
1 Cover plate
2 Supporting rod
3 Base-plate
4 Rubber bellows
5 Filling nozzle (with thread for
attaching radiation cartridge)
6 Absorption foil on hinged
support
1. Safety instructions
• In experiments with radioactive substances, ob-
serve the regulations that currently apply for the
region (e.g., radiation protection regulations).
2. Description
The cloud chamber is used for making the tracks of
ionising radiation visible (especially for α radiation).
The cloud chamber consists of a thick plate of Plexiglas fixed above a base-plate with a gas-tight seal. In
the centre of the base-plate there is a nozzle onto
which a rubber bellows is pushed. There is also a
foam rubber pad recessed into the base-plate, which
provides resistance against the air flow during the
adiabatic expansion of the gas filling. In the chamber
there is an absorption foil (paper) held on a hinged
support. One suitable radiation source for use with
the cloud chamber is the radium radiation cartridge
(U8483110), which can be screwed into an off-centre
threaded hole in the base-plate. A supporting rod on
the side of the cloud chamber allows it to be clamped
to a stand.
The fluid used in the cloud chamber is a mixture of
methanol and water in the proportion 50:50.
A cloud chamber such as this does not need to have
its design licensed, but this model is in fact licensed
as a radiation-proof holder for the radiation cartridge
U8483110. The cloud chamber thus qualifies under
radiation protection provisions (e.g. II. SVO § 9, 4 in
Germany), whereby its design is officially approved
(PTB No. VI B/S 3516) and licensed (licensing document BW 8/65/II).
3. Technical data
Chamber dimensions: 15 mm x 90 mm dia.
Supporting rod: 45 mm x 10 mm dia.
Weight: 600 g approx.
Cloud chamber fluid: methanol/water 30 ml
1
Page 2

4. Operating principle
Experiments by R. von Helmholtz in 1887 showed that
ions in an atmosphere supersaturated with water
vapour act as condensation centres around which
cloud droplets form. The charged particles emitted
from radioactive elements generate large numbers of
ion pairs along their paths in the surrounding atmosphere. If the air is supersaturated with water vapour,
the ions act as condensation centres, and with suitable illumination the tracks of the particles become
visible as fine vapour trails (“condensation trails”).
In the cloud chamber the supersaturation of the surrounding air is produced by sudden expansion and
resultant cooling of the gas filling.
5. Operation
5.1 General instructions
1. When the cloud chamber is being closed, the
knurled screws must be tightened firmly to ensure an
airtight seal. By immersing the chamber under water
and squeezing the rubber bellows, any leakage will
become apparent.
2. It is essential for the cloud chamber to be kept free
of dust particles. When withdrawing the radiation
cartridge from the cloud chamber, the filling nozzle
must be closed with a rubber bung. The risk of contamination is especially great when the chamber is
taken apart. Therefore, do not open the chamber
more often than is necessary, and before reassembling it, clean it thoroughly with a damp chamoisleather.
3. The cloud chamber remains usable for a very long
time if the radiation cartridge remains attached to the
filling nozzle or the nozzle is closed by an air-tight
bung.
4. The radiation cartridge is tightly sealed to prevent
any emanation. Even when it remains in the cloud
chamber for a long time, there is no risk of radioactive contamination.
5. The accurately parallel cover plate allows particle
tracks to be photographed with no optical distortion.
For this the illumination should be arranged, using
apertures, so that the light beam does not fall on the
black base-plate.
6. If a deposit of moisture forms on the Plexiglas plate
during storage or due to uneven heating by the illuminating lights, it can be eliminated by placing a
warm woollen cloth over the plate.
5.2 Experiment procedure
• Using a pipette, introduce the cloud chamber
fluid (about 10 to 20 drops) into the chamber
through the filling nozzle, and distribute it evenly
by shaking.
• Screw the radiation cartridge into the filling noz-
zle, after first using a screwdriver or flat object to
rotate the cartridge shaft so that its flattened end
faces towards the middle of the chamber.
• Align the cloud chamber horizontally by clamping
it on a stand.
• Set up the illumination so that the light beam
enters the chamber from the side at about 90
o
to
the direction of the radiation from the radioactive
source.
• Rub the cover plate with a woollen cloth, without
applying pressure.
• Squeeze the rubber bellows tightly, hold for 1 to 2
seconds, then release.
On releasing the rubber bellows, the tracks of the
α−particles become visible as vapour trails. They
slowly disappear after 1 to 2 seconds. The process can
be repeated after waiting only a few seconds.
• By tilting the cloud chamber, bring the absorp-
tion foil into the path of the radiation and observe the absorption of the α−particles on paper.
5.3 Comments
1. When the cover plate is rubbed, an electric field is
generated between it and the base-plate, which
purges the chamber of residual ions, which would
interfere with the experiment by causing a haze. If the
photographs obtained after repeated operation of the
rubber bellows are blurred, the cover plate needs to
be rubbed again.
2. In the photographs obtained from the cloud chamber, it can clearly be seen that the trails are of different lengths. A large fraction of them are only about
half as long as the longest ones. From the different
lengths of the trails, it can be concluded that the
particles are emitted at differing velocities.
Each α-emitting substance (nuclide) is characterised
by a unique emission energy, and a corresponding
range of penetration through air. The α-particles from
radium 226 have a range of 3.6 cm (at atmospheric
pressure). The α−particles with the long trails are
emitted by a decay product (Ra A, range 6.3 cm). The
radioactive material in the radiation cartridge is surrounded by an extremely thin metal foil. Consequently, the observed ranges are slightly smaller than
the values given in the tables.
If an α−particle collides with an atomic nucleus in its
flight, its direction is changed and the affected nucleus is set in motion, thus producing a trail of its
own. Such collisions are very rare, and therefore you
will be very lucky if you are able to observe such an
event.
2
Page 3

3. If, instead of paper, a very thin film of Hostaphan is
placed in front of the source (thickness 5 to 10 μm, or
0.7 to 1.5 mg/cm
2
), it can be seen that nearly all the
α−particles pass through the film without any significant deviation or shortening of their range. Thus,
α−particles can pass through thin films of materials.
This experiment is analogous in a qualitative way to
Rutherford’s scattering experiment, and shows that
the structure of matter consists largely of gaps. Instead of Hostaphan, thin foils of other materials can
also be used, such as gold leaf. The easiest way to
handle such a foil is to tape it over a hole in a strip of
adhesive tape (Sellotape or Scotch Tape).
Elwe Didactic GmbH • Steinfelsstr. 6 • 08248 Klingenthal • Germany • www.elwedidactic.com
3B Scientific GmbH • Rudorffweg 8 • 21031 Hamburg • Germany • www.3bscientific.com
Subject to technical amendments
© Copyright 2007 3B Scientific GmbH
 Loading...
Loading...