Page 1
[ CARE AND USE MANUAL ]
XSELECT CSH XP 2.5 µm COLUMNS
CONTENTS
I. INTRODUCTION
II. GETTING STARTED
a. Column Connection
b. Column Installation
c. Minimizing Band Spread Volume
d. Measuring Band Spread Volume
e. Measuring System Dwell Volume
f. Column Equilibration
g. eCord Installation
h. Initial Column Efficiency Determination
i. VanGuard Pre-Columns
III. COLUMN USE
a. Sample Preparation
b. pH Range
c. Solvents
d. Pressure
e. Temperature
I. INTRODUCTION
Thank you for choosing a Waters XSelect™ Charged Surface Hybrid
[CSH] eXtended Performance [XP] 2.5 µm Column. The manufacture
of XSelect CSH XP 2.5 µm Columns begins with ultrapure reagents
and are manufactured in a cGMP, ISO 9001 certified facility to
control the chemical composition and purity of the final product.
Well-controlled manufacturing processes result in industry-leading
batch-to-batch reproducibility. Every column is individually tested.
A Performance Chromatogram and Certificate of Batch Analysis are
provided on the eCord™ Intelligent Chip.
XSelect CSH XP 2.5 µm Columns are based on the same base particle
technology and bonded-phase chemistry as 1.7 µm ACQUITY UPLC®
CSH Columns as well as XSelect CSH 3.5 and 5 µm HPLC Columns,
thus enabling seamless transferability between HPLC, UHPLC and
UPLC® platforms.
XSelect CSH XP 2.5 µm Columns will exhibit maximum chromatographic
performance when used on a member of the ACQUITY UPLC
System family.
IV. COLUMN CLEANING, REGENERATION AND STORAGE
a. Cleaning and Regeneration
b. Storage after Reversed-Phase Use
V. eCORD INTELLIGENT CHIP TECHNOLOGY
a. Introduction
b. Installation
c. Manufacturing
d. Column Use
VI. ADDITIONAL INFORMATION
a. Tips for Maximizing XSelect CSH XP 2.5 µm Column Lifetime
b. Troubleshooting Questions
c. Recommended Flow Rates and Anticipated Backpressures for
Reversed-Phase XSelect CSH XP 2.5 µm Columns
XSelect CSH XP 2.5 µm Columns 1
Page 2
[ CARE AND USE MANUAL ]
II. GET TING STARTED
Each XSelect CSH XP 2.5 µm Column comes with a Certificate of
Analysis and a Performance Test Chromatogram embedded within
the eCord intelligent chip. The Certificate of Analysis is specific to
each batch of packing material contained in the XSelect CSH XP 2.5 µm
Column and includes the gel batch number, analysis of unbonded
particles, analysis of bonded particles and chromatographic results
and conditions. The Performance Test Chromatogram is specific to
each individual column and contains such information as: gel batch
number, column serial number, USP plate count, USP tailing factor,
retention factor and chromatographic test conditions. T hese data
should be recorded and stored for future reference or can be accessed
via the ACQUITY UPLC console.
a. Column Connection
XP 2.5 µm Columns are designed to operate on any HPLC, UHPLC or
UPLC System. Due to the absence of an industry standard, please be
aware that the type of fittings and connections on each system will
vary by manufacturer and should be mated specifically to a column
when it is installed.
The chromatographic performance can be negatively impacted,
or leaking can occur, if the style of the column endfitting does
not properly match that of the compression screw/ferrule tubing
depth setting.
b. Column Installation
Note: The flow rates given in the procedure below are described for a 2.1 mm ID
column. Scale the flow rate according to the flow rate and pressure guidelines
described in Section VI (Additional Information).
1. Purge the pumping system of any buffer-containing mobile
phases and connect the inlet of the column.
4. Gradually increase the flow rate as described in step 2.
5. Monitor until a steady backpressure and baseline have been achieved.
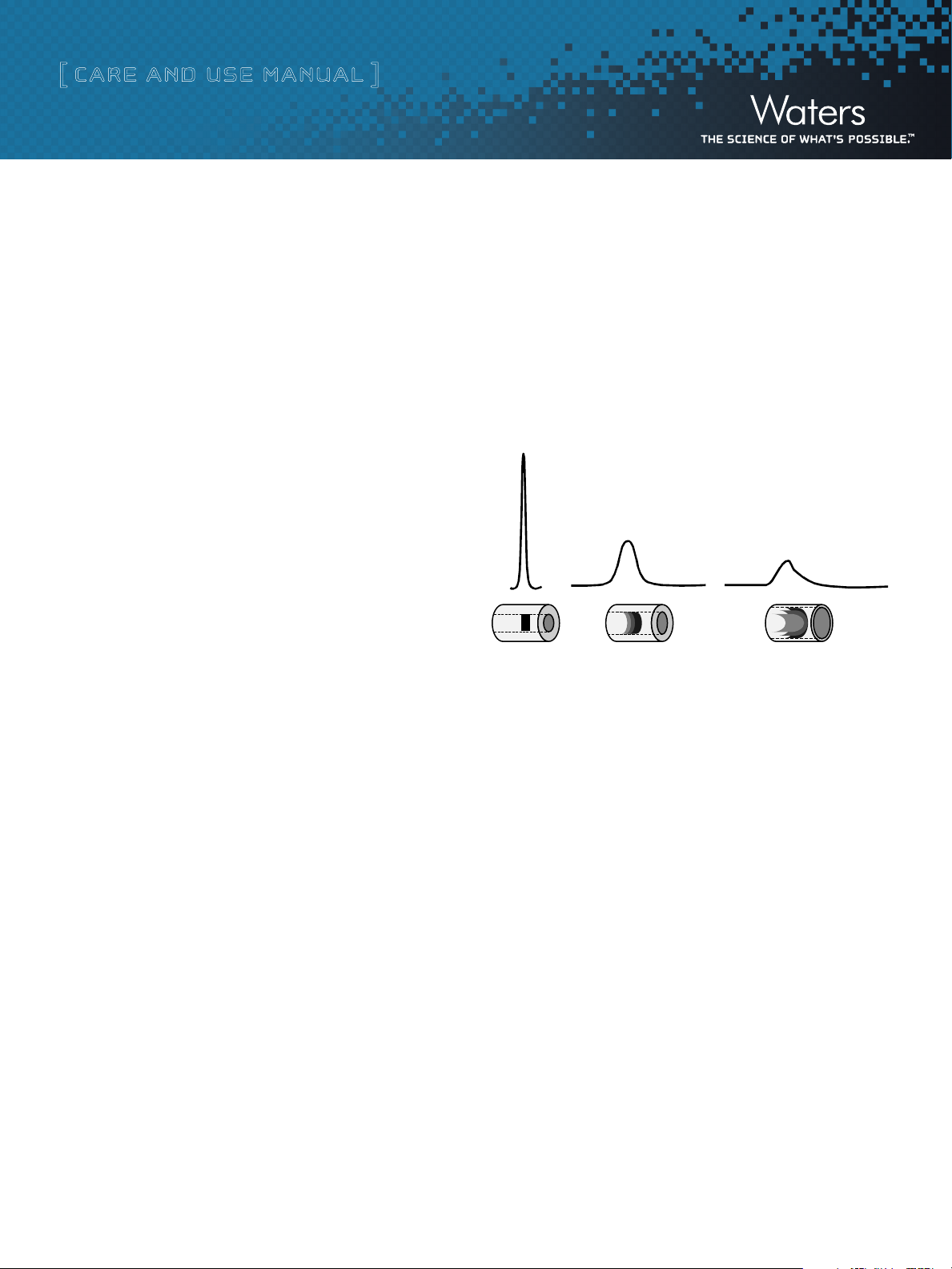
c. Minimizing Band Spread Volume
Band spreading is a measurement of the system dispersion that impacts
the chromatographic performance. Internal tubing diameter and fluidic
connections can significantly impact system band spreading and
chromatographic performance. Larger tubing diameters cause excessive
peak broadening and reduced sensitivity (Figure 1).
0.005 inches
0.020 inches
0.040 inches
Diluted/Distorted Sample Band
Figure 1: Impact of tubing diameter on band spread.
d. Measuring Band Spread Volume
Note: This test should be performed on an LC system equipped with a UV detector.
1. Disconnect the column from the system and replace with a zero
dead volume union.
2. Set the flow rate to 1 mL/min.
3. Use a test mixture (dissolved in the mobile-phase conditions)
that delivers a maximum peak height of 0.5 – 1.0 AU (System
Start Up Test Mixture can be used, Part No. WAT034544).
4. Inject 2 – 5 µL of this solution.
2. Flush the column with 100% organic mobile phase (methanol or
acetonitrile) by setting the pump flow rate to 0.1 mL/min and
increase the flow rate to 0.5 mL/min over 5 minutes.
3. When the mobile phase is flowing freely from the column outlet,
stop the flow and attach the column outlet to the detector. This
prevents air entering the detection system and provides a more
rapid baseline equilibration.
XSelect CSH XP 2.5 µm Columns 2
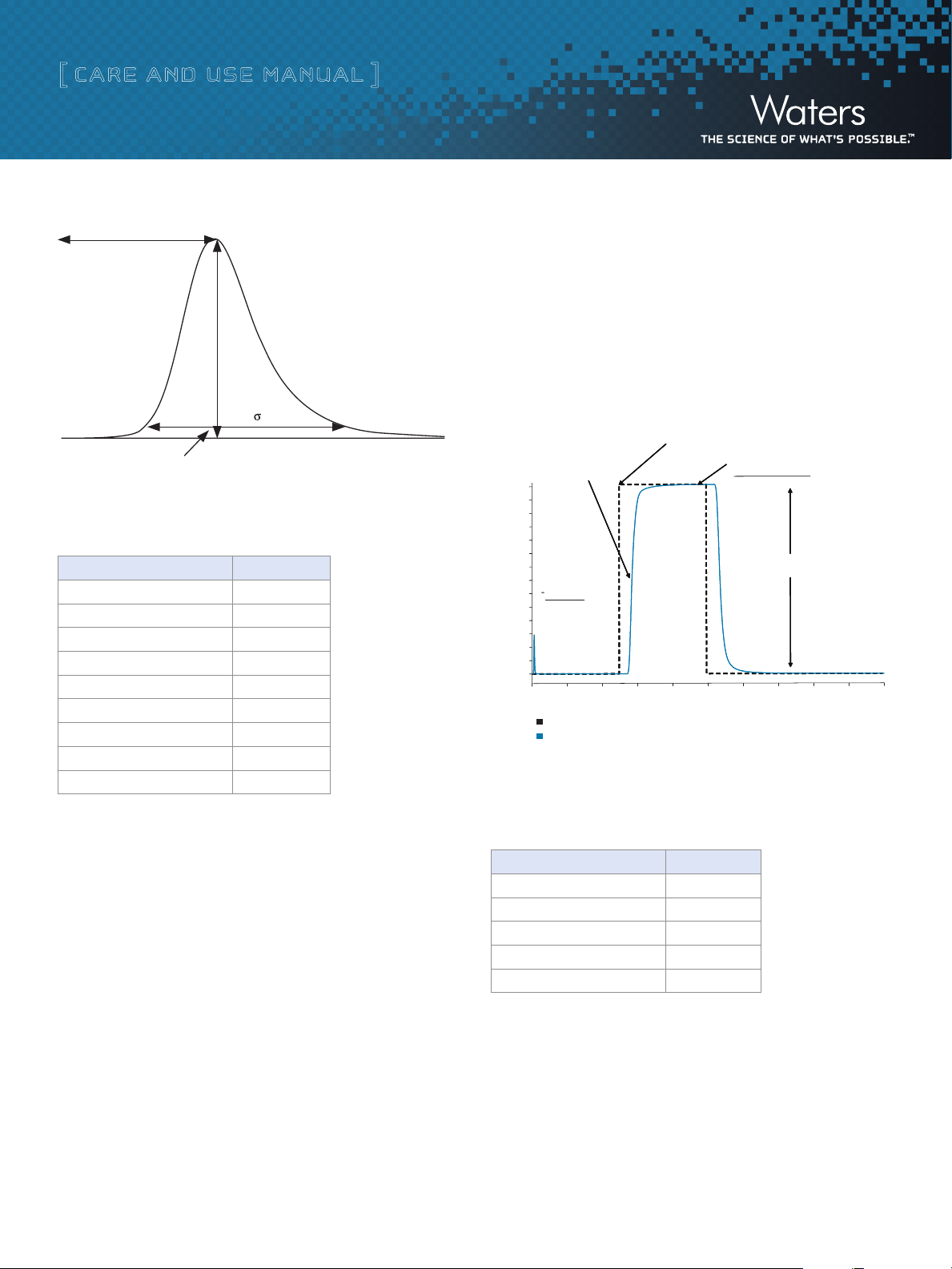
5. Using the 5-Sigma method, measure the peak width at 4.4% of
peak height:
Band Spreading (µL) = Peak Width (min) x Flow Rate (µL/min)
(For example, if peak width = 0.1 min and flow rate = 1000 µL/min,
band spread = 100 µL)
Page 3
[ CARE AND USE MANUAL ]
System Volume
5
4.4 %h
Figure 2: Determination of system band spread volume using 5-Sigma Method.
Table 1: Expected System Band Spread Volumes
System Band Spread
Alliance 2695 HPLC 29 µL
Vendor A HPLC 41 µL
Vendor B UHPLC (600 bar) 28 µL
Vendor C UHPLC 21 µL
Vendor D UHPLC 17 µL
ACQUITY UPLC 12 µL
ACQUITY UPLC H-Class 9 µL
ACQUITY UPLC I-Class (FTN) 7.5 µL
ACQUITY UPLC I-Class (FL) 5.5 µL
6. At 5 minutes, program a step to 100% B, and collect data for an
additional 5 minutes.
7. Measure absorbance difference between 100% A and 100% B.
8. Measure time at 50% of that absorbance difference.
9. Calculate time difference between start of step and 50% point.
10. Multiply time difference by flow rate to calculate system volume.
Programmed time = 5 minutes
50% Absorbance = 0.35852 AU
Time = 5.6953 minutes
0.70
0.65
0.60
0.55
0.50
0.45
0.40
5.69
0.35
0.30
5.00
0.25
0.69 min
0.20
0.15
0.10
0.05
0.00
0.00 2.00
= Programmed Gradient
= Actual Gradient
4.00
6.00 8.00
System Volume
0.69 min x 1.5 mL/min = 1.04 mL
Figure 3: Measuring system band spread volume.
100% Asymptotic
Total absorbance = 0.7164 AU
10.00
12.00 14.00
16.00
18.00 20.00
e. Measuring System Dwell Volume
Dwell volume is different than system band spreading. System dwell
volume is a measurement of the volume it takes for the initial gradient
conditions to reach the head of the column. This calculation is
particularly useful when it is necessary to transfer a method between
different LC systems.
1. Disconnect the column from the system and replace with a zero dead
volume union.
2. Use acetonitrile as mobile phase A, and acetonitrile with 0.05 mg/mL
uracil as mobile-phase B.
3. Monitor UV at 254 nm.
4. Use the flow rate in the original method and the intended flow rate on
the target instrument.
5. Collect 100% A baseline for 5 minutes.
XSelect CSH XP 2.5 µm Columns 3
Table 2: Expected System Dwell Volumes
System Dwell Volume
Alliance 2695 HPLC 900 µL
ACQUITY UPLC 120 µL
ACQUITY UPLC H-Class 350 µL
ACQUITY UPLC I-Class (FTN) 100 µL
ACQUITY UPLC I-Class (FL) 95 µL
f. Column Equilibration
XSelect CSH XP 2.5 µm Columns are shipped in 100% acetonitrile.
It is important to ensure mobile-phase compatibility before changing
to a different mobile-phase system. Equilibrate the column with a
minimum of 10 column volumes of the mobile phase to be used
(refer to Table 3 for a list of column volumes). The column may
be considered fully equilibrated once a constant backpressure
is achieved.
Page 4
[ CARE AND USE MANUAL ]
Table 3: Column Volumes (mL)
Column Length
(mm)
30 0.10 0.21 0.50
50 0.17 0.35 0.83
75 0.26 0.53 1.25
100 0.35 0.71 1.66
2.1 mm 3.0 mm 4.6 mm
Internal Diameter
To avoid precipitating mobile-phase buffers within the column or
system, flush the column with five column volumes of a water/organic
solvent mixture using the same, or lower, solvent content as in the
desired buffered mobile phase (i.e., flush the column and system with
60% methanol in water prior to introducing 60% methanol/40%
buffer mobile phase).
Note: If mobile-phase additives (i.e., ion-pairing reagents) are present in low
concentrations (<0.2% v/v), 100 to 200 column volumes may be required for
complete equilibration. In addition, mobile phases that contain formate (i.e.,
ammonium formate, formic acid) may require extended equilibration times.
g. eCord Installation
eCord™ Technology represents a significant advancement in column
usage tracking management which can be realized if the column is
installed on an ACQUITY UPLC System. T he eCord can be read
by connecting the yellow fob to the reader/writer located on the
right-hand side of the ACQUITY UPLC Column heater module.
Embedded information such as the column manufacturing QC data
and Certificates of Analysis may then be accessed via the ACQUITY
UPLC console.
modified to such an extent that they are not commercially viable and have
limited method flexibility other than isocratic column testing.
2. Determine the number of theoretical plates (N) and use this
value for periodic comparisons.
3. Repeat the test periodically to track column performance over
time. Slight variations may be obtained on different LC systems
due to the quality of the connections, operating environment,
system electronics, reagent quality, column condition and
operator technique.
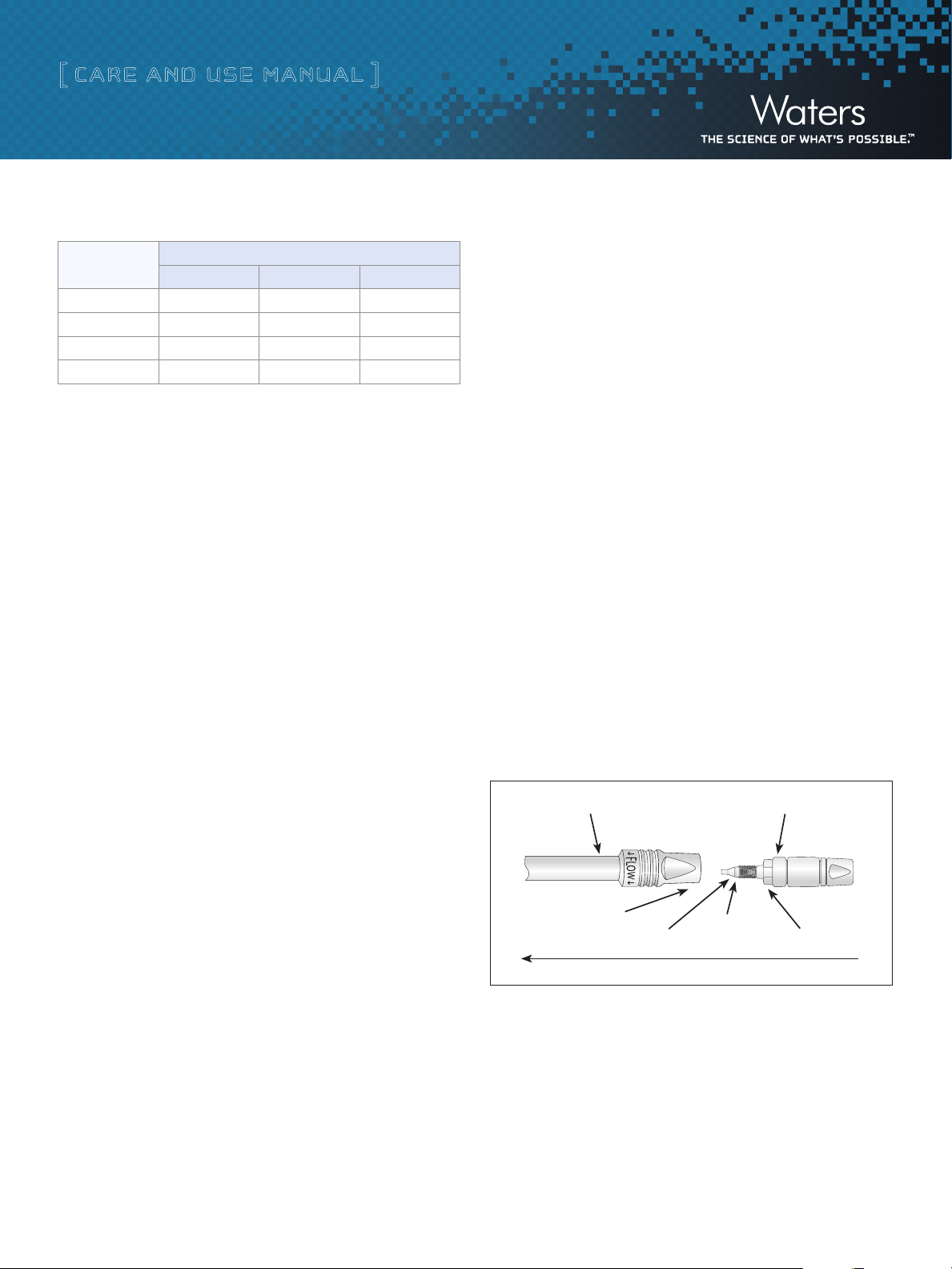
i. VanGuard Pre-Columns
VanGuard™ Pre-Columns are 2.1 mm ID x 5 mm length guard column
devices designed specifically to protect an analytical column while
minimizing the negative dispersion impact of utilizing such a device.
VanGuard Pre-Columns are packed with the same stationary phases
as the XP 2.5 µm Column offering. VanGuard Pre-Columns
are designed to be directly attached to the inlet of a eXtended
Performance [XP] 2.5 µm Column.
Note: VanGuard Pre-Columns are shipped with a collet and ferrule that are NOT
pre-swaged. This enables the end user to mate the VanGuard Pre-Column to a
specific XP 2.5 µm Column and ensures void-free and leak-free connections.
Care must be taken when removing the O-ring that holds these two pieces on
the pre-column tubing.
2.5 µm XP Column
VanGuard Pre-Column
h. Initial Column Efficiency Determination
1. Perform an efficiency test on the column before using it to track column
performance over time. This test may consist of:
a. An analyte test mixture that is commonly used in your laboratory
b. An analyte mixture as found on the “Performance Test
Chromatogram” which can be accessed via the eCord.
Note: If [b] is performed, the isocratic efficiencies measured in your laboratory
may be less than those given on the Waters Performance Test Chromatogram.
This is normal and expected. The Waters isocratic column testing systems have
been modified in order to achieve extremely low system dispersion. This presents
a more challenging test of how well the column was packed. This also guarantees
the highest quality packed column. These special testing systems have been
XSelect CSH XP 2.5 µm Columns 4
Place wrench here
Ferrule
Figure 4: Installing a VanGuard Pre-Column.
Flow
Collet
Place wrench here
VanGuard Pre-Column Installation Instructions
1. Remove the VanGuard Pre-Column from its box and shipping
tube and remove the plastic plug.
2. Orient the pre-column so that the male end is facing up and
carefully remove the black O-ring that holds the collet and
ferrule in place during shipment (collet and ferrule are not
permanently attached).
Page 5

[ CARE AND USE MANUAL ]
3. Orient the XP 2.5 µm Column perpendicular to the work surface
so that the column inlet is on the bottom.
4. From below, insert the VanGuard Pre-Column into the column
inlet; turn the assembled column and pre-column 180° so that
the pre-column is now on top.
5. Tighten with two 5/16” wrenches placed onto the XP 2.5 µm
Column flats and VanGuard Pre-Column hex nut (male end) as
shown in Figure 4.
6. While keeping pressure on the VanGuard Pre-Column against the
XP 2.5 µm Column, tighten turn to set the collet and ferrule.
7. Check that the ferrule is set by loosening the connection and
inspecting the ferrule depth.
8. Reattach the pre-column to the XP 2.5 µm Column, apply flow
and inspect for leaks.
III. COLUMN USE
To ensure the continued high performance of XSelect CSH XP 2.5 µm
Columns, follow these guidelines:
a. Sample Preparation
1. Sample impurities and/or particulates often contribute to column
contamination. One option to avoid column contamination is
to use Waters Oasis® or Sep-Pak® Solid-Phase Extraction (SPE)
devices. To select the appropriate sorbent for a specific sample
type, visit www.waters.com/sampleprep
3. If the sample is not prepared in the mobile phase, ensure that
the sample, solvent and mobile phases are miscible in order to
avoid sample and/or buffer precipitation.
4. Filter sample with a 0.2 µm membrane to remove particulates.
If the sample is dissolved in a solvent that contains an
organic modifier (i.e., acetonitrile, methanol) ensure that the
membrane/filter material is compatible with the solvents in use.
Alternatively, centrifuge the sample for 20 minutes at
8000 rpm, followed by the transfer of the supernatant to an
appropriate vial could be considered.
b. pH Range
Table 4: Recommended pH Range
Chemistry pH Range
XSelect CSH C
XSelect CSH Phenyl-Hexyl 1 - 11
XSelect CSH Fluoro-Phenyl 1 - 8
Column lifetime will vary depending on the combination of
temperature, mobile-phase pH and type of buffer/additive used.
Table 5 lists the recommended buffers and additives for XSelect
CSH XP 2.5 µm Columns.
Note: Working in combinations of extreme pH, temperature and pressure may
result in reduced column lifetime.
18
1 - 11
2. It is preferable to prepare the sample in the initial mobile-
phase conditions or a weaker solvent for the best peak shape
and sensitivity.
XSelect CSH XP 2.5 µm Columns 5
Page 6

[ CARE AND USE MANUAL ]
Table 5. Buffer Recommendations for XSelect CSH XP 2.5 µm Columns
Additive/Buffer pKa Buffer Range
Volatility
(±1 pH unit)
TFA 0.3 - Volatile Yes
Acetic Acid 4.76 - Volatile Yes
Formic Acid 3.75 - Volatile Yes
Acetate (CH
COO-) 4.76 3.76 – 5.76 Volatile Yes
3
Formate (HCOO-) 3.75 2.75 – 4.75 Volatile Yes
Used for
Mass Spec
Comments
Ion pair additive, can suppress MS signal,
used in the 0.02-0.1% range.
Maximum buffering obtained when used with
ammonium acetate salt. Used in 0.1-1.0% range.
Maximum buffering obtained when used with
ammonium formate salt. Used in 0.1-1.0% range.
Used in the 1-10 mM range. Note that sodium or
potassium salts are not volatile.
Used in the 1-10 mM range. Note that sodium or
potassium salts are not volatile.
Phosphate 1 2.15 1.15 – 3.15 Non-volatile No Traditional low pH buffer, good UV transparency.
Phosphate 2 7.2 6.20 – 8.20 Non-volatile No
Phosphate 3 12.3 11.3 – 13.3 Non-volatile No
Above pH 7, reduce temperature/concentration and use
a guard column to maximize lifetime.
Above pH 7, reduce temperature/concentration and use
a guard column to maximize lifetime.
4-Methylmorpholine ~8.4 7.4 – 9.4 Volatile Yes Generally used at 10 mM or less.
Ammonia (NH
) 9.2 8.2 – 10.2 Volatile Yes
4
Keep concentration below 10 mM and
temperatures below 30 ˚C.
Used in the 5-10 mM range (for MS work keep source
Ammonium Bicarbonate
10.3 (HCO
9.2 (NH
-
)
3
+
)
4
8.2 – 11.3 Volatile Yes
>150 ˚C ). Adjust pH with ammonium hydroxide or acetic
acid. Good buffering capacity at pH 10. Note: use ammonium
bicarbonate (NH
HCO3), not ammonium carbonate ((NH4)2CO3).
4
Ammonium (Acetate) 9.2 8.2 – 10.2 Volatile Yes Used in the 1-10 mM range.
Ammonium (Formate) 9.2 8.2 – 10.2 Volatile Yes Used in the 1-10 mM range.
Borate 9.2 8.2 – 10.2 Non-volatile No
CAPSO 9.7 8.7 – 10.7 Non-volatile No
Glycine 2.4, 9.8 8.8 – 10.8 Non-volatile No
Reduce temperature/concentration and use a
guard column to maximize lifetime.
Zwitterionic buffer, compatible with acetonitrile,
used in the 1-10 mM range. Low odor.
Zwitterionic buffer, can give longer lifetimes
than borate buffer.
1-Methylpiperidine 10.2 9.3 – 11.3 Volatile Yes Used in the 1-10 mM range.
CAPS 10.4 9.5 – 11.5 Non-volatile No
Triethylamine
(as acetate salt)
10.7 9.7 – 11.7 Volatile Yes
Zwitterionic buffer, compatible with acetonitrile,
used in the 1-10 mM range. Low odor.
Used in the 0.1-1.0% range. Volatile only when titrated
with acetic acid (not hydrochloric or phosphoric). Used as
ion-pair for DNA analysis at pH 7-9.
Pyrrolidine 11.3 10.3 – 12.3 Volatile Yes Mild buffer, gives long lifetime.
XSelect CSH XP 2.5 µm Columns 6
Page 7

[ CARE AND USE MANUAL ]
c. Solvents
To maintain maximum column performance, use high quality HPLC
or MS grade solvents. Filter all aqueous buffers prior to use through
a 0.2 µm filter. Solvents containing suspended particulate materials
will generally clog the outside surface of the inlet of the column.
This may result in higher backpressure or distorted peak shape.
d. Pressure
XSelect CSH XP 2.5 µm Columns are compatible with HPLC, UHPLC and
UPLC pressures. Table 6 depicts the maximum operation pressure.
Table 6: Maximum Operation Pressure
Column ID Pressure Range
2.1 mm 18,000 psi [1034 bar]
3.0 mm 18,000 psi [1034 bar]
4.6 mm 9000 psi [620 bar]
e. Temperature
XSelect CSH XP 2.5 µm Columns can be used up at intermediate
temperatures to enhance selectivity, reduce solvent viscosity and
increase mass transfer rates.
Chemistry
XSelect CSH C
XSelect CSH Phenyl-Hexyl 80 °C 45 °C
XSelect CSH Fluoro-Phenyl 60 °C 45 °C
Note: Working in combinations of extreme pH, temperature and pressure may
result in reduced column lifetime.
18
Temperature Limit
Low pH
80 °C 45 °C
Temperature Limit
High pH
Use a cleaning routine that matches the properties of the samples,
stationary-phase type (reversed-phase, normal-phase or HILIC) and
will solubilize the suspected contaminate. Flush with 20 column
volumes of solvent at an intermediate temperature of 45 °C. Return
to the initial mobile-phase conditions by reversing the sequence.
If using a reversed-phase column, purge the column with a sequence
of progressively more non-polar solvents (i.e., water-to-methanol-to-
tetrahydrofuran-to-methylene chloride).
If column performance has not improved after regeneration/
cleaning procedures, contact your local Waters representative for
additional support.
b. Storage after Reversed-Phase Use
For periods longer than four days, store the XP 2.5 µm Column in
100% acetonitrile. For separations utilizing elevated temperature,
store immediately after use in 100% acetonitrile. Do not store
columns in buffered eluents. If the mobile phase contained a buffer
salt, flush the column with 10 column volumes of HPLC grade water
(see Table 3 for column volume information) followed by 10 column
volumes of acetonitrile. Failure to perform this intermediate step
could result in precipitation of the buffer salt in the column when
100% acetonitrile is introduced. Completely seal the column to avoid
solvent evaporation and drying out of the chromatographic bed.
Note: If a column has been run with a formate-containing mobile phase
(e.g., ammonium formate, formic acid, etc.) and is purged with 100%
acetonitrile, slightly longer equilibration times may be necessary when the
column is re-installed and re-wetted with that same formate-containing
mobile phase.
IV. COLUMN CLEANING, REGENERATION AND STORAGE
a. Cleaning and Regeneration
Changes in peak shape, peak splitting, shouldering peaks, shifts
in retention, change in resolution or increasing backpressure may
indicate contamination of the column. Flush with a neat organic
solvent to remove the non-polar contaminant(s), taking care not to
precipitate any buffered mobile-phase components. If this flushing
procedure does not solve the problem, purge the column with the
following cleaning and regeneration procedures.
XSelect CSH XP 2.5 µm Columns 7
V. eCORD INTELLIGENT CHIP TECHNOLOGY
a. Introduction
The eCord Intelligent Chip Technology represents a significant
advancement in column usage tracking management which can be
realized if the column is installed on an ACQUITY UPLC System.
The eCord Intelligent Chip provides a paperless tracking history of the
column’s performance and usage throughout its lifetime. The eCord
is permanently attached to the column body via a tether that cannot
be removed. T his ensures that the history of the column is always
accessible to the user of that column.
Page 8

[ CARE AND USE MANUAL ]
Figure 5: eCord Intelligent Chip.
At the time of manufacture, information such as the performance test
chromatogram, column manufacturing QC data and Certificates of
Analysis is downloaded onto the eCord. This information may then be
accessed via the ACQUITY UPLC console once the column is installed.
b. Installation
The eCord can be read by connecting the yellow fob to the reader/
writer located on the right-hand side of the ACQUITY UPLC Column
heater module. Once the eCord is connected to the magnetic catch on
the column heater, column identification and overall column usage
information can be accessed.
Figure 6: Installing the eCord Intelligent Chip.
Figure 7: Manufacturing results stored on an eCord.
c. Manufacturing
The eCord provides the user with the Batch Certificate of Analysis and
Performance Test Chromatogram.
XSelect CSH XP 2.5 µm Columns 8
Page 9

[ CARE AND USE MANUAL ]
d. Column Use Information
The eCord Intelligent Chip provides the user with specific column
information as well as column use data including: chemistry type,
column dimension, serial number and part number. The overall
column use information includes: total number of samples injected,
total number of injections as well as the maximum pressure and
temperature that the column has been exposed to. Additionally,
detailed column history includes the sample set start date, user name
and system name.
3. Always remember to:
Use an in-line filter unit (Part No. 205000343) or a
VanGuard Pre-Column.
Discourage bacterial growth by minimizing the use of
100% aqueous mobile phases where possible.
Discard and re-prepare aqueous mobile phase every
24-48 hours (if 100% aqueous mobile phase is required).
Add 5 – 10% organic modifier to aqueous buffer to
minimize bacterial growth (adjust gradient profile
as necessary).
Filter aqueous portions of mobile phase through a
0.2 µm filter.
Routinely maintain your water purification system to
ensure it is functioning properly.
Only use ultra-pure water (18 MegaOhm-cm) and highest
quality solvent possible.
Consider sample preparation (e.g., solid-phase extraction,
filtration, centrifugation, etc.) when possible.
4. Avoid when possible:
Figure 8: Column use information.
VI. ADDITIONAL INFORMATION
a. Tips for Maximizing XSelect CSH XP 2.5 µm Column Lifetime
1. To maximize XP 2.5 µm Column lifetime, pay close attention to:
Water quality (including water purification systems)
Solvent quality
Mobile-phase preparation, storage and age
Sample, buffer and mobile-phase solubilities
Sample quality and preparation.
2. When problems arise, systematically troubleshoot potential
causes one variable at a time in a systematic fashion.
100% aqueous mobile phases
HPLC-grade bottled water
‘Topping off’ your mobile phases
Using phosphate salt buffer in combination with
high acetonitrile concentrations (e.g., >70%) due
to precipitation.
5. Don’t assume the column is to blame:
Investigate cause of column failure
Monitor backpressure
Mobile-phase age, bacterial contamination,
mobile-phase precipitation...etc.
Peak splitting
Sample quality
Injection solvent strength.
6. Do not prepare excessive amounts of mobile-phase:
To reduce the chances of mobile-phase contamination
or degradation, prepare enough mobile phase to last for
3 – 4 days. Alternatively, store excess bulk quantities
in a refrigerated environment.
XSelect CSH XP 2.5 µm Columns 9
Page 10

[ CARE AND USE MANUAL ]
b. Troubleshooting Questions
1. Are you using 100% aqueous mobile phases?
2. What is the age of the mobile phase?
3. Is the mobile phase filtered through a 0.2 µm membrane?
4. Was the mobile phase prepared fresh or topped off?
7. Is bacterial growth a possibility (pH 7 phosphate buffer is
susceptible to bacterial growth within 24 hours)?
8. If a neat standard is prepared in the initial mobile-phase
conditions and injected, are the problems still observed?
9. If the sample is filtered/purified (i.e., SPE, filtration) is the
problem still observed?
5. Is the water source of adequate quality?
10. Has the quality of the samples changed over time?
6. When was the last time the water system was serviced
or was the bottle of water unopened?
c. Recommended Flow Rates and Anticipated Backpressures for Reversed-Phase XSelect CSH XP 2.5 µm Columns
XP 2.5 µm, 2.1 mm ID Columns (40 °C)
Linear Velocity 3 mm/sec 4 mm/sec 5 mm/sec 6 mm/sec
Column Dimension
2.1 x 30 mm 0.45 1760 0.6 2350 0.75 2940 0.9 3520
2.1 x 50 mm 0.45 2640 0.6 3520 0.75 4400 0.9 5280
2.1 x 75 mm 0.45 3740 0.6 4980 0.75 6230 0.9 7470
2.1 x 100 mm 0.45 4830 0.6 6440 0.75 8055 0.9 9670
Flow Rate
[mL/min]
Backpressure
[psi]
Flow Rate
[mL/min]
Backpressure
[psi]
Flow Rate
[mL/min]
Backpressure
[psi]
Flow Rate
[mL/min]
Backpressure
[psi]
XP 2.5 µm, 3.0 mm ID Columns (40 °C)
Linear Velocity 3 mm/sec 4 mm/sec 5 mm/sec 6 mm/sec
Column Dimension
3.0 x 30 mm 0.9 2180 1.17 2840 1.53 3710 1.8 4360
3.0 x 50 mm 0.9 3040 1.17 3950 1.53 5170 1.8 6080
3.0 x 75 mm 0.9 4120 1.17 5350 1.53 7000 1.8 8230
3.0 x 100 mm 0.9 5190 1.17 6750 1.53 8825 1.8 10380
Linear Velocity 3 mm/sec 4 mm/sec 5 mm/sec 6 mm/sec
Column Dimension
4.6 x 30 mm 2.1 3360 2.8 4480 3.5 5600 4.2 6720
4.6 x 50 mm 2.1 4210 2.8 5620 3.5 7020 4.2 8430
4.6 x 75 mm 2.1 5280 2.8 7040 3.5 8800 4.2 10560
4.6 x 100 mm 2.1 6350 2.8 8460 3.5 10580 4.2 12700
Flow Rate
[mL/min]
Flow Rate
[mL/min]
Backpressure
[psi]
Backpressure
[psi]
Flow Rate
[mL/min]
XP 2.5 µm, 4.6 mm ID Columns (40 °C)
Flow Rate
[mL/min]
Backpressure
[psi]
Backpressure
[psi]
Flow Rate
[mL/min]
Flow Rate
[mL/min]
Backpressure
[psi]
Backpressure
[psi]
Flow Rate
[mL/min]
Flow Rate
[mL/min]
Backpressure
Backpressure
[psi]
[psi]
XSelect CSH XP 2.5 µm Columns 10
Page 11

[ CARE AND USE MANUAL ]
Austria and European Export
(Central South Eastern Europe, CIS
and Middle East) 43 1 877 18 07
Australia 61 2 9933 1777
Belgium 32 2 726 1000
Brazil 55 11 5094-3788
Canada 1 800 252 4752 x2205
China 86 21 6879 5888
CIS/Russia +497 727 4490/290 9737
Czech Republic 420 2 617 1 1384
Denmark 45 46 59 8080
Finland 09 5659 6288
France 33 1 30 48 72 00
Germany 49 6196 400600
Hong Kong 852 2964 1800
The Netherlands 31 76 508 7200
Norway 47 6 384 60 50
Poland 48 22 6393000
Puerto Rico 1 787 747 8445
Singapore 86 21 6879 5888
Spain 34 936 009 300
Sweden 46 8 555 11 500
Switzerland 41 56 676 70 00
Taiwan 886 2 2543 1898
United Kingdom 44 208 238 6100
All other countries:
Waters Corporation U.S.A.
1 508 478 2000
1 800 252 4752
www.waters.com
Hungary 36 1 350 5086
India and India Subcontinent
91 80 2837 1900
Ireland 353 1 448 1500
Italy 39 02 265 0983
Japan 81 3 3471 7191
Korea 82 2 6300 4800
Mexico 52 55 5524 7636
©2012 Waters C orporation. Waters, AC QUIT Y UPLC, UPLC,
Oasis, and Sep- Pak are registered trademarks of Waters
Corporation. XSelect, eC ord, VanGuard, and The Science of
What's Possible are trademarks of Waters Corporation. All
other trademarks are the property of their respective owners.
March 2012 720004164EN Rev B VW-IH-PDF
Waters Corporation
34 Maple Street
Milford, MA 01757 U.S.A.
T: 1 508 478 2000
F: 1 508 872 1990
www.waters.com
XSelect CSH XP 2.5 µm Columns 11
 Loading...
Loading...